An Endophytic Fungal Isolate Paecilomyces lilacinus Produces Bioactive Secondary Metabolites and Promotes Growth of Solanum lycopersicum under Heavy Metal Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of Fungal Endophyte
2.2. Screening of Culture Filtrate for Secondary Metabolites
2.2.1. Determination of IAA and Total Phenolics in the Culture Filtrate
2.2.2. Determination of Total Flavonoids, Proline and Soluble Sugars
2.2.3. DPPH-Free Radical Scavenging Activity (2,2-Diphenylpicrylhydrazyl)
2.3. Antibacterial Activity of Fungal CF by Well Diffusion Method
2.4. Extraction of Genomic DNA and Molecular Identification
2.5. Gas Chromatography Mass Spectrometry Analysis of the CF and Identification of Phytocompounds
2.6. Heavy Metal Tolerance of Fungal Strains
2.7. Determination of Fungal Growth and Screening of Rice Seedlings
2.8. Pot Experiment for Growth Promotion and Stress Alleviation Activity
2.8.1. Estimation of Root Shoot Length and Fresh Dry Weight
2.8.2. Determination of Proline Content and RWC
2.8.3. Estimation of Total Sugar and Protein in Plant Samples
2.8.4. Estimation of Total Phenolics, Flavonoids and IAA Estimation
2.9. Statistical Analysis
3. Results
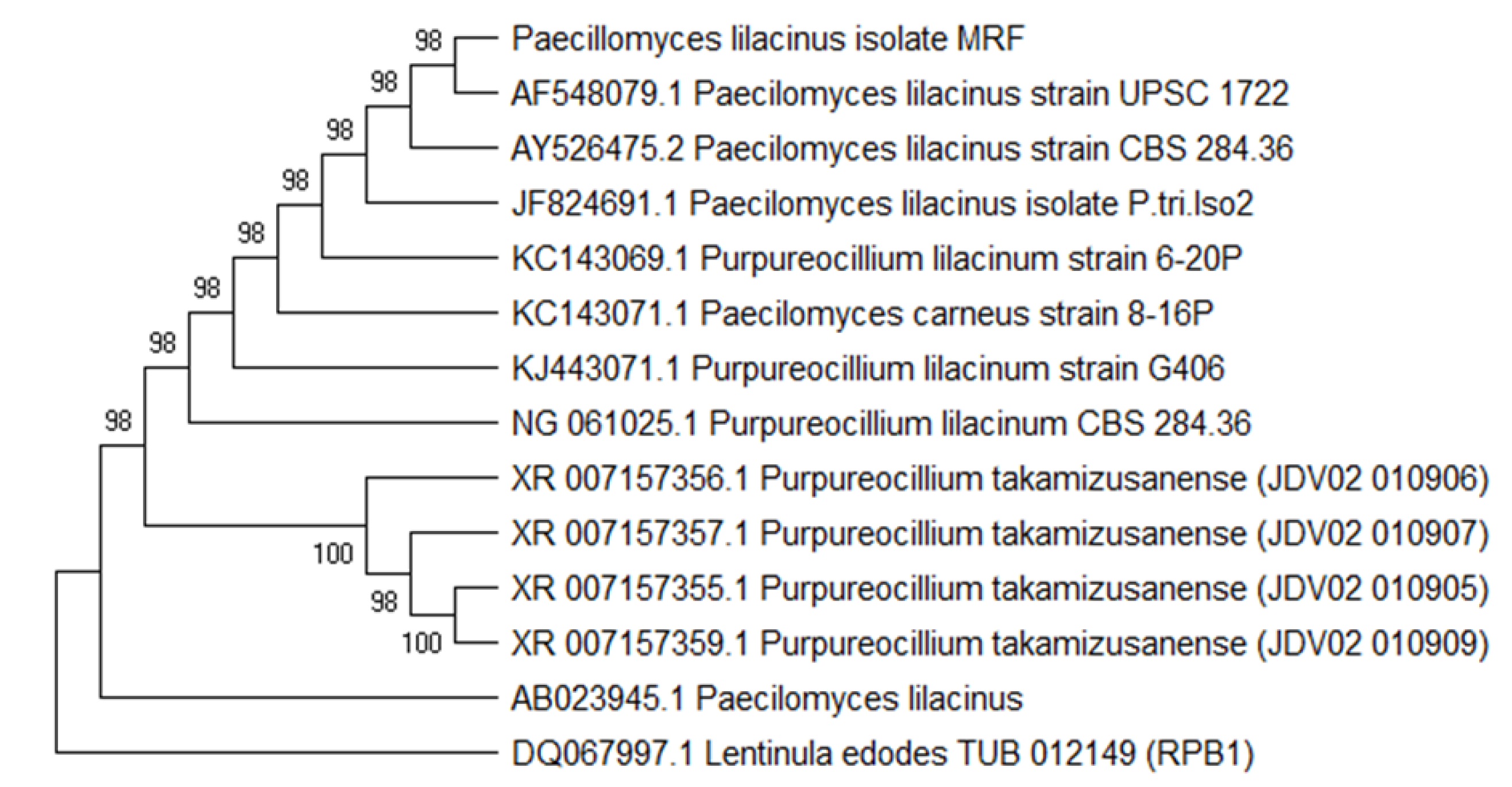
3.1. Isolation, Screening and Identification of Fungal Endophyte
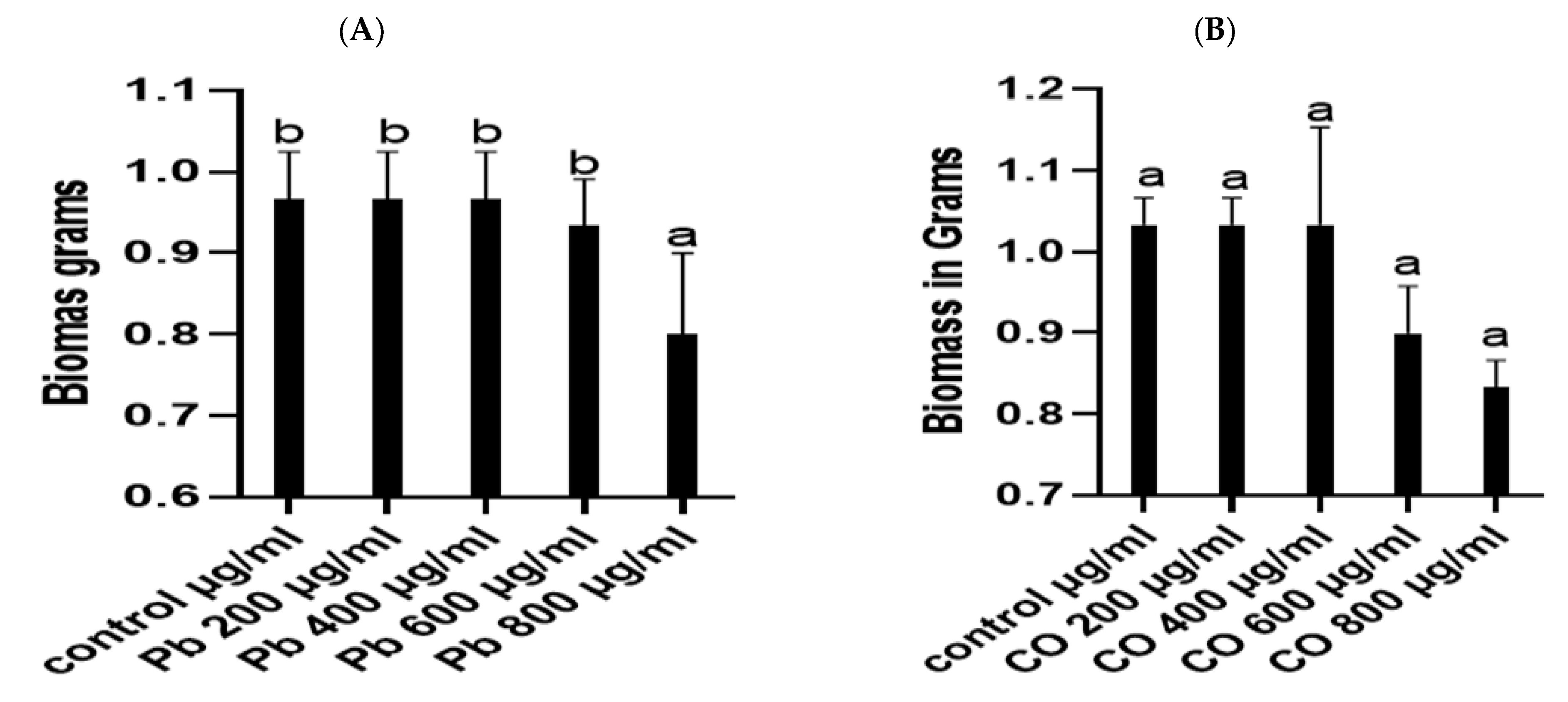
3.2. Heavy Metal Stress Tolerance of P. lilacinus
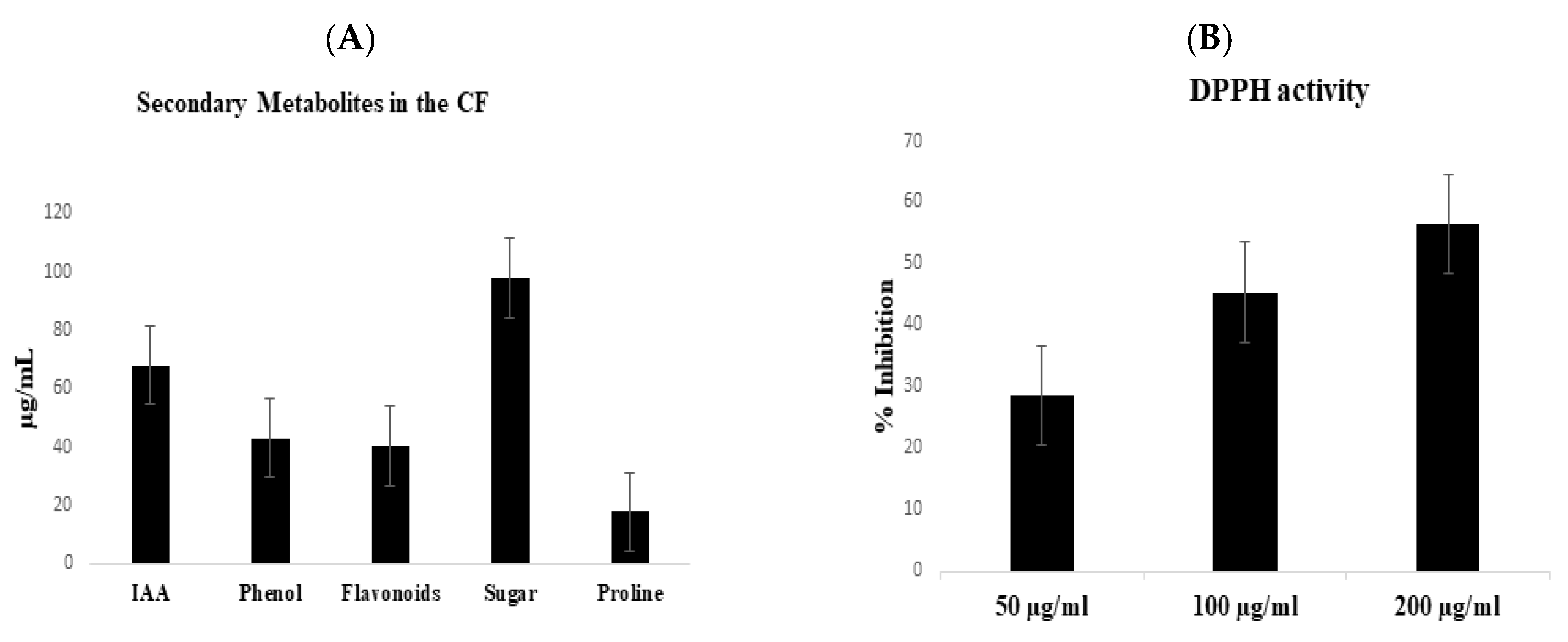
3.3. Antibacterial Activity and Chemical Analysis of the CF
3.4. Effect of P. lilacinus on the Growth of Solanum lycopersicum
3.5. P. lilacinus Enhanced Proline Content and RWC of S. lycopersicum
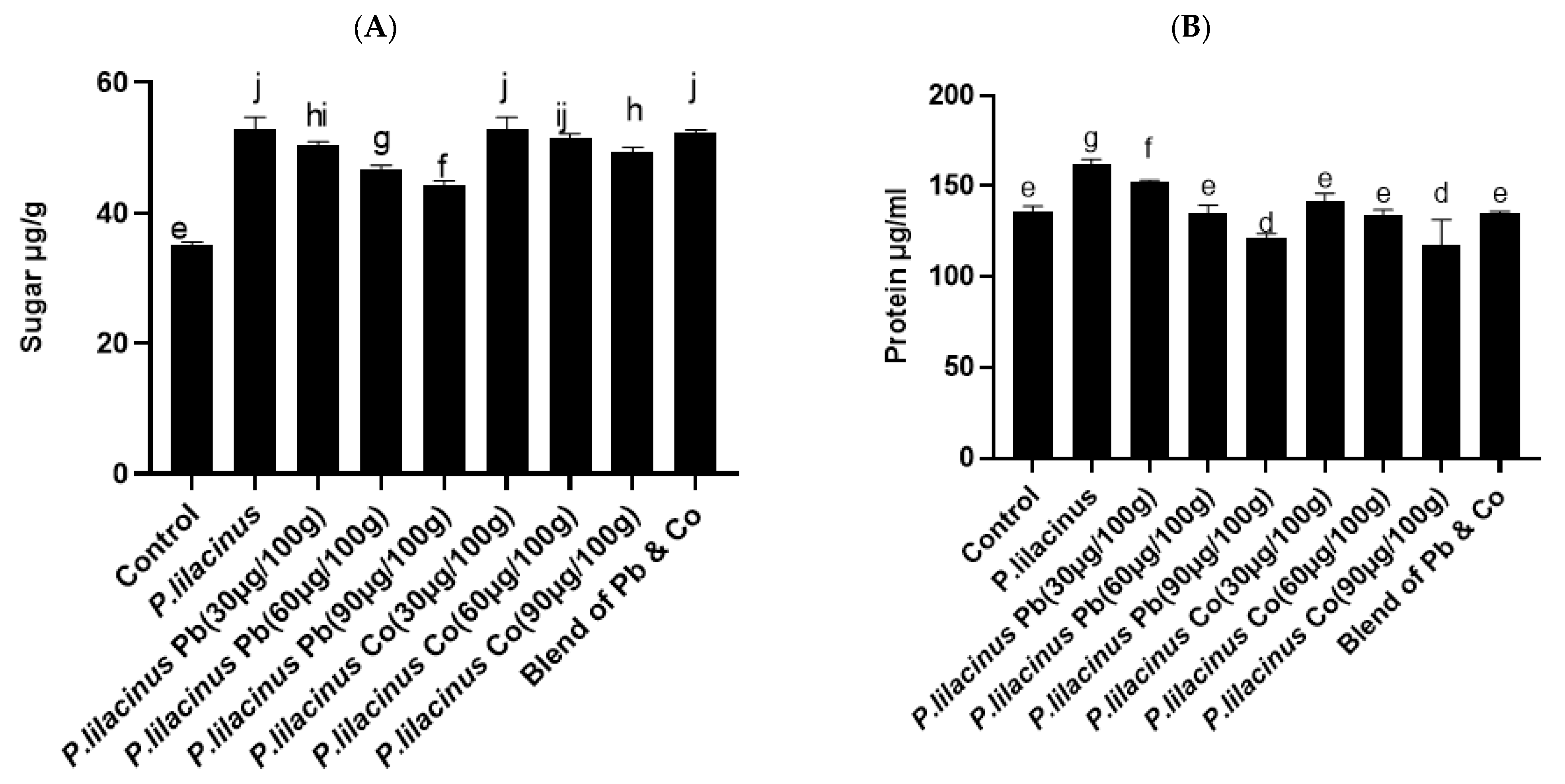
3.6. P. lilacinus Effect on Sugar and Protein Contents in S. lycopersicum
3.7. P. lilacinus Improved Effect on Phenols, Flavonoids and IAA Contents in S. lycopersicum
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, J.; Ren, L.; Zhou, Y.; Gao, J.; Luo, L.; Yang, Y.; Peng, Q.; Huang, H.; Chen, A. Diagnosis of soil contamination using microbiological indices: A review on heavy metal pollution. J. Environ. Manag. 2019, 242, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pogrzeba, M.; Rusinowski, S.; Krzyżak, J. Macroelements and heavy metals content in energy crops cultivated on contaminated soil under different fertilization—Case studies on autumn harvest. Environ. Sci. Pollut. Res. 2018, 25, 12096–12106. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination–A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, e0208150. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Prasad, M.N.V.; Freitas, H.; Fraenzle, S.; Wuenschmann, S.; Markert, B. Knowledge explosion in phytotechnologies for environmental solutions. Environ. Pollut. 2010, 158, 18–23. [Google Scholar] [CrossRef]
- Hussain, A.; Shah, M.; Hamayun, M.; Qadir, M.; Iqbal, A. Heavy metal tolerant endophytic fungi Aspergillus welwitschiae improves growth, ceasing metal uptake and strengthening antioxidant system in Glycine max L. Environ. Sci. Pollut. Res. 2021, 29, 15501–15515. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, Y.H.; Kim, K.W. The ars genotype characterization of arsenic-resistant bacteria from arsenic-contaminated gold–silver mines in the Republic of Korea. Appl. Microbiol. Biotechnol. 2008, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Liu, M.; Shi, X.; Zhao, Z. Dark septate endophyte (DSE) fungi isolated from metal polluted soils: Their taxonomic position, tolerance, and accumulation of heavy metals in vitro. J. Microbiol. 2008, 46, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Luo, S.; Zeng, G.; Wei, W.; Wan, Y.; Chen, L.; Xi, Q. Biosorption of cadmium by endophytic fungus (EF) Microsphaeropsis sp. LSE10 isolated from cadmium hyperaccumulator Solanum nigrum L. Bioresour. Technol. 2010, 101, 1668–1674. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Ali, S.; Kim, J.Y.; Kim, W.-C. Transgenic Arabidopsis expressing acdS gene of Pseudomonas veronii-KJ alleviate the adverse effects of salt and water-logging stress. Plant Breed. Biotechnol. 2018, 6, 221–232. [Google Scholar] [CrossRef]
- Hassen, A.; Saidi, N.; Cherif, M.; Boudabous, A. Resistance of environmental bacteria to heavy metals. Biores. Technol. 1998, 64, 7–15. [Google Scholar] [CrossRef]
- Njoku, K.L.; Akinyede, O.R.; Obidi, O.F. Microbial Remediation of Heavy Metals Contaminated Media by Bacillus megaterium and Rhizopus stolonifer. Sci. Afr. 2020, 10, e00545. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Yadav, A.N. Biodiversity and biotechnological applications of host-specific endophytic fungi for sustainable agriculture and allied sectors. Acta Sci. Microbiol. 2018, 1, 1–5. [Google Scholar]
- Jan, F.G.; Hamayun, M.; Hussain, A.; Jan, G.; Ali, S.; Khan, S.A.; Lee, I.J. Endophytic Candida membranifaciens from Euphorbia milii L. Alleviate Salt Stress Damages in Maize. Agronomy 2022, 12, 2263. [Google Scholar] [CrossRef]
- Khushdil, F.; Jan, F.G.; Jan, G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Bibi, N. Salt stress alleviation in Pennisetum glaucum through secondary metabolites modulation by Aspergillus terreus. Plant Physiol. Biochem. 2019, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.L.; Ali, L.; Rizvi, T.S.; Khan, S.A.; Hussain, J.; Al-Harrasi, A. Enzyme inhibitory metabolites from endophytic Penicillium citrinum isolated from Boswellia sacra. Arch. Microbiol. 2017, 199, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, S.A.; Hamayun, M.; Iqbal, A.; Khan, A.L.; Hussain, A.; Shah, M. Endophytic fungi from Caralluma acutangula can secrete plant growth promoting enzymes. Fresenius Environ. Bull. 2019, 28, 2688–2696. [Google Scholar]
- Rigobelo, E.C.; Baron, N.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2021, 13, 39–55. [Google Scholar] [CrossRef]
- Deepthi, V.C.; Sumathi, S.; Faisal, M.; Elyas, K.K. Isolation and identification of endophytic fungi with antimicrobial activities from the leaves of Elaeocarpus sphaericus (gaertn.) K. Schum and Myristicafragranshoutt. Intern. J. Pharmac. Sci. Res. 2018, 9, 2783–2791. [Google Scholar]
- Khan, S.A.; Hamayun, M.; Khan, A.L.; Lee, I.J.; Shinwari, Z.K.; Kim, J.G. Isolation of plant growth promoting endophytic fungi from dicots inhabiting coastal sand dunes of Korea. Pak. J. Bot. 2012, 44, 1453–1460. [Google Scholar]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Jayanthi, G.; Kamalraj, S.; Karthikeyan, K.; Muthumary, J. Antimicrobial and antioxidant activity of the endophytic fungus Phomopsis sp. GJJM07 isolated from Mesua ferrea. Int. J. Curr. Sci. 2011, 1, 85–90. [Google Scholar]
- Saravanan, S.; Parimelazhagan, T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. Fruit Pulp. Food Sci. Hum. Wellness 2014, 3, 56–64. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lakshmi, A.S.; Narasimha, G. Production of cellulases by fungal cultures isolated from forest litter soil. Ann. For. Res. 2012, 55, 85–92. [Google Scholar]
- Dhayanithy, G.; Subban, K.; Chelliah, J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Yadav, A.; Yadav, J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014, 7, S256–S261. [Google Scholar] [CrossRef] [PubMed]
- Taye, B.; Giday, M.; Animut, A.; Seid, J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac. J. Trop. Biomed. 2011, 1, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizating the principle of protein dyes binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gurupavithra, S.; Jayachitra, A. Isolation and identification of endophytic fungi from Ocimum sanctum and analyse its antioxidant properties. Int. J. Res. Pharmaceut. Biomed. Sci. 2013, 4, 1120–1125. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Husna, H.; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Asim, S.; Lee, I.-J. Stemphylium lycopersici and Stemphylium solani improved antioxidant system of soybean under chromate stress. Front. Microbiol. 2022, 4314. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, N.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Abbas, A.; Hussain, M.; Jiang, Y.; Zhou, P.; Li, Y.; et al. Interplay of higher plants with lithium pollution: Global trends, meta-analysis, and perspectives. Chemosphere 2022, 310, 136663. [Google Scholar] [CrossRef]
- Natasha; Shahid, M.; Saleem, M.; Anwar, H.; Khalid, S.; Tariq, T.Z.; Murtaza, B.; Amjad, M.; Naeem, M.A. A multivariate analysis of comparative effects of heavy metals on cellular biomarkers of phytoremediation using Brassica oleracea. Int. J. Phytoremediation 2020, 22, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Pirhadi, M.; Shariatifar, N.; Bahmani, M.; Manouchehri, A. Heavy metals in wheat grain and its impact on human health: A mini-review. J. Chem. Health Risks 2022, 12, 421–426. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 101865. [Google Scholar] [CrossRef]
- Cheplick, G.P. Symbiotic fungi and clonal plant physiology. New Phytol. 2004, 413–415. [Google Scholar] [CrossRef]
- Ali, S.; Park, S.-K.; Kim, W.-C. The pragmatic introduction and expression of microbial transgenes in plants. J. Microbiol. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Khan, A.R.; Ullah, I.; Waqas, M.; Shahzad, R.; Hong, S.J.; Park, G.S.; Jung, B.K.; Lee, I.J.; Shin, J.H. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J. Microbiol. Biotechnol. 2015, 31, 1461–1466. [Google Scholar] [CrossRef]
- Farhat, H.; Urooj, F.; Tariq, A.; Sultana, V.; Ansari, M.; Ahmad, V.U.; Ehteshamul-Haque, S. Evaluation of antimicrobial potential of endophytic fungi associated with healthy plants and characterization of compounds produced by endophytic Cephalosporium and Fusarium solani. Biocatal. Agric. Biotechnol. 2019, 18, 101043. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Zahmatkesh, A. Phytochemical screening, antioxidant potential, and cytotoxic effects of different extracts of red algae (Laurencia snyderiae) on HT29 cells. Res. Pharm. Sci. 2021, 16, 400. [Google Scholar] [CrossRef]
- Ibrahim, M.; Oyebanji, E.; Fowora, M.; Aiyeolemi, A.; Orabuchi, C.; Akinnawo, B.; Adekunle, A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Compl. Med. Ther. 2021, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.Z.; Wu, A.; Sallam, S.M. Comparative phytochemical constituents of Leucaena leucocephala (Lam.) leaves, fruits, stem barks, and wood branches grown in Egypt using GC-MS method coupled with multivariate approaches. BioResources 2019, 14, 996–1013. [Google Scholar] [CrossRef]
- Banaras, S.; Javaid, A.; Khan, I.H. Potential antifungal constituents of Sonchus oleraceous against Macrophomina phaseolina. Intern. J. Agric. Biol. 2020, 24, 1376–1382. [Google Scholar]
- Saravanan, S.; ParimelazhaganKalaivani, C.S.; Sathish, S.S.; Janakiraman, N.; Johnson, M. GC-MS studies on Andrographis paniculata (Burm. f.) Wall. Ex Nees—A medicinally important plant. Int. J. Med. Arom. Plants 2012, 2, 69–74. [Google Scholar]
- Pamila, U.A.; Karpagam, S. GC-MS Analysis of Ethanolic Extract of Alternanthera philoxeroides and Alternanthera bettzickiana from India. World Res. J. Biol. Biol. Sci. 2017, 2, 2–4. [Google Scholar]
- Khan, A.R.; Ullah, I.; Waqas, M.; Park, G.S.; Khan, A.L.; Hong, S.J.; Ullah, R.; Jung, B.K.; Park, C.E.; Ur-Rehman, S.; et al. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol. Environ. Saf. 2017, 136, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Lee, I.J. Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol. 2013, 13, 86. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-stimulated drought stress improves the attributes of in vitro growth, steviol glycosides production, and antioxidant activities in Stevia rebaudiana Bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef]
- Saradhi, P.P. Proline accumulation under heavy metal stress. J. Plant Physiol. 1991, 138, 554–558. [Google Scholar] [CrossRef]
- Rasheed, F.; Markgren, J.; Hedenqvist, M.; Johansson, E. Modeling to understand plant protein structure-function relationships—Implications for seed storage proteins. Molecules 2020, 25, 873. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Ban, Y.; Tang, M.; Chen, H.; Xu, Z.; Zhang, H.; Yang, Y. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS ONE 2012, 7, e47968. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J. Plant Growth Reg. 2013, 32, 216–232. [Google Scholar] [CrossRef]



| Treatments | IAA | Phenols | Flavonoids |
|---|---|---|---|
| Control | 62.45 ± 1.6 b | 54.34 ± 0.96 c | 40.31 ± 0.29 bc |
| Lead 200 µg/mL | 62.16 ± 3.3 b | 55.47 ± 1.13 c | 41.69 ± 1.24 c |
| Lead 400 µg/mL | 66.07 ± 1.8 b | 54.19 ± 1.64 c | 40.06 ± 0.42 bc |
| Lead 600 µg/mL | 58.87 ± 2.5 ab | 48.93 ± 0.89 b | 37.95 ± 0.58 b |
| Lead 800 µg/mL | 53.04 ± 0.5 a | 40.12 ± 0.74 b | 31.71 ± 0.69 a |
| Treatments | IAA, µg/mL | Phenols, µg/mL | Flavonoids, µg/mL |
|---|---|---|---|
| Control | 62.45 ± 1.3 b | 55.75 ± 1.5 b | 41.55 ± 0.60 c |
| Cobalt 200 µg/mL | 63.39 ± 2.4 b | 56.36 ± 1.2 b | 42.76 ± 0.87 c |
| Cobalt 400 µg/mL | 63.02 ± 5.5 b | 57.1 ± 1.3 b | 43.48 ± 0.38 c |
| Cobalt 600 µg/mL | 59.44 ± 3.0 ab | 52.16 ± 3.0 b | 37.95 ± 1 b |
| Cobalt 800 µg/mL | 51.21 ± 0.75 a | 36.94 ± 1.0 a | 32.54 ± 1.01 a |
| Bacterial Strain | Mean Zone of Inhibition (mm) |
|---|---|
| Salmonella typhi | 9.55 |
| Shigella sonnei | 10.36 |
| Compound | RT | Probability | Chemical Formula |
|---|---|---|---|
| Trichloromethane | 1.33 | 81.31 | CHCl3 |
| Phytol | 79.97 | 20.64 | C20H40O |
| 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 21.27 | 36.23 | C20H40O |
| 1,2-Benzenedicarboxylic acid, diisooctyl ester | 23.12 | 60.35 | C38H56O8 |
| Squalene | 24.45 | 59.22 | C₃₀H₅₀ |
| Cyclotrisiloxane, hexamethyl- | 25.67 | 57.87 | C6H18O5 |
| bis (2-ethylhexyl) ester | 23.45 | 68.49 | (CH2)8(COOC8H17)2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, M.; Jan, F.G.; Hamayun, M.; Jan, G.; Khan, S.A.; Rehman, G.; Ali, S.; Lee, I.-J. An Endophytic Fungal Isolate Paecilomyces lilacinus Produces Bioactive Secondary Metabolites and Promotes Growth of Solanum lycopersicum under Heavy Metal Stress. Agronomy 2023, 13, 883. https://doi.org/10.3390/agronomy13030883
Musa M, Jan FG, Hamayun M, Jan G, Khan SA, Rehman G, Ali S, Lee I-J. An Endophytic Fungal Isolate Paecilomyces lilacinus Produces Bioactive Secondary Metabolites and Promotes Growth of Solanum lycopersicum under Heavy Metal Stress. Agronomy. 2023; 13(3):883. https://doi.org/10.3390/agronomy13030883
Chicago/Turabian StyleMusa, Muhammad, Farzana Gul Jan, Muhammad Hamayun, Gul Jan, Sumera Afzal Khan, Gauhar Rehman, Sajid Ali, and In-Jung Lee. 2023. "An Endophytic Fungal Isolate Paecilomyces lilacinus Produces Bioactive Secondary Metabolites and Promotes Growth of Solanum lycopersicum under Heavy Metal Stress" Agronomy 13, no. 3: 883. https://doi.org/10.3390/agronomy13030883
APA StyleMusa, M., Jan, F. G., Hamayun, M., Jan, G., Khan, S. A., Rehman, G., Ali, S., & Lee, I.-J. (2023). An Endophytic Fungal Isolate Paecilomyces lilacinus Produces Bioactive Secondary Metabolites and Promotes Growth of Solanum lycopersicum under Heavy Metal Stress. Agronomy, 13(3), 883. https://doi.org/10.3390/agronomy13030883








