New Insights on Alternative Hosts of Xanthomonas vasicola pv. vasculorum, the Causal Agent of Bacterial Leaf Streak of Maize
Abstract
:1. Introduction
2. Materials and Methods
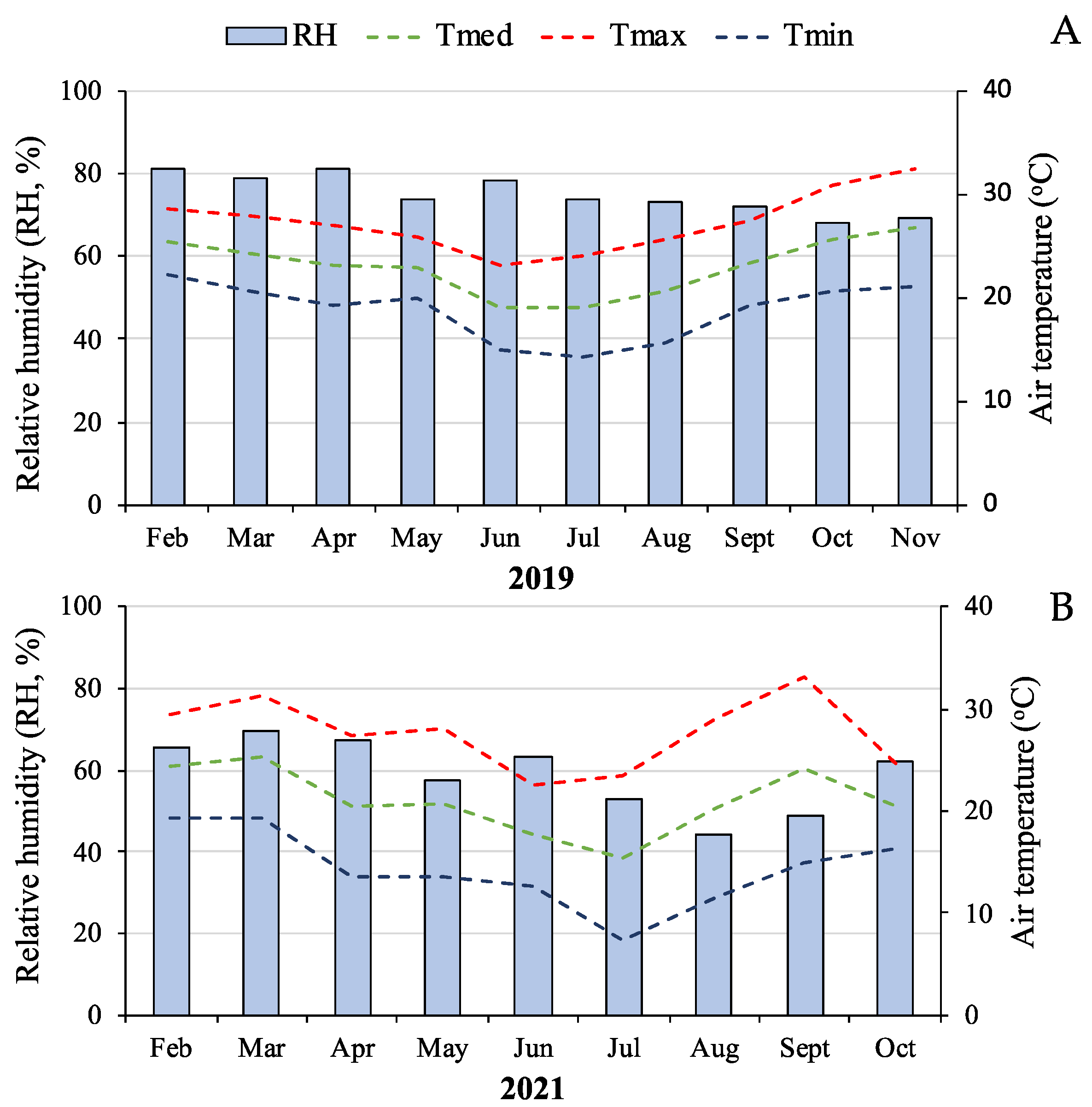
2.1. Origin and Plant Cultivation
2.2. Isolates of Xvv and Plant Inoculation
2.3. Pathogenicity and Epiphytic Colonization of Xvv in Multiple Plant Species
2.4. PCR Assessment for Xvv
2.5. Scanning Electron Microscopy (SEM) Analysis of Plants Colonized with Xvv
3. Results
3.1. Pathogenicity and Epiphytic Colonization of Different Plant Species by Xvv
3.2. Scanning Electron Microscopy (SEM) Analysis of Plants Colonized by Xvv
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leite, R.P., Jr.; Custódio, A.A.P.; Madalosso, T.; Robaina, R.R.; Duin, I.M.; Sugahara, V.H. First report of the occurrence of bacterial leaf streak of corn caused by Xanthomonas vasicola pv. vasculorum in Brazil. Plant Dis. 2019, 103, 145. [Google Scholar] [CrossRef]
- Broders, K. Status of bacterial leaf streak of corn in the United States. In Proceedings of the Integrated Crop Management Conference, Ames, IA, USA, 29–30 November 2017; pp. 111–115. [Google Scholar]
- Damicone, J.; Cevallos, F.; Olson, J. First report of bacterial leaf streak of corn caused by Xanthomonas vasicola pv. vasculorum in Oklahoma. Plant Dis. 2018, 102, 437. [Google Scholar] [CrossRef]
- Jamann, T.M.; Plewa, D.; Mideros, S.X.; Bissonnette, S. First report of bacterial leaf streak of corn caused by Xanthomonas vasicola pv. vasculorum in Illinois. Plant Dis. 2019, 103, 1018. [Google Scholar] [CrossRef]
- Korus, K.A.; Lang, J.M.; Adesemoye, A.O.; Block, C.C.; Pal, N.; Leach, J.E.; Jackson-Ziems, T.A. First report of Xanthomonas vasicola causing bacterial leaf streak on corn in the United States. Plant Dis. 2017, 101, 1030. [Google Scholar] [CrossRef]
- Plazas, M.C.; de Rossi, R.L.; Brücher, E.; Guerra, F.A.; Vilaro, M.L.; Guerra, G.D.; Wu, G.; Ortiz-Castro, M.C.; Broders, K. First report of Xanthomonas vasicola pv. vasculorum causing bacteria leaf streak of maize (Zea mays) in Argentina. Plant Dis. 2018, 102, 1026. [Google Scholar] [CrossRef]
- Leite, R.P., Jr.; Custódio, A.A.P.; Madalosso, T.; Robaina, R.R.; Duin, I.M.; Sugahara, V.H. Estria Bacteriana do Milho no Paraná; IAPAR: Londrina, Brazil, 2018. [Google Scholar]
- North, D.S. The Gumming Disease of the Sugar-Cane, its Dissemination and Control; The Colonial Sugar Refining Co. Ltd.: Sydney, Australia, 1935; p. 149. [Google Scholar]
- Karamura, G.; Smith, J.; Studholme, D.; Kubiriba, J.; Karamura, E. Comparative pathogenicity studies of the Xanthomonas vasicola species on maize, sugarcane and banana. Afr. J. Plant Sci. 2015, 9, 385–400. [Google Scholar]
- Coutinho, T.A.; Van der Westhuizen, L.; Roux, J.; McFarlane, S.A.; Venter, S.N. Significant host jump of Xanthomonas vasicola from sugarcane to a Eucalyptus grandis clone in South Africa. Plant Pathol. 2015, 64, 576–581. [Google Scholar] [CrossRef]
- Lang, J.M.; Du Charme, E.; Ibarra Caballero, J.; Luna, E.; Hartman, T.; Ortiz-Castro, M.; Korus, K.J.; Rascoe, J.; Jackson-Ziems, T.A.; Broders, K.; et al. Detection and characterization of Xanthomonas vasicola pv. vasculorum (Cobb 1894) comb. nov. causing bacterial leaf streak of corn in the United States. Phytopathology 2017, 107, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- Rott, P.; Girard, J.C. Sugarcane producing countries/locations and their diseases. In A Guide to Sugarcane Diseases; Rott, P., Bailey, R.A., Comstock, J.C., Croft, B.J., Saumtally, A.S., Eds.; La Librairie du Cirad: Montpellier, France, 2000; pp. 323–336. [Google Scholar]
- Ricaud, C.; Autrey, L.J.C. Gumming disease. In Diseases of Sugarcane: Major Diseases; Elsevier: Amsterdam, The Netherlands, 1989; pp. 21–38. [Google Scholar]
- Hartman, T.; Tharnish, B.; Harbour, J.; Yuen, G.Y.; Jackson-Ziems, T.A. Alternative hosts in the families Poaceae and Cyperaceae for Xanthomonas vasicola pv. vasculorum, causal agent of bacterial leaf streak of corn. Phytopathology 2020, 110, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.L.; Coyne, D.P. Survival mechanisms of phytopathogenic bacteria. Annu. Rev. Phytopathol. 1974, 12, 199–221. [Google Scholar] [CrossRef]
- Schuster, M.L.; Coyne, D.P. Survival of plant parasitic bacteria of plants grown in tropics with emphasis on beans (Phaseolus vulgaris). Fitopatol. Bras. 1977, 2, 117–130. [Google Scholar]
- Robaina, R.R.; Longhi, T.V.; Zeffa, D.M.; Gonçalves, L.S.A.; Leite, R.P., Jr. Development of a protocol and a diagrammatic scale for quantification of bacterial leaf streak disease on young plants of maize. Plant Dis. 2020, 104, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.P., Jr. The Relationship between Pathogenic and Nonpathogenic Strains of Pseudomonas syringae and Bacterial Brown Spot Disease in Beans. Master’s Thesis, University of Wisconsin, Madison, WI, USA, 1986; p. 107. [Google Scholar]
- Gonçalves, R.M.; Schipanski, C.A.; Koguishi, L.; Soman, J.M.; Sakate, R.K.; Júnior, T.A.; Maringoni, A.C. Alternative hosts of Curtobacterium flaccumfaciens pv. flaccumfaciens, causal agent of bean bacterial wilt. Eur.J. Plant Pathol. 2017, 148, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, E.W.; Leite, B. Curso Introdutório de Microscopia Eletrônica de Varredura; NAP/MEPA ESALQ: Piracicaba, Brazil, 1999; p. 48. [Google Scholar]
- Wasukira, A.; Coulter, M.; Al-Sowayeh, N.; Thwaites, R.; Paszkiewicz, K.; Kubiriba, J.; Smith, J.; Grant, M.; Studholme, D.J. Genome sequencing of Xanthomonas vasicola pathovar vasculorum reveals variation in plasmids and genes encoding lipopolysaccharide synthesis, type-IV pilus and type-III secretion effectors. Pathogens 2014, 3, 211–237. [Google Scholar] [CrossRef]
- Studholme, D.J.; Wicker, E.; Abrare, S.M.; Aspin, A.; Bogdanove, A.; Broders, K.; Dubrow, Z.; Grant, M.; Jones, J.B.; Karamura, G.; et al. Transfer of Xanthomonas campestris pv. arecae and X. campestris pv. musacearum to X. vasicola (Vauterin) as X. vasicola pv. arecae comb. nov. and X. vasicola pv. musacearum comb. nov. and description of X. vasicola pv. vasculorum pv. nov. Phytopathology 2020, 110, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Peros, J.P. Variability in colony type and pathogenicity of the causal agent of sugarcane gumming Xanthomonas campestris pv. vasculorum (Cobb) Dye. J. Plant Dis. Prot. 1988, 95, 541–545. Available online: https://www.jstor.org/stable/43386650 (accessed on 3 November 2022).
- Perez-Quintero, A.L.; Ortiz-Castro, M.; Lang, J.M.; Rieux, A.; Wu, G.; Liu, S.; Chapman, T.A.; Chang, C.; Ziegle, J.; Peng, Z.; et al. Genomic acquisitions in emerging populations of Xanthomonas vasicola pv. vasculorum infecting corn in the United States and Argentina. Phytopathology 2020, 110, 1161–1173. [Google Scholar] [CrossRef]
- Ceccon, G. Consórcio Milho-Braquiária; Embrapa Agropecuária Oeste: Dourados, Brazil, 2013; Available online: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/982597/1/LVCONSORCIOMB.pdf (accessed on 11 September 2022).
- Lorenzi, H. Plantas Daninhas do Brasil: Terrestres, Aquáticas, Parasitas e Tóxicas; Plantarum: Nova Odessa, Brazil, 2008; p. 640. [Google Scholar]
- Adegas, F.S.; Voll, E.R.; Gazziero, D.L.P. Manejo de plantas daninhas em milho safrinha em cultivo solteiro ou consorciado à braquiária ruziziensis. Pesqui. Agropecu. Bras. 2011, 46, 1226–1233. [Google Scholar] [CrossRef] [Green Version]
- Conab. National Supply Company: Agricultural Information System. 2023. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 5 March 2023).
- Gonçalves Netto, A.; Cordeiro, E.M.; Nicolai, M.; de Carvalho, S.J.; Ovejero, R.F.L.; Brunharo, C.A.; Zucchi, M.I.; Christoffoleti, P.J. Population genomics of Digitaria insularis from soybean areas in Brazil. Pest Manag. Sci. 2021, 77, 5375–5381. [Google Scholar] [CrossRef]
- Mercier, J.; Lindow, S.E. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 2000, 66, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Kinkel, L.L.; Wilson, M.; Lindow, S.E. Utility of microcosm studies for predicting phylloplane bacterium population sizes in the field. Appl. Environ. Microbiol. 1996, 62, 3413–3423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.S.; Upper, C.D. Population biology and epidemiology of Pseudomonas syringae. Annu. Rev. Phytopathol. 1990, 28, 155–177. [Google Scholar] [CrossRef]
- Kinkel, L.L.; Lindow, S.E. Invasion and exclusion among coexisting Pseudomonas syringae strains on leaves. Appl. Environ. Microbiol. 1993, 59, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Kinkel, L.L.; Wilson, M.; Lindow, S.E. Effect of sampling scale on the assessment of epiphytic bacterial populations. Microb. Ecol. 1995, 29, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Kinkel, L.L. Microbial population dynamics on leaves. Annu. Rev. Phytopathol. 1997, 35, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.S.; Upper, C.D. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 2000, 64, 624–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveau, J.H. A brief from the leaf: Latest research to inform our understanding of the phyllosphere microbiome. Curr. Opin. Microbiol. 2019, 49, 41–49. [Google Scholar] [CrossRef]
- O’brien, R.D.; Lindow, S.E. Effect of plant species and environmental conditions on epiphytic population sizes of Pseudomonas syringae and other bacteria. Phytopathology 1989, 79, 619–627. [Google Scholar] [CrossRef]
- Toussaint, V.; Benoit, D.L.; Carisse, O. Potential of weed species to serve as a reservoir for Xanthomonas campestris pv. vitians, the causal agent of bacterial leaf spot of lettuce. Crop Prot. 2012, 41, 64–70. [Google Scholar] [CrossRef]
- Denny, T.P. Autoregulator-dependent control of extracellular polysaccharide production in phytopathogenic bacteria. Eur. J. Plant Pathol. 1999, 105, 417–430. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [Green Version]
- Dow, J.M.; Crossman, L.; Findlay, K.; He, Y.Q.; Feng, J.X.; Tang, J.L. Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10995–11000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, N.E.; Cleary, J.M.; Ielpi, L. Genetics and biochemistry of xanthan gum production by Xanthomonas campestris. In Food Biotechnology Microorganism; VCH Publishers Inc.: New York, NY, USA, 1995; pp. 495–514. [Google Scholar]
- Braun, E.J. Colonization of resistant and susceptible maize plants by Erwinia stewartii strains differing in exopolysaccharide production. Physiol. Mol. Plant Pathol. 1990, 36, 363–379. [Google Scholar] [CrossRef]
- Saile, E.; McGarvey, J.A.; Schell, M.A.; Denny, T.P. Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology 1997, 87, 1264–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, M.H.; Torres, P.S.; Oirdi, M.E.; Rigano, L.A.; Gonzalez-Lamothe, R.; Marano, M.R.; Castagnaro, A.P.; Dankert, M.A.; Bouarab, K.; Vojnov, A.A. Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiol. 2006, 141, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Dunger, G.; Relling, V.M.; Tondo, M.L.; Barreras, M.; Ielpi, L.; Orellano, E.G.; Ottado, J. Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch. Microbiol. 2007, 188, 127–135. [Google Scholar] [CrossRef]





| Botanical Group | Scientific Name | Common Name | Cultivar | S1 | EC2 |
|---|---|---|---|---|---|
| Monocotyledonous | |||||
| Poaceae | Avena sativa | Oat | IPR Afrodite | + | N/A |
| A. sativa | Oat | IPR Esmeralda | + | N/A | |
| A. strigosa | Black oat | IPR 161 | + | N/A | |
| Hordeum vulgare | Barley | BRS Cauê | + | N/A | |
| Oryza sativa | Rice | IPR 117 | + | N/A | |
| Pennisetum glaucum | Millet | IPR St. Tereza do Oeste | − | + | |
| Saccharum officinarum | Sugar cane | Not determined | − | + | |
| Secale cereale | Rye | IPR 89 | − | + | |
| Sorghum bicolor | Sorghum | BRS 658 | − | + | |
| ×Triticosecale | Triticale | IPR Caiapó | − | + | |
| Triticum aestivum | Wheat | IPR Potyporã | − | + | |
| Zea mays3 | White maize | IPR 127 | + | N/A | |
| Z. mays3 | Common maize | IPR 164 | + | N/A | |
| Dicotyledonous | |||||
| Asteraceae | Helianthus annuus | Sunflower | BRS 417 | − | + |
| Glycine max | Soybean | Potência | − | + | |
| Lupinus albus | White lupin | Not determined | − | + | |
| L. angustifolius | Blue lupin | IPR 124 | − | + | |
| Fabaceae | Mucuna aterrima cv. Mucuna preta | Black velvet-bean | Not determined | − | + |
| M. pruriens | Bengal velvet-bean | Not determined | − | + | |
| Phaseolus vulgaris | Bean | Carioca | − | + | |
| Malvaceae | Gossypium hirsutum | Cotton | FMT 701 | − | + |
| Polygonaceae | Fagopyrum esculentum Moench | Buckwheat | IPR 91 | − | + |
| Botanical Group | Scientific Name | Common Name | Cultivar | S1 | EC2 |
|---|---|---|---|---|---|
| Monocotyledonous | |||||
| Poaceae | Brachiariabrizantha | Brizantha bread grass | Not determined | + | N/A |
| B. brizantha | Marandu bread grass | Marandu | + | N/A | |
| B. brizantha | Piatã bread grass | BRS Piatã | − | + | |
| B. brizantha | MG-5 Vitória bread grass | MG-5 Vitória | − | + | |
| B. decumbens | Basilisk bread grass | Basilisk | − | + | |
| B. decumbens | Decumbens bread grass | Not determined | − | + | |
| B. humidicola | Humidicola bread grass | Not determined | − | + | |
| B. ruzisiensis | Ruzisiensis bread grass | Not determined | − | + | |
| Panicum maximum | MG-12 Paredão Guinea grass | MG12 Paredão | − | + | |
| P. maximum | Tanzania Guinea grass | Tanzânia-1 | − | + | |
| Zea mays3 | White maize | IPR 127 | + | N/A | |
| Z. mays3 | Common maize | IPR 164 | + | N/A | |
| Dicotyledonous | |||||
| Fabaceae | Cajanus cajan | Pigeon pea | IAPAR 43 | − | + |
| Crotalaria spectabilis | Showy rattlepod | Not determined | − | + | |
| Botanical Group | Scientific Name | Common Name | S1 | EC2 |
|---|---|---|---|---|
| Monocotyledonous | ||||
| Poaceae | Brachiaria plantaginea | Plantain signalgrass | − | + |
| Cenchrus echinatus | Southern sandbur | − | + | |
| Chloris gayana | Rhodes grass | − | + | |
| C. polydactyla | Finger grass | − | − | |
| Digitaria horizontalis | Jamaican crabgrass | + | N/A | |
| D. insularis | Sourgrass | + | N/A | |
| Echinochloa colonum | Jungle rice | + | N/A | |
| Eleusine indica | Crowfootgrass | + | N/A | |
| Lolium multiflorum | Annual ryegrass | − | + | |
| Panicum maximum | Guinea grass | − | + | |
| Pennisetum purpureum | Napier grass | − | + | |
| Rhynchelytrum repens | Natal grass | − | + | |
| Sorghum arundinaceum | Common wild sorghum | + | N/A | |
| Zea mays3 | White maize | + | N/A | |
| Z. mays3 | Common maize | + | N/A | |
| Dicotyledonous | ||||
| Amaranthaceae | Amaranthus hybridus | Smooth pigweed | − | + |
| A. viridis | Slender amaranth | − | + | |
| Asteraceae | Acanthospermum hispidum | Bristly starbur | − | + |
| Bidens pilosa | Beggar’s tick | − | + | |
| Conyza spp. | Hairy fleabane | − | + | |
| Galinsoga parviflora | Gallant soldier | − | − | |
| Tridax procumbens | Coatbuttons | − | + | |
| Brassicaceae | Raphanus raphanistrum | Wild radish | − | + |
| R. sativus | Radish | − | + | |
| Commelinaceae | Commelina benghalensis | Wandering jew | − | + |
| Euphorbiaceae | Euphorbia heterophylla | Wild poinsettia | − | + |
| Malvaceae | Sidarhom bifolia | Arrow-leaf sida | − | + |
| Rubiaceae | Richardia brasiliensis | Tropical Mexican clover | − | − |
| Botanical Group | Scientific Name | Common Name | Xvv | |
|---|---|---|---|---|
| Maximum Recovery Period (DAI) 1 | CFU (Log10·g−1) 2 | |||
| Cultivated plants and cover crops | ||||
| Poaceae | Pennisetum glaucum | Millet | 30 | 5.46 |
| Saccharum officinarum | Sugarcane | 21 | 4.40 | |
| Secale cereale | Rye | 30 | 6.36 | |
| Sorghum bicolor | Sorghum | 21 | 5.18 | |
| ×Triticosecale | Triticale | 21 | 5.40 | |
| Triticum aestivum | Wheat | 30 | 6.71 | |
| Asteraceae | Glycine max | Soybean | 21 | 3.30 |
| Helianthus annuus | Sunflower | 21 | 6.02 | |
| Lupinus albus | White lupin | 21 | 7.00 | |
| L. angustifolius | Blue lupin | 21 | 4.00 | |
| Fabaceae | Mucuna pruriens | Bengal velvet-bean | 21 | 4.30 |
| M. aterrima cv. Mucuna preta | Black velvet-bean | 21 | 6.01 | |
| Phaseolus vulgaris | Bean | 30 | 5.00 | |
| Malvaceae | Gossypium hirsutum | Cotton | 21 | 5.04 |
| Polygonaceae | Fagopyrum esculentum Moench | Buckwheat | 21 | 5.93 |
| Forages and grasses | ||||
| Poaceae | Brachiaria brizantha | Piatã bread grass | 21 | 3.48 |
| B. brizantha | MG-5 Vitória bread grass | 21 | 3.65 | |
| B. decumbens | Decumbensbread grass | 21 | 4.74 | |
| B. decumbens | Basilisk bread grass | 30 | 2.70 | |
| B. humidicola | Humidicola bread grass | 21 | 4.00 | |
| B. ruzisiensis | Ruzisiensis bread grass | 21 | 5.00 | |
| Panicum maximum | Tanzânia Guinea grass | 15 | 4.00 | |
| P. maximum | MG-12 Paredã Guinea grass | 21 | 4.81 | |
| Fabaceae | Cajanus cajan | Pigeon pea | 21 | 4.18 |
| Crotalaria spectabilis | Showy rattlepod | 30 | 5.23 | |
| Weeds | ||||
| Poaceae | B. plantaginea | Plantain signalgrass | 21 | 5.95 |
| Cenchrus echinatus | Southern sandbur | 30 | 5.00 | |
| Chloris gayana | Rhodes grass | 30 | 6.27 | |
| Lolium multiflorum | Annual ryegrass | 21 | 7.00 | |
| P. maximum | Guinea grass | 30 | 5.18 | |
| Pennisetum purpureum | Napier grass | 30 | 5.92 | |
| Rhynchelytrum repens | Natal grass | 30 | 6.40 | |
| Amaranthaceae | Amaranthus hybridus | Smooth pigweed | 30 | 3.85 |
| A. viridis | Slender amaranth | 30 | 3.98 | |
| Asteraceae | Acanthospermum hispidum | Bristly starbur | 30 | 5.18 |
| Bidens pilosa | Beggar’s tick | 21 | 5.00 | |
| Conyza spp. | Hairy fleabane | 15 | 4.00 | |
| Tridax procumbens | Coatbuttons | 30 | 3.70 | |
| Brassicaceae | Raphanus raphanistrum | Wild radish | 30 | 5.48 |
| Raphanus sativus | Radish | 15 | 4.28 | |
| Commelinaceae | Commelina benghalensis | Wandering jew | 30 | 5.18 |
| Euphorbiaceae | Euphorbia heterophylla | Wild poinsettia | 30 | 3.93 |
| Malvaceae | Sidarhombifolia | Arrow-leaf sida | 30 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longhi, T.V.; Robaina, R.R.; de Carvalho, D.U.; de Oliveira, A.G.; Leite Junior, R.P.; Balbi-Peña, M.I. New Insights on Alternative Hosts of Xanthomonas vasicola pv. vasculorum, the Causal Agent of Bacterial Leaf Streak of Maize. Agronomy 2023, 13, 1073. https://doi.org/10.3390/agronomy13041073
Longhi TV, Robaina RR, de Carvalho DU, de Oliveira AG, Leite Junior RP, Balbi-Peña MI. New Insights on Alternative Hosts of Xanthomonas vasicola pv. vasculorum, the Causal Agent of Bacterial Leaf Streak of Maize. Agronomy. 2023; 13(4):1073. https://doi.org/10.3390/agronomy13041073
Chicago/Turabian StyleLonghi, Talita Vigo, Renata Rodrigues Robaina, Deived Uilian de Carvalho, Admilton Gonçalves de Oliveira, Rui Pereira Leite Junior, and Maria Isabel Balbi-Peña. 2023. "New Insights on Alternative Hosts of Xanthomonas vasicola pv. vasculorum, the Causal Agent of Bacterial Leaf Streak of Maize" Agronomy 13, no. 4: 1073. https://doi.org/10.3390/agronomy13041073
APA StyleLonghi, T. V., Robaina, R. R., de Carvalho, D. U., de Oliveira, A. G., Leite Junior, R. P., & Balbi-Peña, M. I. (2023). New Insights on Alternative Hosts of Xanthomonas vasicola pv. vasculorum, the Causal Agent of Bacterial Leaf Streak of Maize. Agronomy, 13(4), 1073. https://doi.org/10.3390/agronomy13041073









