The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Sites and Design
2.2. Muskmelon Wilt Investigation and Sample Collection
2.3. DNA Extraction and High-Throughput Sequencing
2.4. Sequence Processing and Data Analysis
2.5. Soil Physicochemical Properties’ Measurements
2.6. Soil Metabolomics Analysis
2.7. Statistical Analysis of Metabolome Data
3. Results
3.1. Muskmelon Wilt Disease Index and the T. viride T23 Control Effect on Muskmelon Wilt
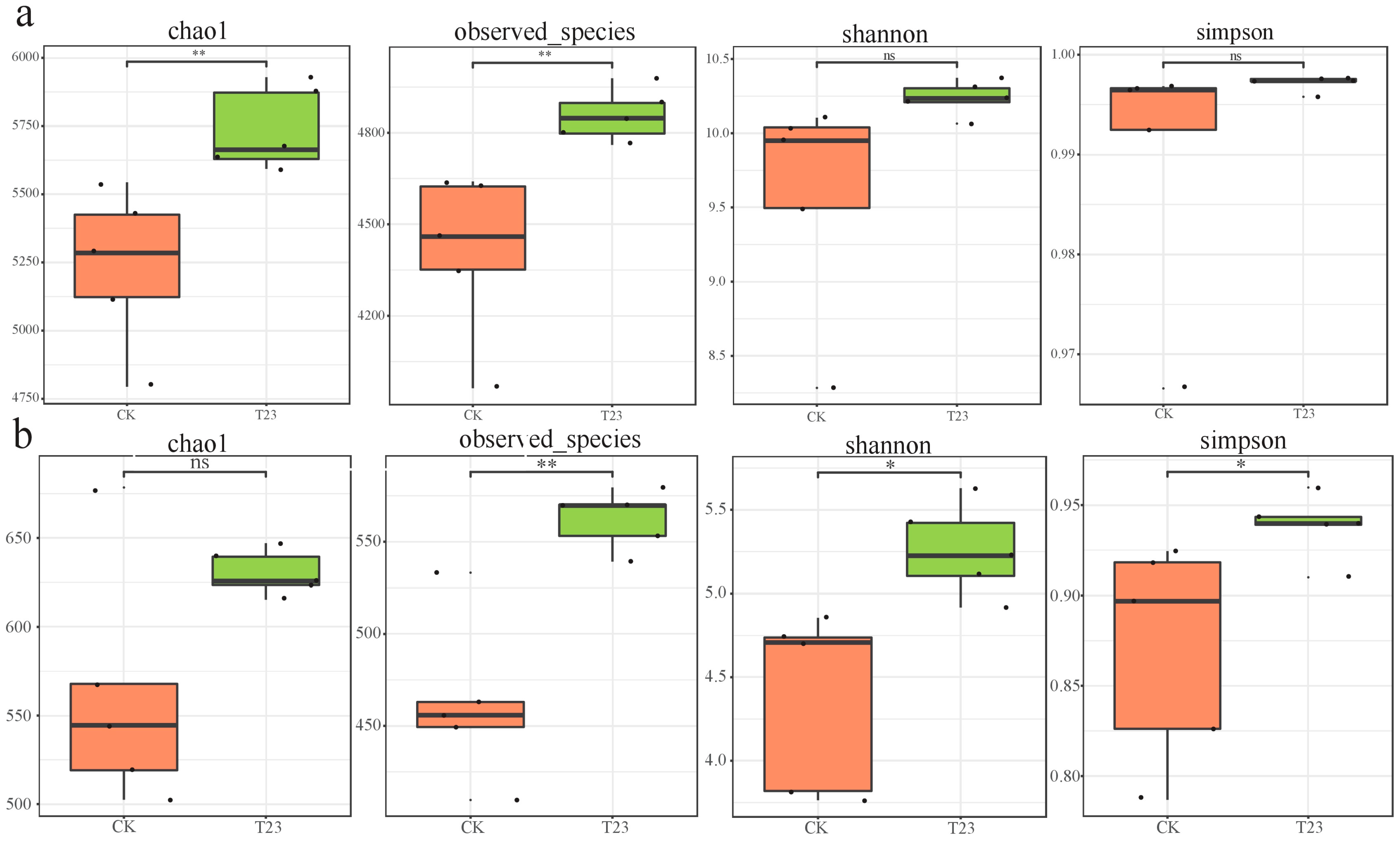
3.2. Effect of T. viride T23 on Microbial Diversity in the Muskmelon Rhizosphere
3.2.1. The Alpha Diversity of the Microbial Communities
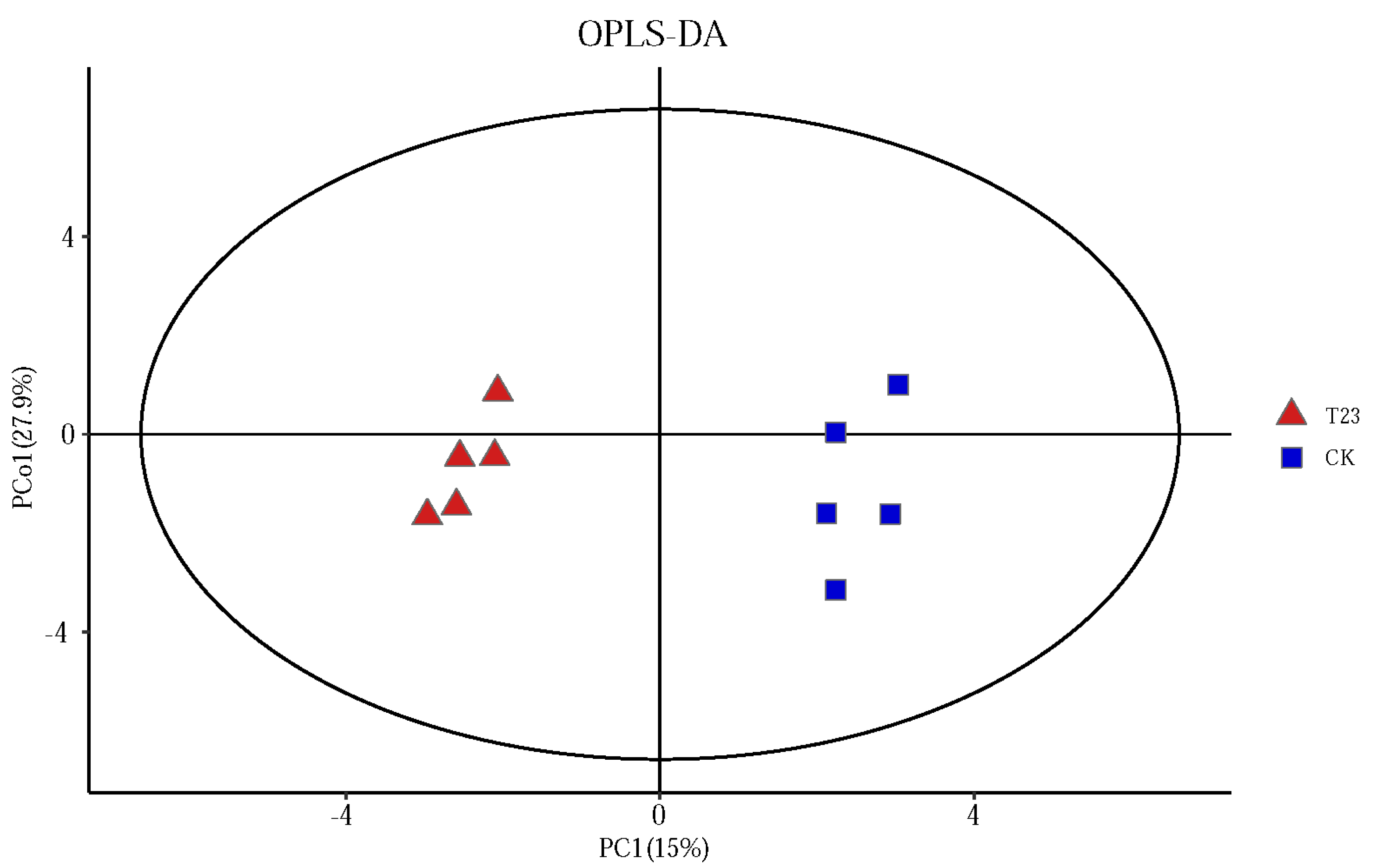
3.2.2. Beta Diversity of Microbial Communities
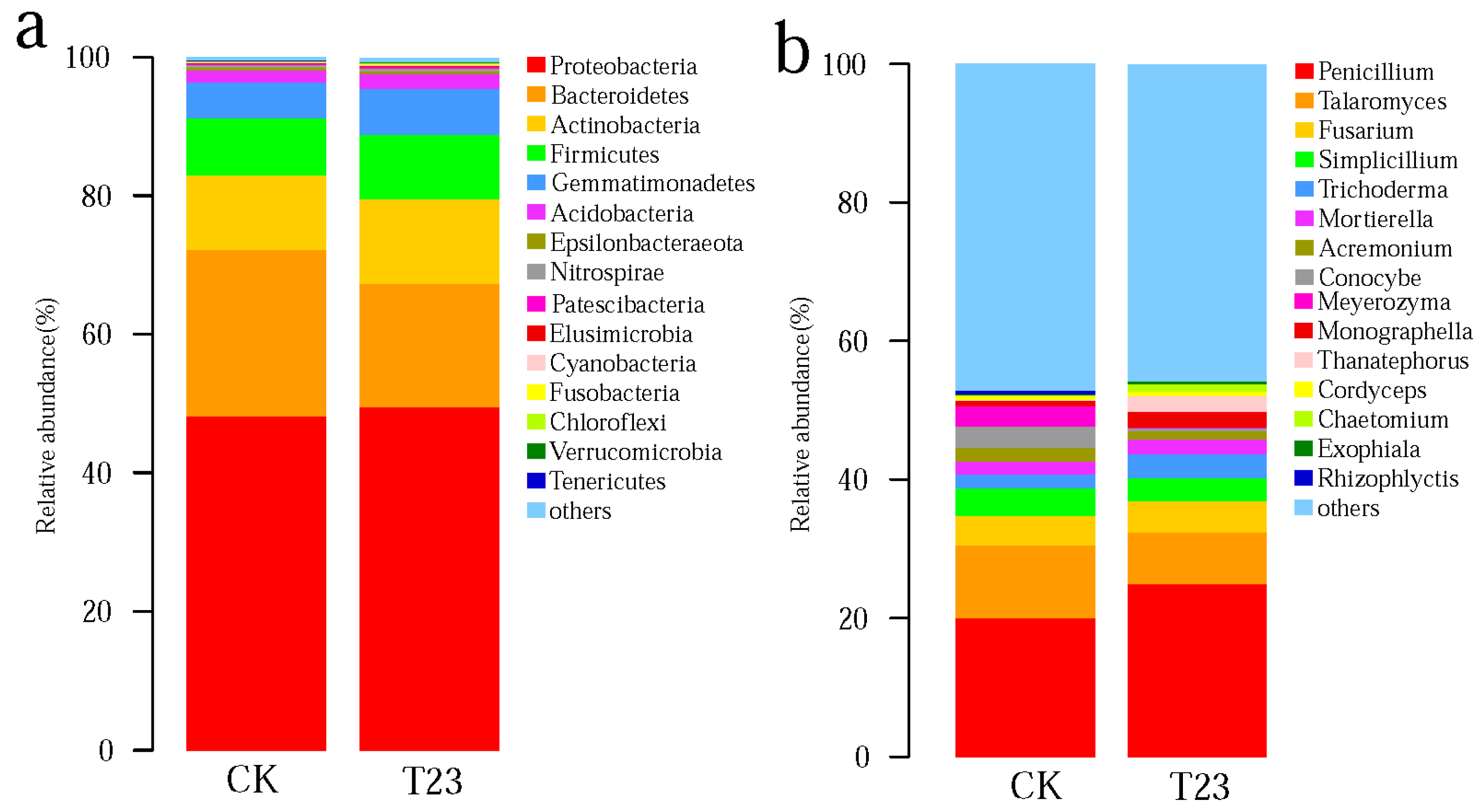
3.3. Relative Abundance of Microbial Communities
3.4. Soil Physicochemical Properties
3.5. Effect of T. viride T23 on Rhizosphere Metabolism
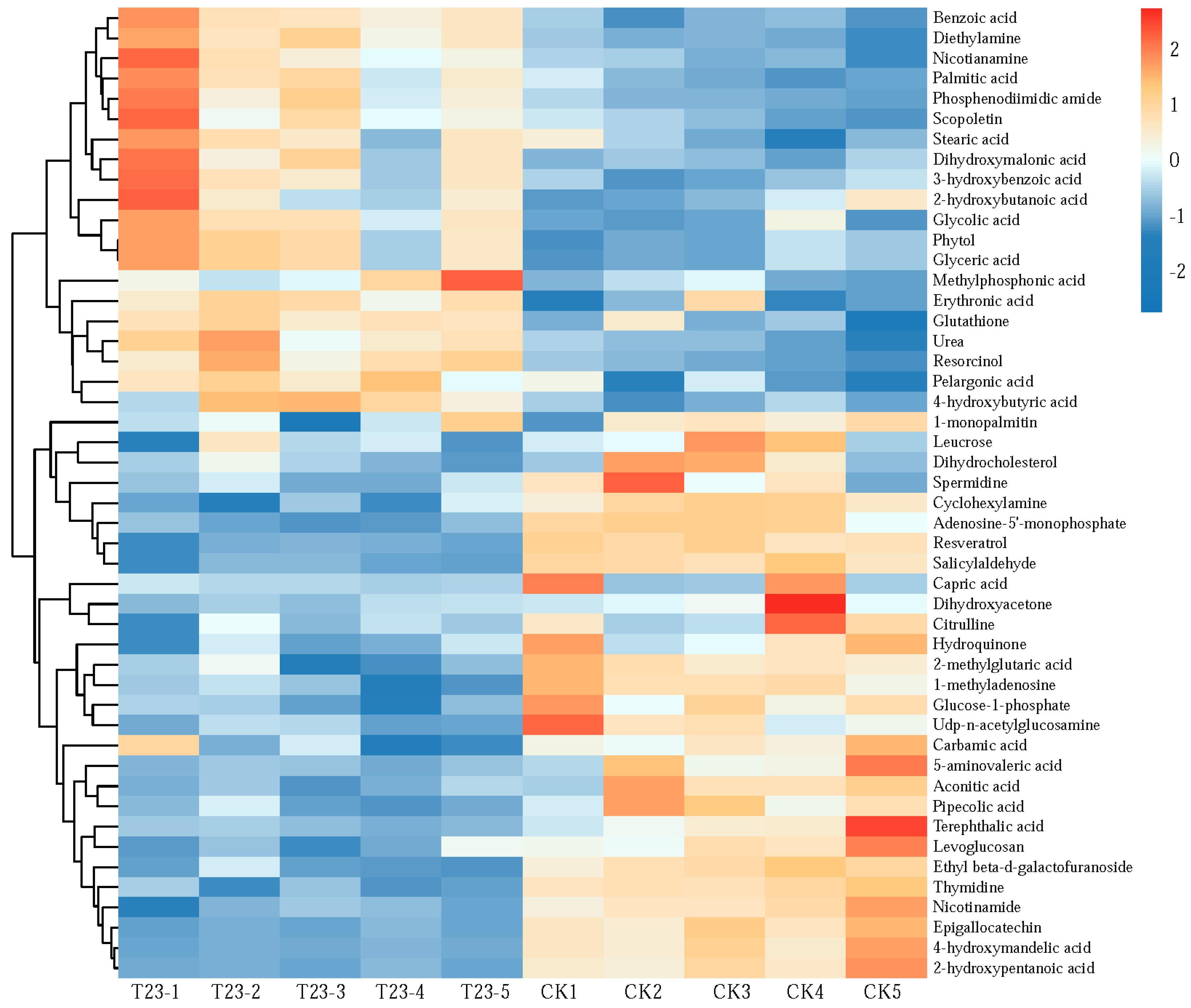
3.6. Differential Metabolite Analysis
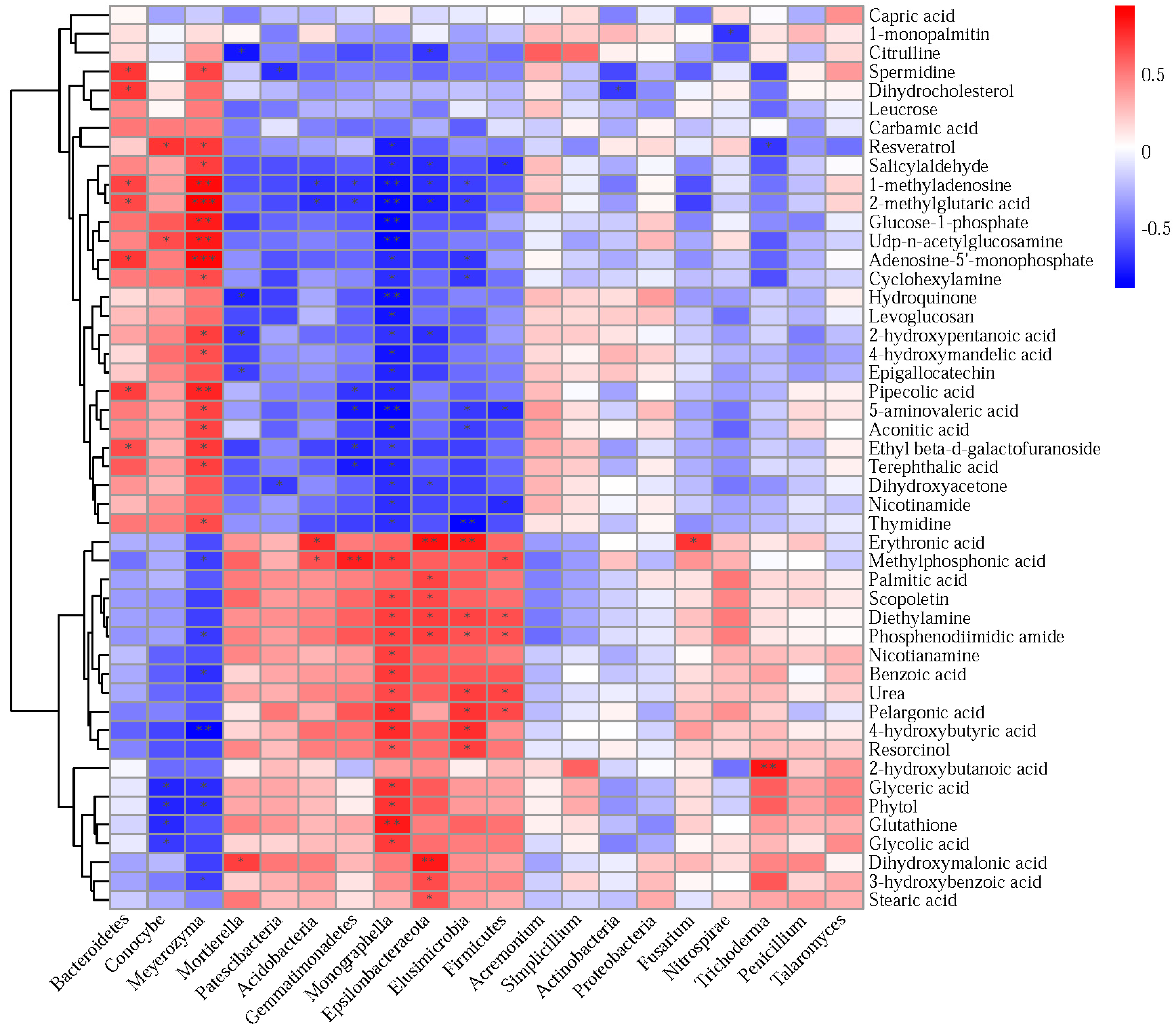
3.7. Correlations of Soil Microbial Communities with Differential Metabolites
4. Discussion
4.1. Control Effect of T. viride T23 on Muskmelon Wilt
4.2. Effects of T. viride T23 on Soil Physicochemical Properties and Soil Microbial Communities
4.3. Differential Metabolite Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Tian, H.; Wang, P.; Xiao, Q.; Zhu, S.; Jiang, H. Variations in pH significantly affect cadmium uptake in grafted muskmelon (Cucumis melo L.) plants and drive the diversity of bacterial communities in a seedling substrate. Plant Physiol. Biochem. 2019, 139, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, S.; Xia, L.; Gao, P.; Luan, F.; Liu, Z. Advances of melon breeding technology and method. China Cucurbits Veg. 2022, 35, 1–8. [Google Scholar]
- Wang, S.N.; Gao, Y.; Yang, R.X.; Zhang, Z.R.; Cai, X.T.; Liu, B.; Gao, Z.G.; Liu, X.; Yao, Y. Screening and using allelopathic crops to increase the yield of muskmelon (Cucumis melon L.). Allelopath. J. 2017, 40, 117–131. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiao, H.; Gao, H.; Li, S. Effect of Melon Continuous Cropping on Rhizosphere Soil Microorganisms and Enzyme Activities. North Hortic. 2015, 19, 158–161. [Google Scholar]
- Xu, X.; Zhang, G.; Zhou, Y.; Hu, J.; Xu, Y. Studies on the physical-chemical and biological properties of soils cropped continuously with melon under protected cultivation condition. J. Fruit Sci. 2016, 33, 1131–1138. [Google Scholar]
- Yan, H.; Wu, G.; Cao, B.; Wan, H.; Wang, X.; Wang, L.; Wang, G.; Qian, D.; Ge, M. Effects of Microbial Fertilizer Ningdun on Muskmelon in Continuous Cropping Field and Noncontinuous Cropping Field. J. Chang. Veg. 2018, 452, 75–77. [Google Scholar]
- Yang, R.X.; Gao, Z.G.; Liu, X.; Yao, Y.; Cheng, Y. Root exudates from muskmelon (Cucumis melon L.) induce autotoxicity and promote growth of Fusarium oxysporum f. sp melonis. Allelopath. J. 2014, 33, 175–187. [Google Scholar]
- Ruangwong, O.-U.; Wonglom, P.; Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.-I.; Sunpapao, A. Biological control activity of Trichoderma asperelloides PSU-P1 against gummy stem blight in muskmelon (Cucumis melo). Physiol. Mol. Plant Pathol. 2021, 115, 101663. [Google Scholar] [CrossRef]
- Gava, C.A.T.; Pinto, J.M. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control 2016, 97, 13–20. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Jiang, L.; Zhang, L.; Cui, S.; Meng, F.; Wang, Q.; Li, X.; Zhou, Y. Changes of soil microbial community under different degraded gradients of alpine meadow. Agric. Ecosyst. Environ. 2016, 222, 213–222. [Google Scholar] [CrossRef]
- Li, M.; Ma, G.-S.; Lian, H.; Su, X.-L.; Tian, Y.; Huang, W.-K.; Mei, J.; Jiang, X.-L. The effects of Trichoderma on preventing cucumber fusarium wilt and regulating cucumber physiology. J. Integr. Agric. 2019, 18, 607–617. [Google Scholar] [CrossRef]
- Cong, Y.; Fan, H.; Ma, Q.; Lu, Y.; Xu, L.; Zhang, P.; Chen, K. Mixed culture fermentation between Rhizopus nigricans and Trichoderma pseudokoningii to control cucumber Fusarium wilt. Crop Prot. 2019, 124, 104857. [Google Scholar] [CrossRef]
- Shi, R.; Gu, H.; He, S.; Xiong, B.; Huang, Y.; Horowitz, A.R.; He, X. Comparative Metagenomic and Metabolomic Profiling of Rhizospheres of Panax notoginseng Grown under Forest and Field Conditions. Agronomy 2021, 11, 2488. [Google Scholar] [CrossRef]
- Nossa, C.W.; Oberdorf, W.E.; Yang, L.; Aas, J.A.; Paster, B.J.; Desantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135–4144. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Retuerto, M.; Sikaroodi, M.; Brown, R.E.; Jurevic, R.; Salata, R.A.; Lederman, M.M.; Gillevet, P.M.; Ghannoum, M.A. Oral mycobiome analysis of HIV-infected patients: Identification of Pichia as an antagonist of opportunistic fungi. PLoS Pathog. 2014, 10, e1003996. [Google Scholar] [CrossRef] [PubMed]
- Foden, J.A.; Khayter, C.; Tsai, S.Q.; Joung, J.K.; Reyon, D.; Sander, J.D. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 2012, 30, 460–465. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Kodadinne Narayana, N.; Kingery, W.L.; Shankle, M.W.; Ganapathi Shanmugam, S. Differential Response of Soil Microbial Diversity and Community Composition Influenced by Cover Crops and Fertilizer Treatments in a Dryland Soybean Production System. Agronomy 2022, 12, 618. [Google Scholar] [CrossRef]
- Li, N.; Chang, R.; Chen, S.; Lei, J.; Liu, Y.; Cui, W.; Chen, Q.; Wu, F. The role of the biogas slurry microbial communities in suppressing fusarium wilt of cucumber. Waste Manag. 2022, 151, 142–153. [Google Scholar] [CrossRef]
- Li, J.; Shao, X.; Huang, D.; Liu, K.; Shang, J.; Zhang, Q.; Zhao, T.; Yang, X. Short-term biochar effect on soil physicochemical and microbiological properties of a degraded alpine grassland. Pedosphere 2022, 32, 426–437. [Google Scholar] [CrossRef]
- Manoj, S.; Thirumurugan, M.; Elango, L. Determination of distribution coefficient of uranium from physical and chemical properties of soil. Chemosphere 2020, 244, 125411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; White, J.C.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]
- Ren, H.; Wang, H.; Wang, Q.; Qi, X.; Zhang, S.; Yu, Z.; Ijaz, M.; Zhang, M.; Ahmed, T.; El-Sharnouby, M.; et al. Effect of Fungicides on Bayberry Decline Disease by Modulating Rhizosphere Soil Properties, Microflora, and Metabolites. Agronomy 2022, 12, 677. [Google Scholar] [CrossRef]
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma names in the year 2015. IMA Fungus 2015, 6, 263–295. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Zehra, A.; Aamir, M.; Dubey, M.K.; Akhtar Ansari, W.; Meena, M.; Swapnil, P.; Upadhyay, R.S.; Ajmal Ali, M.; Ahmed Al-Ghamdi, A.; Lee, J. Enhanced protection of tomato against Fusarium wilt through biopriming with Trichoderma harzianum. J. King Saud Univ.-Sci. 2023, 35, 102466. [Google Scholar] [CrossRef]
- Li, X.; Lewis, E.E.; Liu, Q.; Li, H.; Bai, C.; Wang, Y. Effects of long-term continuous cropping on soil nematode community and soil condition associated with replant problem in strawberry habitat. Sci. Rep. 2016, 6, 30466. [Google Scholar] [CrossRef]
- Xu, J.; Meng, J.; Meng, X.; Zhao, Y.; Liu, J.; Sun, T.; Liu, Y.; Wang, Q.; Zhang, S. Pathogen-Responsive MPK3 and MPK6 Reprogram the Biosynthesis of Indole Glucosinolates and Their Derivatives in Arabidopsis Immunity. Plant Cell 2016, 28, 1144–1162. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Tian, M.; Shah Jahan, M.; Shu, S.; Sun, J.; Li, P.; Ahammed, G.J.; Guo, S. Mixing of biochar, vinegar and mushroom residues regulates soil microbial community and increases cucumber yield under continuous cropping regime. Appl. Soil Ecol. 2021, 161, 103883. [Google Scholar] [CrossRef]
- Ding, S.; Zhou, D.; Wei, H.; Wu, S.; Xie, B. Alleviating soil degradation caused by watermelon continuous cropping obstacle: Application of urban waste compost. Chemosphere 2021, 262, 128387. [Google Scholar] [CrossRef]
- Ma, Y.; Min, W.; Wang, X. Variation of microflora and enzyme activity in continuous cropping cucumber soil in solar greenhouse. Ying Yong Sheng Tai Xue Bao 2004, 15, 1005. [Google Scholar] [PubMed]
- Liu, L.; Yan, Y.; Ali, A.; Zhao, J.; Cai, Z.; Dai, C.; Huang, X.; Zhou, K. Deciphering the Fusarium-wilt control effect and succession driver of microbial communities managed under low-temperature conditions. Appl. Soil Ecol. 2022, 171, 104334. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.; Shu, S.; Sun, J.; Li, S.; Guo, S. Paenibacillus polymyxa NSY50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep. 2017, 7, 41234. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Wang, B.; Zhong, S.; Su, L.; Li, R.; Shen, Q. Effect of biofertilizer for suppressing Fusarium wilt disease of banana as well as enhancing microbial and chemical properties of soil under greenhouse trial. Appl. Soil Ecol. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Wang, H.-W.; Xu, M.; Cai, X.-Y.; Feng, T.; Xu, W.-L. Application of spent mushroom substrate suppresses Fusarium wilt in cucumber and alters the composition of the microbial community of the cucumber rhizosphere. Eur. J. Soil Biol. 2020, 101, 103245. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Q.; Li, S.; Lu, X.; Dong, L.; Wang, P.; Zhang, X.; Su, Z.; Ma, P. Application of Bacillus subtilis NCD-2 can suppress cotton verticillium wilt and its effect on abundant and rare microbial communities in rhizosphere. Biol. Control 2022, 165, 104812. [Google Scholar] [CrossRef]
- Han, L.; Wang, Z.; Li, N.; Wang, Y.; Feng, J.; Zhang, X. Bacillus amyloliquefaciens B1408 suppresses Fusarium wilt in cucumber by regulating the rhizosphere microbial community. Appl. Soil Ecol. 2019, 136, 55–66. [Google Scholar] [CrossRef]
- Vargas, L.K.; da Costa, P.B.; Beneduzi, A.; Lisboa, B.B.; Passaglia, L.M.P.; Granada, C.E. Soil fertility level is the main modulator of prokaryotic communities in a meta-analysis of 197 soil samples from the Americas and Europe. Appl. Soil Ecol. 2023, 186, 104811. [Google Scholar] [CrossRef]
- Zheng, H.; Dietrich, C.; Radek, R.; Brune, A. Endomicrobium proavitum, the first isolate of Endomicrobia class. nov. (phylum Elusimicrobia)—An ultramicrobacterium with an unusual cell cycle that fixes nitrogen with a Group IV nitrogenase. Environ. Microbiol. 2016, 18, 191–204. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Cao, W.; Atio Abalori, T.; Liu, Y.; Xin, Y.; Wang, S.; Zhang, D. Soil bacterial community responses to short-term grazing exclusion in a degraded alpine shrubland–grassland ecotone. Ecol. Indic. 2021, 130, 108043. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Bian, Y.; Gu, C.; Yang, X.; Wang, F.; Jiang, X. Insights into the mechanisms underlying efficient Rhizodegradation of PAHs in biochar-amended soil: From microbial communities to soil metabolomics. Environ. Int. 2020, 144, 105995. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Yan, C.; Chen, J.; Wang, Y.; Zhu, F.; Liu, Y.; Fan, Y.; Duan, X. Induced resistance of soybean to gray leaf spot by scopoletin coated seeds. Chin. J. Oil Crop Sci. 2019, 41, 243–249. [Google Scholar] [CrossRef]
- Peterson, J.K.; Harrision, H.F.; Jackson, D.M. Biological Activities and Contents of Scopolin and Scopoletin in Sweetotato Clones. HortScience 2003, 38, 1129–1133. [Google Scholar] [CrossRef]

| Treatments | The Whole Disease Index | Samples’ Disease Index |
|---|---|---|
| T. viride T23 | 20.86 ± 23.98 b | 18.00 ± 15.33 b |
| CK | 70.29 ± 25.95 a | 65.86 ± 17.68 a |
| Control effect on wilt | 70.32% | 72.67% |
| Treatments | pH | Organic Matter (g/kg) | Total N (g/kg) | Available P (mg/kg) | Available K (mg/kg) | Slow Acting K (mg/kg) |
|---|---|---|---|---|---|---|
| CK | 6.06 b | 22.04 a | 1.105 a | 13.27 a | 126.3 a | 600.6 a |
| T. viride T23 | 6.40 a | 21.46 a | 1.216 a | 12.9 a | 120.1 a | 570 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Tang, S.; Liu, X.; Ren, X.; Wang, S.; Gao, Z. The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping. Agronomy 2023, 13, 1092. https://doi.org/10.3390/agronomy13041092
Zhang Z, Tang S, Liu X, Ren X, Wang S, Gao Z. The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping. Agronomy. 2023; 13(4):1092. https://doi.org/10.3390/agronomy13041092
Chicago/Turabian StyleZhang, Zhaoran, Shuangshuang Tang, Xiaodi Liu, Xuelian Ren, Suna Wang, and Zenggui Gao. 2023. "The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping" Agronomy 13, no. 4: 1092. https://doi.org/10.3390/agronomy13041092
APA StyleZhang, Z., Tang, S., Liu, X., Ren, X., Wang, S., & Gao, Z. (2023). The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping. Agronomy, 13(4), 1092. https://doi.org/10.3390/agronomy13041092






