Managing Bacterial Spot of Tomato: Do Chemical Controls Pay Off?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Method
2.2.1. Statistical Analysis of Disease and Yield
2.2.2. Profitability Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, Z.; Biswas, T.; Wu, F. The U.S. Tomato Industry: An Overview of Production and Trade. EDIS 2018. [Google Scholar] [CrossRef]
- Huang, K.-M.; Guan, Z.; Hammami, A. The U.S. Fresh Fruit and Vegetable Industry: An Overview of Production and Trade. Agriculture 2022, 12, 1719. [Google Scholar] [CrossRef]
- USDA. Vegetables 2019 Summary; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Wu, F.; Qushim, B.; Calle, M.; Guan, Z. Government Support in Mexican Agriculture. Choices 2018, 33, 1–11. [Google Scholar] [CrossRef]
- Wu, F.; Guan, Z.; Suh, D.H. The Effects of Tomato Suspension Agreements on Market Price Dynamics and Farm Revenue. Appl. Econ. Perspect. Policy 2018, 40, 316–332. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Guan, Z.; Luo, T. How trade affects the US produce industry: The case of fresh tomatoes. Int. Food Agribus. Manag. Rev. 2021, 25, 121–133. [Google Scholar] [CrossRef]
- Guan, Z.; Wu, F.; Roka, F.; Whidden, A. Agricultural Labor and Immigration Reform. CHOICES 2015, 4, 9. [Google Scholar] [CrossRef]
- Roka, F.; Guan, Z. Farm Labor Management Trends in Florida, USA—Challenges and Opportunities. Int. J. Agric. Manag. 2018, 7, 79–87. [Google Scholar] [CrossRef]
- Cao, X.; Guan, Z.; Vallad, G.E.; Wu, F. Economics of fumigation in tomato production: The impact of methyl bromide phase-out on the Florida tomato industry. Int. Food Agribus. Manag. Rev. 2019, 22, 589–600. [Google Scholar] [CrossRef]
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas diversity, virulence and plant–pathogen interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Strayer-Scherer, A.; Liao, Y.-Y.; Abrahamian, P.; Timilsina, S.; Paret, M.; Momol, T.; Jones, J.; Vallad, G. Integrated Management of Bacterial Spot on Tomato in Florida; Plant Pathology Department, UF/IFAS Extension: Gainesville, FL, USA, 2019; pp. 1–8. [Google Scholar]
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015, 16, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ivey, M.L.L.; Miller, S.A. First Report of Xanthomonas gardneri Causing Bacterial Spot of Tomato in Ohio and Michigan. Plant Dis. 2011, 95, 1584. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.E. Yield reduction in tomato caused by bacterial spot and disease control with copper sprays. Proc. Fla. State Hortic. Soc. 1978, 91, 291–293. [Google Scholar]
- Pohronezny, K.; Volin, R. Effect of bacterial spot on yield and quality of fresh market tomatoes. HortScience 1983, 18, 69–70. [Google Scholar] [CrossRef]
- Klein-Gordon, J.M.; Xing, Y.; Garrett, K.A.; Abrahamian, P.; Paret, M.L.; Minsavage, G.V.; Strayer-Scherer, A.L.; Fulton, J.C.; Timilsina, S.; Jones, J.B.; et al. Assessing Changes and Associations in the Xanthomonas perforans Population Across Florida Commercial Tomato Fields Via a Statewide Survey. Phytopathology 2021, 111, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Hamilton, V.A.; Kopittke, R.A. Copper tolerance in Australian populations of Xanthomonas campestris pv. vesicatoria contributes to Poor Field Control of Bacterial Spot of Pepper. Plant Dis. 2004, 9, 921–924. [Google Scholar] [CrossRef] [Green Version]
- Pohronezny, K.; Moss, M.; Dankers, W.; Schenk, J. Dispersal and Management of Xanthomonas campestris pv. vesicatoria During Thinning of Direct-Seeded Tomato. Plant Dis. 1990, 74, 800–805. [Google Scholar] [CrossRef]
- Abrahamian, P.; Jones, J.B.; Vallad, G.E. Efficacy of copper and copper alternatives for management of bacterial spot on tomato under transplant and field production. Crop. Prot. 2019, 126, 104919. [Google Scholar] [CrossRef]
- Abrahamian, P.; Sharma, A.; Jones, J.B.; Vallad, G.E. Dynamics and Spread of Bacterial Spot Epidemics in Tomato Transplants Grown for Field Production. Plant Dis. 2021, 105, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.F. Bacterial spot of pepper and tomato. Plant Health Instr. 2000, 10. [Google Scholar] [CrossRef]
- Kuchler, F.; Tegene, A. Asset Fixity and the Distribution of Rents from Agricultural Policies. Land Econ. 1993, 4, 428–437. [Google Scholar] [CrossRef]
- Mirik, M.; Aysan, Y.; Cinar, O. Copper-resistant strains of Xanthomonas axonopodis pv. vesicatoria (doidge) dye in the eastern mediterranea. J. Plant Pathol. 2007, 1, 153–154. [Google Scholar]
- Obradovic, A.; Jones, J.B.; Balogh, B.; Momol, M.T. Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; pp. 211–223. [Google Scholar] [CrossRef]
- Adhikari, P.; Adhikari, T.B.; Timilsina, S.; Meadows, I.; Jones, J.B.; Panthee, D.R.; Louws, F.J. Phenotypic and Genetic Diversity of Xanthomonas perforans Populations from Tomato in North Carolina. Phytopathology 2019, 109, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Egel, D.S.; Jones, J.B.; Minsavage, G.V.; Creswell, T.; Ruhl, G.; Maynard, E.; Marchino, C. Distribution and Characterization of Xanthomonas Strains Causing Bacterial Spot of Tomato in Indiana. Plant Health Prog. 2018, 19, 319–321. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Khabbaz, S.E.; Weselowski, B.; Zhang, L. Occurrence of Copper-Resistant Strains and a Shift in Xanthomonas Spp. Causing Tomato Bacterial Spot in Ontario. Can. J. Microbiol. 2015, 61, 753–761. [Google Scholar] [CrossRef]
- Horvath, D.M.; Stall, R.E.; Jones, J.B.; Pauly, M.H.; Vallad, G.E.; Dahlbeck, D.; Staskawicz, B.J.; Scott, J.W. Transgenic Resistance Confers Effective Field Level Control of Bacterial Spot Disease in Tomato. PLoS ONE 2012, 7, e42036. [Google Scholar] [CrossRef] [PubMed]
- Conover, R.; Gerhold, N. Mixtures of copper and maneb or mancozeb for control of bacterial spot of tomato and their compatibility for control fo fungus diseases. Proc. Fla. Hortic. Sci. 1981, 94, 154–156. [Google Scholar]
- Huang, C.-H.; Vallad, G.E.; Zhang, S.; Wen, A.; Balogh, B.; Figueiredo, J.F.L.; Behlau, F.; Jones, J.B.; Momol, M.T.; Olson, S.M. Effect of Application Frequency and Reduced Rates of Acibenzolar-S-Methyl on the Field Efficacy of Induced Resistance Against Bacterial Spot on Tomato. Plant Dis. 2012, 96, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trueman, C.L.; Loewen, S.A.; Goodwin, P.H. Can the inclusion of uniconazole improve the effectiveness of acibenzolar-S-methyl in managing bacterial speck (Pseudomonas syringae pv. tomato) and bacterial spot (Xanthomonas gardneri) in tomato? Eur. J. Plant Pathol. 2019, 155, 927–942. [Google Scholar] [CrossRef]
- Pontes, N.d.C.; Nascimento, A.d.R.; Golynski, A.; Maffia, L.A.; Oliveira, J.R.d.; Quezado-Duval, A.M. Intervals and Number of Applications of Acibenzolar-S-Methyl for the Control of Bacterial Spot on Processing Tomato. Plant Dis. 2016, 100, 2126–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunwar, S.; Iriarte, F.; Fan, Q.; Silva, E.E.d.; Ritchie, L.; Nguyen, N.S.; Freeman, J.H.; Stall, R.E.; Jones, J.B.; Minsavage, G.V.; et al. Transgenic Expression of EFR and Bs2 Genes for Field Management of Bacterial Wilt and Bacterial Spot of Tomato. Phytopathology 2018, 108, 1402–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louws, F.J.; Wilson, M.; Campbell, H.L.; Cuppels, D.A.; Jones, J.B.; Shoemaker, P.B.; Sahin, F.; Miller, S.A. Field Control of Bacterial Spot and Bacterial Speck of Tomato Using a Plant Activator. Plant Dis. 2001, 85, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for using bacteriophages for plant disease control. Bacteriophage 2012, 2, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obradovic, A.; Mavridis, A.; Rudolph, K.; Janse, J.D.; Arsenijevic, M.; Jones, J.B.; Minsavage, G.V.; Wang, J.-F. Characterization and PCR-based Typing of Xanthomonas campestris pv. vesicatoria from Peppers and Tomatoes in Serbia. Eur. J. Plant Pathol. 2004, 110, 285–292. [Google Scholar] [CrossRef]
- Bock, C.H.; Gottwald, T.R.; Parker, P.E.; Cook, A.Z.; Ferrandino, F.; Parnell, S.; Bosch, F.v.d. The Horsfall-Barratt scale and severity estimates of citrus canker. Eur. J. Plant Pathol. 2009, 125, 23–38. [Google Scholar] [CrossRef]
- USDA. United States Standards for Grades of Fresh Tomatoes; USDA: Washington, DC, USA, 1991. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Kay, R.D.; Edwards, W.; Duffy, P.A. Farm Management; McGraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- Wossink, G.A.A.; Osmond, D.L. Farm economics to support the design of cost-effective best management practice (BMP) programs to improve water quality: Nitrogen control in the Neuse River Basin, North Carolina | Journal of Soil and Water Conservation. J. Soil Water Conserv. 2002, 4, 213–220. [Google Scholar]
- USDA. Farm Labor; USDA: Washington, DC, USA, 2019. [Google Scholar]
- Stoddard, C.S.; Lestrange, M.; Agerter, B.; Klonsky, K.M.; De Moura, R.I. Sample Costs to Produce Fresh Market Tomatoes–San Joaquin Valley; University of California Cooperative Extension: Oakland, CA, USA, 2007. [Google Scholar]
- EIA. U.S. Gasoline and Diesel Retail Prices; EIA: Washington, DC, USA, 2020. [Google Scholar]
- US-BLS. Producer Price Index Industry Data; US-BLS: Washington, DC, USA, 2018. [Google Scholar]
- USDA. Fruits and Vegetables Market News; USDA: Washington, DC, USA, 2020. [Google Scholar]
- Roberts, P.D.; Momol, M.T.; Ritchie, L.; Olson, S.M.; Jones, J.B.; Balogh, B. Evaluation of spray programs containing famoxadone plus cymoxanil, acibenzolar-S-methyl, and Bacillus subtilis compared to copper sprays for management of bacterial spot on tomato. Crop. Prot. 2008, 27, 1519–1526. [Google Scholar] [CrossRef]
- Garcia, R.M.; de Carvalho Pontes, N.; Quezado-Duval, A.M. Optimizing the application of acibenzolar-S-methyl and copper to control bacterial spot on fresh-market tomato. Crop. Prot. 2023, 164, 106137. [Google Scholar] [CrossRef]
- Byrne, J.M.; Dianese, A.C.; Campbell, H.L.; Cuppels, D.A.; Louws, F.J.; Miller, S.A.; Jones, J.B.; Wilson, M. Biological control of bacterial spot of tomato under Weld conditions at several locations in North America. Biol. Control. 2005, 32, 408–418. [Google Scholar] [CrossRef]
- Thayer, P.L.; Stall, R.E. Streptomycin resistance of the bacterial spot pathogen and control with streptomycin. Plant Dis. Rep. 1962, 45, 389–392. [Google Scholar]
- Thayer, P.L.; Stall, R.E. A survey of Xanthomonas vesicatoria resistance to streptomycin. Fla. State Hortic. Soc. 1962, 75, 163–165. [Google Scholar]
- Jones, J.P. Tolerance of tomato to manual defoliation. Proc. Fla. State Hortic. Soc. 1979, 92, 99–100. [Google Scholar]
- An, S.-Q.; Potnis, N.; Dow, M.; Vorhölter, F.-J.; He, Y.-Q.; Becker, A.; Teper, D.; Li, Y.; Wang, N.; Bleris, L.; et al. Mechanistic insights into host adaptation, virulence and epidemiology of the phytopathogen Xanthomonas. FEMS Microbiol. Rev. 2020, 44, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, S.; Bonas, U. How Xanthomonas type III effectors manipulate the host plant. Curr. Opin. Microbiol. 2009, 12, 37–43. [Google Scholar] [CrossRef] [PubMed]
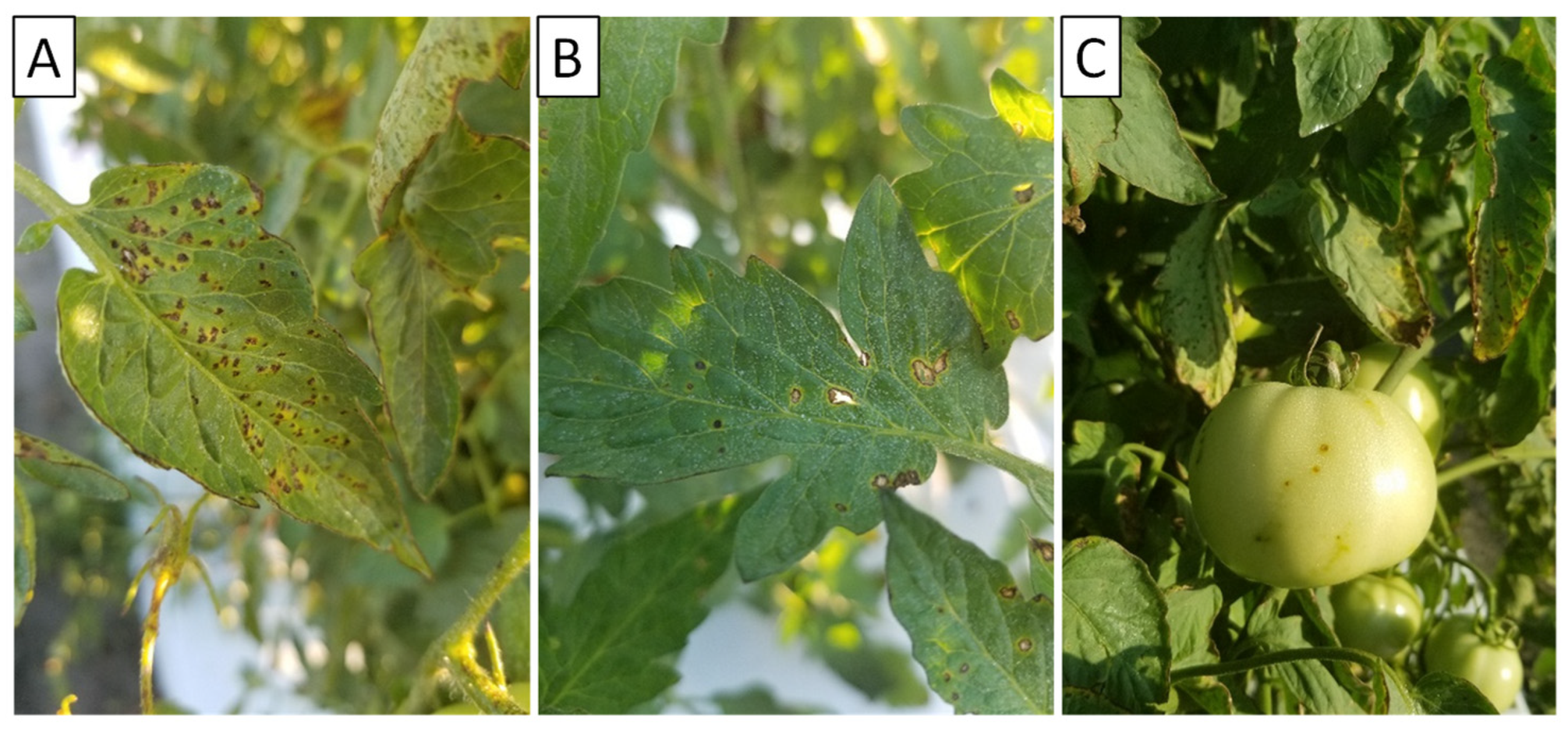

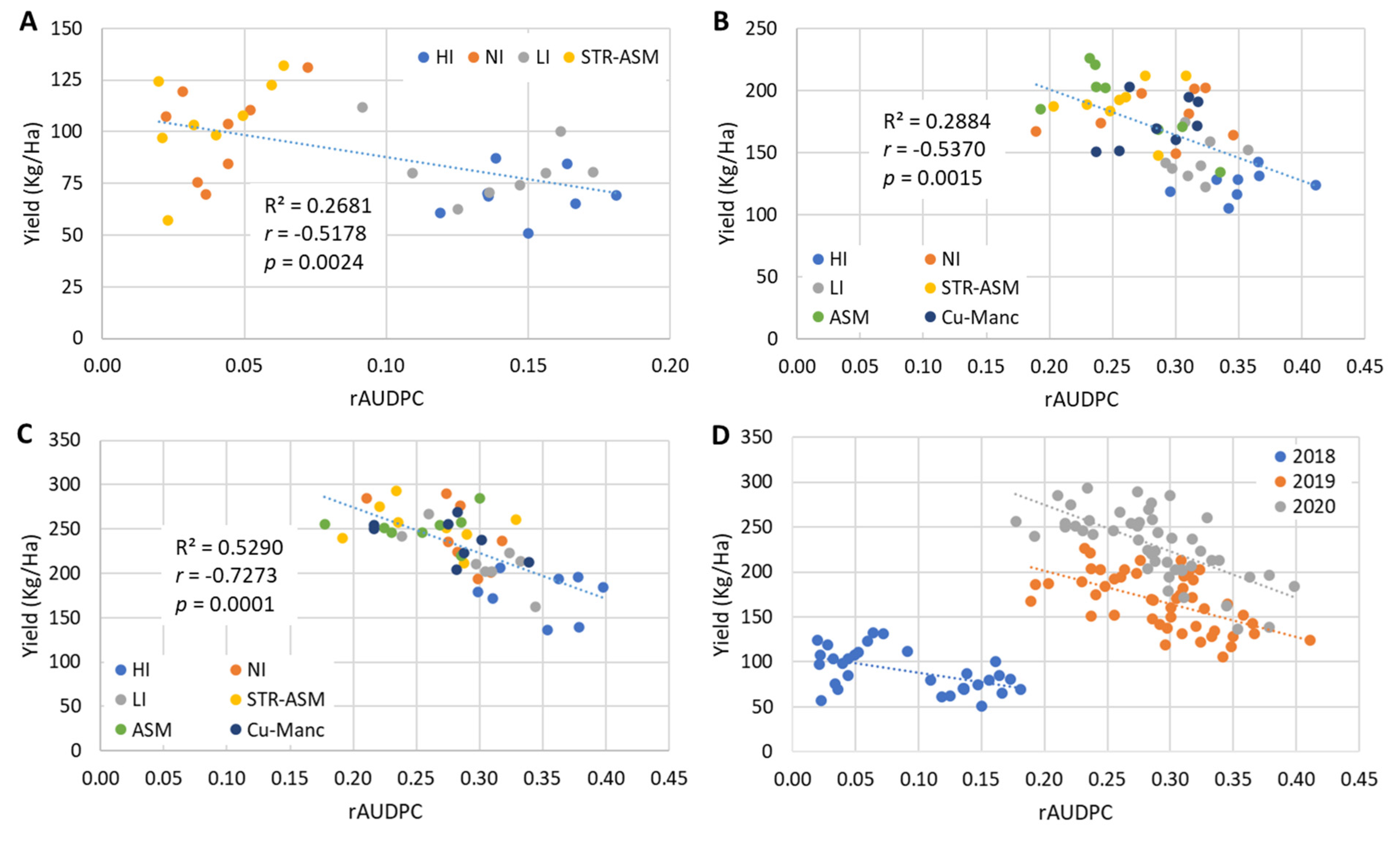
| 2018 | 2019 | 2020 | ||||
|---|---|---|---|---|---|---|
| Treatments 1 | rAUDPC 2 | Yield 3 | rAUDPC | Yield | rAUDPC | Yield |
| NI | 0.042 b 4 | 34,917 a | 0.287 bc | 53,726 a | 0.282 c | 65,893 a |
| LI | 0.138 a | 28,808 ab | 0.317 ab | 42,967 b | 0.301 b | 58,452 ab |
| HI | 0.149 a | 24,294 b | 0.352 a | 36,998 b | 0.350 a | 47,697 b |
| STR-ASM | 0.039 b | 36,475 a | 0.258 c | 56,674 a | 0.258 de | 69,001 a |
| Cu-Manc | n.d. | n.d. | 0.286 bc | 52,073 a | 0.275 cd | 64,639 a |
| ASM | n.d. | n.d. | 0.259 c | 55,976 a | 0.253 e | 68,364 a |
| Season | Material Costs | Labor Costs | Machinery Costs | Total Costs |
|---|---|---|---|---|
| STR-ASM treatment1 | ||||
| 2018 | 895.07 | 56.93 | 41.51 | 993.51 |
| 2019 | 969.67 | 63.26 | 46.13 | 1079.06 |
| 2020 | 895.07 | 56.93 | 41.51 | 993.51 |
| Cu-Manc treatment | ||||
| 2019 | 392.21 | 63.26 | 46.13 | 501.62 |
| 2020 | 362.03 | 56.93 | 41.51 | 460.51 |
| ASM treatment | ||||
| 2019 | 572.13 | 63.26 | 46.13 | 681.52 |
| 2020 | 528.12 | 56.93 | 41.51 | 626.57 |
| Treatment 1 | Season 2018 | Season 2019 | Season 2020 |
|---|---|---|---|
| NI | 9666.56 | 14,873.54 | 18,242.06 |
| LI | 7975.25 | 11,895.23 | 16,181.97 |
| HI | 6725.84 | 10,242.62 | 13,204.40 |
| STR-ASM | 10,097.96 | 15,689.71 | 19,102.73 |
| Cu-Manc | n.d. 2 | 14,415.88 | 17,894.85 |
| ASM | n.d. | 15,496.62 | 18,925.95 |
| Season | Treatment 1 | Revenue 2 | Treatment Related Costs 3 | Harvest Profit Margin 4 |
|---|---|---|---|---|
| 2018 | NI | 39,830.47 | 9,666.56 | 30,163.91 a 5 |
| LI | 32,845.74 | 7,975.25 | 24,870.49 a | |
| HI | 27,703.90 | 6,725.84 | 20,978.06 a | |
| STR-ASM | 41,628.34 | 11,091.47 | 30,536.86 a | |
| 2019 | NI | 61,267.46 | 14,873.54 | 46,393.89 ab |
| LI | 49,025.51 | 11,895.23 | 37,130.28 bc | |
| HI | 42,184.30 | 10,242.62 | 31,941.66 c | |
| STR-ASM | 64,630.41 | 16,768.77 | 47,861.62 a | |
| Cu-Manc | 59,365.21 | 14,917.48 | 44,447.71 ab | |
| ASM | 63,869.80 | 16,178.14 | 47,691.66 ab | |
| 2020 | NI | 80,649.51 | 18,242.06 | 62,407.45 a |
| LI | 71,512.74 | 16,181.97 | 55,330.77 ab | |
| HI | 58,334.37 | 13,204.40 | 45,129.94 b | |
| STR-ASM | 84,479.40 | 20,096.27 | 64,383.13 a | |
| Cu-Manc | 79,125.44 | 18,355.36 | 60,770.08 a | |
| ASM | 83,696.35 | 19,552.54 | 64,143.81 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Caro, A.; Vallad, G.E.; Xavier, K.V.; Abrahamian, P.; Wu, F.; Guan, Z. Managing Bacterial Spot of Tomato: Do Chemical Controls Pay Off? Agronomy 2023, 13, 972. https://doi.org/10.3390/agronomy13040972
Soto-Caro A, Vallad GE, Xavier KV, Abrahamian P, Wu F, Guan Z. Managing Bacterial Spot of Tomato: Do Chemical Controls Pay Off? Agronomy. 2023; 13(4):972. https://doi.org/10.3390/agronomy13040972
Chicago/Turabian StyleSoto-Caro, Ariel, Gary E. Vallad, Katia V. Xavier, Peter Abrahamian, Feng Wu, and Zhengfei Guan. 2023. "Managing Bacterial Spot of Tomato: Do Chemical Controls Pay Off?" Agronomy 13, no. 4: 972. https://doi.org/10.3390/agronomy13040972
APA StyleSoto-Caro, A., Vallad, G. E., Xavier, K. V., Abrahamian, P., Wu, F., & Guan, Z. (2023). Managing Bacterial Spot of Tomato: Do Chemical Controls Pay Off? Agronomy, 13(4), 972. https://doi.org/10.3390/agronomy13040972





