Enhanced Herbicidal Action of Clopyralid in the Form of a Supramolecular Complex with a Gemini Surfactant
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Surface Properties and Aggregation Processes in Solutions of Surfactants
2.2. Plants and Treatments
2.3. Digital Phenotyping
2.4. Data Representation and Statistical Analysis
3. Results and Discussion
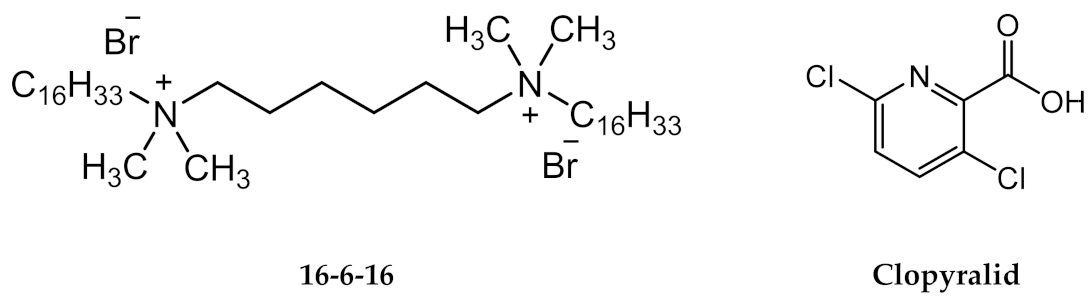
3.1. Characterization of the Solution of Clopyralid and Gemini Surfactant 16-6-16
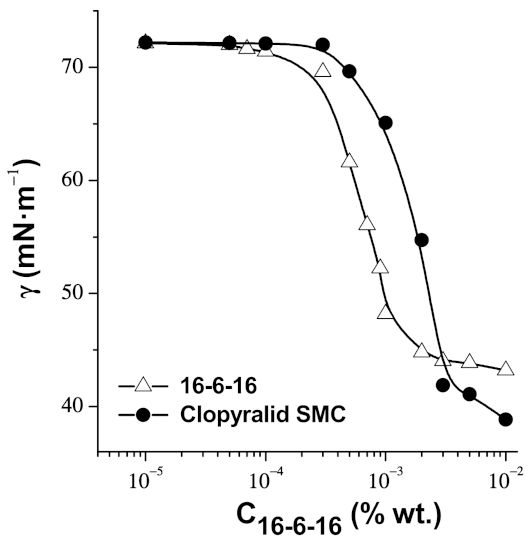
3.1.1. Wetting and the Surface Properties of the Solution of Clopyralid and Gemini Surfactant 16-6-16
3.1.2. The Clopyralid/16-6-16 Supramolecular Complex
3.2. Clopyralid SMC, Hacker WG, and Hacker 300 SL Caused Abnormalities in Cocklebur That Are Characteristic of Auxin-Mimetic Herbicides
3.3. Clopyralid SMC Has the Highest Herbicidal Effect Based on the Changes in Morphological Parameters of Cocklebur
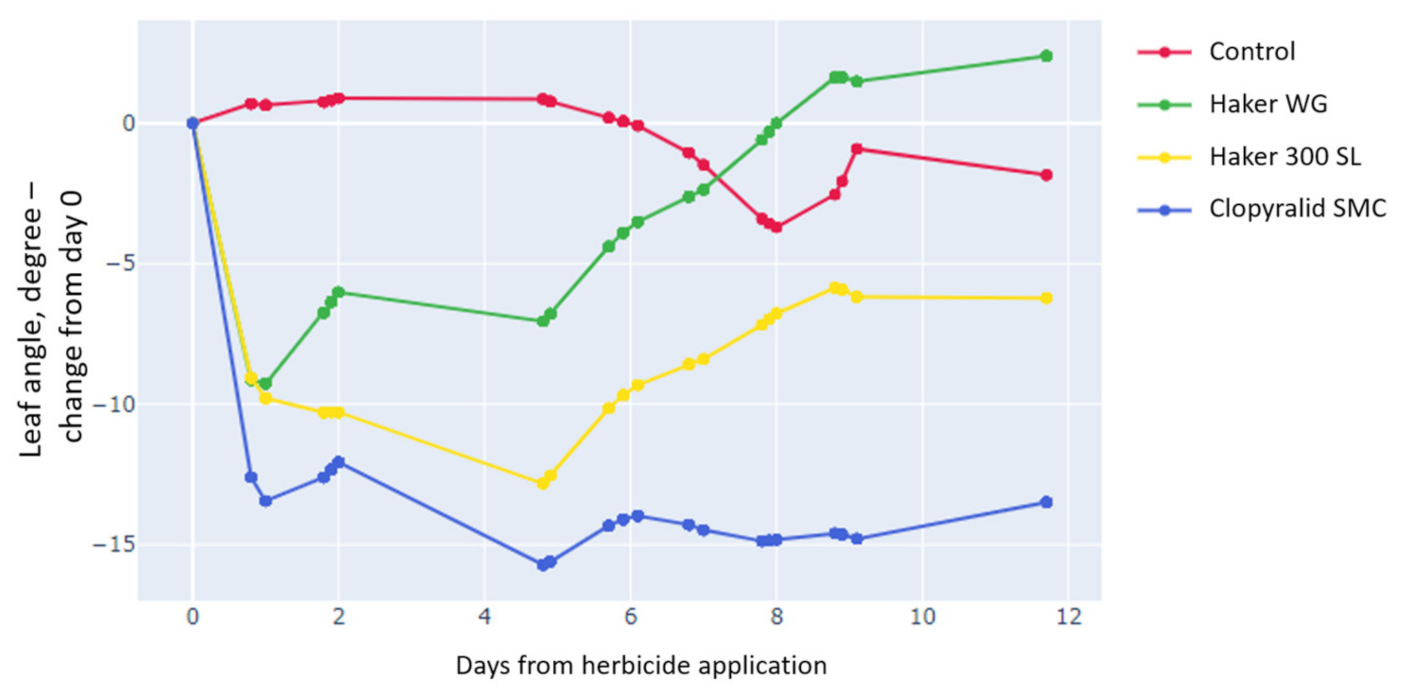
3.3.1. Leaf Angle Decreased with All Formulations, but the Effect of Clopyralid Was Maintained throughout the Experiment Only with the Clopyralid SMC
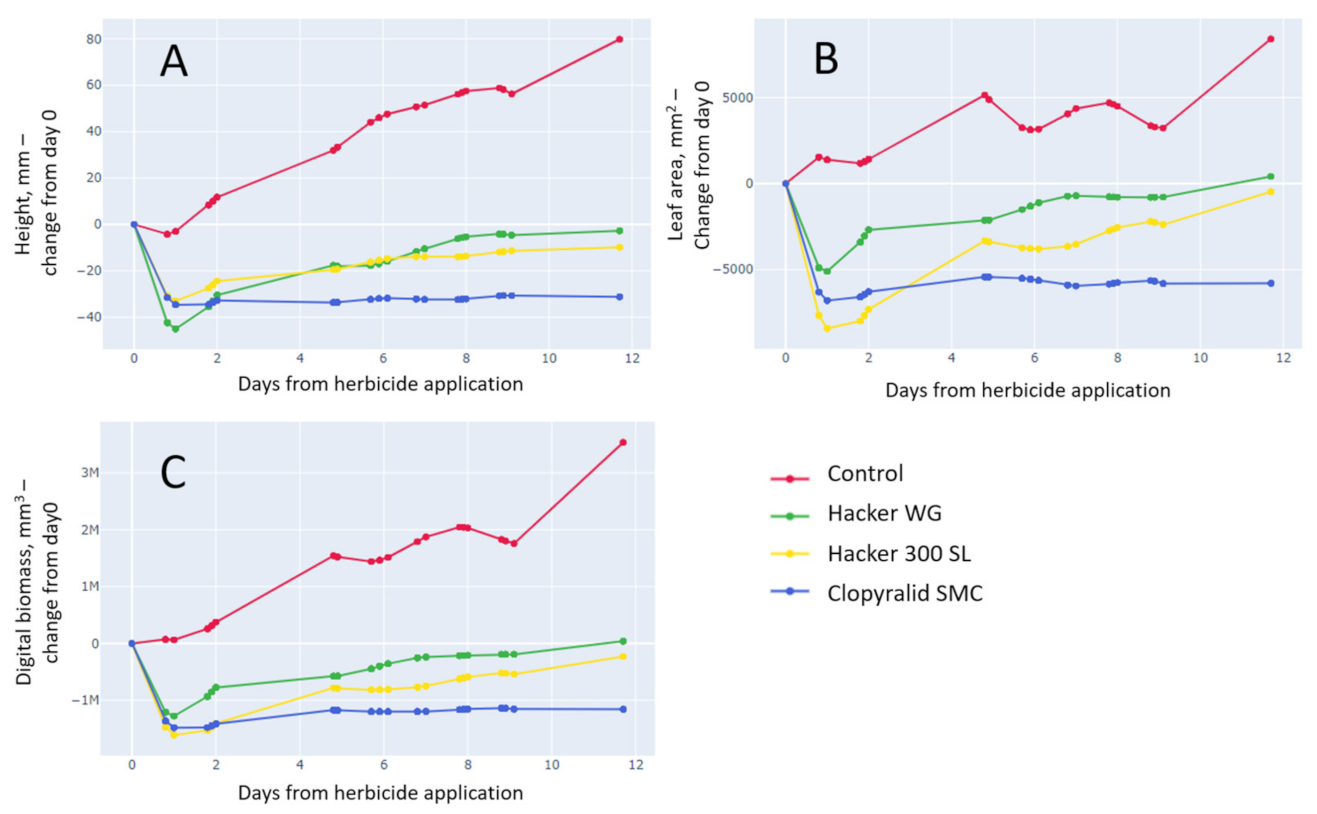
3.3.2. Clopyralid SMC Shows the Strongest Effect on Plant Height, Leaf Area, and Digital Biomass
3.4. Clopyralid SMC Has the Highest Herbicidal Effect Based on the Changes of Spectral Parameters of Cocklebur
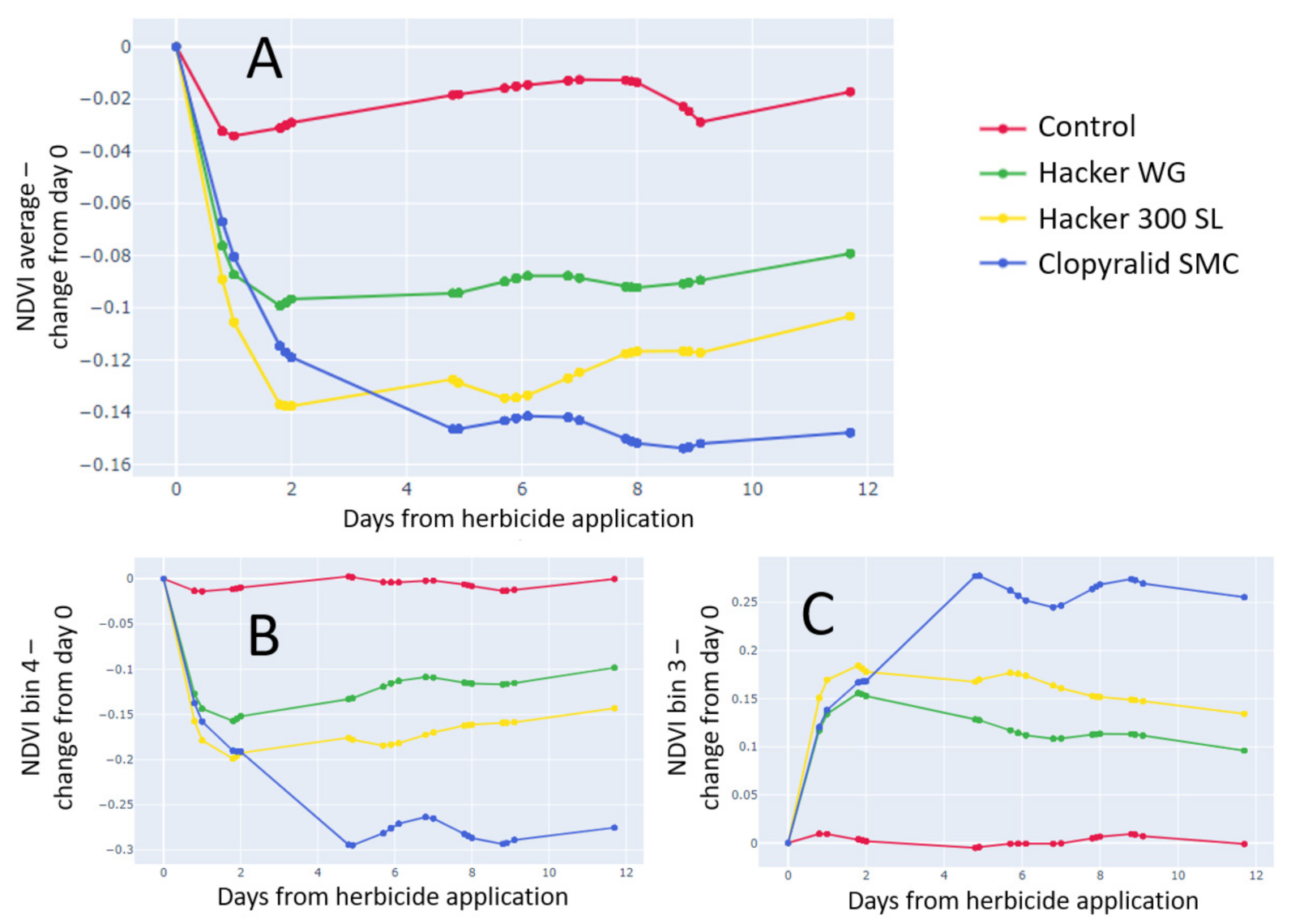
3.4.1. Normalized Differential Vegetation Index (NDVI)
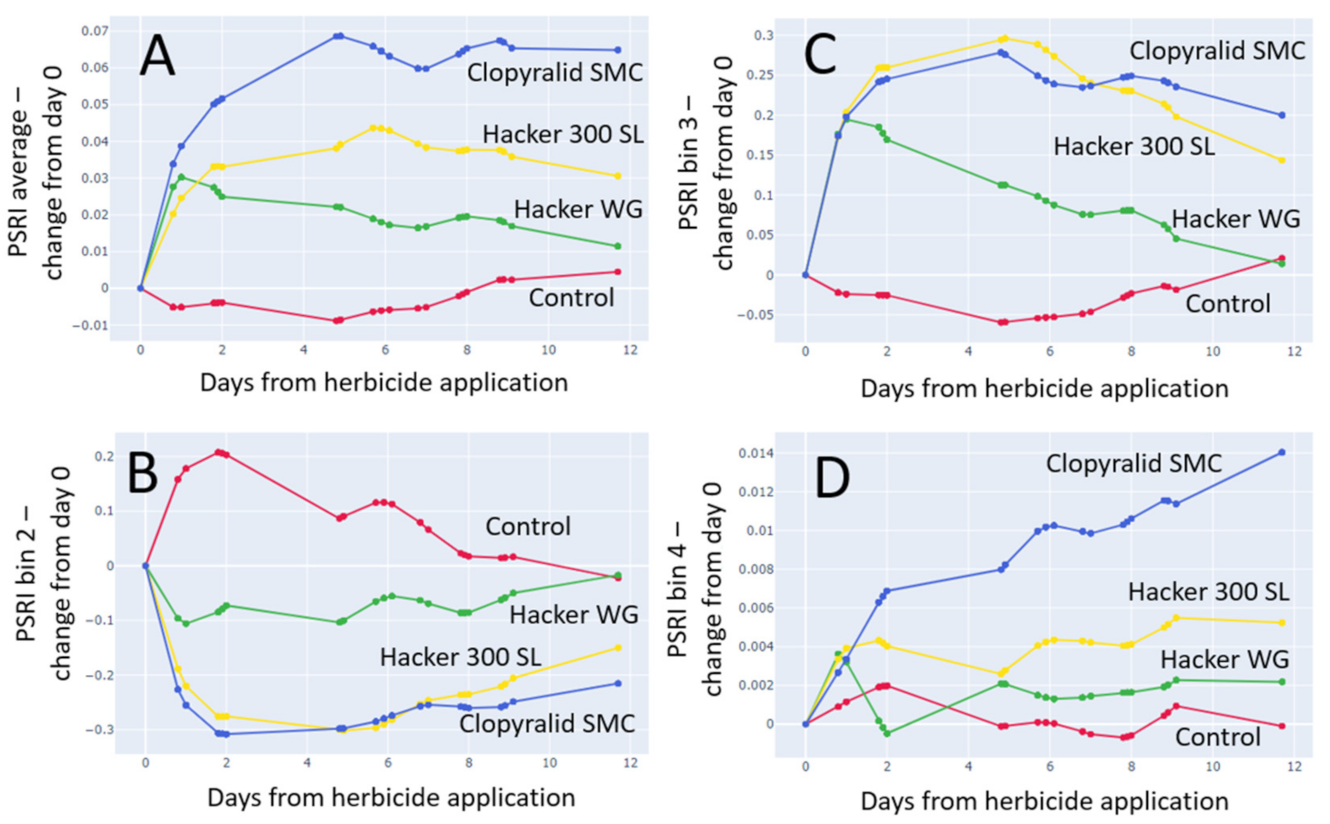
3.4.2. Plant Senescence Reflectance Index (PSRI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castro, M.J.L.; Ojeda, C.; Cirelli, A. Advances in surfactants for agrochemicals (Review). Environ. Chem. Lett. 2014, 12, 85–95. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Simmons, M.J.H. Surfactant-mediated wetting and spreading: Recent advances and applications. Curr. Opin. Colloid Interface Sci. 2021, 51, 101375. [Google Scholar] [CrossRef]
- Dollinger, J.; Schacht, V.J.; Gaus, C.; Grant, S. Effect of surfactant application practices on the vertical transport potential of hydrophobic pesticides in agrosystems. Chemosphere 2018, 209, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Palma-Bautista, C.; Vazquez-Garcia, J.G.; Travlos, I.; Tataridas, A.; Kanatas, P.; Domínguez-Valenzuela, J.A.; De Prado, R. Effect of adjuvant on glyphosate effectiveness, retention, absorption and translocation in Lolium rigidum and Conyza canadensis. Plants 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gong, H.; Li, Z.; Ruane, S.; Liu, H.; Pambou, E.; Bawn, C.; King, S.; Ma, K.; Li, P.; et al. What happens when pesticides are solubilized in nonionic surfactant micelles. J. Colloid Interface Sci. 2019, 541, 175–182. [Google Scholar] [CrossRef]
- Räsch, A.; Hunsche, M.; Maila, M.; Burkhardt, J.; Noga, G.; Pariyar, S. Agricultural adjuvants may impair leaf transpiration and photosynthetic activity. Plant Physiol. Biochem. 2018, 132, 229–237. [Google Scholar] [CrossRef]
- Krogh, K.; Halling-Sørensen, B.; Mogensen, B.; Vejrup, K. Environmental properties and effects of nonionic surfactant adjuvants in pesticides: A review. Chemosphere 2003, 50, 871–901. [Google Scholar] [CrossRef]
- Bao, Z.; Wu, Y.; Song, R.; Gao, Y.; Zhang, S.; Zhao, K.; Wu, T.; Zhang, C.; Du, F. The simple strategy to improve pesticide bioavailability and minimize environmental risk by effective and ecofriendly surfactants. Sci. Total Environ. 2022, 851, 158169. [Google Scholar] [CrossRef]
- Hu, X.; Gong, H.; Liu, H.; Wang, X.; Wang, W.; Liao, M.; Li, Z.; Ma, K.; Li, P.; Rogers, S.; et al. Contrasting impacts of mixed nonionic surfactant micelles on plant growth in the delivery of fungicide and herbicide. J. Colloid Interface Sci. 2022, 618, 78–87. [Google Scholar] [CrossRef]
- He, L.; Ding, L.; Li, B.; Mu, W.; Li, P.; Liu, F. Regulating droplet wetting and pinning behaviors on pathogen-modified hydrophobic surfaces: Strategies and working mechanisms. J. Agric. Food Chem. 2021, 69, 39, 11720–11732. [Google Scholar] [CrossRef]
- Baratella, V.; Renzaglia, M.; Trinchera, A. Effect of surfactant as adjuvant for irrigation/fertigation in vegetables production: Preliminary results on lettuce. Acta Hortic. 2016, 1123, 157–163. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Krupa, B.; Syguda, A.; Walkiewicz, F.; Wilkowska, M.; Kozak, M.; Materna, K. Surface activity and phytotoxicity of morpholinium herbicidal ionic liquids. J. Mol. Liq. 2022, 362, 119750. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary ammonium compounds (QACs) and ionic liquids (ILs) as biocides: From simple antiseptics to tunable antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Sharonova, N.L.; Rakhmaeva, A.M.; Tyryshkina, A.A.; Kuznetsov, D.M.; Nikitin, E.N.; Zakharova, L.Y. New piperidinium surfactants with a benzyl fragment in the head group: Aggregation properties and the possibility of using to control plant pathogens. Russ. Chem. Bull. 2022, 71, 1679–1686. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Van Ginkel, C.G. Biodegradability of cationic surfactants. In Biodegradability of Surfactants; Karsa, D.R., Porter, M.R., Eds.; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Hosseinzadeh, P.; Solouk, A.; Akbari, S.; Szulc, A.M.; Brycki, B.E. Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: A review. Adv. Colloid Interface Sci. 2022, 299, 102581. [Google Scholar] [CrossRef]
- Sharma, R.; Kamal, R.; Abdinejad, M.A.; Mahajan, R.K.; Kraatz, H.-B. Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interface Sci. 2017, 248, 35–68. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Gao, L.; Wang, F.; Ma, J. Heterocyclic cationic Gemini surfactants for efficient antibacterial, dispersion and fixation. Process Saf. Environ. Prot. 2022, 159, 168–177. [Google Scholar] [CrossRef]
- Amerkhanova, S.K.; Voloshina, A.D.; Mirgorodskaya, A.B.; Lyubina, A.P.; Kuznetsova, D.A.; Kushnazarova, R.A.; Mikhailov, V.A.; Zakharova, L.Y. Antimicrobial properties and cytotoxic effect of imidazolium geminis with tunable hydrophobicity. Int. J. Mol. Sci. 2021, 22, 13148. [Google Scholar] [CrossRef]
- Pisárčik, M.; Pupák, M.; Lukáč, M.; Devínsky, F.; Hubčík, L.; Bukovský, M.; Horváth, B. The Synthesis, Self-assembled structures, and microbicidal activity of cationic gemini surfactants with branched tridecyl chains. Molecules 2019, 24, 4380. [Google Scholar] [CrossRef] [PubMed]
- Frolov, N.; Detusheva, E.; Fursova, N.; Ostashevskaya, I.; Vereshchagin, A. Microbiological evaluation of novel bis-quaternary ammonium compounds: Clinical strains, biofilms, and resistance study. Pharmaceuticals 2022, 15, 514. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, K.; Sobiech, Ł.; Kuliszewska, E.; Skrzypczak, G. Effect of gemini surfactants on the glyphosate efficacy. Przem. Chem. 2014, 93, 911–913. [Google Scholar]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Nikitin, E.N.; Sinyashin, K.O.; Nesterova, L.M.; Zakharova, L.Y. Carbamate-bearing surfactants as effective adjuvants promoted the penetration of the herbicide into the plant. Colloids Surf. A 2020, 586, 124252. [Google Scholar] [CrossRef]
- Todd, O.E.; Figueiredo, M.R.; Morran, S.; Soni, N.; Preston, C.; Kubeš, M.F.; Napier, R.; Gaines, T.A. Gaines. Synthetic auxin herbicides: Finding the lock and key to weed resistance. Plant Sci. 2020, 300, 110631. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.A. Clopyralid. In Pesticide Synthesis Handbook; Elsevier: Amsterdam, The Netherlands, 1996; p. 530. [Google Scholar]
- Boyd, N.S.; Dittmar, P.J. Impact of application time and clopyralid rate on strawberry growth and yield. Weed Technol. 2015, 29, 29821–29826. [Google Scholar] [CrossRef]
- Bobadilla, L.; Hulting, A.; Curtis, D.; Mallory-Smith, C. Application of synthetic auxin herbicides to suppress seed viability of Italian ryegrass (Lolium perenne ssp multiflorum) in tall fescue seed production. Weed Technol. 2020, 34, 489–497. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Kudryavtseva, L.A.; Pankratov, V.A.; Lukashenko, S.S.; Rizvanova, L.Z.; Konovalov, A.I. Gemini alkylammonium surfactants: Aggregation properties and catalytic activity. Russ. J. Gen. Chem. 2006, 76, 1625–1631. [Google Scholar] [CrossRef]
- Gao, X.; Wang, D.; Jiang, Z.; Li, X.; Chen, G. Effect of adjuvants on the wetting behaviors of bifenthrin droplets on tea leaves. Appl. Sci. 2022, 12, 4217. [Google Scholar] [CrossRef]
- Song, Y.; Huang, Q.; Huang, G.; Liu, M.; Cao, L.; Li, F.; Zhao, P.; Cao, C. The Effects of adjuvants on the wetting and deposition of insecticide solutions on hydrophobic wheat leaves. Agronomy 2022, 12, 2148. [Google Scholar] [CrossRef]
- Schreiber, L. Review of sorption and diffusion of lipophilic molecules in cuticular waxes and the effects of accelerators on solute mobilities. J. Exp. Bot. 2006, 57, 2515–2523. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Damato, T.; Carrasco, L.D.M.; Carmona-Ribeiro, A.M.; Luiz, R.V.; Godoy, R.; Petri, D.F.S. The interactions between surfactants and the epicuticular wax on soybean or weed leaves: Maximal crop protection with minimal wax solubilization. Crop. Prot. 2017, 91, 57–65. [Google Scholar] [CrossRef]
- Vadez, V.; Kholová, J.; Hummel, G.; Zhokhavets, U.; Gupta, S.K.; Hash, C.T. LeasyScan: A novel concept combining 3D imaging and lysimetry for high-throughput phenotyping of traits controlling plant water budget. J. Exp. Bot. 2015, 66, 5581–5593. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, K.H.; Ottosen, C.-O. 3D Laser Triangulation for Plant Phenotyping in Challenging Environments. Sensors 2015, 15, 13533–13547. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Liebisch, F.; Hund, A. Plant phenotyping: From bean weighing to image analysis. Plant Methods 2015, 11, 14. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Hein, N.T.; Ciampitti, I.A.; Jagadish, S.K. Bottlenecks and opportunities in field-based high-throughput phenotyping for heat and drought stress. J. Exp. Bot. 2021, 72, 5102–5116. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Yao, Y.; Li, M.; Liu, L. Modern imaging techniques in plant nutrition analysis: A review. Comput. Electron. Agric. 2020, 174, 105459. [Google Scholar] [CrossRef]
- Tanner, F.; Tonn, S.; de Wit, J.; Van den Ackerveken, G.; Berger, B.; Plett, D. Sensor-based phenotyping of above-ground plant-pathogen interactions. Plant Methods 2022, 18, 35. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Li, F.; He, L.; Harrington, K.C. Characterization of clopyralid resistance in lawn burweed (Soliva sessilis). PLoS ONE 2021, 16, e0253934. [Google Scholar] [CrossRef] [PubMed]
- Amaç, E.; Liman, R. Cytotoxic and genotoxic effects of clopyralid herbicide on Allium cepa roots. Environ. Sci. Pollut Res. 2021, 28, 48450–48458. [Google Scholar] [CrossRef]
- Bloem, E.; Gerighausen, H.; Chen, X.; Schnug, E. The Potential of Spectral Measurements for Identifying Glyphosate Application to Agricultural Fields. Agronomy 2020, 10, 1409. [Google Scholar] [CrossRef]
- Ranjan, P.N.; Ram, C.J.; Anurag, T.; Nilesh, J.; Kumar, P.B.; Suresh, Y.; Rahul, K. Breeding for herbicide tolerance in crops: A review. Res. J. Biotechnol. 2020, 15, 4. [Google Scholar]
- Abou-Khater, L.; Maalouf, F.; Patil, S.B.; Balech, R.; Nacouzi, D.; Rubiales, D.; Kumar, S. Identification of tolerance to metribuzin and imazethapyr herbicides in faba bean. Crop Sci. 2021, 61, 2593–2611. [Google Scholar] [CrossRef]


| Name | Composition |
|---|---|
| Hacker WG | Clopyralid potassium salt, 0.03% wt. by acid, from water-dispersible granules |
| Hacker 300 SL | Clopyralid monoethanolamine salt, 0.03% wt. by acid, from soluble liquid |
| Clopyralid SMC | Clopyralid acid (0.03% wt.), 16-6-16 (0.01% wt.), supramolecular complex |
| Solution | Si/S0 1 | θ, ° | γ, mN m−1 |
|---|---|---|---|
| Water | 1 | 101.3 | 72.2 |
| Clopyralid 0.03% wt. | 1 | 106.3 | 68.6 |
| 16-6-16 0.01% wt. | 3.1 | 83 | 43.6 |
| Clopyralid SMC | 3.4 | 74 | 38.8 |
| Solution | CMC, %wt. | Dh, nm | PdI |
|---|---|---|---|
| 16-6-16 0.01% wt. | 0.0013 | 3.5 | 0.648 |
| Clopyralid SMC | 0.0029 | 7.2 | 0.325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirgorodskaya, A.B.; Kushnazarova, R.A.; Zakharova, L.Y.; Ulyanova, A.A.; Litvinov, D.Y.; Blinkov, A.O.; Divashuk, M.G.; Kochanova, I.A.; Nesterova, L.M. Enhanced Herbicidal Action of Clopyralid in the Form of a Supramolecular Complex with a Gemini Surfactant. Agronomy 2023, 13, 973. https://doi.org/10.3390/agronomy13040973
Mirgorodskaya AB, Kushnazarova RA, Zakharova LY, Ulyanova AA, Litvinov DY, Blinkov AO, Divashuk MG, Kochanova IA, Nesterova LM. Enhanced Herbicidal Action of Clopyralid in the Form of a Supramolecular Complex with a Gemini Surfactant. Agronomy. 2023; 13(4):973. https://doi.org/10.3390/agronomy13040973
Chicago/Turabian StyleMirgorodskaya, Alla B., Rushana A. Kushnazarova, Lucia Ya. Zakharova, Alana A. Ulyanova, Dmitry Y. Litvinov, Andrey O. Blinkov, Mikhail G. Divashuk, Irina A. Kochanova, and Liliya M. Nesterova. 2023. "Enhanced Herbicidal Action of Clopyralid in the Form of a Supramolecular Complex with a Gemini Surfactant" Agronomy 13, no. 4: 973. https://doi.org/10.3390/agronomy13040973
APA StyleMirgorodskaya, A. B., Kushnazarova, R. A., Zakharova, L. Y., Ulyanova, A. A., Litvinov, D. Y., Blinkov, A. O., Divashuk, M. G., Kochanova, I. A., & Nesterova, L. M. (2023). Enhanced Herbicidal Action of Clopyralid in the Form of a Supramolecular Complex with a Gemini Surfactant. Agronomy, 13(4), 973. https://doi.org/10.3390/agronomy13040973







