Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Vernalization Treatment
2.2. RNA Extraction and qRT-PCR
2.3. Determination of Phytohormone Contents
2.4. RNA-Seq and Differential Expression Analysis
2.5. Bioinformatics
2.6. Exogenous Phytohormones Treated for Sugar Beet Seedlings
3. Results
3.1. Changes in Bolting-Related Gene Expression and the Bolting Rate after Vernalization
3.2. The Effect of Vernalization on the Levels of Phytohormone
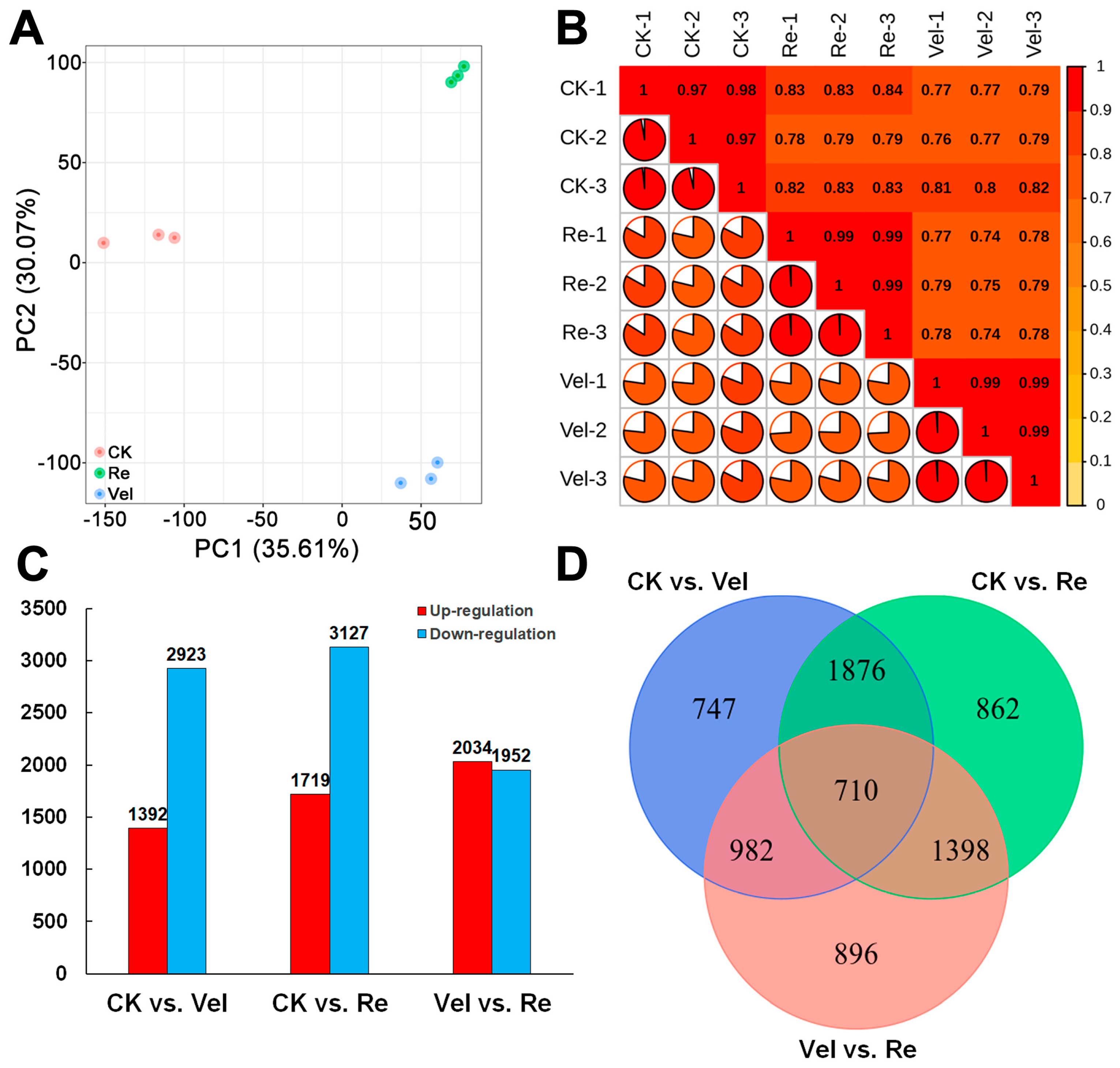
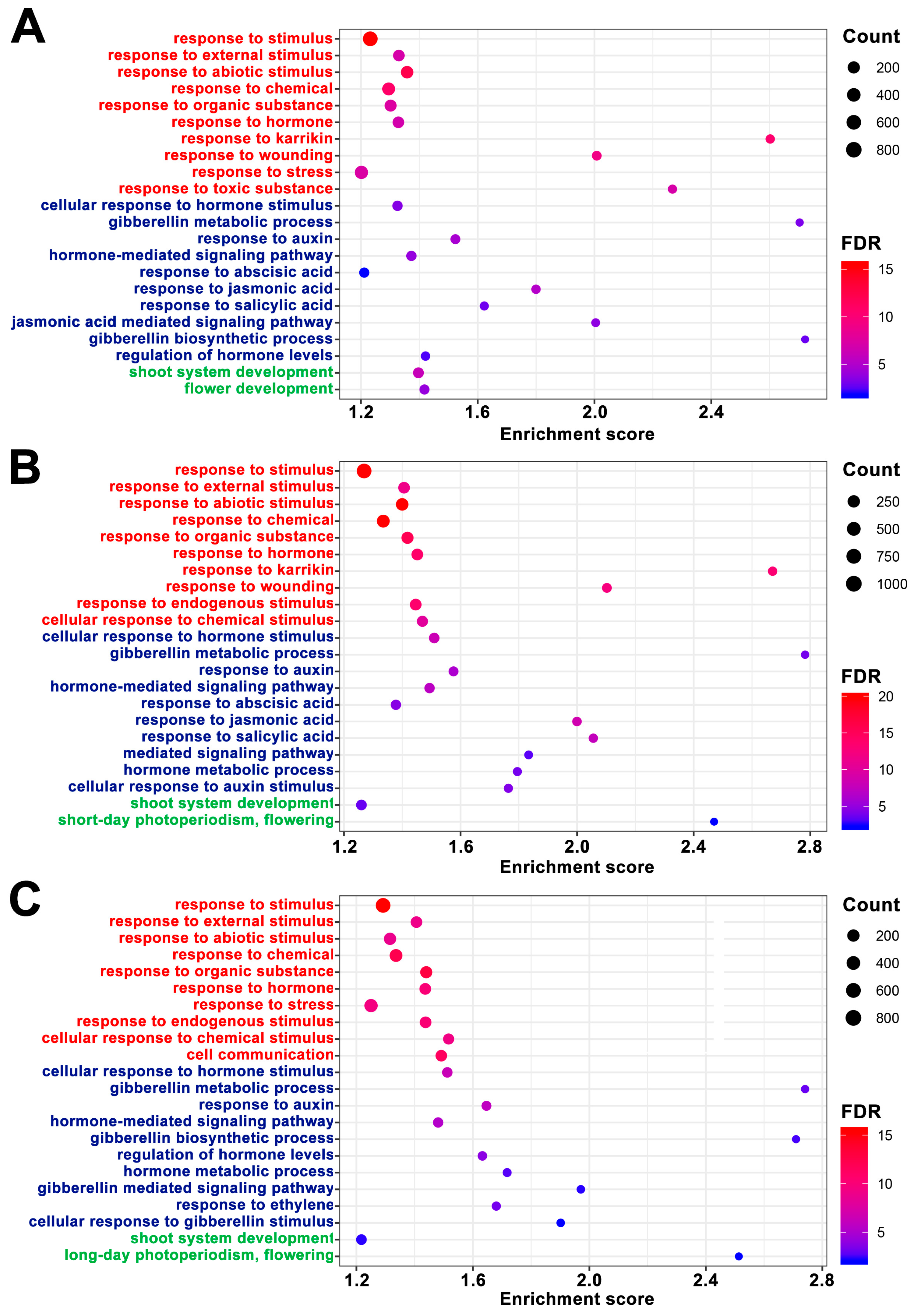
3.3. Transcriptome Changes in the Shoot Apex of Sugar Beet during Vernalization
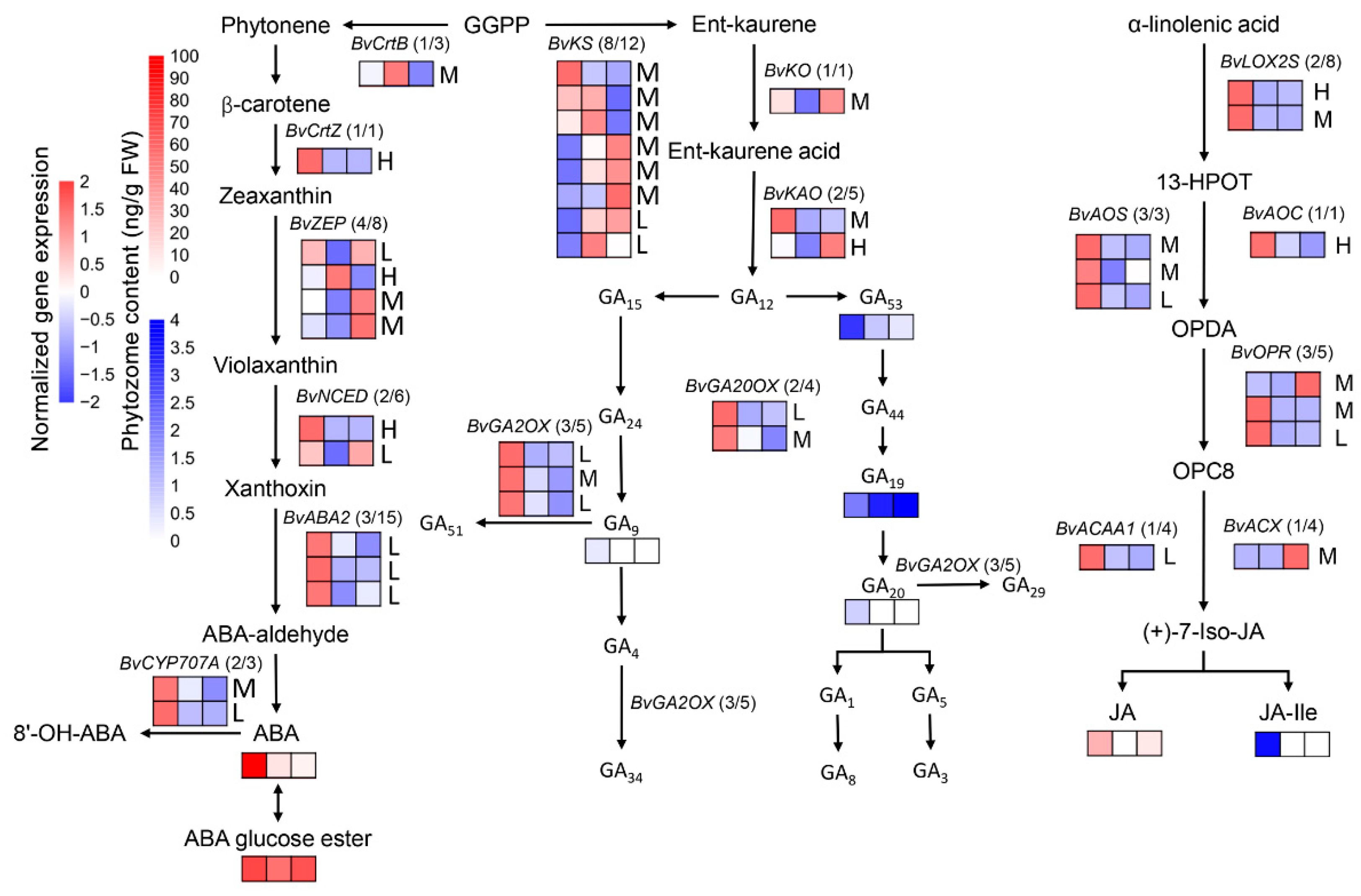
3.4. The Change of DEGs Involved in Phytohormone Metabolism
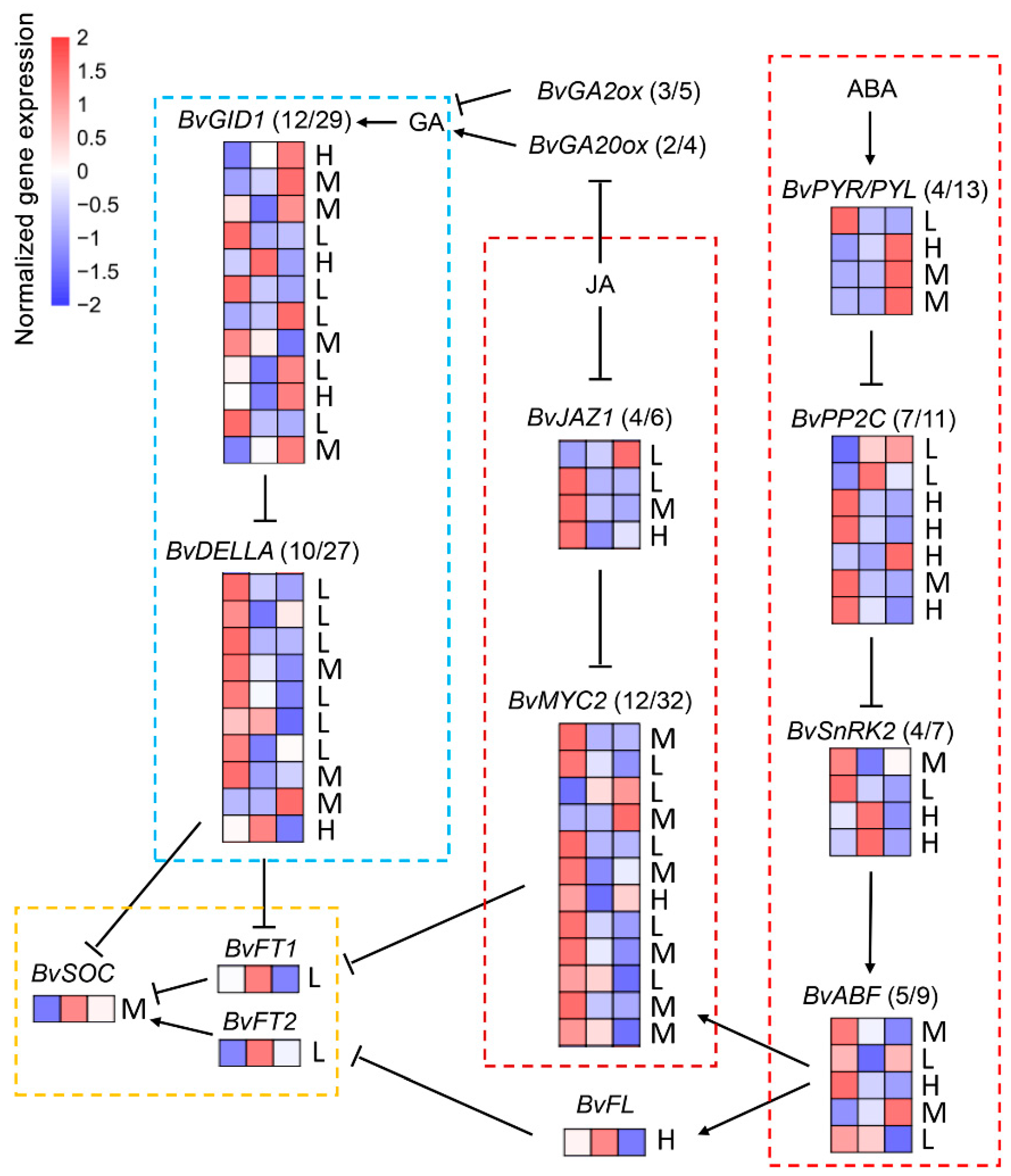
3.5. The Change of DEGs Involved in Phytohormone Signalling
3.6. qRT-PCR-Based Experimental Validation of Select Genes Involved in ABA, JA and GA Biosynthesis and Signaling
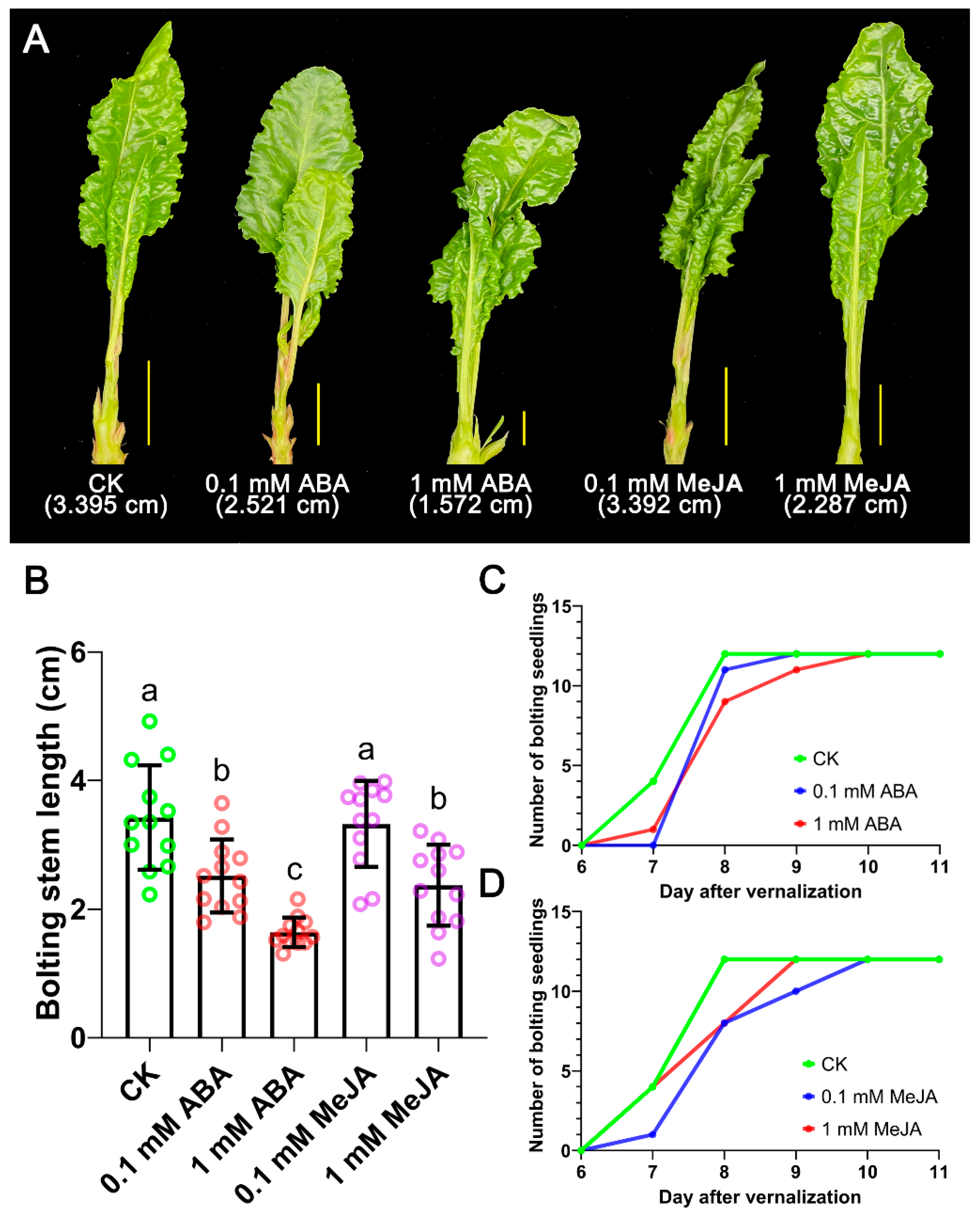
3.7. Bolting Traits of Seedlings Treated by Combinations of GA3, ABA and MeJA
4. Discussion
4.1. Vernalization Suppressed Biosynthesis of ABA and JA, Alleviating the Inhibitory Effect on GA-Induced Bolting
4.2. Vernalization Directly Repressed the Expression of BvDELLAs for Bolting, Although GA Biosynthesis Was Partially Repressed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The Genome of the Recently Domesticated Crop Plant Sugar Beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Müdsam, C.; Keller, I.; Zierer, W.; Czarnecki, O.; Corral, J.M.; Reinhardt, F.; Nieberl, P.; Fiedler-Wiechers, K.; Sommer, F.; et al. Vernalization Alters Sink and Source Identities and Reverses Phloem Translocation from Taproots to Shoots in Sugar Beet. Plant Cell 2020, 32, 3206–3223. [Google Scholar] [CrossRef] [PubMed]
- Dally, N.; Xiao, K.; Holtgräwe, D.; Jung, C. The B2 Flowering Time Locus of Beet Encodes a Zinc Finger Transcription Factor. Proc. Natl. Acad. Sci. USA 2014, 111, 10365–10370. [Google Scholar] [CrossRef]
- Pin, P.A.; Zhang, W.; Vogt, S.H.; Dally, N.; Büttner, B.; Schulze-Buxloh, G.; Jelly, N.S.; Chia, T.Y.P.; Mutasa-Göttgens, E.S.; Dohm, J.C.; et al. The Role of a Pseudo-Response Regulator Gene in Life Cycle Adaptation and Domestication of Beet. Curr. Biol. 2012, 22, 1095–1101. [Google Scholar] [CrossRef]
- Pin, P.A.; Benlloch, R.; Bonnet, D.; Wremerth-Weich, E.; Kraft, T.; Gielen, J.J.L.; Nilsson, O. An Antagonistic Pair of FT Homologs Mediates the Control of Flowering Time in Sugar Beet. Science 2010, 330, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Langridge, J. Effect of Day-Length and Gibberellic Acid on the Flowering of Arabidopsis. Nature 1957, 180, 36–37. [Google Scholar] [CrossRef]
- Guan, H.; Huang, X.; Zhu, Y.; Xie, B.; Liu, H.; Song, S.; Hao, Y.; Chen, R. Identification of DELLA Genes and Key Stage for GA Sensitivity in Bolting and Flowering of Flowering Chinese Cabbage. Int. J. Mol. Sci. 2021, 22, 12092. [Google Scholar] [CrossRef]
- Jung, H.; Jo, S.H.; Jung, W.Y.; Park, H.J.; Lee, A.; Moon, J.S.; Seong, S.Y.; Kim, J.K.; Kim, Y.S.; Cho, H.S. Gibberellin Promotes Bolting and Flowering via the Floral Integrators RsFT and RsSOC1-1 under Marginal Vernalization in Radish. Plants 2020, 9, 594. [Google Scholar] [CrossRef]
- Liu, X.; Lv, S.; Liu, R.; Fan, S.; Liu, C.; Liu, R.; Han, Y. Transcriptomic Analysis Reveals the Roles of Gibberellin-Regulated Genes and Transcription Factors in Regulating Bolting in Lettuce (Lactuca Sativa L.). PLoS ONE 2018, 13, e0191518. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.S.; Joshi, A.; Holmes, H.F.; Hedden, P.; Göttgens, B. A New RNASeq-Based Reference Transcriptome for Sugar Beet and Its Application in Transcriptome-Scale Analysis of Vernalization and Gibberellin Responses. BMC Genom. 2012, 13, 99. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Huang, G.; Cheng, P.; Gong, X.; Shen, L.; Yu, H. Molecular Basis of Natural Variation in Photoperiodic Flowering Responses. Dev. Cell 2019, 50, 90–101.e3. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New Insights into Gibberellin Signalling in Regulating Flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.N.; Heckman, J.W.; Somerville, C.R. Gibberellin is Required for Flowering in Arabidopsis Thaliana under Short Days. Plant Physiol. 1992, 100, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, R.; Zhang, H.; Wang, S.; Tang, S.; Yang, W.; Xie, Q. ABSCISIC ACID-INSENSITIVE 4 Negatively Regulates Flowering through Directly Promoting Arabidopsis FLOWERING LOCUS C Transcription. J. Exp. Bot. 2016, 67, 195–205. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Lu, Y.; Chen, X.; Wu, Y. The Inhibitory Effect of ABA on Floral Transition Is Mediated by ABI5 in Arabidopsis. J. Exp. Bot. 2013, 64, 675–684. [Google Scholar] [CrossRef]
- Zhang, X.; Garreton, V.; Chua, N.H. The AIP2 E3 Ligase Acts as a Novel Negative Regulator of ABA Signalling by Promoting ABI3 Degradation. Genes. Dev. 2005, 19, 1532–1543. [Google Scholar] [CrossRef]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High Temperature-Induced Abscisic Acid Biosynthesis and Its Role in the Inhibition of Gibberellin Action in Arabidopsis Seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef]
- Hong, G.; Xue, X.; Mao, Y.; Wang, L.; Chen, X. Arabidopsis MYC2 Interacts with DELLA Proteins in Regulating Sesquiterpene Synthase Gene Expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhang, X.; Wu, F.; Feng, H.; Deng, L.; Xu, L.; Zhang, M.; Wang, Q.; Li, C. Transcriptional Mechanism of Jasmonate Receptor COI1-Mediated Delay of Flowering Time in Arabidopsis. Plant Cell 2015, 27, 2814–2828. [Google Scholar] [CrossRef]
- Cheng, H.; Song, S.; Xiao, L.; Soo, H.M.; Cheng, Z.; Xie, D.; Peng, J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 2009, 5, e1000440. [Google Scholar] [CrossRef]
- Wijayanti, L.; Fujioka, S.; Kobayashi, M.; Sakurai, A. Involvement of Abscisic Acid and Indole-3-Acetic Acid in the Flowering of Pharbitis nil. J. Plant Growth Regul. 1997, 16, 115–1119. [Google Scholar] [CrossRef]
- Frigerio, M.; Alabadí, D.; Pérez-Gómez, J.; García-Cárcel, L.; Phillips, A.L.; Hedden, P.; Blázquez, M.A. Transcriptional Regulation of Gibberellin Metabolism Genes by Auxin Signalling in Arabidopsis. Plant Physiol. 2006, 142, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Harberd, N.P. Auxin Promotes Arabidopsis Root Growth by Modulating Gibberellin Response. Nature 2003, 421, 740–743. [Google Scholar] [CrossRef]
- Erwin, J.E.; Warner, R.M.; Smith, A.G. Vernalization, Photoperiod and GA3 Interact to Affect Flowering of Japanese Radish (Raphanus Sativus Chinese Radish Jumbo Scarlet). Physiol. Plant. 2002, 115, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Hazebroek, J.P.; Metzger, J.D.; Mansager, E.R. Thermoinductive Regulation of Gibberellin Metabolism in Thlaspi Arvense L. (II. Cold Induction of Enzymes in Gibberellin Biosynthesis). Plant Physiol. 1993, 102, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Zanewich, K.P.; Rood, S.B. Vernalization and Gibberellin Physiology of Winter Canola (Endogenous Gibberellin (GA) Content and Metabolism of [3H]GA1 and [3H]GA20. Plant Physiol. 1995, 108, 615–621. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.S.; Qi, A.; Zhang, W.; Schulze-Buxloh, G.; Jennings, A.; Hohmann, U.; Müller, A.E.; Hedden, P. Bolting and Flowering Control in Sugar Beet: Relationships and Effects of Gibberellin, the Bolting Gene B and Vernalization. AoB Plants 2010, 2010, plq012. [Google Scholar] [CrossRef]
- Mateos, J.L.; Madrigal, P.; Tsuda, K.; Rawat, V.; Richter, R.; Romera-Branchat, M.; Fornara, F.; Schneeberger, K.; Krajewski, P.; Coupland, G. Combinatorial Activities of SHORT VEGETATIVE PHASE and FLOWERING LOCUS C Define Distinct Modes of Flowering Regulation in Arabidopsis. Genome Biol. 2015, 16, 31. [Google Scholar] [CrossRef]
- Li, M.; An, F.; Li, W.; Ma, M.; Feng, Y.; Zhang, X.; Guo, H. DELLA Proteins Interact with FLC to Repress Flowering Transition: DELLAs-FLC Interactions in Regulating Flower Time. J. Integr. Plant Biol. 2016, 58, 642–655. [Google Scholar] [CrossRef]
- Reeves, P.A.; He, Y.; Schmitz, R.J.; Amasino, R.M.; Panella, L.W.; Richards, C.M. Evolutionary Conservation of the FLOWERING LOCUS C-Mediated Vernalization Response: Evidence from the Sugar Beet (Beta Vulgaris). Genetics 2007, 176, 295–307. [Google Scholar] [CrossRef]
- Vogt, S.H.; Weyens, G.; Lefèbvre, M.; Bork, B.; Schechert, A.; Müller, A.E. The FLC-like Gene BvFL1 is Not a Major Regulator of Vernalization Response in Biennial Beets. Front. Plant. Sci. 2014, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, O.A.; Elsheery, N.I.; Karkour, A.M.; Elhamouly, N.; Arafa, R.A.; Mahmoud, G.A.; Dawood, M.F.; Hussein, W.E.; Mansour, A.; Amin, D.H.; et al. Jasmonic Acid Regulates Plant Development and Orchestrates Stress Response during Tough Times. Environ. Exp. Bot. 2023, 208, 105260. [Google Scholar] [CrossRef]
- Pi, Z.; Xing, W.; Zhu, X.; Long, J.; Zou, Y.; Wu, Z. Temporal Expression Pattern of Bolting-Related Genes During Vernalization in Sugar Beet. Sugar Tech 2021, 23, 146–157. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Van Bel, M.; Silvestri, F.; Weitz, E.M.; Kreft, L.; Botzki, A.; Coppens, F.; Vandepoele, K. PLAZA 5.0: Extending the Scope and Power of Comparative and Functional Genomics in Plants. Nucleic Acids Res. 2022, 50, D1468–D1474. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Freytes, S.N.; Canelo, M.; Cerdán, P.D. Regulation of Flowering Time: When and Where? Curr. Opin. Plant Biol. 2021, 63, 102049. [Google Scholar] [CrossRef]
- Liang, N.G.; Cheng, D.Y.; Liu, Q.H.; Luo, C.F.; Dai, C.H. Vernalization and Photoperiods Mediated IAA and ABA Synthesis Genes Expression in Beta vulgaris. Russ. J. Plant Physiol. 2018, 65, 642–650. [Google Scholar] [CrossRef]
- Diallo, A.O.; Agharbaoui, Z.; Badawi, M.A.; Ali-Benali, M.A.; Moheb, A.; Houde, M.; Sarhan, F. Transcriptome Analysis of an mvp Mutant Reveals Important Changes in Global Gene Expression and a Role for Methyl Jasmonate in Vernalization and Flowering in Wheat. J. Exp. Bot. 2014, 65, 2271–2286. [Google Scholar] [CrossRef]
- Shang, M.; Wang, X.; Zhang, J.; Qi, X.; Ping, A.; Hou, L.; Xing, G.; Li, G.; Li, M. Genetic Regulation of GA Metabolism during Vernalization, Floral Bud Initiation and Development in Pak Choi (Brassica Rapa ssp. Chinensis Makino). Front. Plant Sci. 2017, 8, 1533. [Google Scholar] [CrossRef]
- Deng, W.; Casao, M.C.; Wang, P.; Sato, K.; Hayes, P.M.; Finnegan, E.J.; Trevaskis, B. Direct Links between the Vernalization Response and Other Key Traits of Cereal Crops. Nat. Commun. 2015, 6, 5882. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA Signalling in Stress-response and Seed Development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and SignallingPathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Riboni, M.; Robustelli Test, A.; Galbiati, M.; Tonelli, C.; Conti, L. ABA-Dependent Control of GIGANTEA Signalling Enables Drought Escape via Up-regulation of FLOWERING LOCUS T in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6309–6322. [Google Scholar] [CrossRef] [PubMed]
- Nir, I.; Moshelion, M.; Weiss, D. The Arabidopsis GIBBERELLIN METHYL TRANSFERASE 1 Suppresses Gibberellin Activity, Reduces Whole-Plant Transpiration and Promotes Drought Tolerance in Transgenic Tomato: GAMT1 Promotes Drought Tolerance. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Zhang, Q.; Liu, N.; Xu, Q.; Hu, L. Differential Physiological and Metabolic Response to Low Temperature in Two Zoysiagrass Genotypes Native to High and Low latitude. PLoS ONE 2018, 13, e0198885. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Hettenhausen, C.; Lange, T.; Wünsche, H.; Fang, J.; Baldwin, I.T.; Wu, J. High Levels of Jasmonic Acid Antagonize the Biosynthesis of Gibberellins and Inhibit the Growth of Nicotiana attenuata Stems. Plant J. 2013, 73, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Yazdanian, E.; Golkar, P.; Vahabi, M.R.; Taghizadeh, M. Elicitation Effects on Some Secondary Metabolites and Antioxidant Activity in Callus Cultures of Allium jesdianum Boiss. & Buhse.: Methyl Jasmonate and Putrescine. Appl. Biochem. Biotech. 2022, 194, 601–619. [Google Scholar]
- Sorce, C.; Stevanato, P.; Biancardi, E.; Lorenzi, R. Physiological Mechanisms of Floral Stem Elongation (Bolting) Control in Sugar Beet (Beta vulgaris ssp. Vulgaris L.). Agroindustria 2002, 1, 87–91. [Google Scholar]
- Hedden, P.; Phillips, A.L. Gibberellin Metabolism: New Insights Revealed by the Genes. Trends Plant Sci. 2000, 12, 523–530. [Google Scholar] [CrossRef]
- Hernández-García, J.; Briones-Moreno, A.; Blázquez, M.A. Origin and Evolution of Gibberellin Signalling and Metabolism in Plants. Semin. Cell Dev. Biol. 2021, 109, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Arana, M.V.; Marín-de la Rosa, N.; Maloof, J.N.; Blázquez, M.A.; Alabadí, D. Circadian Oscillation of Gibberellin Signalling in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2011, 108, 9292–9297. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, S.; Yu, Q.; Zhang, C.; Wang, L.; Jiang, Y.; Wu, Z.; Pi, Z. Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis. Agronomy 2023, 13, 1251. https://doi.org/10.3390/agronomy13051251
Zhao L, Li S, Yu Q, Zhang C, Wang L, Jiang Y, Wu Z, Pi Z. Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis. Agronomy. 2023; 13(5):1251. https://doi.org/10.3390/agronomy13051251
Chicago/Turabian StyleZhao, Lijuan, Shengnan Li, Qingyang Yu, Chunxue Zhang, Liumin Wang, Yichen Jiang, Zedong Wu, and Zhi Pi. 2023. "Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis" Agronomy 13, no. 5: 1251. https://doi.org/10.3390/agronomy13051251
APA StyleZhao, L., Li, S., Yu, Q., Zhang, C., Wang, L., Jiang, Y., Wu, Z., & Pi, Z. (2023). Vernalization Promotes GA-Mediated Bolting Initiation via the Inhibition of ABA and JA Biosynthesis. Agronomy, 13(5), 1251. https://doi.org/10.3390/agronomy13051251





