Effect of Applying an Organic Amendment on the Persistence of Tebuconazole and Fluopyram in Vineyard Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Organic and Inorganic Amendments
2.3. Experimental Setup
2.4. Fungicide Application and Soil Sampling
2.5. Fungicides Extraction and Analysis
2.6. Adsorption Experiments
2.7. Data Analysis
3. Results and Discussion
3.1. Weather Conditions during the Field Experiment
3.2. Adsorption of Fungicides by Unamended and Amended Soils
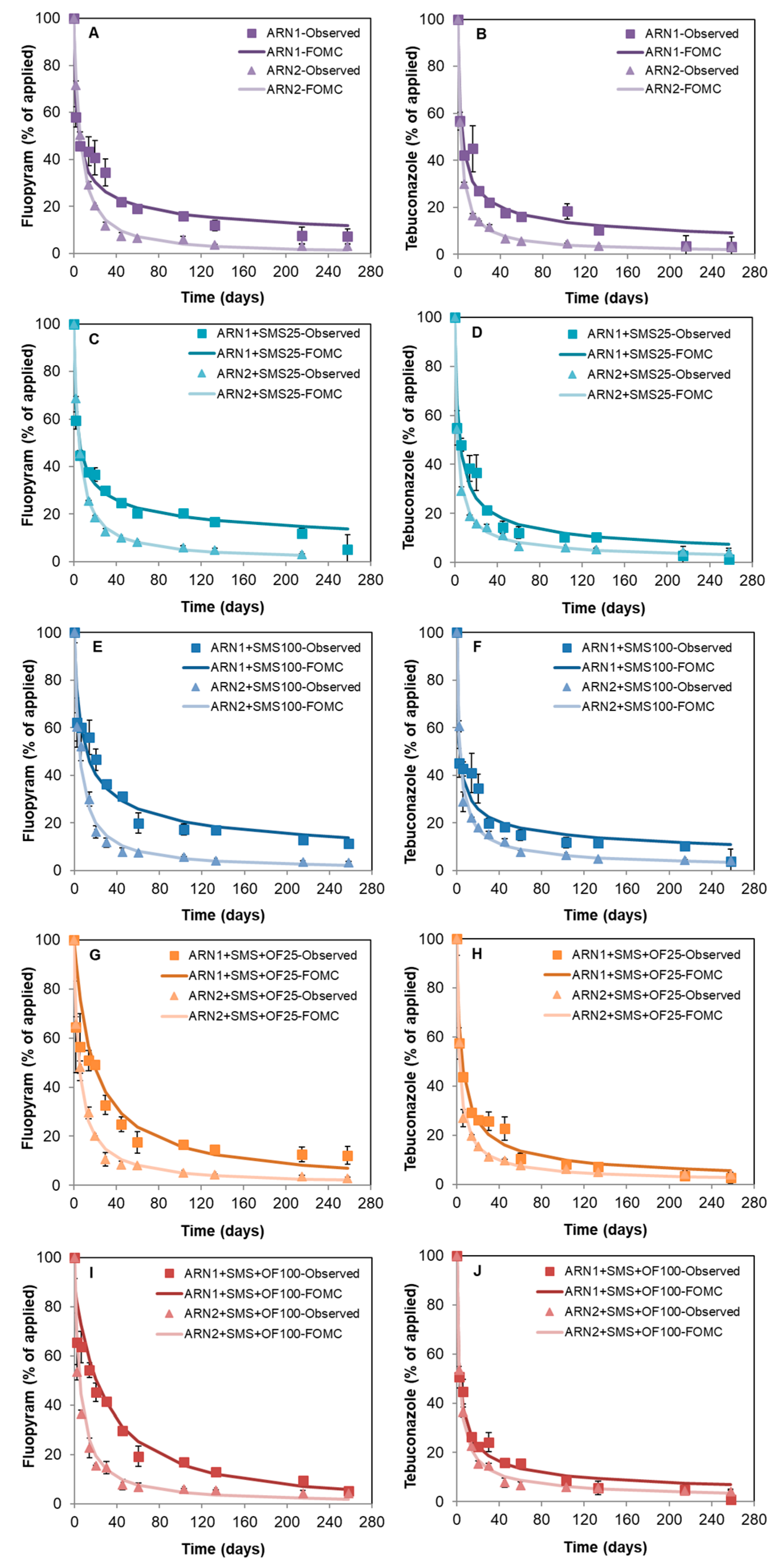
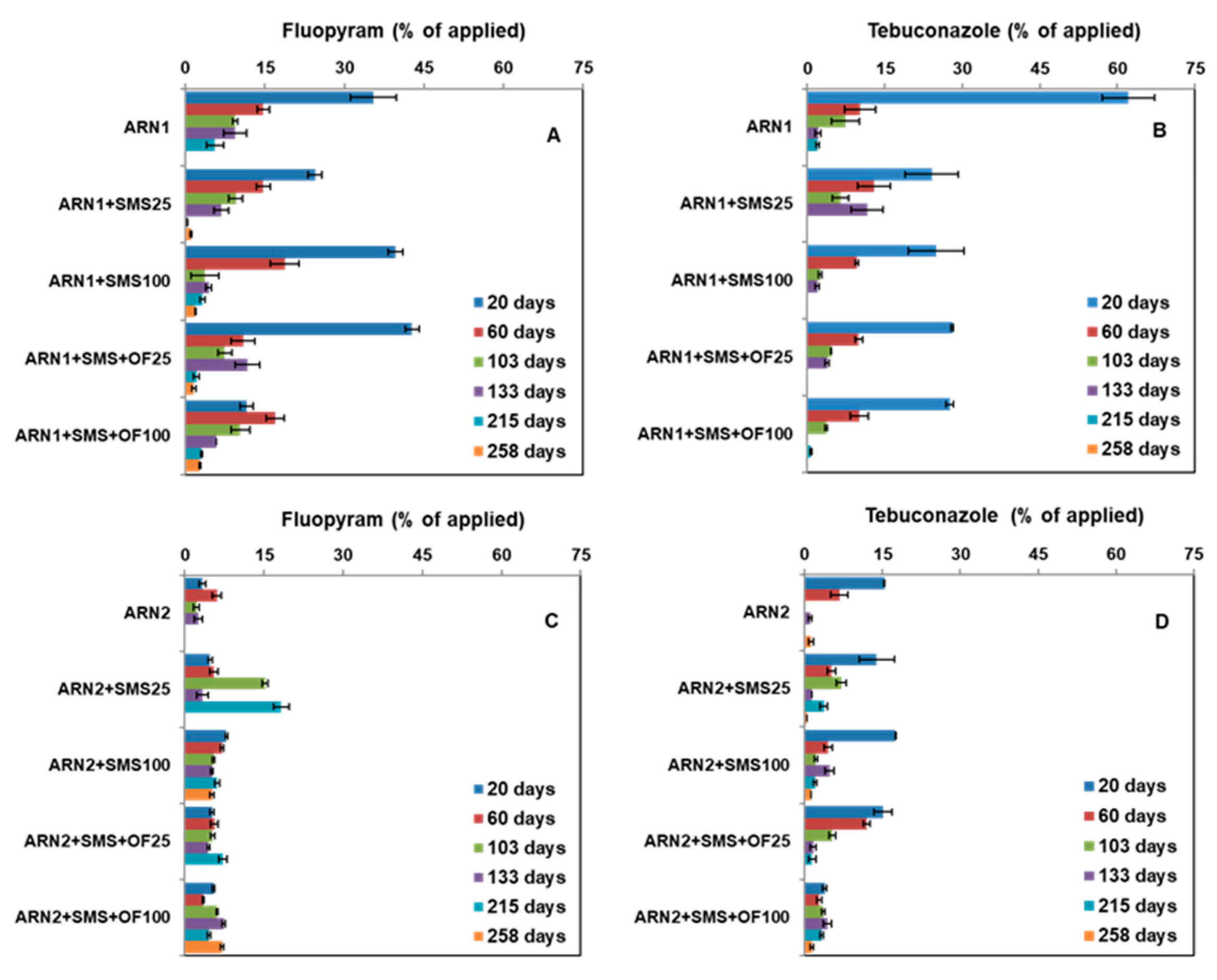
3.3. Dissipation of Fungicides in Unamended and Amended Soils
3.4. Mobility of Fungicides at 15–30 cm Depth in Unamended and Amended Soils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Agri-Environmental Indicator—Consumption of Pesticides. April 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agri-environmental_indicator_-_consumption_of_pesticides (accessed on 1 February 2023).
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Bruhl, C.A.; Imfeld, G.; Knabel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmuller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of fungicides and factors affecting their fate and removal efficacy: A review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance fluopyram. EFSA J. 2013, 11, 3052. [Google Scholar]
- Rathod, P.H.; Shah, P.G.; Parmar, K.D.; Kalasariya, R.L. The fate of fluopyram in the soil–water–plant ecosystem: A review. Rev. Environ. Contam. Toxicol. 2022, 260, 1. [Google Scholar] [CrossRef]
- Matadha, N.Y.; Mohapatra, S.; Siddamallaiah, L.; Udupi, V.R.; Gadigeppa, S.; Raja, D.P.; Donagar, S.P.; Hebbar, S.S. Persistence and dissipation of fluopyram and tebuconazole on bell pepper and soil under different environmental conditions. Int. J. Environ. Anal. Chem. 2021, 101, 2408–2427. [Google Scholar] [CrossRef]
- PPDB (Pesticide Properties DataBase). University of Hertfordshire. 2023. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed on 30 January 2023).
- EFSA (European Food Safety Authority). Conclusion on the peer review of the pesticide risk assessment of the active substance tebuconazole. EFSA J. 2014, 12, 3485. [Google Scholar]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S. Intra-annual trends of fungicide residues in waters from vineyard areas in La Rioja region of northern Spain. Environ. Sci. Pollut. Res. 2016, 23, 22924–22936. [Google Scholar] [CrossRef]
- Pinasseau, L.; Wiest, L.; Fildier, A.; Volatier, L.; Fones, G.R.; Mills, G.A.; Mermillod-Blondin, F.; Vulliet, E. Use of passive sampling and high resolution mass spectrometry using a suspect screening approach to characterise emerging pollutants in contaminated groundwater and runoff. Sci. Total Environ. 2019, 672, 253–263. [Google Scholar] [CrossRef]
- Sjerps, R.; Kooij, P.J.; van Loon, A.; Van Wezel, A.P. Occurrence of pesticides in Dutch drinking water sources. Chemosphere 2019, 235, 510–518. [Google Scholar] [CrossRef]
- Manjarres-López, D.P.; Andrades, M.S.; Sánchez-González, S.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Herrero-Hernández, E. Assessment of pesticide residues in waters and soils of a vineyard region and its temporal evolution. Environ. Pollut. 2021, 284, 116473. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mayán, L.; Ramil, M.; Cela, R.; Rodríguez, I. Multiresidue procedure to assess the occurrence and dissipation of fungicides and insecticides in vineyard soils from Northwest Spain. Chemosphere 2020, 261, 127696. [Google Scholar] [CrossRef] [PubMed]
- Carpio, M.J.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J.; Marín-Benito, J.M. Pesticide fate in soils under different agricultural management practices. In Pesticides in Soils: Ocurrence, Fate, Control and Remediation. The Handbook of Environmental Chemistry; Rodríguez-Cruz, M.S., Sánchez-Martín, M.J., Eds.; Springer: Cham, Switzerland, 2022; Volume 113, pp. 251–286. [Google Scholar]
- Lohithaswan, E.N.; George, T.; Subhachandrakumar, V.K. Persistence of fluopyram under varying moisture conditions in laterite and red loam soils of Kerala, India. Int. J. Environ. Sci. Technol. 2022, in press. [Google Scholar] [CrossRef]
- Dong, B.; Hu, J. Dissipation and residue determination of fluopyram and tebuconazole residues in watermelon and soil by GC-MS. Int. J. Environ. Anal. Chem. 2014, 94, 493–505. [Google Scholar] [CrossRef]
- Podbielska, M.; Szpyrka, E.; Piechowicz, B.; Zwolak, A.; Sadło, S. Behavior of fluopyram and tebuconazole and some selected pesticides in ripe apples and consumer exposure assessment in the applied crop protection framework. Environ. Monit. Assess. 2017, 189, 350. [Google Scholar] [CrossRef]
- Boletín de Avisos Fitosanitarios. Estación de Avisos Agrícolas de La Rioja, Sección de Protección de Cultivos de la Consejería de Agricultura, Ganadería, Mundo Rural, Territorio y Población, Gobierno de La Rioja. 2022. Available online: https://www.larioja.org/agricultura/es/publicaciones-agricultura/boletin-avisos-fitosanitarios-2022 (accessed on 13 February 2023).
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Sun, C.; Yang, K.; Zheng, J. Differences in soil physical properties caused by applying three organic amendments to loamy clay soil under field conditions. J. Soils Sediments 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Cunha, G.O.M.; de Almeida, J.A.; Medeiros Coelho, C.M. Chemical composition of soybean seeds subjected to fertilization with rock dusts. Acta Sci. Agron. 2022, 44, e53312. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Andrades, M.S.; Villalba Eguren, G.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Organic Amendment for the Recovery of Vineyard Soils: Effects of a Single Application on Soil Properties over Two Years. Processes 2022, 10, 317. [Google Scholar] [CrossRef]
- Carpio, M.J.; Andrades, M.S.; Herrero-Hernández, E.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Changes in vineyard soil parameters after repeated application of organic-inorganic amendments based on spent mushroom substrate. Environ. Res. 2023, 221, 115339. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Kuenz, A.; Rahmann, G. Integration of mushroom production into circular food chains. Org. Agr. 2021, 11, 309–317. [Google Scholar] [CrossRef]
- Kulshreshtha, S. Removal of pollutants using spent mushrooms substrates. Environ. Chem. Lett. 2019, 17, 833–847. [Google Scholar] [CrossRef]
- Centro Tecnológico de Investigación del Champiñon de La Rioja. Available online: https://www.ctich.com (accessed on 30 January 2023).
- VITIREG. Grupo Operativo Viticultura Regenerativa. Available online: http://vitireg.org/ (accessed on 30 January 2023).
- Herrero-Hernández, E.; Andrades, M.S.; Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Field-scale dissipation of tebuconazole in a vineyard soil amended with spent mushroom substrate and its potential environmental impact. Ecotoxicol. Environ. Saf. 2011, 74, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Dissipation of fungicides in a vineyard soil amended with different spent mushroom substrates. J. Agric. Food Chem. 2012, 60, 6936–6945. [Google Scholar] [CrossRef]
- Herrero-Hernández, E.; Marín-Benito, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Field versus laboratory experiments to evaluate the fate of azoxystrobin in an amended vineyard soil. J. Environ. Manag. 2015, 163, 78–86. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Pose-Juan, E.; Rodríguez-Cruz, M.S. Effect of different rates of spent mushroom substrate on the dissipation and bioavailability of cymoxanil and tebuconazole in an agricultural soil. Sci. Total Environ. 2016, 550, 495–503. [Google Scholar] [CrossRef]
- Carpio, M.J.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S.; Marín-Benito, J.M. Effect of Organic Residues on Pesticide Behavior in Soils: A Review of Laboratory Research. Environments 2021, 8, 32. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Impact of spent mushroom substrates on the fate of pesticides in soil, and their use for preventing and/or controlling soil and water contamination: A review. Toxics 2016, 4, 17. [Google Scholar] [CrossRef]
- García-Gómez, A.; Szmidt, R.A.K.; Roig, S.A. Enhancing of the composting rate of spent mushroom substrate by rock dust. Compost Sci. Utilization 2002, 10, 99–104. [Google Scholar] [CrossRef]
- Li, J.; Mavrodi, D.V.; Dong, Y. Effect of rock dust-amended compost on the soil properties, soil microbial activity, and fruit production in an apple orchard from the Jiangsu province of China. Arch. Agron. Soil Sci. 2020, 67, 1313–1326. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; United States Department of Agriculture (USDA)—Natural Resources Conservation Service: Washington, DC, USA, 2010. [Google Scholar]
- FOCUS (Forum for Co-Ordination of Pesticide Fate Models and Their Use). Guidance Document on Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, Report of the FOCUS Work Group on Degradation Kinetics. EC Documents Reference Sanco/10058/2005 version 2.0; FOCUS Work Group on Degradation Kinetics: Brussels, Belgium, 2006; 434p. [Google Scholar]
- Zhou, J.; Liang, S.; Cui, Y.; Rong, Y.; Song, J.; Lv, D. Study on environmental behaviour of fluopyram in different banana planting soil. Sci. Rep. 2021, 11, 15346. [Google Scholar] [CrossRef] [PubMed]
- Cadková, E.; Komárek, M.; Kaliszová, R.; Vanek, A.; Balíková, M. Tebuconazole sorption in contrasting soil types. Soil Sediment Contam. 2013, 22, 404–414. [Google Scholar] [CrossRef]
- Škulcová, L.; Neuwirthová, N.; Šimek, Z.; Trojan, M.; Bielská, L. Enantioselective behavior of the fungicide tebuconazole in soil. Environ. Process. 2020, 7, 173–188. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in the sorption–desorption of fungicides over time in an amended sandy clay loam soil under laboratory conditions. J. Soils Sediments 2012, 12, 1111–1123. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Rodríguez-Cruz, M.S.; Andrades, M.S.; Sánchez-Martín, M.J. Application of a biosorbent to soil: A potential method for controlling water pollution by pesticides. Environ. Sci. Pollut. Res. 2016, 23, 9192–9203. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Nogales, R.; Romero, E. Wastes from the olive oil production in sustainable bioremediation systems to prevent pesticides water contamination. Int. J. Environ. Sci. Technol. 2017, 14, 2471–2484. [Google Scholar] [CrossRef]
- Wei, P.; Liu, Y.; Li, W.; Qian, Y.; Nie, Y.; Kim, D.; Wang, M. Metabolic and dynamic profiling for risk assessment of fluopyram, a typical phenylamide fungicide widely applied in vegetable ecosystem. Sci Rep. 2016, 6, 33898. [Google Scholar] [CrossRef]
- Papadopoulou, E.S.; Karas, P.A.; Nikolaki, S.; Storck, V.; Ferrari, F.; Trevisan, M.; Tsiamis, G.; Martin-Laurent, F.; Karpouzas, D.G. Dissipation and adsorption of isoproturon, tebuconazole, chlorpyrifos and their main transformation products under laboratory and field conditions. Sci. Total Environ. 2016, 569–570, 86–96. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Gao, J.; Liu, C.; Cui, L.; Li, A. Dissipation, residues, and safety evaluation of trifloxystrobin and tebuconazole on ginseng and soil. Environ. Monit. Assess. 2015, 187, 344. [Google Scholar] [CrossRef]
- Matadha, N.Y.; Mohapatra, S.; Siddamallaiah, L.; Udupi, V.R.; Gadigeppa, S.; Raja, D.P. Uptake and distribution of fluopyram and tebuconazole residues in tomato and bell pepper plant tissues. Environ. Sci. Poll. Res. 2019, 26, 6077–6086. [Google Scholar] [CrossRef] [PubMed]
- Bielská, L.; Hale, S.E.; Škulcová, L. A review on the stereospecific fate and effects of chiral conazole fungicides. Sci Total Environ. 2021, 750, 141600. [Google Scholar] [CrossRef] [PubMed]
- Bošković, N.; Bílková, Z.; Šudoma, M.; Bielská, L.; Škulcová, L.; Ribitsch, D.; Soja, G.; Vrana, B.; Hofman, J. Effects of biochar on the fate of conazole fungicides in soils and their bioavailability to earthworms and plants. Environ. Sci. Poll. Res. 2022, 29, 23323–23337. [Google Scholar] [CrossRef]
- Gámiz, B.; López-Cabeza, R.; Facenda, G.; Velarde, P.; Hermosín, M.C.; Cox, L.; Celis, R. Effect of synthetic clay and biochar addition on dissipation and enantioselectivity of tebuconazole and metalaxyl in an agricultural soil: Laboratory and field experiments. Agric. Ecosyst. Environ. 2016, 230, 32–41. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Sánchez-Martín, M.J.; Ordax, J.M.; Marín-Benito, J.M.; Rodríguez-Cruz, M.S. Leaching of two fungicides in spent mushroom substrate amended soil: Influence of amendment rate, fungicide aging and flow condition. Sci. Total Environ. 2017, 584–585, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, M.; Barba, V.; Ordax, J.M.; Andrades, M.S.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Application of green compost as amendment in an agricultural soil: Effect on the behaviour of triasulfuron and prosulfocarb under field conditions. J. Environ. Manag. 2018, 207, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Delgado-Moreno, L.; Rodríguez-Liébana, J.A. A review of the impact of wastewater on the fate of pesticides in soils: Effect of some soil and solution properties. Sci. Total Environ. 2020, 718, 134468. [Google Scholar] [CrossRef]
- Fenoll, J.; Ruiz, E.; Flores, P.; Vela, N.; Hellín, P.; Navarro, S. Use of farming and agro-industrial wastes as versatile barriers in reducing pesticide leaching through soil columns. J. Hazard. Mat. 2011, 187, 206–212. [Google Scholar] [CrossRef]


| Properties | Fluopyram | Tebuconazole |
|---|---|---|
| Chemical structure | 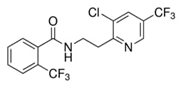 | 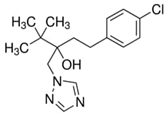 |
| Molecular weight (MW) | 396.76 | 307.82 |
| Solubility in water (mg L−1) | 16 (low) | 36 (low) |
| log Kow | 3.3 (high) | 3.7 (high) |
| Kf/Kfoc (mL g−1) | 4.41/278.9 (moderately mobile) | 12.69/769 (slightly mobile) |
| Field DT50 (days) | 118.8 (persistent) | 47.1 (moderately persistent) |
| Field DT90 (days) | 833 (very persistent) | 177 (persistent) |
| GUS index | 3.23 (high leachability) | 1.86 (transition state) |
| Parameters | SMS | OF | SMS + OF | ARN1 | ARN2 |
|---|---|---|---|---|---|
| Texture | Silty loam | Sandy loam | |||
| Sand (%) | 27.8 | 56.7 | |||
| Silt (%) | 55.3 | 27.0 | |||
| Clay (%) | 16.9 | 16.2 | |||
| pH | 7.60 ± 0.04 | 8.37 ± 0.34 | 7.48 ± 0.01 | 8.10 ± 0.10 | 8.20 ± 0.02 |
| Electrical conductivity (dS m−1) | 10.9 ± 0.08 | 0.25 ± 0.00 | 10.6 ± 0.27 | 0.30 ± 0.01 | 0.17 ± 0.04 |
| CaCO3 (%) | 14.6 ± 0.27 | 2.38 ± 0.14 | 10.7 ± 0.33 | 9.85 ± 0.78 | 12.9 ± 0.28 |
| OM (%) | 41.9 ± 0.34 | 0.09 ± 0.04 | 31.2 ± 0.68 | 1.67 ± 0.34 | 1.53 ± 0.38 |
| OC (%) | 24.3 ± 0.20 | 0.05 ± 0.02 | 18.1 ± 0.39 | 0.97 ± 0.20 | 0.89 ± 0.22 |
| Total N (%) | 2.05 ± 0.00 | 0.02 ± 0.00 | 1.67 ± 0.03 | 0.12 ± 0.02 | 0.10 ± 0.00 |
| C/N | 11.9 ± 0.09 | 3.21 ± 1.55 | 10.8 ± 0.07 | 8.08 ± 0.33 | 8.90 ± 2.90 |
| CEC (cmol + kg−1) | 42.9 ± 1.01 | 1.66 ± 0.03 | 32.9 ± 1.84 | 7.50 ± 0.90 | 6.56 ± 0.75 |
| Soil | ARN | +SMS25 | +SMS100 | +SMS + OF25 | +SMS + OF100 |
|---|---|---|---|---|---|
| ARN1 (0–15 cm) | 0.97 ± 0.06 | 1.76 ± 0.14 | 3.13 ± 0.26 | 2.58 ± 0.15 | 5.43 ± 0.51 |
| ARN1 (15–30 cm) | 1.00 ± 0.15 | 1.20 ± 0.11 | 1.70 ± 0.13 | 1.20 ± 0.09 | 2.50 ± 0.21 |
| ARN2 (0–15 cm) | 0.89 ± 0.03 | 1.53 ± 0.09 | 2.58 ± 0.40 | 1.27 ± 0.12 | 3.94 ± 0.29 |
| ARN2 (15–30 cm) | 0.60 ± 0.02 | 0.96 ± 0.11 | 2.20 ± 0.32 | 1.10 ± 0.08 | 1.40 ± 0.02 |
| Soils/Amendments | +SMS25 | +SMS100 | +SMS + OF25 | +SMS + OF100 | |
|---|---|---|---|---|---|
| Fluopyram | |||||
| ARN1 | 1.39 ± 0.22 | 1.95 ± 0.06 | 3.59 ± 0.13 | 3.91 ± 0.33 | 5.70 ± 0.28 |
| ARN2 | 0.45 ± 0.13 | 0.71 ± 0.03 | 1.61 ± 1.26 | 1.66 ± 0.37 | 4.73 ± 0.18 |
| SMS | 57.4 ± 6.93 | ||||
| SMS + OF | 104 ± 0.37 | ||||
| Tebuconazole | |||||
| ARN1 | 7.26 ± 0.00 | 8.25 ± 0.27 | 15.0 ± 0.58 | 17.8 ± 1.82 | 27.3 ± 0.73 |
| ARN2 | 2.21 ± 0.21 | 3.19 ± 0.20 | 4.82 ± 1.40 | 6.42 ± 0.91 | 16.4 ± 0.80 |
| SMS | 239 ± 10.2 | ||||
| SMS + OF | 277 ± 4.64 | ||||
| Soils | M0 (%) | α | β | DT50 (days) | DT90 (days) | ꭓ2 | r2 |
|---|---|---|---|---|---|---|---|
| Fluopyram | |||||||
| ARN1 | 99.0 | 0.38 | 0.93 | 4.9 | 408.5 | 13.0 | 0.984 |
| ARN1 + SMS25 | 99.2 | 0.34 | 0.78 | 5.2 | 677.2 | 13.0 | 0.982 |
| ARN1 + SMS100 | 93.8 | 0.44 | 3.50 | 13.1 | 617.8 | 13.3 | 0.970 |
| ARN1 + SMS + OF25 | 101.4 | 0.95 | 16.7 | 17.9 | 170.7 | 10.9 | 0.987 |
| ARN1 + SMS + OF100 | 88.1 | 1.35 | 39.3 | 26.3 | 176.5 | 15.2 | 0.971 |
| ARN2 | 99.2 | 1.18 | 7.09 | 5.7 | 42.9 | 5.6 | 0.998 |
| ARN2 + SMS25 | 99.9 | 0.93 | 4.18 | 4.7 | 46.0 | 3.0 | 1.000 |
| ARN2 + SMS100 | 97.6 | 0.91 | 4.25 | 4.8 | 48.4 | 13.4 | 0.991 |
| ARN2 + SMS + OF25 | 98.7 | 0.94 | 4.52 | 4.9 | 47.9 | 7.7 | 0.997 |
| ARN2 + SMS + OF100 | 98.5 | 1.01 | 5.06 | 5.0 | 44.1 | 9.0 | 0.996 |
| Tebuconazole | |||||||
| ARN1 | 99.1 | 0.43 | 1.02 | 4.1 | 214.3 | 15.1 | 0.981 |
| ARN1 + SMS25 | 97.9 | 0.50 | 1.55 | 4.7 | 153.2 | 16.0 | 0.979 |
| ARN1 + SMS100 | 99.4 | 0.34 | 0.39 | 2.6 | 339.7 | 16.3 | 0.975 |
| ARN1 + SMS + OF25 | 90,6 | 0.63 | 3.16 | 6.3 | 116.6 | 6.6 | 0.984 |
| ARN1 + SMS + OF100 | 99.3 | 0.47 | 0.87 | 2.9 | 117.7 | 13.4 | 0.989 |
| ARN2 | 100.2 | 0.79 | 1.77 | 2.5 | 30.6 | 3.8 | 0.999 |
| ARN2 + SMS25 | 100.2 | 0.63 | 1.13 | 2.3 | 43.3 | 4.6 | 0.999 |
| ARN2 + SMS100 | 100.4 | 0.65 | 1.46 | 2.8 | 48.2 | 7.5 | 0.997 |
| ARN2 + SMS + OF25 | 100.4 | 0.69 | 1.41 | 2.4 | 38.1 | 6.9 | 0.998 |
| ARN2 + SMS + OF100 | 99.7 | 0.64 | 1.32 | 2.6 | 47.6 | 5.8 | 0.998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Hernández, E.; Andrades, M.S.; Sánchez-Martín, M.J.; Marín-Benito, J.M.; Rodríguez-Cruz, M.S. Effect of Applying an Organic Amendment on the Persistence of Tebuconazole and Fluopyram in Vineyard Soils. Agronomy 2023, 13, 1270. https://doi.org/10.3390/agronomy13051270
Herrero-Hernández E, Andrades MS, Sánchez-Martín MJ, Marín-Benito JM, Rodríguez-Cruz MS. Effect of Applying an Organic Amendment on the Persistence of Tebuconazole and Fluopyram in Vineyard Soils. Agronomy. 2023; 13(5):1270. https://doi.org/10.3390/agronomy13051270
Chicago/Turabian StyleHerrero-Hernández, Eliseo, María Soledad Andrades, María J. Sánchez-Martín, Jesús M. Marín-Benito, and María Sonia Rodríguez-Cruz. 2023. "Effect of Applying an Organic Amendment on the Persistence of Tebuconazole and Fluopyram in Vineyard Soils" Agronomy 13, no. 5: 1270. https://doi.org/10.3390/agronomy13051270






