Petal Morphology Is Correlated with Floral Longevity in Paeonia suffruticosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Species
2.2. Flower Longevity
2.3. Fresh Weight and Dry Weight of Petals
2.4. Scanning Electron Microscope
2.5. Light Microscope
2.6. Statistical Analysis
3. Results
3.1. Flower Longevity
3.2. Epidermal Morphological Characteristics of Petals
3.3. Analysis of the Petal Cross-Sectional Anatomy
3.4. The Correlations between Water-Related Traits and Floral Longevity
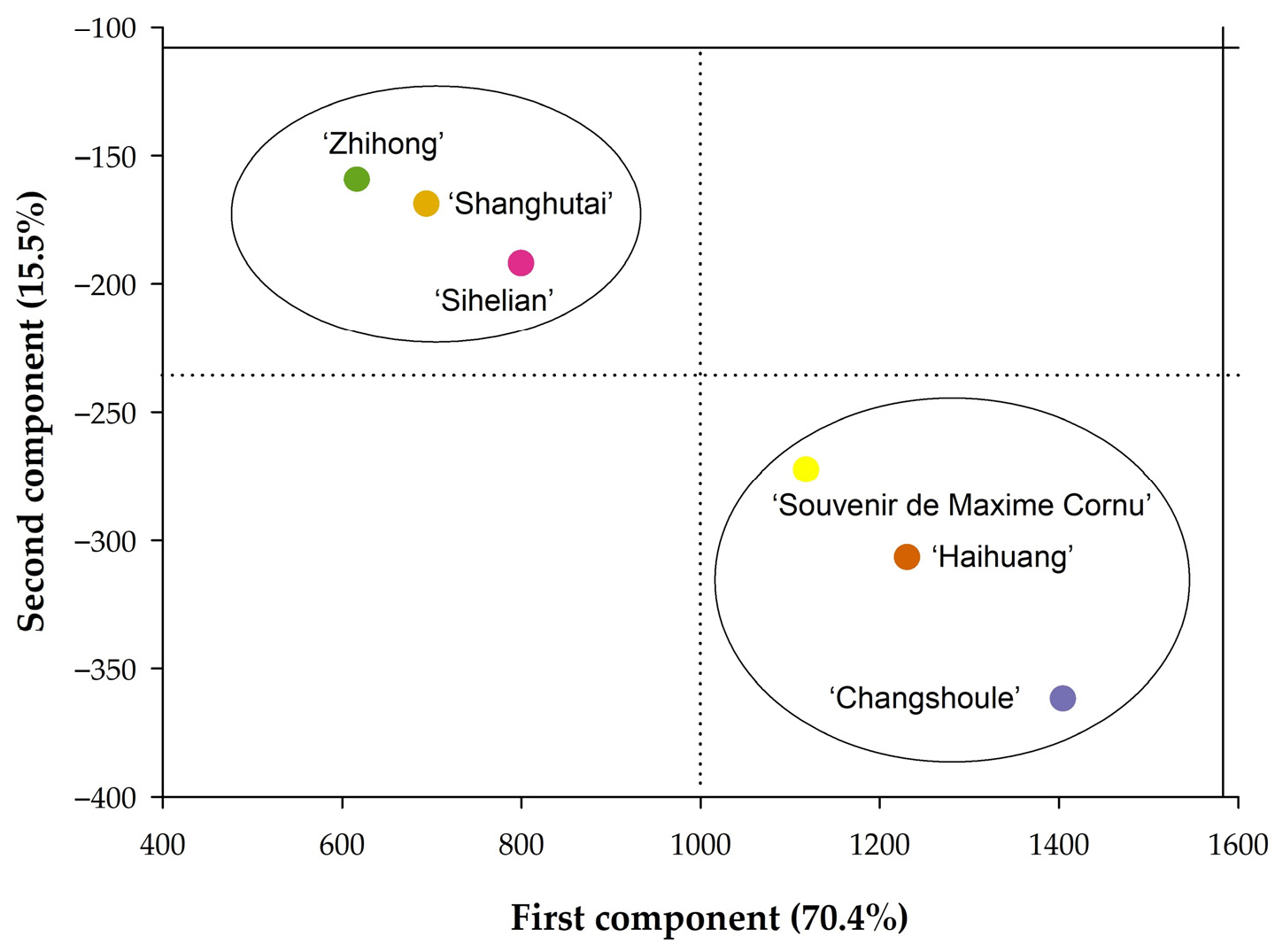
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ashman, T.L.; Schoen, D.J. Floral Longevity: Fitness Consequences. Floral Biology: Studies on Floral Evolution in Animal-Pollinated Plants; Springer Science & Business Media: Berlin, Germany, 1996; p. 112. [Google Scholar]
- Ashman, T.-L.; Schoen, D.J. How Long Should Flowers Live? Nature 1994, 371, 788–791. [Google Scholar] [CrossRef]
- Ishii, H.S.; Sakai, S. Effects of Display Size and Position on Individual Floral Longevity in Racemes of Narthecium asiaticum (Liliaceae). Funct. Ecol. 2001, 15, 396–405. [Google Scholar] [CrossRef]
- Sargent, R.; Roitberg, B. Seasonal Decline in Male-phase Duration in a Protandrous Plant: A Response to Increased Mating Opportunities? Funct. Ecol. 2000, 14, 484–489. [Google Scholar] [CrossRef]
- Arathi, H.S.; Rasch, A.; Cox, C.; Kelly, J.K. Autogamy and Floral Longevity in Mimulus guttatus. Int. J. Plant Sci. 2002, 163, 567–573. [Google Scholar] [CrossRef]
- Itagaki, T.; Sakai, S. Relationship between Floral Longevity and Sex Allocation among Flowers within Inflorescences in Aquilegia buergeriana var. oxysepala (Ranunculaceae). Am. J. Bot 2006, 93, 1320–1327. [Google Scholar] [CrossRef]
- Johnson, S.D.; Neal, P.R.; Harder, L.D. Pollen Fates and the Limits on Male Reproductive Success in an Orchid Population. Biol. J. Linn. Soc. 2005, 86, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, R.; Arathi, H.S. Floral Longevity and Autonomous Selfing are Altered by Pollination and Water Availability in Collinsia Heterophylla. Ann. Bot. 2013, 112, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Rathcke, B.J. Floral Longevity and Reproductive Assurance: Seasonal Patterns and an Experimental Test with Kalmia latifolia (Ericaceae). Am. J. Bot. 2003, 90, 1328–1332. [Google Scholar] [CrossRef]
- Vesprini, J.L.; Pacini, E. Temperature-Dependent Floral Longevity in Two Helleborus Species. Plant Syst. Evol. 2005, 252, 63–70. [Google Scholar] [CrossRef]
- Weber, J.J.; Goodwillie, C. Variation in Floral Longevity in the Genus Leptosiphon: Mating System Consequences. Plant Biol. 2013, 15, 220–225. [Google Scholar] [CrossRef]
- Yasaka, M.; Nishiwaki, Y.; Konno, Y. Plasticity of Flower Longevity in Corydalis ambigua. Ecol. Res. 1998, 13, 211–216. [Google Scholar] [CrossRef]
- Giblin, D.E. Variation in Floral Longevity between Populations of Campanula Rotundifolia (Campanulaceae) in Response to Fitness Accrual Rate Manipulation. Am. J. Bot. 2005, 92, 1714–1722. [Google Scholar] [CrossRef]
- Abdala-Roberts, L.; Parra-Tabla, V.; Navarro, J. Is Floral Longevity Influenced by Reproductive Costs and Pollination Success in Cohniella ascendens (Orchidaceae)? Ann. Bot. 2007, 100, 1367–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stead, A.D. Pollination-Induced Flower Senescence: A Review. Plant Growth Regul. 1992, 11, 13–20. [Google Scholar] [CrossRef]
- Nobel, P.S. Water Relations of Flowering of Agave deserti. Bot. Gaz. 1977, 138, 1–6. [Google Scholar] [CrossRef]
- Ichimura, K.; Azuma, M. Characterization of Petal Senescent Types in Cut Dahlia and Extension of Their Vase Life by Treatment with Silver Thiosulfate Complex Followed by Glucose Plus Germicides. Horticulturae 2022, 8, 922. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Shi, J.L.; Du, X.H.; Dong, S.H.; Li, J.P. Preservation Effects of Different Preserv watives on Eustoma grandiflorum Cut Flower. Mod. Agric. Sci. Technol. 2022, 13, 80–84. [Google Scholar]
- Sun, J.; Guo, H.; Tao, J. Effects of Harvest Stage, Storage, and Preservation Technology on Postharvest Ornamental Value of Cut Peony (Paeonia lactiflora) Flowers. Agronomy 2022, 12, 230. [Google Scholar] [CrossRef]
- Van Doorn, W. The Postharvest Quality of Cut Lily Flowers and Potted Lily Plants. II Int. Symp. Genus 2011, 900, 255–264. [Google Scholar] [CrossRef]
- Zhang, F.-P.; Feng, J.-Q.; Huang, J.-L.; Huang, W.; Fu, X.-W.; Hu, H.; Zhang, S.-B. Floral Longevity of Paphiopedilum and Cypripedium Is Associated with Floral Morphology. Front. Plant Sci. 2021, 12, 737. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, T.; Kaffarnik, F.; Riederer, M.; Schreiber, L. Cuticular Permeability of the Three Tree Species Prunus Iaurocerasus L., Ginkgo biloba L. and Juglans regia L.: Comparative In-vestigation of the Transport Properties of Intact Leaves, Isolated Cuticles and Reconstituted Cuticular Waxes. J. Exp. Bot. 1997, 48, 1035–1045. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.; Zhang, S.-B.; Li, S.-Y.; Hu, H. Ecophysiological Significance of Leaf Traits in Cypripedium and Paphiopedilum. Physiol. Plant. 2011, 141, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Zhang, F.-P.; Zhang, Y.-B.; Li, W.; Wang, Q.; Yang, D.; Zhang, J.-L.; Cao, K.-F. Convergent relationships between flower economics and hydraulic traits across aquatic and terrestrial herbaceous plants. Plant Divers. 2023, 1, 6. [Google Scholar] [CrossRef]
- Zhang, F.-P.; Yang, Y.-J.; Yang, Q.-Y.; Zhang, W.; Brodribb, T.J.; Hao, G.-Y.; Hu, H.; Zhang, S.-B. Floral Mass per Area and Water Maintenance Traits Are Correlated with Floral Longevity in Paphiopedilum (Orchidaceae). Front. Plant Sci. 2017, 8, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, H.Y.M.; Rao, I.V.R. Physiology of flower bud growth and opening. Proc. Indian Acad. Sci. 1984, 93, 253–274. [Google Scholar] [CrossRef]
- Patiño, S.; Grace, J. The cooling of convolvulaceous flowers in a tropical environment. Plant Cell Environ. 2002, 25, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Galen, C. It never rains but then it pours: The diverse effects of water on flower integrity and function. In Reproductive Allocation in Plants; Reekie, E., Bazzaz, F.A., Eds.; Elsevier Press: San Diego, CA, USA, 2005; pp. 77–95. [Google Scholar]
- Roddy, A.B.; Dawson, T.E. Determining the water dynamics of flowering using miniature sap flow sensors. Acta Hortic. 2012, 951, 47–54. [Google Scholar] [CrossRef]
- Roddy, A.B.; Guilliams, C.M.; Lilittham, T.; Farmer, J.; Wormser, V.; Pham, T.; Fine, P.V.; Feild, T.S.; Dawson, T.E. Uncorrelated evolution of leaf and petal venation patterns across the angiosperm phylogeny. J. Exp. Bot. 2013, 64, 4081–4088. [Google Scholar] [CrossRef] [Green Version]
- Roddy, A.B.; Brodersen, C.R.; Dawson, T.E. Hydraulic conductance and the maintenance of water balance in flowers. Plant Cell Environ. 2016, 39, 2123–2132. [Google Scholar] [CrossRef]
- Hong, D.Y.; Pan, K.Y. Paeonia Cathayana D.Y. Hong & K.Y. Pan, A new tree peony, with revision of P. suffruticosa ssp. yinpingmudan. Acta Phytotax Sin. 2007, 45, 285–288. [Google Scholar]
- Chavarria, M.R.; Wherley, B.; Jessup, R.; Chandra, A. Leaf Anatomical Responses and Chemical Composition of Warm-Season Turfgrasses to Increasing Salinity. Curr. Plant Biol. 2020, 22, 100147. [Google Scholar] [CrossRef]
- Olsen, J.T.; Caudle, K.L.; Johnson, L.C.; Baer, S.G.; Maricle, B.R. Environmental and Genetic Variation in Leaf Anatomy among Populations of Andropogon gerardii (Poaceae) Along a Precipitation Gradient. Am. J. Bot. 2013, 100, 1957–1968. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Shi, L.; Feng, Y.; Wei, Q.; Chen, H.; Liu, D.; Qin, F.; Xu, H.; Qiu, Y.; Hu, B. The Observation of Ultra-Structure during the Petal Senescence of Dracocephalum argunense Fisch. Ex Link. J. Food Agric. Environ. 2012, 10, 732–735. [Google Scholar]
- Jia, W.; Wang, Y.; Qi, Q.; He, S.; Mi, Z.; Zhu, X. Leaf Epidermal Morphology of Ten Wild Tree Peonies in China and Its Taxonomic Significance. Horticulturae 2022, 8, 502. [Google Scholar] [CrossRef]
- Costa, V.B.S.; Pimentel, R.M.M.; Chagas, M.G.S.; Alves, G.D.; Castro, C.C. Petal micromorphology and its relationship to pollination. Plant Biol. 2017, 19, 115–122. [Google Scholar] [CrossRef]
- Mamrutha, H.M.; Mogili, T.; Lakshmi, K.J.; Rama, N.; Kosma, D.; Kumar, M.U.; Jenks, M.A.; Nataraja, K.N. Leaf cuticular wax amount and crystal morphology regulate post-harvest water loss in mulberry (Morus species). Plant Physiol. Biochem. 2010, 48, 690–696. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z. Comparative Morphology of the Leaf Epidermis in Ligusticum (Apiaceae) from China. Am. J. Plant Sci. 2018, 9, 1105–1123. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Sawhney, V.K.; Steeves, T.A. Staining of paraffin-embedded plant material in safranin and fast green without prior removal of the paraffin. Can. J. Bot. 1993, 71, 996–999. [Google Scholar] [CrossRef]
- Richards, A.G.; Korda, F.H. Studies on arthropod cuticle. II. electron microscope studies of extracted cuticle. Biol. Bull. 1948, 94, 212–235. [Google Scholar] [CrossRef]
- Harrap, M.J.M.; Rands, S.A. The Role of Petal Transpiration in Floral Humidity Generation. Planta 2022, 255, 78. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.-C.; Burghardt, M.; Riederer, M. The Ecophysiology of Leaf Cuticular Transpiration: Are Cuticular Water Permeabilities Adapted to Ecological Conditions? J. Exp. Bot. 2017, 68, 5271–5279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Du, T.; Wang, L. Isotope Signature of Maize Stem and Leaf and Investigation of Transpiration and Water Transport. Agric. Water Manag. 2021, 247, 106727. [Google Scholar] [CrossRef]
- Ding, F.; Wang, G.; Wang, M.; Zhang, S. Exogenous Melatonin Improves Tolerance to Water Deficit by Promoting Cuticle Formation in Tomato Plants. Molecules 2018, 23, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the Architecture, Biosynthesis and Functional Aspects of the Plant Cuticle: There is more Scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Gorb, E.V.; Gorb, S.N. Anti-Adhesive Effects of Plant Wax Coverage on Insect Attachment. J. Exp. Bot. 2017, 68, 5323–5337. [Google Scholar] [CrossRef] [Green Version]
- Ristic, Z.; Jenks, M.A. Leaf Cuticle and Water Loss in Maize Lines Differing in Dehydration Avoidance. J. Plant Physiol. 2002, 159, 645–651. [Google Scholar] [CrossRef]
- Trunschke, J.; Stöcklin, J. Plasticity of Flower Longevity in Alpine Plants is Increased in Populations from High Elevation Compared to low Elevation Populations. Alp. Bot. 2017, 127, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, D.W.; Robertson, G.W.; Shepherd, T.; Birch, A.N.E.; Gordon, S.C.; Woodford, J.T. A comparison of the Composition of Epicuticular Wax from Red Raspberry (Rubus idaeus L.) and Hawthorn (Crataegus monogyna Jacq.) Flowers. Phytochemistry 2000, 55, 111–116. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Biosynthesis and Secretion of Plant Cuticular Wax. Prog. Lipid Res. 2003, 42, 51–80. [Google Scholar] [CrossRef]
- Tomaszewski, D.; Zieliński, J. Epicuticular Wax Structures on Stems and Comparison between Stems and Leaves–A Survey. Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 215–232. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.-J. The Hydrophobic Coatings of Plant Surfaces: Epicuticular Wax Crystals and Their Morphologies, Crystallinity and Molecular Self-Assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against Water Loss: Analysis of the Barrier Properties of Plant Cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Schönherr, J. Characterization of Aqueous Pores in Plant Cuticles and Permeation of Ionic Solutes. J. Exp. Bot. 2006, 57, 2471–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagadish, S.K.; Way, D.A.; Sharkey, T.D. Plant Heat Stress: Concepts Directing Future Research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Van Meeteren, U. Flower Opening and Closure: A Review. J. Exp. Bot. 2003, 54, 1801–1812. [Google Scholar] [CrossRef]
- Zhu, G.L.; Deng, R.H.; Wei, X.Z. Leaf epidermal micromorphology of Ziziphus jujuba var. spinosa in response to a gradient of drought stress. Acta Ecol. Sin. 2016, 36, 5193–5203. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Fu, P.-L.; Jiang, Y.-J.; Wang, A.-Y.; Brodribb, T.; Zhang, J.-L.; Zhu, S.-D.; Cao, K.-F. Stem Hydraulic Traits and Leaf Water-Stress Tolerance are Coordinated with the Leaf Phenology of Angiosperm Trees in an Asian Tropical Dry karst Forest. Ann. Bot. 2012, 110, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Cal, A.J.; Sanciangco, M.; Rebolledo, M.C.; Luquet, D.; Torres, R.O.; McNally, K.L.; Henry, A. Leaf Morphology, rather than Plant Water Status, Underlies Genetic Variation of Rice Leaf Rolling under Drought. Plant Cell Environ. 2019, 42, 1532–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, K.; Hayakawa, K.; Kawasaki, M.; Taniguchi, M.; Miyake, H. Bundle Sheath Chloroplasts of Rice are more Sensitive to drought Stress than Mesophyll Chloroplasts. J. Plant Physiol. 2003, 160, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A New Look at Water Transport Regulation in Plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, N.A.; Shafiq, S.; Ashraf, M.; Aisha, R.; Sajid, M.A. Drought-Induced Anatomical Changes in Radish (Raphanus sativus L.) Leaves Supplied with Trehalose through Different Modes. Arid. Land Res. Manag. 2016, 30, 412–420. [Google Scholar] [CrossRef]
- Primack, R.B. Longevity of Individual Flowers. Annu. Rev. Ecol. Syst. 1985, 16, 15–37. [Google Scholar] [CrossRef]
- Steinacher, G.; Wagner, J. Flower Longevity and Duration of Pistil Receptivity in High Mountain Plants. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 376–387. [Google Scholar] [CrossRef]




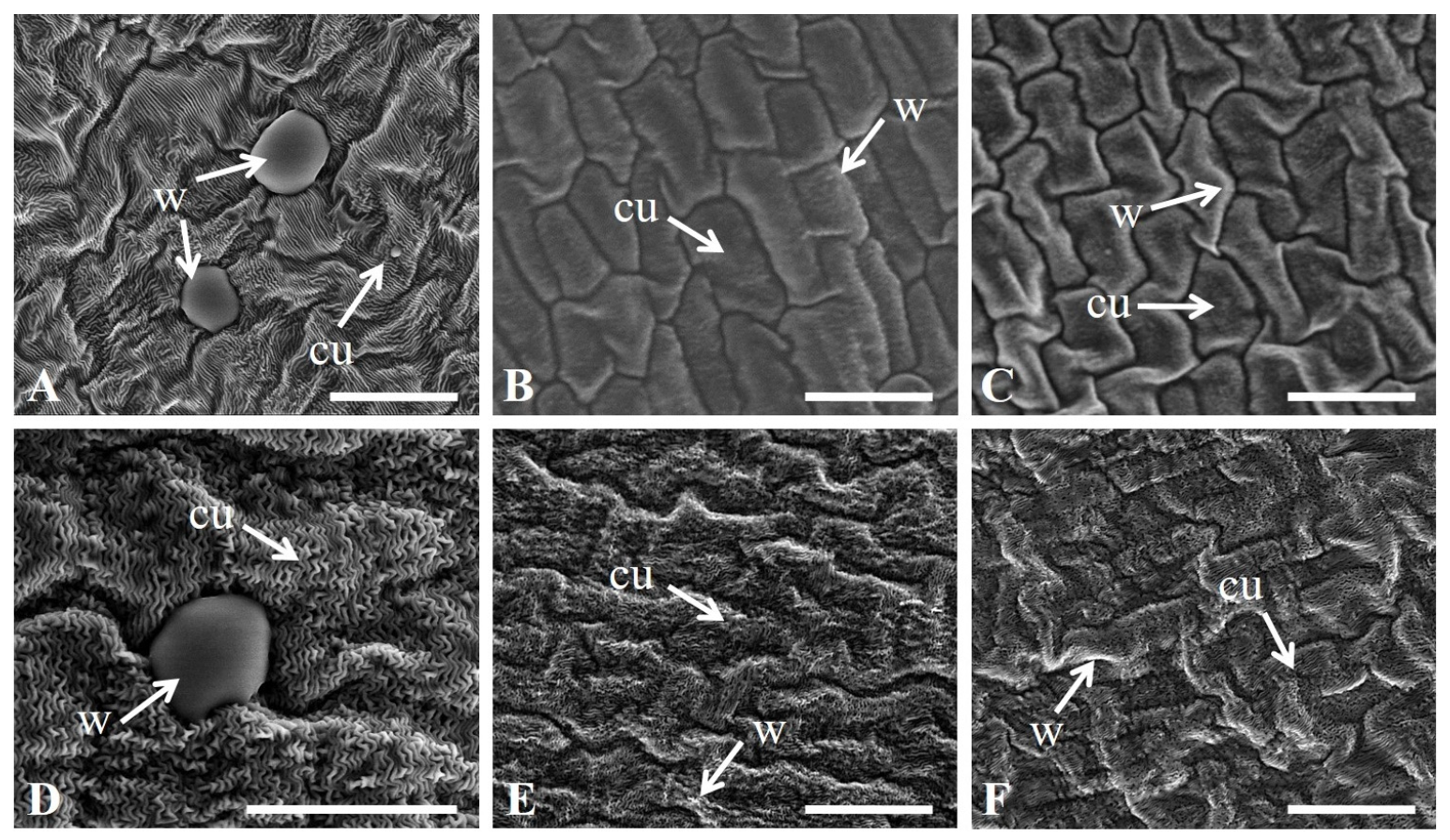
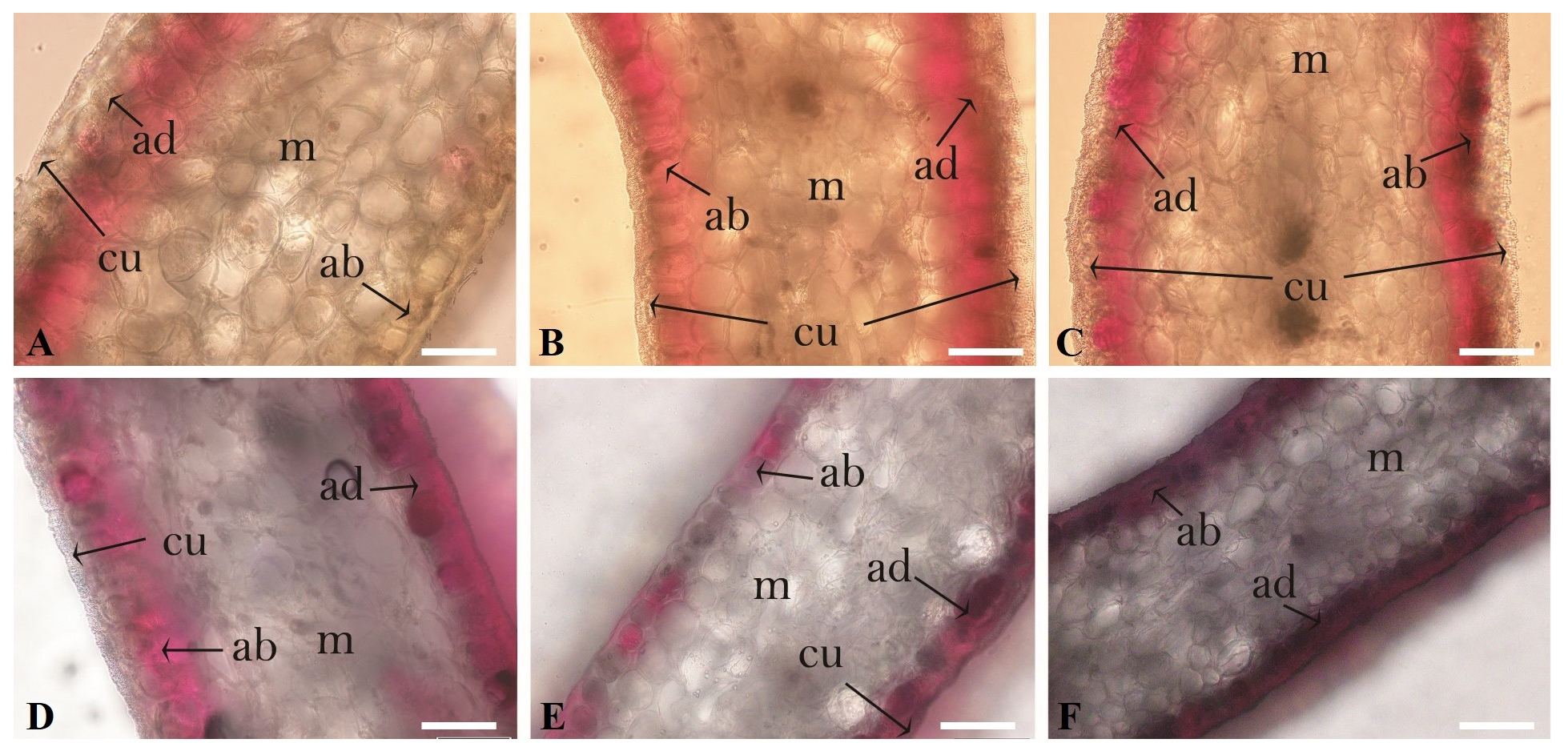
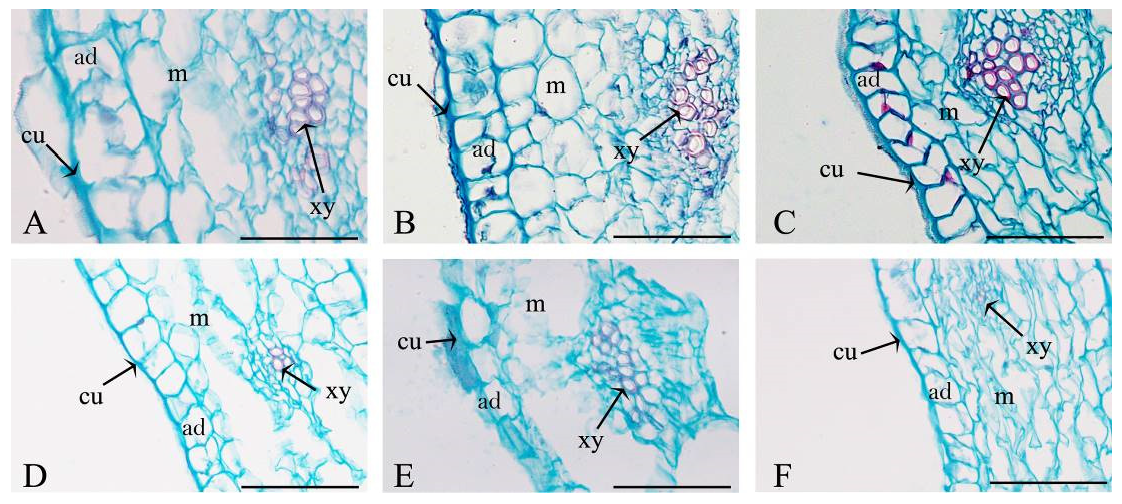
| Code | Name | Flower Color | Flower Type | FL (Days) |
|---|---|---|---|---|
| 1 | ‘Souvenir de Maxime’ Cornu’ | Yellowish orange | double-petaled | 8.5 ± 1.5 a |
| 2 | ‘Haihuang’ | Yellow | single-petaled | 9.0 ± 1.2 a |
| 3 | ‘Changshoule’ | Light pink | double-petaled | 8.9 ± 1.1 a |
| 4 | ‘Sihelian’ | Pink | single-petaled | 4.0 ± 1.1 b |
| 5 | ‘Zhihong’ | Rouge red | double-petaled | 3.5 ± 1.5 b |
| 6 | ‘Shanhutai’ | Pink | double-petaled | 3.7 ± 1.3 b |
| Traits | ‘Souvenir de Maxime Cornu’ | ‘Haihuang’ | ‘Changshoule’ | ‘Sihelian’ | ‘Zhihong’ | ‘Shanhutai’ |
|---|---|---|---|---|---|---|
| Petal thickness (μm) | 183.3 ± 10.9 ab | 198.1 ± 9.2 a | 188.9 ± 16.8 ab | 164.8 ± 8.3 b | 75.3 ± 5.1 d | 136.8 ± 8.2 c |
| Cuticle thickness (μm) | 11.8 ± 1.6 a | 4.2 ±0.6 c | 6.3 ± 0.5 b | 3.7 ± 1.2 cd | 1.6 ± 0.6 e | 2.6 ± 1.0 d |
| Number of cell layers | 10.5 ± 1.2 b | 11.3 ± 1.2 b | 18.2 ± 2.0 a | 8.3 ± 2.0 bc | 9.2 ± 2.0 bc | 9.0 ± 2.0 bc |
| Mesophyll thickness (μm) | 127.6 ± 3.8 ab | 142.3 ± 3.0 a | 137.0 ± 3.2 ab | 120.3± 1.8 b | 50.4 ± 1.2 d | 98.5 ± 3.5 c |
| Adaxial epidermis thickness (μm) | 23.5 ± 0.6 b | 26.7 ± 1.3 a | 22.7 ± 0.8 b | 19.7 ± 0.8 bc | 12.3 ± 0.6 d | 18.9 ± 3.5 c |
| Abaxial epidermis thickness (μm) | 20.3 ± 1.2 b | 25.4 ± 2.2 a | 22.1 ± 1.3 ab | 18.2 ± 2.1 bc | 11.0 ± 1.2 d | 16.7 ± 1.2 c |
| Vein density (mm/mm2) | 2.3 ± 0.2 a | 2.6 ± 0.21 a | 2.0 ± 0.3 ab | 1.6 ± 0.1 b | 1.4 ± 0.1 c | 1.5 ± 0.1 c |
| Vessel number per vein | 8.0 ± 1.00 b | 8.7 ± 0.6 b | 12.2 ± 0.10 a | 7.7 ± 1.15 bc | 5.6 ± 0.58 c | 7.9 ± 0.5 bc |
| Vessel diameter (μm) | 7.8 ± 0.2 a | 6.7 ± 0.25 b | 6.2 ± 0.4 b | 4.8 ± 0.5 c | 5.3 ± 0.3 c | 3.9 ± 0.2 d |
| Petal fresh mass (mg/cm2) | 24.6 ± 0.3 a | 23.8 ± 0.4 a | 19.8 ± 0.9 b | 16.0 ± 0.8 c | 12.9 ± 0.1 d | 13.5 ± 0.3 d |
| Petal dry mass (mg/cm2) | 4.0 ± 0.1 a | 4.2 ± 0.1 a | 3.5 ± 0.2 b | 3.0 ± 0.1 c | 2.4 ± 0.1 d | 2.2 ± 0.2 d |
| Traits | Floral Longevity | Petal Thickness | Cuticle Thickness | Mesophyll Thickness | Epidermis Thickness | Vein Density | Vessel Number | Vessel Diameter | Fresh Weight | Dry Weight |
|---|---|---|---|---|---|---|---|---|---|---|
| Floral longevity | 1 | |||||||||
| Petal thickness | 0.54 * | 1 | ||||||||
| Cuticle thickness | 0.40 * | 0.32 | 1 | |||||||
| Mesophyll thickness | 0.82 ** | 0.92 ** | 052 * | 1 | ||||||
| Epidermis thickness | 0.63 * | 0.94 ** | 0.22 | 0.68 * | 1 | |||||
| Vein density | 0.81 ** | 0.57 * | 0.54 * | 0.95 ** | 0.50 * | 1 | ||||
| Vessel number per vein | 0.42 * | 0.51 * | 0.11 | 0.74 ** | 0.43 * | 0.70 | 1 | |||
| Vessel diameter | 0.74 ** | 0.29 | 0.63 * | 0.76 ** | 0.32 | 0.54 * | 0.07 | 1 | ||
| Petal fresh mass | 0.80 ** | 0.75 ** | 0.43 * | 0.86 ** | 0.82 ** | 0.58 * | 0.23 | 0.73 ** | 1 | |
| Petal dry mass | 0.79 ** | 0.67 * | 0.42 * | 0.69 * | 0.73 ** | 0.56 * | 0.20 | 0.78 ** | 0.90 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Qiu, Y.; Hu, H.; Wang, Y.; Mi, Z.; Zhang, S.; He, S.; Jia, W. Petal Morphology Is Correlated with Floral Longevity in Paeonia suffruticosa. Agronomy 2023, 13, 1372. https://doi.org/10.3390/agronomy13051372
Guo Y, Qiu Y, Hu H, Wang Y, Mi Z, Zhang S, He S, Jia W. Petal Morphology Is Correlated with Floral Longevity in Paeonia suffruticosa. Agronomy. 2023; 13(5):1372. https://doi.org/10.3390/agronomy13051372
Chicago/Turabian StyleGuo, Yingzi, Yongjie Qiu, Huan Hu, Yanli Wang, Zhaorong Mi, Shulin Zhang, Songlin He, and Wenqing Jia. 2023. "Petal Morphology Is Correlated with Floral Longevity in Paeonia suffruticosa" Agronomy 13, no. 5: 1372. https://doi.org/10.3390/agronomy13051372




