Abstract
Spring durum wheat is an important raw material for producing diverse products such as couscous, bulgur, bread, and pasta. The quality of the dough is significantly influenced by high-molecular-weight glutenins, the allelic status of which depends on the region and breeding program. In this study, a collection of 69 cultivars and promising lines of durum wheat were analyzed for the allelic state of Glu-A1 and Glu-B1 using SDS-PAGE and KASP PCR markers. Protein and gluten content, volume increase index, pasta breaking strength, general pasta estimation, SDS, and gluten index were measured for each accession based on a two-year field experiment in the Krasnodar region. The analysis revealed that the Glu-B1al, Glu-B1d, and Glu-B1z* alleles positively influence gluten index, with Glu-B1al increasing protein, gluten, and SDS content, whereas Glu-B1d decreased these traits. Glu-B1e, on average, decreased the gluten index and SDS value but did not affect protein or gluten content. The role of alleles affecting the gluten index and protein content in ensuring the quality of pasta in durum wheat breeding is discussed.
1. Introduction
Durum wheat (Triticum turgidum L. subsp. durum (Desf.) Husn), a tetraploid wheat species (2n = 28) with the genomic composition BBAA, is widely cultivated around the world. The total estimated cropping area of durum wheat is 13.5 million hectares, with the estimated/projected production ranging from 31.3 to 32.8 million tons in 2021–2023 years [1,2]. The main global producers of durum wheat are countries in the Mediterranean basin (Southern Europe, Turkey, and North Africa), Canada, Mexico, Kazakhstan, USA, Argentina, Russia, India, Syria, Australia, and other countries [1,3,4].
The quality of durum wheat plays a vital role in producing various products such as pasta, couscous, bulgur, and bread. Cultivation practices [5,6], weather and climatic conditions [7,8,9,10], and genetic factors [11,12] affect the quality of these durum wheat products. Genetic factors determine the protein content, dough strength, and levels of yellow pigments. Modern breeding has significantly improved the gluten index, SDS values, yellow pigment index, and gliadin/glutenin ratio [13,14,15,16]. Advances in the gluten quality of durum wheat varieties owe much to the development of instrumental gluten quality assessment tools applied at the early stages of breeding, including classic devices such as the alveograph, mixograph, and systems for SDS and gluten index estimation, as well as more modern tools such as the glutograph, glutopeak, and mixolab [17]. Improvements in gluten strength are most likely associated with the replacement of the Glu-B1e allele (Bx20 + By20) with the Glu-B1b (Bx7 + By9) and Glu-B1d (Bx6 + By8) high-molecular-weight glutenins (HMW-GS), the positive effect of low-molecular-weight glutenin (LMW-GS) subunits of the LMW-2 group, and the γ-45 subunit of gliadin associated with high firmness and viscoelasticity of pasta [11,12,13,15,18,19,20]. Identifying alleles (including novel ones) with a positive impact on quality and implementing them into the breeding process is facilitated by classic methods of protein (SDS-PAGE) and molecular genetic analysis (PCR), as well as modern approaches such as Lab-on-a-Chip [21], KASP [22,23], and GWAS-based markers [24,25].
The leading breeding center in Russia, P.P. Lukyanenko National Grain Centre, has been developing high-yielding adaptive durum wheat cultivars for regional producers for a long time [26,27,28]. Despite the commercial success of these cultivars, the breeding of durum wheat in Russia has long been conducted without the use of the gluten index determination method. To enter the global market and increase competitiveness, breeders started using gluten index evaluation methodology. The aim of this study was to assess the effect of HMW-GS allele status on the grain and pasta quality parameters including the gluten index in commercial cultivars and stable lines from the competitive variety testing at the P.P. Lukyanenko National Grain Centre.
2. Materials and Methods
2.1. Plant Materials
Commercial cultivars and non-segregating perspective breeding lines from the competitive variety trial nursery (CVT, prefinal stage of cultivar assessment preceding its state variety trial and registration) distinguished by stability, uniformity, and distinctness (69 accessions) are presented in Supplementary Table S1; their origin and year of release are indicated.
2.2. Field Experiment
The field experiment was performed on a plot of land at the P.P. Lukyanenko National Grain Centre (45°03′40″ N 38°54′23″ E) in 2020 and 2021. The sowing of spring durum wheat was carried out with peas as a forecrop. Harrowing was performed using a two-disc Catros harrow (Amazone, Osnabrück, Germany). Under the main tillage, the complex fertilizer Azofoska was applied (N16:P16:K16, 200 kg/ha). Plowing was performed to a depth of 25–30 cm with a turn-wrest plow (Lemken GmbH & Co., Alpen, Germany), followed by double cultivation using KPS-4.0 (Tehmash, Lida, Belarus). Sowing was performed using a self-propelled Plotseed TC seed drill (Wintersteiger, Ried, Austria) in 5 m2 plots in double replication. After seedling establishment, top dressing was carried out using ammonia nitrate (100 kg/ha). Harvesting was performed by direct combining using a Classic harvesting machine (Wintersteiger, Ried, Austria).
2.3. Grain and Pasta Quality Estimation
Grain and pasta quality estimation, high molecular glutenin characterization, Glu-A1/Glu-B1 genotyping, and statistical analyses were carried out as described in detail in our recent publication [29]. Therefore, here, we briefly describe the general points. The quality parameters, namely SDS, protein content, and gluten content, were determined using the Infratec™ 1241 grain analyzer (Foss Analytical, Hillerød, Denmark). Gluten index was determined using the Perten Glutomatic® 2100 System (PerkinElmer, Waltham, MA, USA). Pasta breaking strength was determined using the IPM-1 device (VNIIZ, Moscow, Russia). The volume increase index was calculated using a standard method as the ratio of the volume of dry pasta to the volume of pasta after cooking. The overall pasta evaluation assigned scores from “2” (lowest) to “5” (highest) considering pasta color, pasta breaking strength (directly proportional), and volume increase index (inversely proportional).
2.4. High Molecular Glutenin Characterization and Glu-A1/Glu-B1 Genotyping
The allelic state of Glu-B1 and Glu-A1 genes was determined using SDS-PAGE analysis as described in [30] with modifications and KASP markers Glu-Ax1/2*_SNP, distinguishing Glu-A1c (subunit Ax-null) from Glu-A1a/Glu-A1b (subunits Ax1/Ax2*) and BX7OE_866_SNP, distinguishing the Glu-B1al allele (subunit Bx7OE) from the others developed in [31].
2.5. Statistical Analyses
For all observed values expressed in percent (gluten content, protein content, gluten index), logit transformation was applied, and all statistical calculations were performed with the transformed values [32]. The mean observed values for each genotype/HMW GS subunit subcategory were grouped according to Tukey’s criterion. Cross-correlations and internal correlations of the observed values and the input categories, ordered and unordered, were estimated using the Φk coefficient [33]. Correlation and significance matrices were obtained via phik library (https://github.com/KaveIO/PhiK, accessed on 17 May 2023) in Python.
The principal component analysis (PCA) was performed via prince library (https://github.com/MaxHalford/prince, accessed on 17 May 2023) after the logit transformation of percentage data using scipy library. The first three components were used to create 3D reports in html via plotly (https://plotly.com/, accessed on 17 May 2023), showing both data points plotted in the coordinates of principal components and projections of initial numeric variables. The biplots were interpreted based on the angles between resulted vectors visible in html files in Supplementary Figures S1 and S2. The values of angles are shown in Supplementary Table S5. The angle between two vectors (traits) approximates the correlation between the vectors: an angle less than 90° corresponds to a positive correlation; an angle equal to 90° shows no correlation; an angle more than 90° corresponds to a negative correlation.
3. Results
3.1. Collection Structure
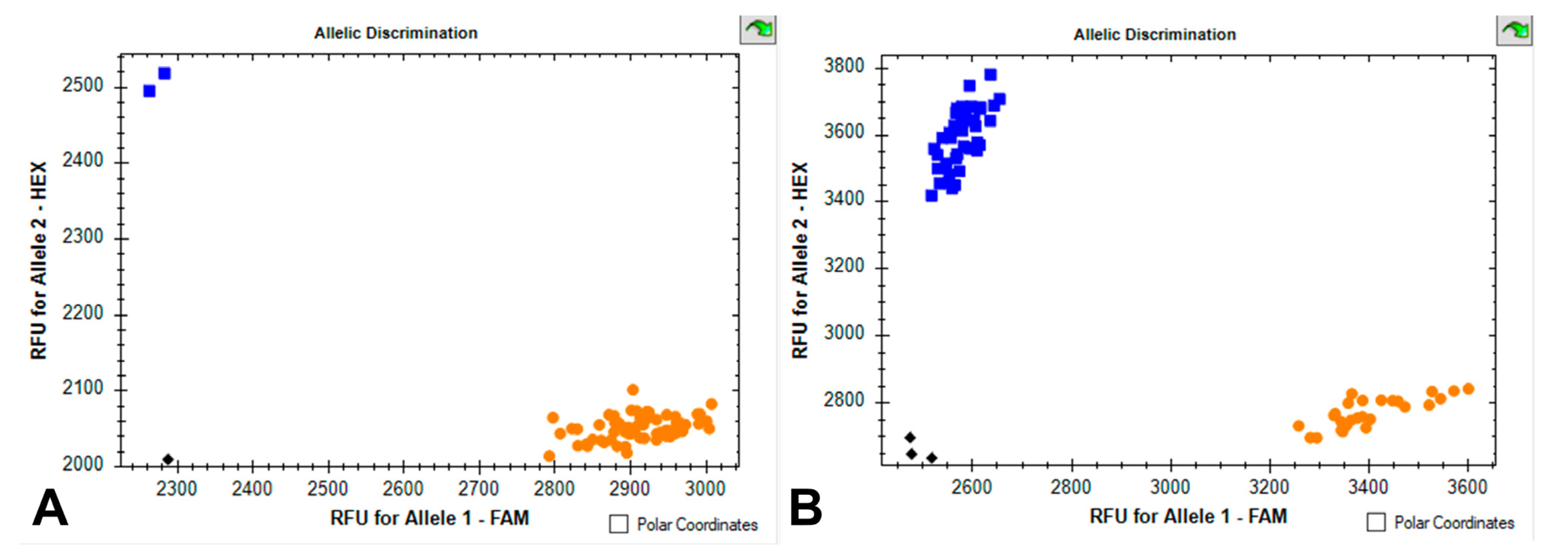
Based on the identification of the allelic state of genes encoding high-molecular-weight glutenins using KASP and SDS PAGE assays, we found that 67 accessions carry the Glu-A1c allele, and 2 accessions carry the Glu-A1b allele (Figure 1). Therefore, we do not describe the influence of the Glu-A1 locus on grain and pasta quality (although the statistical data are shown in Table 1 and Supplementary Materials). The studied collection had the following allelic composition for the Glu-B1 locus: Glu-B1al (7OE + 8), 38 accessions; Glu-B1d (6 + 8), 13 accessions; Glu-B1e (20), 9 accessions; Glu-B1b (7 + 8), 2 accessions. Additionally, in seven accessions, SDS-PAGE analysis revealed Bx7 + By15 subunits, while using the KASP marker BX7OE_866_SNP designed for SNP in the Glu-B1 promoter region showed the “G” call (corresponding to alleles other than Glu-B1al) in two of them and the “C” call (corresponding to Glu-B1al encoding the Bx7OE subunit) in five of them (Figure 1). Consequently, two accessions with “G” were assigned to the Glu-B1z genotype (Bx7 + By15) while the Bx subunit in the other five accessions with “C” was designated as Bx7C, and the coding novel non-typical allele was tentatively designated as Glu-B1z* (Supplementary Table S1).
Figure 1.
Scatter plots for Glu-Ax1/x2*_SNP and BX7OE_866_SNP assays showing clustering of durum wheat accessions on the X-(FAM) and Y-(HEX) axes. (A) Orange dots, Ax1/Ax2* allele; blue dots, Ax-null allele. (B) Orange dots, non-Bx7OE allele; blue dots, Bx7OE allele. Black dots show negative control (water).

Table 1.
Mean values of quality traits of 69 spring durum wheat accessions. Mean values designated with the same letters have no significant differences within each group, as calculated using Tukey’s criterion. N, number of accessions of a corresponding group.
3.2. Correlations between Quality Traits
In both years of the trials, a strong correlation was found between protein and gluten content. The correlation between SDS value and protein content (Φk 0.87) and between SDS value and gluten content (0.78) in 2021 was stronger than in 2020 (0.36 and 0.51, respectively). As it can be seen from the PCA biplots (Supplementary Figures S1 and S2), all three vectors/traits—protein content, gluten content, and SDS—have a similar direction; the pairwise angle values between them do not exceed 6°, which corresponds to a positive correlation (Supplementary Table S5). In 2021, the pasta breaking strength showed a tendency towards a positive correlation with protein content and gluten content (Φk 0.39 and 0.44, respectively, not statistically significant) and a negative correlation with the gluten index (Φk 0.38; Supplementary Tables S2 and S3). The most reliable correlation between traits within observation years was demonstrated by gluten content (Φk 0.53 at a significance level of 3.11); general pasta evaluation (Φk 0.56), protein content (Φk 0.44), volume increase index (Φk 0.34), and SDS (Φk 0.32) showed a moderate level of correlation at a low level of statistical significance (Supplementary Table S4).
3.3. Effects of HMW-GS on Grain and Pasta Quality
The Glu-B1 gene had a statistically significant influence on SDS value, protein content, and gluten content only in 2020. Genotypes with Glu-B1al (51.2 units) and Glu-B1d (48.3 units) alleles differed significantly in SDS value by 5.7%, while others had intermediate values. The highest gluten content was found in the accessions with Glu-B1z and Glu-B1al genotypes (31.6% and 30.3%, respectively, no significant differences), and the lowest was in the Glu-B1d genotype (28.6%), while other genotypes showed intermediate positions. The maximum protein content was found in the carriers of Glu-B1z, Glu-B1e, and Glu-B1al (16.9%, 16.4%, and 16.2%, respectively, no significant differences), while the minimum was in the Glu-B1d genotype (15.5%), and other genotypes showed intermediate positions (Table 1). In the PCA biplot, dots corresponding to Glu-B1d genotypes are located in the area opposite to the vectors/traits corresponding to gluten and protein content and SDS, which demonstrates a negative effect of Glu-B1d on these parameters (Supplementary Figure S1).
The highest values of gluten index were shown by the genotypes Glu-B1d, Glu-B1al, and Glu-B1z* in both observation years, although statistically significant differences between means were only shown in 2021. The difference between carriers of Glu-B1z*, Glu-B1d, and Glu-B1al alleles, on the one hand, and Glu-B1e, on the other hand, was 17.7 pp, 16.0 pp, and 15.7 pp, respectively; other accessions showed intermediate values (Table 1, Supplementary Figure S1).
4. Discussion
The influence of high-molecular-weight glutenins (HMW-GS) on dough properties and pasta quality is an important factor that needs to be considered in the breeding process. Noteworthy, although the main tendencies of HMW-GS to affect grain quality kept stable between years of the present field experiment, in 2020, HMW-GS influenced the gluten index, while in 2021, their effect on protein content, gluten content, and SDS was the most pronounced. This demonstrates that the HMW-GS genetic system may have different ways of impacting grain and protein quality depending on weather conditions that may be explained by the environmental influence on gluten polymerization at grain filling [8,34].
The structure of the world collections based on the allelic composition of Glu-A1 and Glu-B1 genes confirms the impact of certain alleles of HMW-GS on grain quality and final products. Apparently, the more positive influence of the allele on gluten strength and quality parameters, the higher frequency the allele has in the studied collection, especially when modern cultivars are studied. In the present study, for example, almost all accessions had the Glu-A1c allele (Ax-Null), while only two accessions carried Ax2*, which is rare for durum wheat and characteristic of common bread wheat, known for its positive effect on breadmaking quality and dough properties [35]. In the studies of collections of durum wheat cultivars and landraces, all or the vast majority of the accessions also had the Glu-A1c allele, with only a few accessions carrying the Ax2* subunits of Glu-A1b or Ax1 of Glu-A1a alleles [13,18,19,36,37,38,39,40,41]. However, in our recent study of winter durum wheat, 14.5% of the accessions had the Glu-A1a allele [29]; in the collection of varieties from different countries, the Glu-A1b and Glu-A1a alleles accounted for 18.5% and 22.4%, respectively [42], whereas 38.1% of the Mediterranean landrace collection had the Glu-A1a allele [13]. In the present study, Glu-A1b was associated with the Glu-B1al allele (Bx7OE + By8), while in the winter durum wheat collection obtained from NGC, it was mainly associated with the Glu-B1b allele (Bx7 + By8) [29]; in the world collection of durum wheat cultivars, Glu-A1b was associated with the By8 subunit in the absence of Bx [42], whereas in the collection of Mediterranean landraces and old varieties, Glu-A1a was more frequently associated with Glu-B1e and bands 2 + 4 + 15 + 19 at Glu-B3 [43]. Thus, it can be hypothesized that non-null Glu-A1 alleles are still in demand in the breeding of durum wheat and may have a certain advantage if combined with other high- and low-molecular-weight glutenin alleles.
According to the previous study, among Glu-B1 alleles, Glu-B1b (Bx7 + By8), Glu-B1d (Bx6 + By8), and Glu-B1z (Bx7 + By15) are usually indicated as having a positive effect on gluten strength, dough, and pasta quality in durum wheat [12]. In the present study, it was shown that the presence of Glu-B1d, Glu-B1al, and Glu-B1z* alleles positively affects the gluten index in both years of the trials. These genotypes were characterized by the highest gluten index values in both years of the trials, although statistical differences were only detected in 2021.
The Glu-B1al allele has the unique feature of possessing a double copy of the gene encoding the Bx7 subunit [44]. Glu-B1al is generally associated with greater gluten strength (gluten index, SDS sedimentation), extensibility (alveograph L and P/L), and protein content [35,45]. Its possible “predecessor”, the Glu-B1b allele, is associated with medium to strong gluten, high SDS values, gluten index, dough strength, and mixing development time in durum wheat [13,18,19,29,38,39,40,46,47,48,49]. In many studies, the structure of the durum wheat collection was determined without differentiation of Glu-B1b (Bx7 + By8) and Glu-B1al (B7OE + By8), i.e., the Glu-B1b allele in these collections was exclusively determined through SDS-PAGE electrophoresis. To compare our results with literature data, when combining the Glu-B1b and Glu-B1al genotypes into one group, “Glu-B1b”, it turns out that the predominant allele is Glu-B1b (60%), followed by Glu-B1d (18.8%) and Glu-B1e (13%). The significant predominance of the Glu-B1b allele over Glu-B1d by 2–5-fold has also been observed in breeding lines and cultivars from Australia [18], Mediterranean modern cultivars [50], exotic germplasm from various countries [36], and Moroccan modern cultivars [41]. The opposite situation is observed in collections of modern varieties from Canada and the United States, where the frequency of Glu-B1b exceeds that of Glu-B1d by 4–5-fold [18,41], and in Iberian landraces [51]. In the analysis of global collections of modern varieties, the difference between the frequencies of Glu-B1b and Glu-B1d alleles usually is less than twice [19,39,46,48]. Possible differences in the frequencies between “positive” alleles Glu-B1b and Glu-B1d in regional collections may be explained by their different influence on gluten quality under various climatic conditions and in different combinations with Glu-A1 and low-molecular-weight gluten alleles [18].
Due to the difficulty in distinguishing Bx7 and Bx7OE at SDS-PAGE electropherogram, differentiation is possible using MALDI-TOF-MS technology [52], Lab-on-a-Chip [21], high-performance liquid chromatography (RP-HPLC) [53], or with the use of more accessible classical PCR markers or KASP technology markers with primers developed at the 7OE locus [22,31,44,54]. When considering the frequencies of the Glu-B1al and Glu-B1b alleles obtained in the present study separately, it is obvious that the former (55.1%) has an advantage over the latter (2.9%) in the studied spring durum wheat collection of NCC. In our recent study of winter durum wheat from the same breeding center, Glu-B1al was identified in 17.1% and Glu-B1b was detected in 53.9% of accessions [29]. In the study where MALDI-TOF-MS was applied for the identification of the Bx7OE subunit in the collection of durum wheat from different regions, Bx7OE + By8 was found in 16.4% of accessions, Bx7 + By8 in 2.6%, and 9.0% were heterogeneous in Bx7 + By8/Bx7OE + By8, including accessions from the Russian Federation and the former Soviet Union [42]. Thus, it can be concluded that the Glu-B1al allele has been successfully introduced and is being used in the breeding of durum wheat at NCC, owing to its positive effect on the quantity and quality of gluten.
In our study, the Glu-B1d allele (Bx6 + By8) was detected in 13 out of 69 accessions (18.8%), which is consistent with the frequency found in modern varieties from Australia [18], the Mediterranean [50], and Morocco [41]. Glu-B1d had a similar effect in both years of the present experiment: as the gluten index increased, values for SDS, gluten content, and protein decreased (significant in 2020, as a general tendency in 2021). According to the literature, Glu-B1d generally has a positive effect on gluten quality and characteristics, SDS, and gluten index values [18,38,46,47,48] and negatively affects breadmaking quality in hexaploid bread wheat [35].
In the current study, a new allele, tentatively designated as Glu-B1z*, was identified in five accessions, one of which was represented by the Yarina cultivar, while the remaining four were the lines of the competitive variety trial nurseries. On the one hand, they showed bands typical of Bx7 + By15 in SDS-PAGE. On the other hand, the KASP marker BX7OE_866_SNP designed for the SNP G/C in the promoter of the gene encoding the Bx7 subunit [31] showed a call “C” associated with the Glu-B1al allele. The Glu-B1al allele carries a double dose of the gene encoding Bx7, which explains its overexpression [44]. Although the KASP marker BX7OE_866_SNP showed efficiency in detecting Glu-B1al in screening wheat collections [55], it is not causal, i.e., it was not developed directly on the border regions of the Bx7 duplication. Thus, in our atypical case, it cannot be directly concluded that the identified five accessions carry two copies of the Bx7 gene, but only stated the presence of an allele that differs from Glu-B1z in promoter region. It cannot be ruled out that the identified allele Glu-B1z* is a variant of Glu-B1z with a normal Bx copy number, as the promoter of this allele has not been studied before our research as SNPs in the promoter region of Bx are quite common phenomena [54].
The Glu-B1z allele generally shows a relatively low frequency of occurrence among old, intermediate, and modern genotypes in durum wheat germplasm from Argentina, France, Italy, CIMMYT, and Iran [19,37,56,57], as well as in Turkish landraces [58] and synthetic hexaploid cross-combinations [59]. In the collection of 313 rivet wheat accessions (T. turgidum ssp. turgidum), the Glu-B1z allele was detected only in three accessions, whereas among 77 accessions of khorasan wheat (T. turgidum ssp. turanicum), it was found in 52 genotypes [60]. The rareness of this allele in durum wheat might be explained by the frequent use of triticale, emmer, Aegilops, and other related species in breeding programs at NGC, which may lead to the introgression of Glu-B1z in the durum wheat germplasm. Furthermore, Glu-B1z has shown the highest mean values in the SDSS test [19]. In the present study, Glu-B1z and Glu-B1z* alleles have also shown a positive effect on the quality parameters of gluten in spring durum wheat germplasm, as was also shown in other studies [19,61]. The utilization of Glu-B1z and its modification Glu-B1z* in breeding programs may help to increase the genetic diversity of durum wheat and to improve its technological qualities.
In our study, Glu-B1e demonstrated (although not always statistically significant) either minimal or the lowest values for SDS and gluten index in both years of trials. This regularity is consistent with the results of many studies examining SDS and gluten index directly, as well as breadmaking properties and mixograph, alveograph, and farinograph parameters [13,18,19,29,36,38,39,40,46,47,48,49,62]. In our study, which included modern varieties and breeding lines, the frequency of this allele was 13.0%, while in our recent research on winter durum wheat from NGC, it was 14.5%. Subunits Bx20 + By20 have been shown to be progressively replaced by subunits Bx6 + By8 and Bx7 + By8 in Italian-bred varieties and were absent in old Spanish cultivars [13]. The frequency of Glu-B1e in the worldwide collections of wheat varieties or from individual countries is on average about 20%, ranging from 7% to 27% [18,19,39,41,46,48,50]; in landrace collections, its frequency is usually higher and ranges from 34% to 45% [36,41,50,51]. The lack of complete elimination of subunit 20x + 20y from the breeding process can be explained by the interaction of these high-molecular-weight glutenins with low-molecular-weight ones, which can even lead to outstanding SDS values [43,50]. Indeed, despite the generally low levels of SDS and gluten index among carriers of Glu-B1e, accessions x-15 and OSU-3910103/DUREX//OSU were consistently distinguished among other Glu-B1e genotypes by their high values for these traits, as well as their high general past estimation (integrating pasta breaking strength, volume increase index, and color) for both years of observation. Thus, the Glu-B1e allele remains in demand in the breeding process of durum wheat, but it is necessary to take into account the contribution of other genes for storage proteins and starch.
In our present and previous studies of durum wheat genotypes grown under Krasnodar conditions [29], SDS showed a positive correlation with protein content, which is consistent with the results of other studies [63,64]. Indeed, the Glu-B1al allele showed the highest values of SDS, protein, and gluten content, while Glu-B1d showed the lowest values of these parameters in 2019–2020. At the same time, we demonstrated the absence of any significant correlation between SDS and gluten index, whereas such a correlation has been found in many studies [37,49,63,64,65]. Probably, this is due to the fact that the SDS variation in the studied accessions is quite low, despite the overall high values since the breeding for this trait has been directed for many years at NGC, thus achieving its possible maximum. At the same time, evaluation of the gluten index started relatively recently, and selection for this trait was not carried out, which accounts for its high variation. In addition to high-molecular-weight glutenins, low-molecular-weight glutenins and the glutenin/gliadin ratio have been found to affect the gluten index significantly [14,18,49].
Gluten index values should be considered with caution, as selection based solely on this trait does not guarantee the production of pasta varieties with superior technological quality. It has been shown that pasta texture is better only with high protein content in addition to a high gluten index [66]. In the present study in 2021, a weak trend correlation was shown between protein level and pasta breaking strength, a parameter positively affecting the general pasta estimation. However, selection based solely on gluten index cannot lead to higher protein content, as gluten index does not depend on protein content according to many studies [17,39,63]. Modern Spanish and Italian cultivars with higher gluten index and SDS values have lower protein and gluten content compared to old cultivars and landraces [13,15,16]. At the same time, technological qualities such as bread volume, height, and specific volume can be high in old cultivars and landraces even with low gluten index values [67]. Bread baked from ancient Sicilian durum genotypes showed a more pleasant appearance as well as greater diversity in organoleptic and aromatic estimates and the characteristics of the processed products compared to modern cultivars [16]. In our case, for example, in 2021, both high (Glu-B1al and Glu-B1z*)- and low (Glu-B1e)-gluten-index genotypes showed comparable general pasta estimation scores close to 4.8 from a maximum of 5.0. Apparently, there may be an optimum value of gluten index that provides a proper interaction with starch and other macromolecules, resulting in the best cooking and organoleptic qualities of pasta.
Different effects of Glu-B1 on grain and pasta quality are due to different physical and chemical properties of the subunits provided by differences in their amino acid sequences. The presence/absence of extra cystein residues plays a major role in glutenin polymerization and thus may determine dough properties [68,69]. Glu-B1e has been shown to have a detrimental effect on gluten quality due to the substitution of two cysteine residues in the N-terminal domain of subunit Bx20 by tyrosines [70]. Interestingly, the Glu-B1 subunit of durum wheat contains a significantly lower number of negatively and positively charged residues than that of durum wheat [40], which may be associated with the differences in requirements for pasta- and breadmaking technology. Therefore, functional characterization of molecular mechanisms determining the differences between the studies’ alleles would greatly contribute to understanding their role in the breeding process and providing a quality end-product.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13061510/s1, Figure S1: Principal component analysis of quality traits in the studied spring durum wheat accessions estimated in 2020. Dots indicate Glu-B1 genotypes; vectors indicate protein content (protein), gluten content (gluten), gluten index (GI), general pasta estimation (GPE), pasta breaking strength (PBS), SDS sedimentation volume (SDS), and volume increase index (VII); Figure S2: Principal component analysis of quality traits in the studied spring durum wheat accessions estimated in 2021. Dots and vectors indicate the same as in Figure S1; Supplementary Table S1: Accessions of the studied durum wheat collection, their Glu-A1 and Glu-B1 allelic state, gluten index, and general pasta estimation, as measured in 2020 and 2021 during the field experiment, with origin and year of release; Supplementary Table S2: Φk correlation indices (A) and their significance (B) between grain quality of durum wheat grown in 2020 and the allelic state of Glu-A1 and Glu-B1; Supplementary Table S3: Φk correlation indices (A) and their significance (B) between grain quality of durum wheat grown in 2021 and the allelic state of Glu-A1 and Glu-B1; Supplementary Table S4: Correlation coefficient between parameters of spring durum wheat measured in 2020 and 2021 during the field experiment; Supplementary Table S5: The values of angles between vectors in principal component analysis biplot.
Author Contributions
Conceptualization, L.A.B., A.S.Y. and M.G.D.; methodology, A.Y.K., A.S.Y., V.A.K. and M.G.D.; software, D.S.U.; validation, A.Y.K., A.S.Y., V.A.K. and D.S.U.; formal analysis, D.S.U.; investigation, A.Y.K., A.S.Y. and V.A.K.; resources, L.A.B., A.S.Y., G.I.K. and M.G.D.; data curation, D.S.U.; writing—original draft preparation, P.Y.K., A.Y.K., A.S.Y. and D.S.U.; writing—review and editing, P.Y.K., L.A.B., A.S.Y., G.I.K. and M.G.D.; visualization, A.Y.K. and D.S.U.; supervision, L.A.B., A.S.Y., G.I.K. and M.G.D.; project administration, L.A.B., G.I.K. and M.G.D.; funding acquisition, M.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant 21-16-00121.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martínez-Moreno, F.; Ammar, K.; Solís, I. Global Changes in Cultivated Area and Breeding Activities of Durum Wheat from 1800 to Date: A Historical Review. Agronomy 2022, 12, 1135. [Google Scholar] [CrossRef]
- Cereals Market Situation. Available online: https://circabc.europa.eu/sd/a/98826879-f6a2-4931-b2fc-4780ee466338/cereals-market-situation.pdf (accessed on 17 May 2023).
- Tedone, L.; Ali, S.A.; De Mastro, G. Optimization of Nitrogen in Durum Wheat in the Mediterranean Climate: The Agronomical Aspect and Greenhouse Gas (GHG) Emissions. In Nitrogen in Agriculture—Updates; InTech: Vienna, Austria, 2018. [Google Scholar]
- Zingale, S.; Spina, A.; Ingrao, C.; Fallico, B.; Timpanaro, G.; Anastasi, U.; Guarnaccia, P. Factors Affecting the Nutritional, Health, and Technological Quality of Durum Wheat for Pasta-Making: A Systematic Literature Review. Plants 2023, 12, 530. [Google Scholar] [CrossRef]
- Banach, J.K.; Majewska, K.; Żuk-Gołaszewska, K. Effect of Cultivation System on Quality Changes in Durum Wheat Grain and Flour Produced in North-Eastern Europe. PLoS ONE 2021, 16, e0236617. [Google Scholar] [CrossRef] [PubMed]
- Bouatrous, A.; Harbaoui, K.; Karmous, C.; Gargouri, S.; Souissi, A.; Belguesmi, K.; Cheikh Mhamed, H.; Gharbi, M.S.; Annabi, M. Effect of Wheat Monoculture on Durum Wheat Yield under Rainfed Sub-Humid Mediterranean Climate of Tunisia. Agronomy 2022, 12, 1453. [Google Scholar] [CrossRef]
- Flagella, Z.; Giuliani, M.M.; Giuzio, L.; Volpi, C.; Masci, S. Influence of Water Deficit on Durum Wheat Storage Protein Composition and Technological Quality. Eur. J. Agron. 2010, 33, 197–207. [Google Scholar] [CrossRef]
- Gagliardi, A.; Carucci, F.; Masci, S.; Flagella, Z.; Gatta, G.; Giuliani, M.M. Effects of Genotype, Growing Season and Nitrogen Level on Gluten Protein Assembly of Durum Wheat Grown under Mediterranean Conditions. Agronomy 2020, 10, 755. [Google Scholar] [CrossRef]
- De Santis, M.A.; Soccio, M.; Laus, M.N.; Flagella, Z. Influence of Drought and Salt Stress on Durum Wheat Grain Quality and Composition: A Review. Plants 2021, 10, 2599. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mohammadi, R.; Etminan, A.; Shooshtari, L.; Maleki-Tabrizi, N.; Poczai, P. Effects of Drought Stress on Some Agronomic and Morpho-Physiological Traits in Durum Wheat Genotypes. Sustainability 2020, 12, 5610. [Google Scholar] [CrossRef]
- Saini, P.; Kaur, H.; Tyagi, V.; Saini, P.; Ahmed, N.; Dhaliwal, H.S.; Sheikh, I. Nutritional Value and End-Use Quality of Durum Wheat. Cereal Res. Commun. 2022, 51, 283–294. [Google Scholar] [CrossRef]
- Giraldo, P.; Ruiz, M.; Ibba, M.I.; Morris, C.F.; Labuschagne, M.T.; Igrejas, G. Durum Wheat Storage Protein Composition and the Role of LMW-GS in Quality. In Wheat Quality for Improving Processing and Human Health; Springer International Publishing: Cham, Switzerland, 2020; pp. 73–108. [Google Scholar]
- Subira, J.; Peña, R.J.; Álvaro, F.; Ammar, K.; Ramdani, A.; Royo, C. Breeding Progress in the Pasta-Making Quality of Durum Wheat Cultivars Released in Italy and Spain during the 20th Century. Crop Pasture Sci. 2014, 65, 16–26. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in Gluten Protein Composition between Old and Modern Durum Wheat Genotypes in Relation to 20th Century Breeding in Italy. Eur. J. Agron. 2017, 87, 19–29. [Google Scholar] [CrossRef]
- Mefleh, M.; Conte, P.; Fadda, C.; Giunta, F.; Piga, A.; Hassoun, G.; Motzo, R. From Ancient to Old and Modern Durum Wheat Varieties: Interaction among Cultivar Traits, Management, and Technological Quality. J. Sci. Food Agric. 2019, 99, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Ruisi, P.; Ingraffia, R.; Urso, V.; Giambalvo, D.; Alfonzo, A.; Corona, O.; Settanni, L.; Frenda, A.S. Influence of Grain Quality, Semolinas and Baker’s Yeast on Bread Made from Old Landraces and Modern Genotypes of Sicilian Durum Wheat. Food Res. Int. 2021, 140, 110029. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, C.; Bresciani, A.; Menesatti, P.; Pagani, M.A.; Marti, A. Assessing the Rheological Properties of Durum Wheat Semolina: A Review. Foods 2021, 10, 2947. [Google Scholar] [CrossRef]
- Sissons, M.J.; Ames, N.P.; Hare, R.A.; Clarke, J.M. Relationship between Glutenin Subunit Composition and Gluten Strength Measurements in Durum Wheat. J. Sci. Food Agric. 2005, 85, 2445–2452. [Google Scholar] [CrossRef]
- Roncallo, P.F.; Guzmán, C.; Larsen, A.O.; Achilli, A.L.; Dreisigacker, S.; Molfese, E.; Astiz, V.; Echenique, V. Allelic Variation at Glutenin Loci (Glu-1, Glu-2 and Glu-3) in a Worldwide Durum Wheat Collection and Its Effect on Quality Attributes. Foods 2021, 10, 2845. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum Wheat Breeding in the Mediterranean Region: Current Status and Future Prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Shin, D.; Cha, J.-K.; Lee, S.-M.; Kabange, N.R.; Lee, J.-H. Rapid and Easy High-Molecular-Weight Glutenin Subunit Identification System by Lab-on-a-Chip in Wheat (Triticum aestivum L.). Plants 2020, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Jin, H.; Xiao, Y.; Zhang, Y.Y.; Hao, Y.; Zhang, Y.Y.; Hickey, L.T.; Morgounov, A.I.; Xia, X.; He, Z. Allelic Effects and Variations for Key Bread-Making Quality Genes in Bread Wheat Using High-Throughput Molecular Markers. J. Cereal Sci. 2019, 85, 305–309. [Google Scholar] [CrossRef]
- Nikitina, E.A.; Arkhipov, A.A.; Min’kova, Y.V.; Yanovskiy, A.S.; Korobkova, V.A.; Samarina, M.A.; Chernook, A.G.; Krupin, P.Y.; Karlov, G.I.; Divashuk, M.G. Competitive Allele Specific PCR (KASP): Features, the Interpretation of the Results. Izv. Timirazevsk. Selʹskohozajstvennoj Akad. 2022, 1, 79–93. [Google Scholar] [CrossRef]
- Johnson, M.; Kumar, A.; Oladzad-Abbasabadi, A.; Salsman, E.; Aoun, M.; Manthey, F.A.; Elias, E.M. Association Mapping for 24 Traits Related to Protein Content, Gluten Strength, Color, Cooking, and Milling Quality Using Balanced and Unbalanced Data in Durum Wheat [Triticum turgidum L. var. durum (Desf).]. Front. Genet. 2019, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- Requena-Ramírez, M.D.; Rodríguez-Suárez, C.; Flores, F.; Hornero-Méndez, D.; Atienza, S.G. Marker-Trait Associations for Total Carotenoid Content and Individual Carotenoids in Durum Wheat Identified by Genome-Wide Association Analysis. Plants 2022, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- Malchikov, P.N.; Sidorenko, V.S.; Bespalova, L.A.; Mudrova, A.A.; Myasnikova, M.G.; Chakheeva, T.V.; Starikova, Z.V.; Tugareva, F.V. Spring Durum Wheat Cultivar Triada, Proposed for Economic Use in the 5th Region of Russia. Legum. Groat Crop. 2020, 3, 112–120. [Google Scholar] [CrossRef]
- Mudrova, A.A.; Yanovsky, A.S.; Bespalova, L.A.; Borovik, A.N. Breeding High-Quality Spring Durum Wheat. In Proceedings of the IV International Scientific Conference “Current State, Problems and Prospects of the Development of Agrarian Science”, Yalta, Russia, 9–13 September 2019; pp. 178–179. [Google Scholar]
- Mudrova, A.A.; Yanovsky, A.S.; Bespalova, L.A.; Borovik, A.N. Yasenka’ Is a New Word in Obtaining the ‘Golden Grain’ of Durum Wheat. Grain Econ. Russ. 2021, 3, 41–45. [Google Scholar] [CrossRef]
- Kroupina, A.Y.; Yanovsky, A.S.; Korobkova, V.A.; Bespalova, L.A.; Arkhipov, A.V.; Bukreeva, G.I.; Voropaeva, A.D.; Kroupin, P.Y.; Litvinov, D.Y.; Mudrova, A.A.; et al. Allelic Variation of Glu-A1 and Glu-B1 Genes in Winter Durum Wheat and Its Effect on Quality Parameters. Foods 2023, 12, 1436. [Google Scholar] [CrossRef]
- Singh, N.K.; Shepherd, K.W.; Cornish, G.B. A Simplified SDS—PAGE Procedure for Separating LMW Subunits of Glutenin. J. Cereal Sci. 1991, 14, 203–208. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and Validation of KASP Assays for Genes Underpinning Key Economic Traits in Bread Wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef]
- Warton, D.I.; Hui, F.K.C. The Arcsine Is Asinine: The Analysis of Proportions in Ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef]
- Baak, M.; Koopman, R.; Snoek, H.; Klous, S. A New Correlation Coefficient between Categorical, Ordinal and Interval Variables with Pearson Characteristics. Comput. Stat. Data Anal. 2018, 152, 107043. [Google Scholar] [CrossRef]
- Fois, S.; Schlichting, L.; Marchylo, B.; Dexter, J.; Motzo, R.; Giunta, F. Environmental Conditions Affect Semolina Quality in Durum Wheat (Triticum turgidum ssp. durum L.) Cultivars with Different Gluten Strength and Gluten Protein Composition. J. Sci. Food Agric. 2011, 91, 2664–2673. [Google Scholar] [CrossRef]
- Guzmán, C.; Crossa, J.; Mondal, S.; Govindan, V.; Huerta, J.; Crespo-Herrera, L.; Vargas, M.; Singh, R.P.; Ibba, M.I. Effects of Glutenins (Glu-1 and Glu-3) Allelic Variation on Dough Properties and Bread-Making Quality of CIMMYT Bread Wheat Breeding Lines. Field Crops Res. 2022, 284, 108585. [Google Scholar] [CrossRef]
- Raciti, C.N.; Doust, M.A.; Lombardo, G.M.; Boggini, G.; Pecetti, L. Characterization of Durum Wheat Mediterranean Germplasm for High and Low Molecular Weight Glutenin Subunits in Relation with Quality. Eur. J. Agron. 2003, 19, 373–382. [Google Scholar] [CrossRef]
- Lerner, S.E.; Cogliatti, M.; Ponzio, N.R.; Seghezzo, M.L.; Molfese, E.R.; Rogers, W.J. Genetic Variation for Grain Protein Components and Industrial Quality of Durum Wheat Cultivars Sown in Argentina. J. Cereal Sci. 2004, 40, 161–166. [Google Scholar] [CrossRef]
- del Carmen Martinez, M.; Ruiz, M.; Carrillo, J.M. Effects of Different Prolamin Alleles on Durum Wheat Quality Properties. J. Cereal Sci. 2005, 41, 123–131. [Google Scholar] [CrossRef]
- Edwards, N.M.; Gianibelli, M.C.; McCaig, T.N.; Clarke, J.M.; Ames, N.P.; Larroque, O.R.; Dexter, J.E. Relationships between Dough Strength, Polymeric Protein Quantity and Composition for Diverse Durum Wheat Genotypes. J. Cereal Sci. 2007, 45, 140–149. [Google Scholar] [CrossRef]
- Al-Khayri, J.; Alshegaihi, R.; Mahgoub, E.I.; Mansour, E.; Atallah, O.; Sattar, M.; Al-Mssallem, M.; Alessa, F.; Aldaej, M.; Hassanin, A. Association of High and Low Molecular Weight Glutenin Subunits with Gluten Strength in Tetraploid Durum Wheat (Triticum turgidum spp. durum L.). Plants 2023, 12, 1416. [Google Scholar] [CrossRef] [PubMed]
- Chegdali, Y.; Ouabbou, H.; Essamadi, A.; Cervantes, F.; Ibba, M.I.; Guzmán, C. Assessment of the Glutenin Subunits Diversity in a Durum Wheat (T. turgidum ssp. durum) Collection from Morocco. Agronomy 2020, 10, 957. [Google Scholar] [CrossRef]
- Elfatih, S.; Peng, Y.; Ma, J.; Peng, J.; Sun, D.; Ma, W. High Frequency of Unusual High Molecular Weight Glutenin Alleles in 232 Tetraploid Durum Wheat Accessions (Triticum turgidum L. ssp. durum Desf). Cereal Res. Commun. 2013, 41, 583–592. [Google Scholar] [CrossRef]
- Nazco, R.; Peña, R.J.; Ammar, K.; Villegas, D.; Crossa, J.; Royo, C. Durum Wheat (Triticum durum Desf.) Mediterranean Landraces as Sources of Variability for Allelic Combinations at Glu-1/Glu-3 Loci Affecting Gluten Strength and Pasta Cooking Quality. Genet. Resour. Crop Evol. 2014, 61, 1219–1236. [Google Scholar] [CrossRef]
- Ragupathy, R.; Naeem, H.A.; Reimer, E.; Lukow, O.M.; Sapirstein, H.D.; Cloutier, S. Evolutionary Origin of the Segmental Duplication Encompassing the Wheat GLU-B1 Locus Encoding the Overexpressed Bx7 (Bx7OE) High Molecular Weight Glutenin Subunit. Theor. Appl. Genet. 2008, 116, 283–296. [Google Scholar] [CrossRef]
- D’Ovidio, R.; Masci, S.; Porceddu1, E.; Kasarda, D.D. Duplication of the Bx7 High-Molecular-Weight Glutenin Subunit Gene in Bread Wheat (Triticum aestivum L.) Cultivar’Red River 68′. Plant Breed. 1997, 116, 525–531. [Google Scholar] [CrossRef]
- Magallanes-López, A.M.; Ammar, K.; Morales-Dorantes, A.; González-Santoyo, H.; Crossa, J.; Guzmán, C. Grain Quality Traits of Commercial Durum Wheat Varieties and Their Relationships with Drought Stress and Glutenins Composition. J. Cereal Sci. 2017, 75, 1–9. [Google Scholar] [CrossRef]
- Peña, R.J.; Zarco-Hernandez, J.; Amaya-Celis, A.; Mujeeb-Kazi, A. Relationships Between Chromosome 1B-Encoded Glutenin Subunit Compositions and Bread-Making Quality Characteristics of Some Durum Wheat (Triticum turgidum) Cultivars. J. Cereal Sci. 1994, 19, 243–249. [Google Scholar] [CrossRef]
- Ammar, K.; Kronstad, W.E.; Morris, C.F. Breadmaking Quality of Selected Durum Wheat Genotypes and Its Relationship with High Molecular Weight Glutenin Subunits Allelic Variation and Gluten Protein Polymeric Composition. Cereal Chem. J. 2000, 77, 230–236. [Google Scholar] [CrossRef]
- Brites, C.; Carrillo, J.M. Influence of High Molecular Weight (HMW) and Low Molecular Weight (LMW) Glutenin Subunits Controlled by Glu-1 and Glu-3 Loci on Durum Wheat Quality. Cereal Chem. J. 2001, 78, 59–63. [Google Scholar] [CrossRef]
- Nazco, R.; Peña, R.J.; Ammar, K.; Villegas, D.; Crossa, J.; Moragues, M.; Royo, C. Variability in Glutenin Subunit Composition of Mediterranean Durum Wheat Germplasm and Its Relationship with Gluten Strength. J. Agric. Sci. 2014, 152, 379–393. [Google Scholar] [CrossRef]
- Moragues, M.; Zarco-Hernández, J.; Moralejo, M.A.; Royo, C. Genetic Diversity of Glutenin Protein Subunits Composition in Durum Wheat Landraces [Triticum turgidum ssp. turgidum convar. durum (Desf.) MacKey] from the Mediterranean Basin. Genet. Resour. Crop Evol. 2006, 53, 993–1002. [Google Scholar] [CrossRef]
- Liu, L.; Wang, A.; Appels, R.; Ma, J.; Xia, X.; Lan, P.; He, Z.; Bekes, F.; Yan, Y.; Ma, W. A MALDI-TOF Based Analysis of High Molecular Weight Glutenin Subunits for Wheat Breeding. J. Cereal Sci. 2009, 50, 295–301. [Google Scholar] [CrossRef]
- Li, J.; Han, C.; Zhen, S.; Li, X.; Yan, Y. Characterization of HMW Glutenin Subunit Bx7 OE and Its Distribution in Common Wheat and Related Species. Plant Genet. Resour. 2014, 12, 191–198. [Google Scholar] [CrossRef]
- Ravel, C.; Faye, A.; Ben-Sadoun, S.; Ranoux, M.; Dardevet, M.; Dupuits, C.; Exbrayat, F.; Poncet, C.; Sourdille, P.; Branlard, G. SNP Markers for Early Identification of High Molecular Weight Glutenin Subunits (HMW-GSs) in Bread Wheat. Theor. Appl. Genet. 2020, 133, 751–770. [Google Scholar] [CrossRef]
- Shin, D.; Cha, J.-K.; Lee, S.-M.; Ko, J.-M.; Lee, J.-H. Validation and Selection of Functional Allele-Specific Molecular Markers to Analyze High-Molecular-Weight Glutenin Subunit Composition in Wheat. Korean J. Breed. Sci. 2020, 52, 235–243. [Google Scholar] [CrossRef]
- Bellil, I.; Hamdi, O.; Khelifi, D. Diversity of Five Glutenin Loci within Durum Wheat (Triticum turgidum L. ssp. durum (Desf.) Husn.) Germplasm Grown in Algeria. Plant Breed. 2014, 133, 179–183. [Google Scholar] [CrossRef]
- Hernández-Espinosa, N.; Payne, T.; Huerta-Espino, J.; Cervantes, F.; Gonzalez-Santoyo, H.; Ammar, K.; Guzmán, C. Preliminary Characterization for Grain Quality Traits and High and Low Molecular Weight Glutenins Subunits Composition of Durum Wheat Landraces from Iran and Mexico. J. Cereal Sci. 2019, 88, 47–56. [Google Scholar] [CrossRef]
- Temizgul, R.; Akbulut, M. Allele Frequency of Glutenin Subunits and Glu-1 Quality Scores in Some Turkish Bread Wheat Landraces. Trak. Univ. J. Nat. Sci. 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Rasheed, A.; Mahmood, T.; Kazi, A.G.; Ghafoor, A.; Mujeeb-Kazi, A. Allelic Variation and Composition of HMW-GS in Advanced Lines Derived from d-Genome Synthetic Hexaploid / Bread Wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. 2012, 15, 1–7. [Google Scholar] [CrossRef]
- Carmona, S.; Caballero, L.; Martín, L.M.; Alvarez, J.B. Genetic Diversity in Khorasan and Rivet Wheat by Assessment of Morphological Traits and Seed Storage Proteins. Crop Pasture Sci. 2010, 61, 938. [Google Scholar] [CrossRef]
- Trad, H.; Ayed, S.; Rhazi, L.; Slim, A.; Teixeira da Silva, J.A.; Hellal, R.; Sghaier, M.; Amara, H.S. Comparative Quality Analysis of Gluten Strength and the Relationship with High Molecular Weight Glutenin Subunits of 6 Tunisian Durum Wheat Genotypes. Food Sci. Biotechnol. 2014, 23, 1363–1370. [Google Scholar] [CrossRef]
- Sapirstein, H.D.; David, P.; Preston, K.R.; Dexter, J.E. Durum Wheat Breadmaking Quality: Effects of Gluten Strength, Protein Composition, Semolina Particle Size and Fermentation Time. J. Cereal Sci. 2007, 45, 150–161. [Google Scholar] [CrossRef]
- Clarke, F.R.; Clarke, J.M.; Ames, N.A.; Knox, R.E.; Ross, R.J. Gluten Index Compared with SDS-Sedimentation Volume for Early Generation Selection for Gluten Strength in Durum Wheat. Can. J. Plant Sci. 2010, 90, 1–11. [Google Scholar] [CrossRef]
- Cha, J.-K.; Shin, D.; Park, H.; Kwon, Y.; Lee, S.-M.; Ko, J.-M.; Lee, J.-H. Effects of High-Molecular-Weight Glutenin Subunits and Agronomic Traits on Bread Wheat Quality Parameters. Korean J. Crop Sci. 2022, 67, 111–120. [Google Scholar] [CrossRef]
- Gaines, C.S.; Reid, J.F.; Vander Kant, C.; Morris, C.F. Comparison of Methods for Gluten Strength Assessment. Cereal Chem. J. 2006, 83, 284–286. [Google Scholar] [CrossRef]
- Ames, N.P.; Clarke, J.M.; Marchylo, B.A.; Dexter, J.E.; Schlichting, L.M.; Woods, S.M. The Effect of Extra-Strong Gluten on Quality Parameters in Durum Wheat. Can. J. Plant Sci. 2003, 83, 525–532. [Google Scholar] [CrossRef]
- Spina, A.; Dinelli, G.; Palumbo, M.; Whittaker, A.; Cambrea, M.; Negri, L.; Bosi, S. Evaluation of Standard Physico-chemical and Rheological Parameters in Predicting Bread-making Quality of Durum Wheat (Triticum turgidum L. ssp. durum [Desf.] Husn.). Int. J. Food Sci. Technol. 2021, 56, 3278–3288. [Google Scholar] [CrossRef]
- Gao, X.; Appelbee, M.J.; Mekuria, G.T.; Chalmers, K.J.; Mather, D.E. A Second ‘Overexpression’ Allele at the Glu-B1 High-Molecular-Weight Glutenin Locus of Wheat: Sequence Characterisation and Functional Effects. Theor. Appl. Genet. 2012, 124, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Q.; Newberry, M.P.; Chalmers, K.J.; Mather, D.E. A Cysteine in the Repetitive Domain of a High-Molecular-Weight Glutenin Subunit Interferes with the Mixing Properties of Wheat Dough. Amino Acids 2013, 44, 1061–1071. [Google Scholar] [CrossRef]
- Shewry, P.; Gilbert, S.; Savage, A.; Tatham, A.; Wan, Y.-F.; Belton, P.; Wellner, N.; D’Ovidio, R.; Békés, F.; Halford, N. Sequence and Properties of HMW Subunit 1Bx20 from Pasta Wheat (Triticum durum) Which Is Associated with Poor End Use Properties. Theor. Appl. Genet. 2003, 106, 744–750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).