Abstract
The variation of gene expression of seeds or leaves of Arabidopsis thaliana was investigated by irradiation with oxygen and air plasmas. The irradiation with oxygen plasma reported on the growth promotion and induced the consequence of gene expression in plant cells by neutral active oxygen species. The increase in leaf area ratio by oxygen plasma to seeds was due to epigenetics such as activation of DNA demethylation transcription factors and the growth enhancement effect induced by the plasma irradiation of seeds was inherited by next-generation cells through cell division even after germination. In oxygen irradiation for 10 s, expression of each de-DNA methylation-related gene increased, and DNA methylation-related genes decreased in expression. DNA acetylation that induces gene expressions was suppressed. However, irradiated for 20 s by oxygen, both demethylation suppression and promotion and methylation/acetylation suppression and promotion were obtained. On the other hand, methylation and demethylation may occur at the same time but were not significant and the acetylation was suppressed by air plasma irradiation. In both cases, active oxygen species was the key factor for the variation of gene expression.
1. Introduction
A living body’s response and inheritance of acquired traits to the next generation are observed when external stimuli, such as ultraviolet rays and radiation, are applied. The bonds between bases that make up genes are capable of being dissociated by ultraviolet rays or radiation with energies of several eV or more. As a result, biological changes caused by high-energy external stimuli such as ultraviolet rays and radiation are generally a result of changes in protein synthesis and changes in gene sequences. In recent years, however, it was discovered that functions and heredity epigenetics are not mediated by mutations in the base sequence of genes [1,2,3,4,5,6,7,8,9,10]. The epigenetic process does not change the base sequence but rather alters the structure of the chromatin or nucleosome (for example, histone modification and DNA methylation), which is a larger structure of the gene, to change the expression of the function or inherit the acquired function. It is considered to be related [1,2]. Recent research indicates that plasma irradiation of a living body causes a variety of biological reactions in cells, as well as physical changes on its surface, and can promote plant growth, increase cell proliferation, and induce apoptosis among other biological functions [3,4]. Yet, many parts of the expression process and inheritance mechanism of biological functions by plasma irradiation remain unclear. This paper describes the gene expression effect induced in plant cells by neutral active oxygen species when the seeds or leaves of Arabidopsis thaliana are irradiated with oxygen plasma. We also report an application example of the growth promotion method using oxygen plasma.
2. Experimental Apparatus and Methods
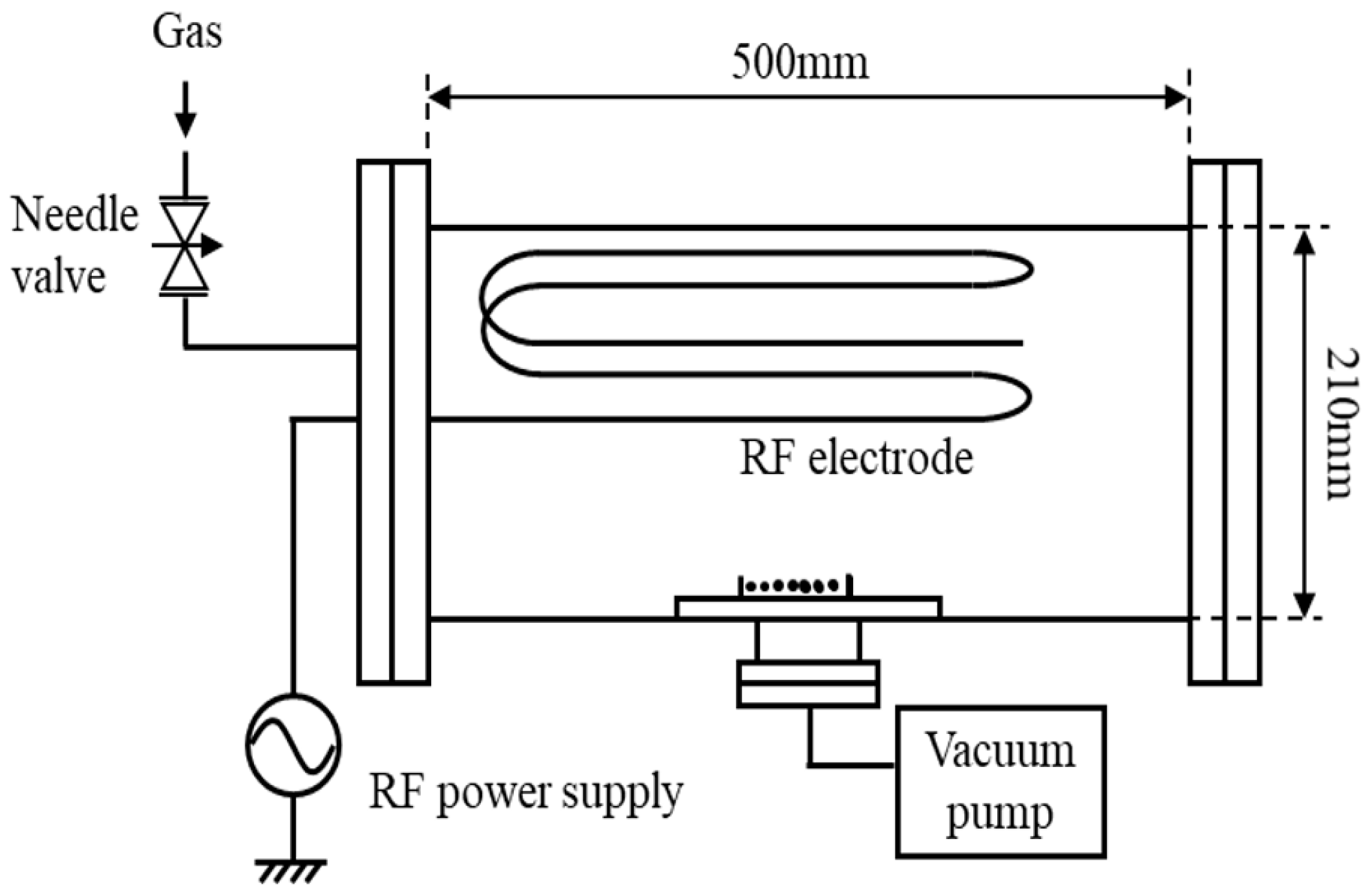
The inside of a vacuum vessel with a volume of 20 L, as shown in Figure 1, was filled with oxygen gas with a pressure of 20 to 80 Pa, and a high-frequency power with a frequency of 13.56 MHz and a power of 20 to 60 W was installed along the inner wall of the vacuum vessel. It was charged into a linear discharge electrode to generate a capacitance-coupled oxygen plasma [5,6,11,12,13,14,15,16]. At this time, an electric discharge occurred in a space of 1 to 2 cm between the electrode and the inner wall of the vacuum vessel, and the generated oxygen ions became neutral active species by charge exchange while diffusing toward the center of the vacuum vessel. Figure 2 shows a picture of seed treatment in oxygen plasma. In the afterglow region, seeds were placed 150 mm away from the linear discharge electrode fixed on the inner surface of the vacuum vessel. The electron density at the seed position was 106 cm−3 or less. Neutral reactive oxygen species (excited oxygen atoms O (1D), O (5P), singlet oxygen molecules 1∑g+, etc.) in oxygen plasma were measured by UV-Vis light emission spectroscopic analysis. A neutral active oxygen species (oxygen atom O (5P), singlet oxygen molecule 1∑g+, etc.) in oxygen plasma was allowed to act on seeds. Seeds were bound in a non-woven bag so that high-energy ions in oxygen plasma were blocked.
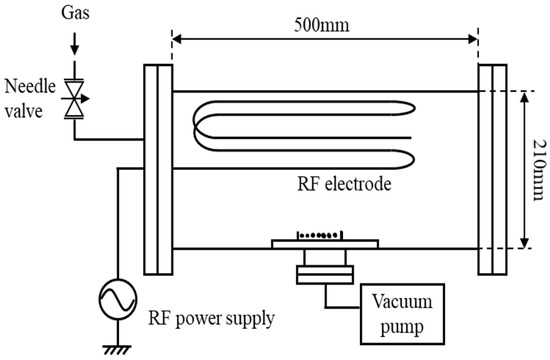
Figure 1.
Schematic of low-pressure plasma device.

Figure 2.
Irradiation of plant seeds with oxygen plasma.
In this study, Arabidopsis thaliana wild type (Columbia-0) was used, which was collected from the Institute of Physical and Chemical Research, RIKEN BRC. Arabidopsis thaliana is grown in a pot containing artificial soil as per the guideline of plant growth of the RIKEN bio-resource research center. Seeds of Arabidopsis thaliana were irradiated with oxygen pressure of 20, 40, 60, and 80 Pa. Every condition consisted of 5 pots and 10 seeds in each pot. Thinning was performed after germination and 1 plant was allowed to grow in each pot for the experiment. The plant was irrigated daily. To grow plants, an artificial climate chamber was used for one month after sowing, which was irradiated with the fluorescent light of 3000 Lux. After one month, plants were transferred to a stainless-steel container along with the pot and soil.
For plasma irradiation to the leaf under atmospheric pressure, a torch-type dielectric barrier discharge (DBD) device was used [17,18,19,20]. A ceramic tube used as torch tubes with dimensions of 6 mm in outer diameter, 4 mm in inner diameter, and 100 mm in length were employed as dielectrics for discharge, and copper films wound on the outside surface were used as grounded electrodes. As a cathode, a cylindrical shaped stainless-steel mesh was set inside the tube touching the inner wall of the tube. The DBD occurred in the tube with the application of high-frequency electrical power of 10 kHz to the mesh electrode. Discharged voltage and current were measured using the high-voltage probe and the Rogowski coil with an oscilloscope, respectively. The voltage and frequency applied on the cathode were set at 5.2 kV and 10 kHz, respectively. Oxygen gas or air was flown into the torch tube with a flow rate of 1.0 L/min. DBD in the oxygen gas generated plasma and also ozone. However, air at atmospheric pressure generated a plasma including nitrogen oxides. Ozone possessed an adequately long lifetime to reach plants from the discharge region. The plant was irradiated with oxygen plasma for 10 s 3 times within 24 h to avoid serious ozone damage to the plant. The other two treatments were: one was plant irradiated with oxygen plasma for 20 s and another was plant irradiated with air plasma for 30 s. In both cases, the same CT value of ozone, 1 ppm-min, was adjusted. The ozone CT value [ppm-min] was defined as the product of the ozone concentration, C, and the contact time, T with ozone [21,22]. Chemical indicators (CIs) were used to measure the amount of active oxygen produced by plasmas both at low pressure and atmospheric pressure which were almost proportional to CT values [23,24].
Gene expression was assessed by creating a microarray using a reagent by extracting RNA from seeds and leaves after irradiation with oxygen plasma and identifying genes whose expression fluctuated in a microarray scanner (Agilent SurePrint G3 GE 8 × 60 K v2). In addition, gene enrichment analysis by means of a bioinformatics database (DAVID by LHRI/ADRD at Frederick National Laboratory, Washington, DC, USA) [25,26] and heat maps revealed the functions of gene clusters that fluctuate in response to plasma irradiation. Based on the database for annotation, visualization, and integrated discovery, annotations of gene expression due to plasma irradiation were obtained based on expression genes with a p-value less than 0.05. DNA methylation, which is one of the indicators of epigenetics, was evaluated by quantifying the amount of 5-methylcytosine (5-mC) produced with a reagent. It was confirmed by germination tests and genetic analysis that the seed surface temperature during plasma irradiation was about 10 °C higher than room temperature, but no significant thermal shock occurred in the plants at this time.
3. Experimental Results and Discussion
3.1. Gene Expression of Plant Seeds Irradiated with Plasma
It was found that plant seed irradiation with plasma for a short period of time improved plant growth and antioxidant activity in plants [3,9]. However, there were many unclear points such as the mechanism by which the plasma irradiation effect was hereditary from seeds to leaves and stems by cell division and the mechanism of inheritance to second-generation seeds. Seeds collected from plasma-irradiated plants were cultivated and investigated the inheritance to the second generation due to the effect of plasma irradiation. (a) The effect of plasma irradiation appeared only for one generation, and acquired traits such as methylation of DNA and modification of histone were not inherited, and (b) in the second generation (by the seeds obtained from the first generation), growth promotion effects due to plasma irradiation were not observed. It was clarified that germination and growth were enhanced even in the second generation that was cultivated and that the effect of plasma irradiation was inherited across generations [3,9]. In either case, the promotion of germination and growth from irradiated seeds of the first generation continued even after cell division, thus passing on the effect of oxygen plasma irradiation to future generations.
Epigenetics is a concept that explains the inherited characteristics of acquired traits by irradiation with oxygen plasma. Information about biological functions, such as the growth-promoting effect of plasma irradiation, is primarily stored in DNA chromatin structures and is subsequently passed on to the cells after division. However, the details of the mechanism of inheritance due to the effect of plasma irradiation between cells that underwent cell division remain unclear. So far, it was revealed that the consequences of inclusive gene expression analysis of plasma-irradiated plant seeds show the induction in expression of epigenetics-related genes [10,11]. On the other hand, when the growth-promoting effect can be obtained even in the second generation, there are cases where the gene sequence itself was changed by oxygen plasma, and epigenetics such as DNA methylation were inherited by the next generation. In this study, we explored the possibility of inducing and controlling epigenetic gene expression effects by oxygen plasma.
3.2. Growth Characteristics of Plants
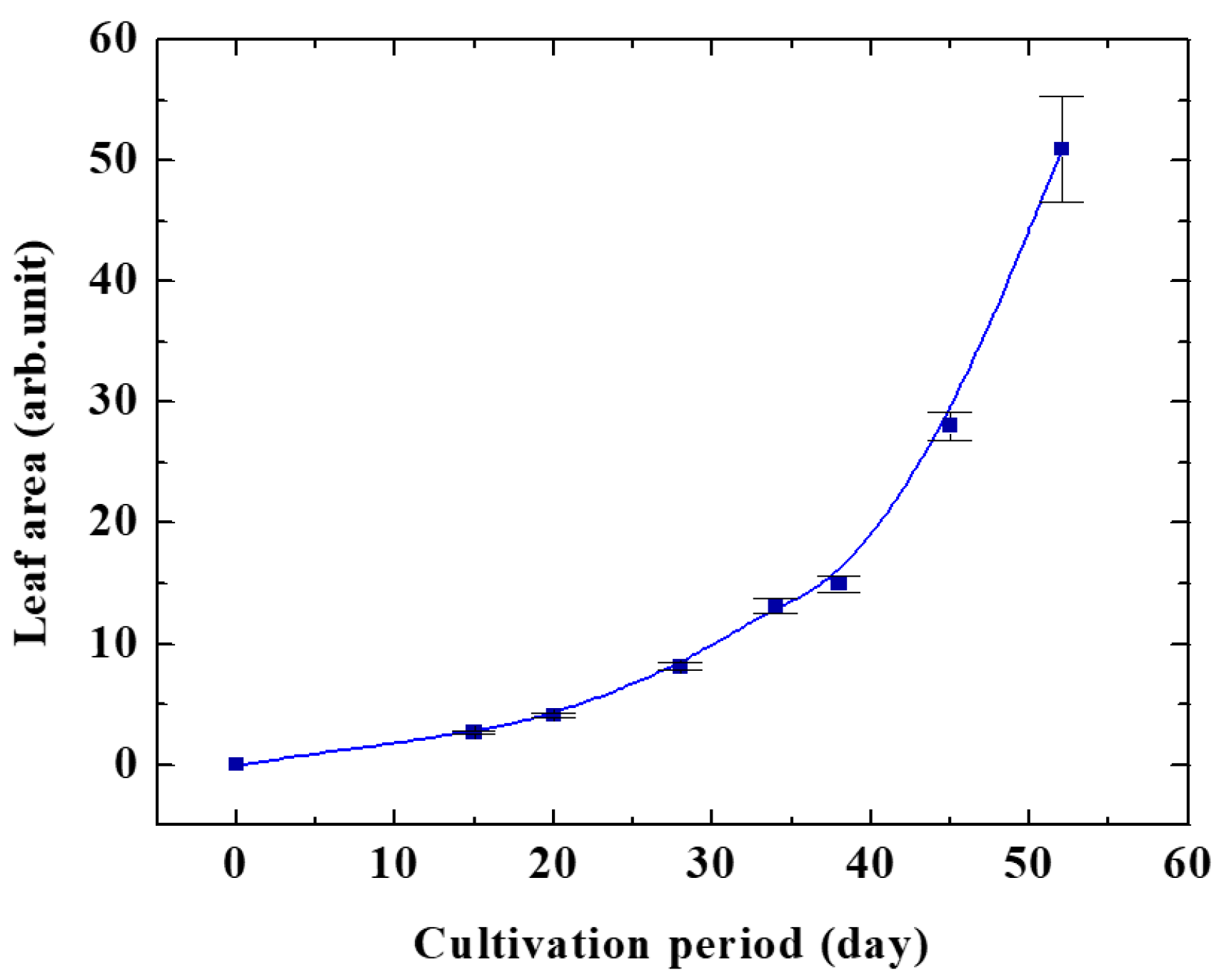
Figure 3 shows the effect of oxygen plasma on the growth of the Arabidopsis plant at different pressures. It was clearly identified from this figure that oxygen pressure of 20 Pa gave maximum plant growth. The leaf area of Arabidopsis in control with changing the cultivation period is shown in Figure 4.

Figure 3.
Oxygen pressure dependence photographs on the growth of Arabidopsis thaliana.
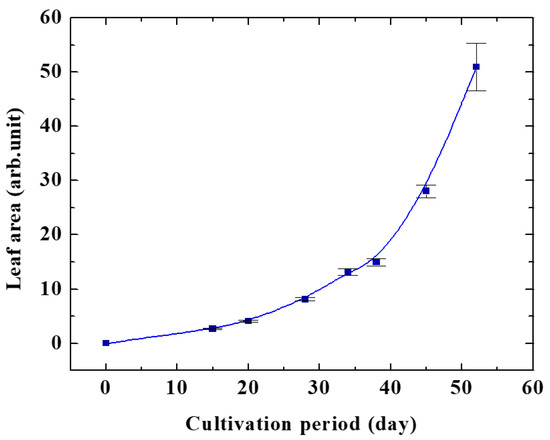
Figure 4.
Leaf area of Arabidopsis plant in control.
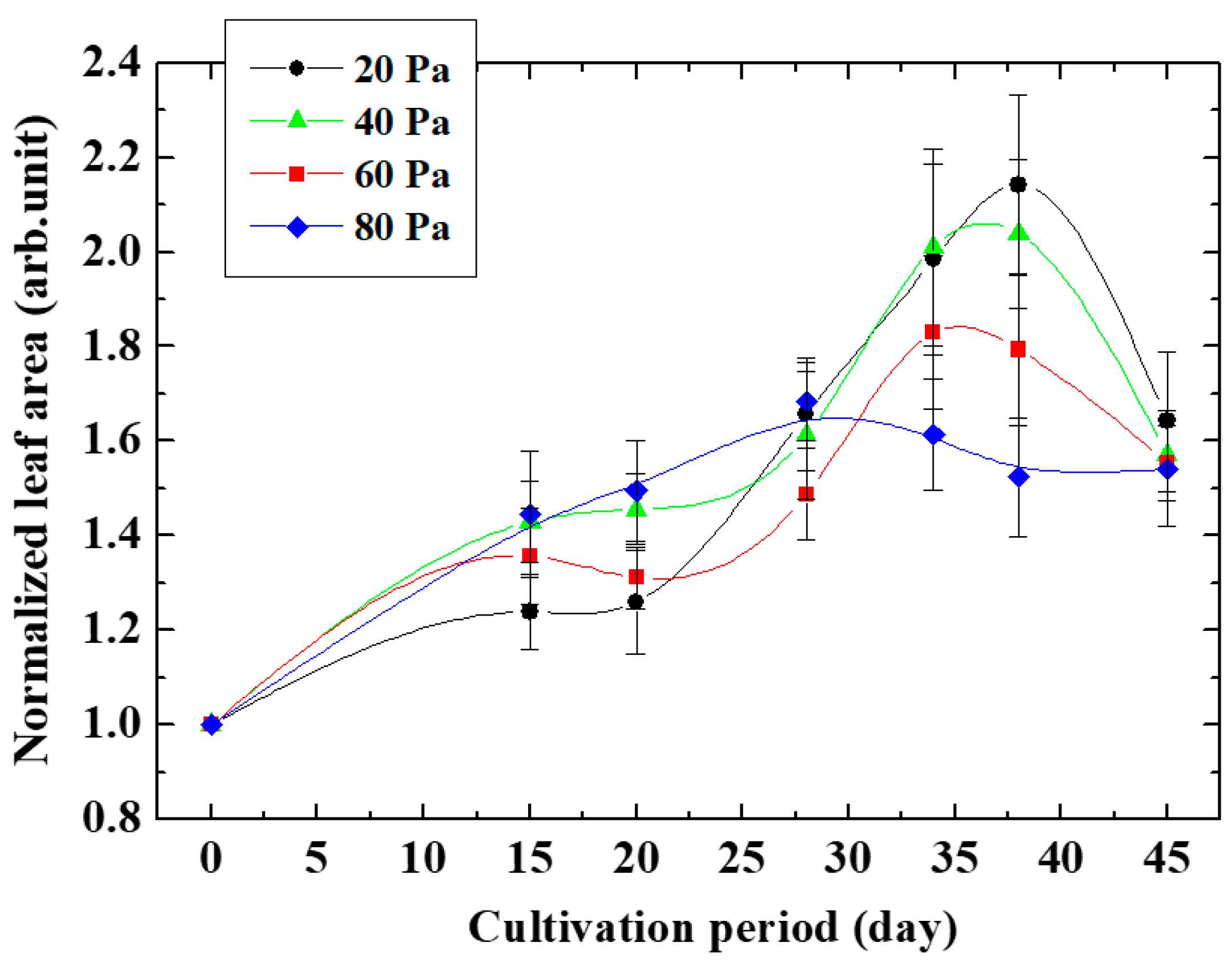
Figure 5 shows the measurement results of the area of leaves of Arabidopsis thaliana grown from plasma-irradiated seeds changing the cultivation period. The ratio of the leaf area irradiated with oxygen plasma to the unirradiated leaf area represents the leaf growth enhancement effect. The pressure of oxygen gas, which is the raw material gas of the plasma irradiated to the seeds, was varied. It was found that the size of the leaf area ratio increased with cultivation time from the beginning of cultivation, and was maximized when the cultivation days were 36 days at an oxygen pressure of 20 Pa and 26 days at an oxygen pressure of 80 Pa. This result indicates that the growth enhancement effect lasted for 36 days at an oxygen gas pressure of 20 Pa, and it was maintained for 26 days at an oxygen gas pressure of 80 Pa.
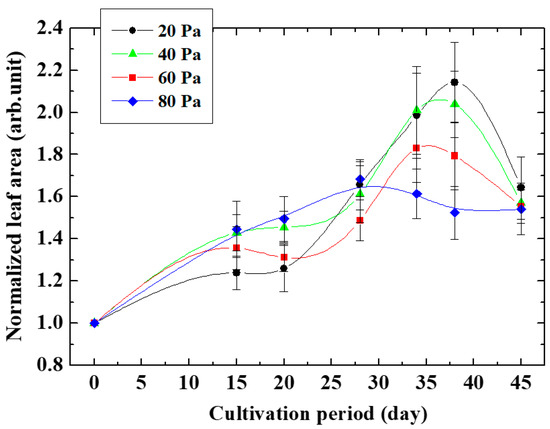
Figure 5.
Growth of normalized leaf area changing oxygen pressure.
At any gas pressure, the leaf area ratio increased with the number of cultivation days, peaked at a certain point, and then decreased. In other words, the growth-promoting effect persisted until the number of cultivation days reached its peak, and after reaching the peak, the growth-promoting effect gradually disappeared, and the growth rate approached that of non-plasma-irradiated seeds. It is possible that the increase in leaf area ratio by oxygen plasma to seeds was due to epigenetics such as activation of DNA demethylation transcription factors. At a certain point, the loss of DNA demethylation became prominent, and the growth-promoting effect was thought to decrease. As a result, the leaf area ratio was considered to have a peak at a certain cultivation time.
From this result, the growth enhancement effect induced by the plasma irradiation of seeds was inherited by next-generation cells through cell division even after germination. It can be seen that the size of the leaf area ratio decreased after reaching the maximum value. This decrease suggests that the expression level of cell growth-related genes was decreased. It was found so far that an enzyme that catalyzes a reaction related to DNA demethylation is expressed by plasma irradiation during growth promotion [6,10]. On the other hand, the activity of the transcription factor of genes encoding this enzyme such as AT1G would decrease over time to a level equivalent to that of non-irradiated plasma, and the gene expression that was enhanced would decrease due to the increase in methylated DNA such as AT4G.
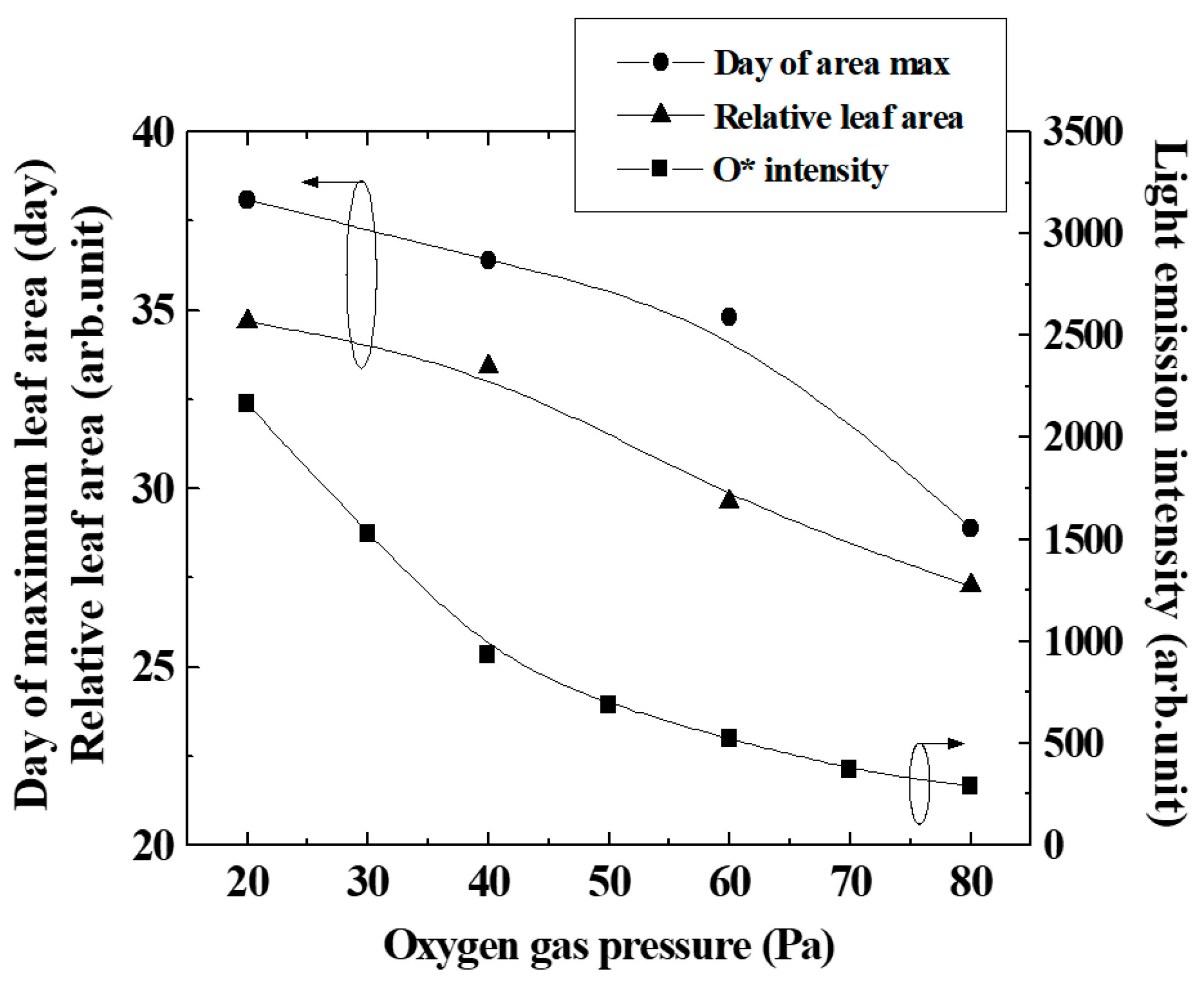
Figure 6 shows the number of cultivation days when the leaf area ratio reached its maximum (peak in Figure 5), the maximum value of the leaf area normalized by the control (leaf area ratio), and the intensity of light emission from atomic oxygen, changing the oxygen gas pressure. It can be seen that the number of cultivation days, at which the leaf area ratio shown by the peak in Figure 5 was maximum, increased as the gas pressure of the plasma irradiated to the seeds decreased. In other words, the duration when the DNA demethylation was effective was thought to be longer when the oxygen gas pressure was lower, and the duration became shorter when the pressure was higher.
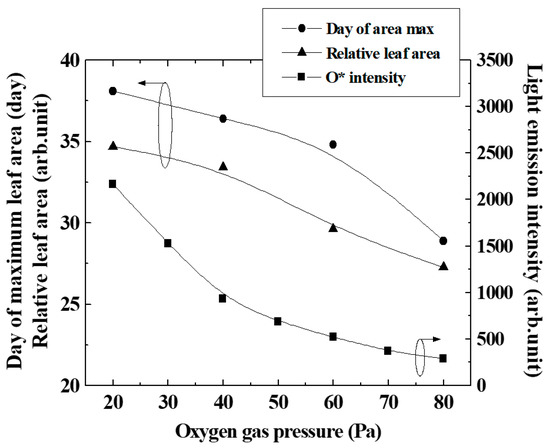
Figure 6.
Light emission intensity of excited oxygen particles and day of maximum leaf area changing oxygen pressure.
Since the flux of atomic oxygen reaching the seeds in the afterglow region increased as the pressure decreased, the number of cells affected by oxygen plasma irradiation at 20 Pa and 40 Pa was larger than 60 Pa and 80 Pa, as shown in Figure 6. Assuming that DNA demethylation is induced by atomic oxygen, the greater the number of cells irradiated with oxygen plasma, the longer the demethylation will continue. It is thought that the effect of promoting proliferation was maintained for a long time by the treatment.
As shown in Figure 6, the relative leaf area decreased slightly with the oxygen gas pressure, even though the O* intensity varied significantly. From our experiments so far, the maximum growth promotion effect was about 1.5–2 times. In this experiment, the growth enhancement effect probably would reach the maximum at pressures of 20 and 40 Pa, and it was inferred that the leaf size did not change even with excessive irradiation of active oxygen. The oxygen gas pressure dependence of the maximum leaf area showed the same trend as the number of cultivation days when the leaf area ratio was maximized. As a result, the area of the leaves was considered to increase.
3.3. Expression of Epigenetics-Related Genes in Seeds
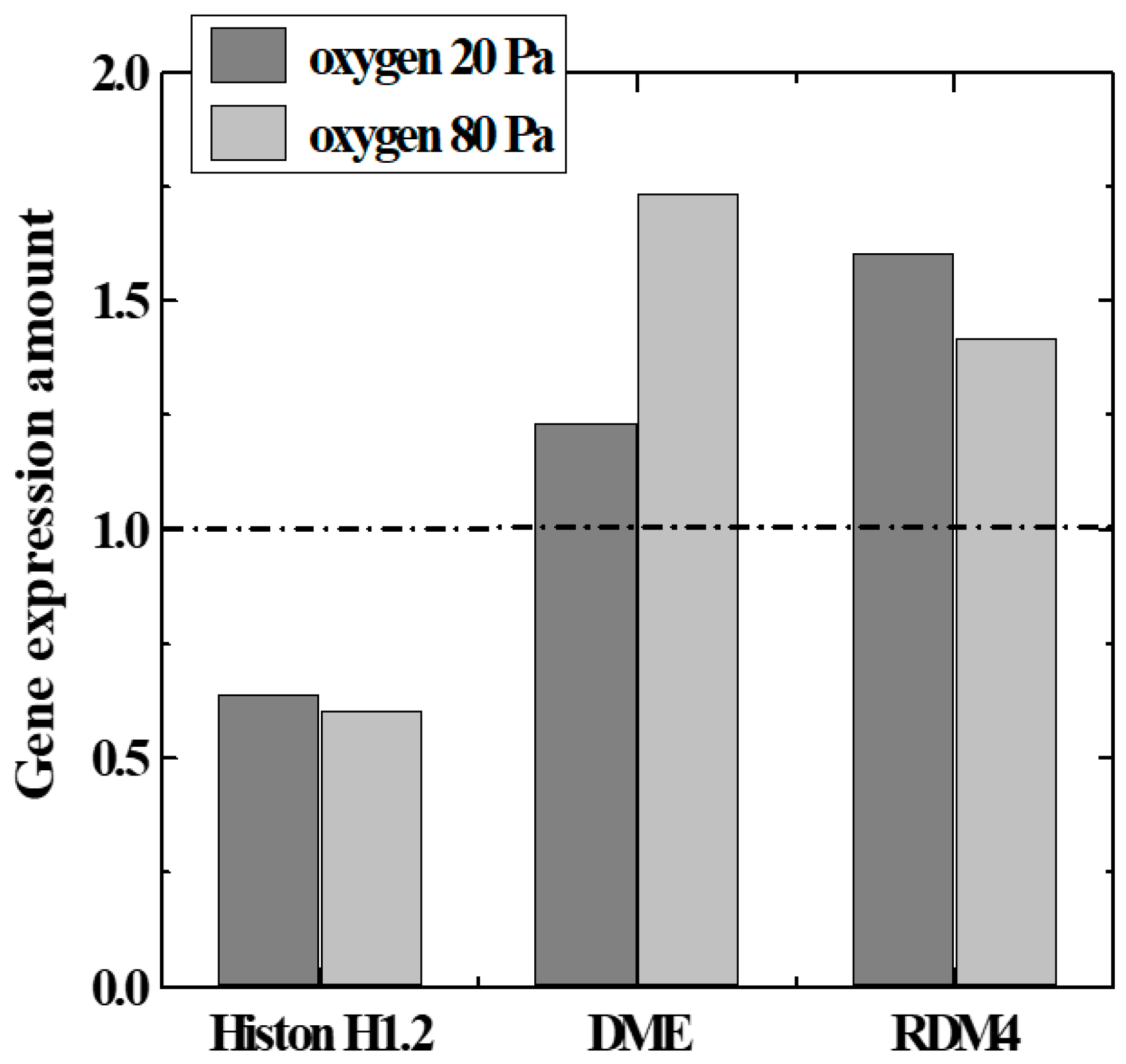
To confirm the above hypothesis, we focused on genes related to modification and chromatin structure (histone H1.2 [12]) among genes with remarkable expression fluctuations. Expression levels of epigenetics-related genes (DME, RDM4, etc. [13]) were measured by the microarray method and the real-time PCR method. The genes encode enzyme production that catalyzes the demethylation reaction in DNA, and histones which regulate the structure of the chromosomal region surrounding the genes. A quantitative comparison of Histone 1.2, DME, and RDM4 expression levels with various parameters of plasma irradiation is shown in Figure 7. The data in the figure satisfied a T-test by a p-value of 0.05 or less. DME and RDM4 expression was upwardly regulated by irradiation with oxygen plasma, whereas histone H1.2 expression was downwardly regulated. This result suggests that histone methylation was suppressed by irradiation with oxygen plasma. It is possible to obtain gene expression profiles of seeds obtained by cultivating plasma-irradiated seeds (second-generation seeds). Unlike the first-generation seeds directly irradiated with plasma, DME and RDM4 expression levels were about the same as those without irradiation, and the histone H1.2 expression levels were considered to be increased [5].
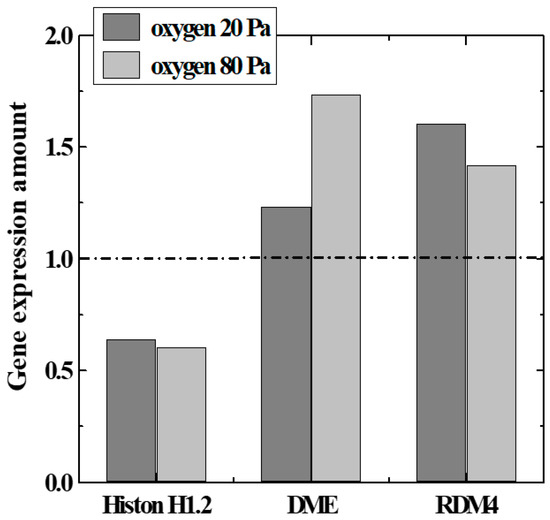
Figure 7.
Gene expression variation related to DNA methylation and histone modification.
The change in the expression level of each gene was different from the changes in gas type and plasma conditions. When comparing Air and O2 plasmas, the total amount of change in the expression level of DME and RDM4 was larger in the O2 plasma. Even if the oxygen pressure was increased, the expression level of linker histone gene methylation was common in both cases, and the total amount of changes in gene expression related to DNA demethylation increased. This confirmed that the epigenetic gene modification was regulated by irradiation of seeds with the oxygen plasma and that gene expression related to growth could be improved.
3.4. Quantitative Analysis of DNA Methylation
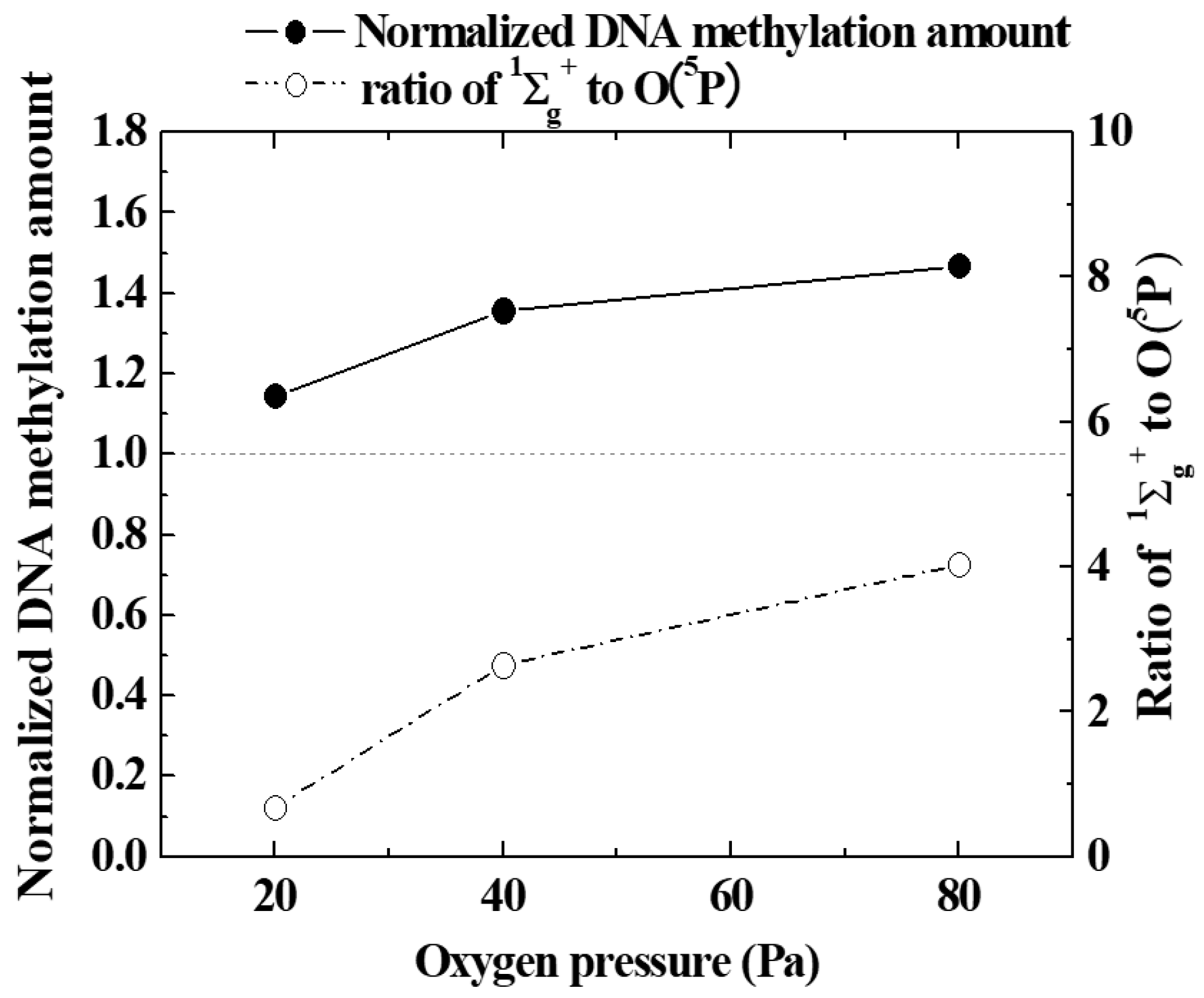
In order to quantitatively evaluate the promotion of epigenetic gene expression by oxygen plasma irradiation, the amount of DNA methylation (methylation of cytosine 5-position) was measured with a quantitative reagent. A black circle (●) in Figure 8 shows how the amount of methylated DNA in seeds irradiated with oxygen plasma generated by changing oxygen pressure was influenced by oxygen gas pressure. It can be seen that the higher the oxygen pressure, in the range of 20 to 80 Pa, the greater the methylation of seed nuclear DNA by irradiation with oxygen plasma. Therefore, it was confirmed that epigenetic gene expression was promoted at lower oxygen pressures. This was consistent with the tendency of DNA demethylation predicted in Figure 6 and Figure 7. At the same time, the emission intensity ratio of 1∑g+ and O (5P) obtained from the emission spectroscopic spectrum is indicated by a white circle (○). This can be roughly considered as the abundance ratio of 1∑g+ and O (5P). The amount of methylated DNA increases with the pressure of oxygen gas. As this tendency was similar to the pressure change of the ratio of 1∑g+ to O (5P), it is possible that 1∑g+ was a major factor controlling DNA methylation as well as the excited oxygen atom.
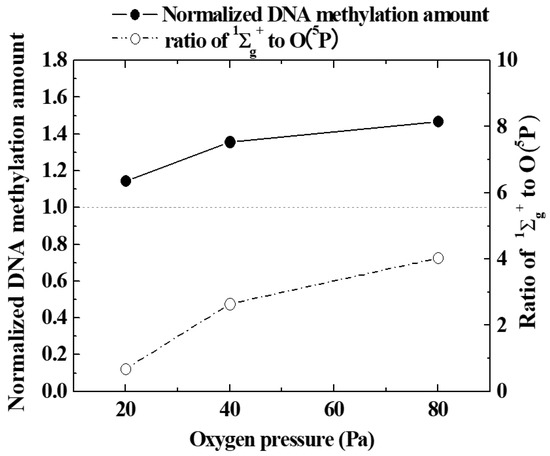
Figure 8.
Variation of the amount of Normalized DNA methylation and the ratio of 1∑g+ and O(5P) with oxygen pressure.
As a result of oxygen plasma irradiation, we identified epigenetic genes that increase their expression levels and estimated the pathway of these expressed genes from oxygen plasma irradiation to plant seeds to epigenetic regulation. Details were not clarified yet.
- (1)
- Active oxygen permeates the cell membrane and reaches the nucleus;
- (2)
- In histone modification and DNA methylation, active oxygen binds to and activates the oxygen receptor (-SH) on the transcription factor of the gene encoding the enzyme catalyzing the rate-determining reaction;
- (3)
- Increased expression level of epigenetics-related genes;
- (4)
- Promotion of DNA methylation and histone modification;
- (5)
- Such a route is expected in the plasma sterilization of agricultural products. It is, therefore, essential to clarify details of ozone damage in order to advance plasma sterilization technology.
4. Atmospheric Pressure Plasma Irradiation on Plant Leaves
4.1. Atmospheric Plasma Irradiation of Plant Leaves
To investigate the plasma irradiation effects on plant leaves that indicate the growth characteristics of leaves, the atmospheric discharge plasma irradiates Arabidopsis plant leaves with various irradiation parameters. In atmospheric pressure plasma irradiation on leaves, the raw material gas is oxygen or air. In this experiment, three irradiation methods were used: (i) oxygen plasma irradiation for 10 s was repeated three times to reduce the damage, (ii) oxygen plasma irradiation for 20 s, and (iii) air plasma irradiation for 30 s. These irradiation methods were adjusted so that the same ozone CT value was applied to the leaves. In all irradiation methods, the area of Arabidopsis leaves after irradiation decreased to about 80% compared to the unirradiated one. This was damage to leaves caused by ozone. From the gene expression analysis, it was clarified that the chaperone gene expression was upwardly regulated as the expression level of the photosynthesis-related gene increased.
4.2. DNA Methylation Analysis
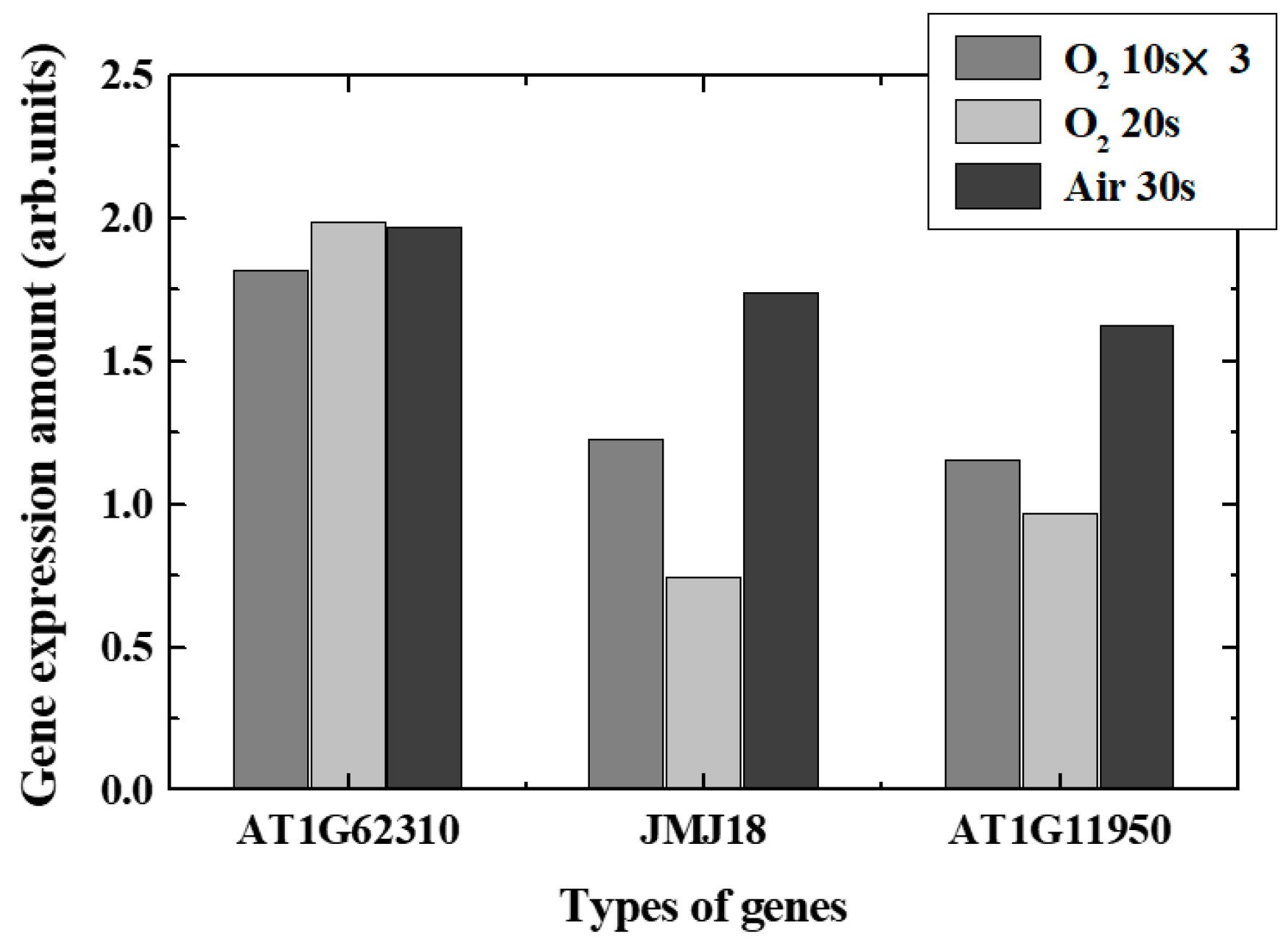
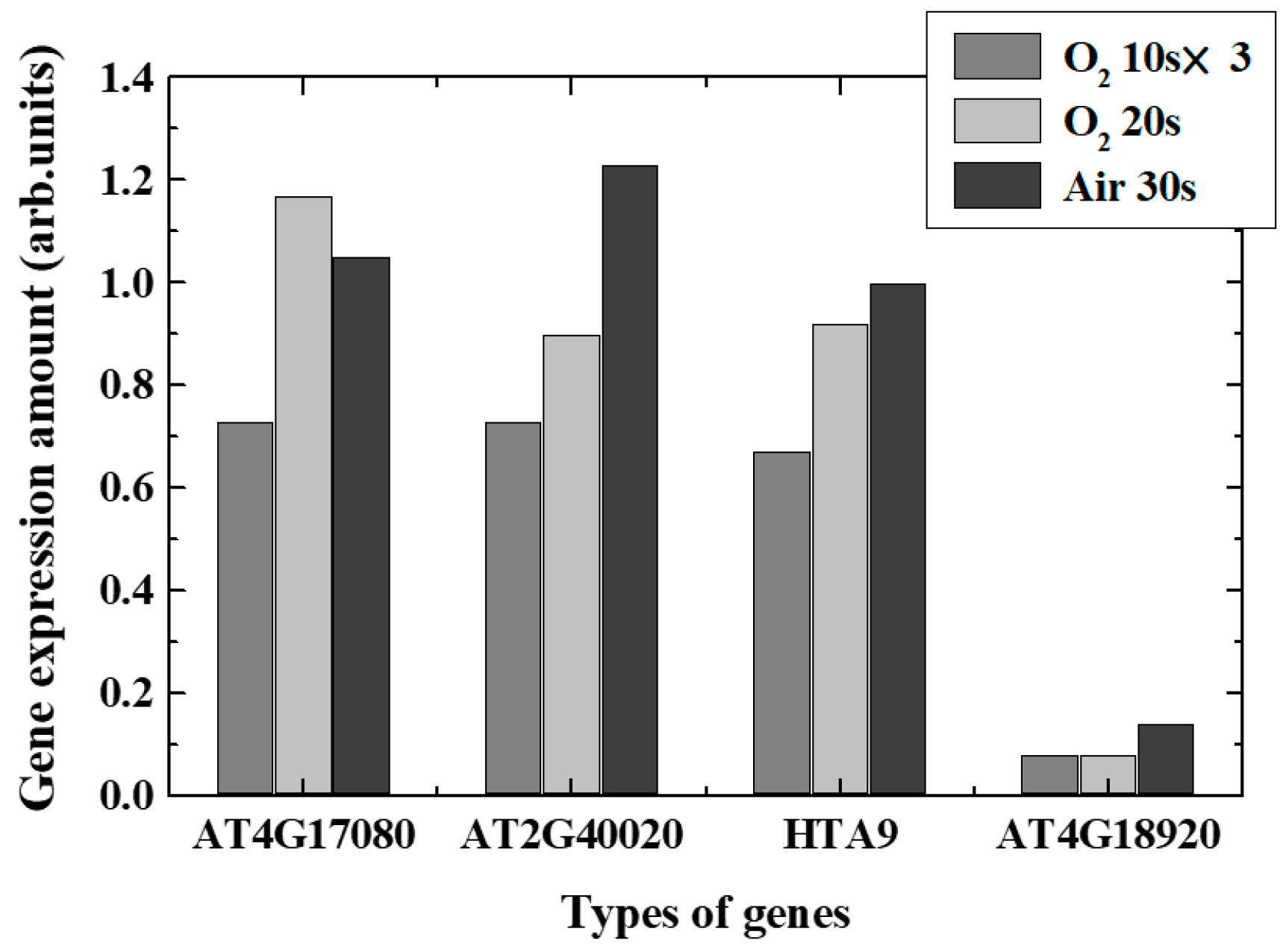
Real-time PCR revealed changes in typical gene expression associated with epigenetics-related genes, especially DNA demethylation, DNA methylation, and acetylation, in plasma-irradiated leaves. Figure 9 and Figure 10 show the expression level variations of genes encoding enzymes that catalyzed DNA demethylation and DNA methylation/acetylation in each irradiation method, respectively. The remarkable characteristics obtained in each case are shown below.
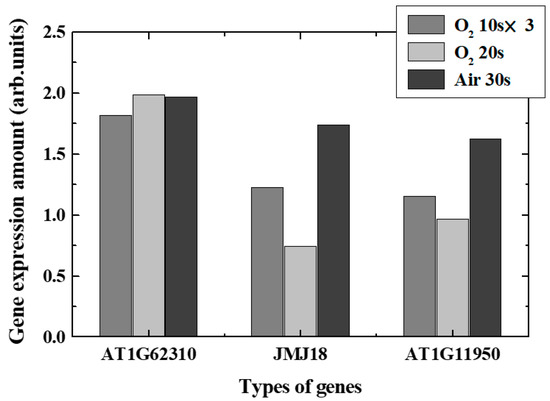
Figure 9.
Variation of gene expression ratios of the demethylation-related genes.
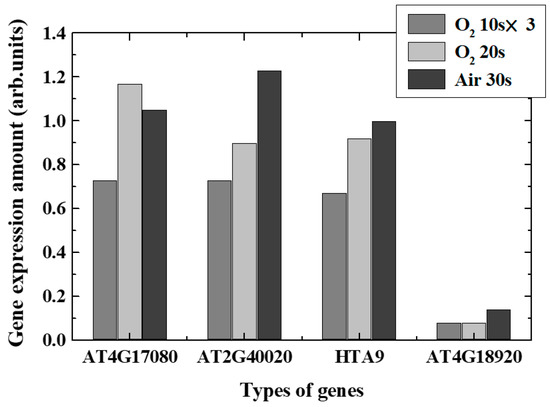
Figure 10.
Variation of gene expression ratios of the methyltransferase and acetyltransferase.
- (i)
- O2 plasma, 10 s, three times irradiation: Gene expressions varied significantly. Expression of each de-DNA methylation-related genes increased and DNA methylation-related genes decreased in expression. It was found that DNA acetylation that induces gene expressions was suppressed. Comprehensively, it is expected that plasma irradiation will promote gene expression.
- (ii)
- O2 plasma, irradiation for 20 s: Suppression and promotion were obtained for both demethylation and methylation/acetylation. Actual gene expression was considered to be determined by the balance between suppression and promotion of DNA methylation.
- (iii)
- Air plasma, irradiation for 30 s: It was shown that demethylation promotion may occur. The methylation would not be significant in this case, and the acetylation was suppressed. Since active nitrogen species were also generated in addition to active oxygen species in air plasma, it was presumed that characteristics different from those in oxygen plasma were obtained.
From the above, demethylation was promoted and methylation/acetylation was suppressed in the leaf cell genes when oxygen plasma irradiation for 10 s was performed three times, even though there were some fluctuations of expression amounts in each gene. As a result, gene expression is thought to be enhanced by demethylation. On the other hand, when air plasma was irradiated for 30 s, methylation and demethylation may have occurred at the same time.
5. Conclusions
Arabidopsis thaliana seeds and leaves were treated with oxygen and air plasma to determine the plant growth enhancement mechanism. The effect of oxygen plasma irradiation on gene expression in plant cells was investigated by gene expression analysis. A growth enhancement effect lasted for 36 days at 20 Pa oxygen gas pressure and 26 days at 80 Pa oxygen gas pressure. A significant increase in leaf area ratio by oxygen plasma to seeds may be due to epigenetic factors such as DNA demethylation transcription factors being activated. As a result of exposure to ozone, the area of Arabidopsis leaves after irradiation decreased to approximately 80% compared to the unirradiated leaves. In addition, the growth enhancement effect induced by plasma irradiation of seeds was transmitted to next-generation cells through cell division. A growth-promoting effect can be obtained even when the gene sequence itself was changed by oxygen plasma, and epigenetics, such as DNA methylation, were passed on to the next generation. Demethylation is thought to enhance gene expression in oxygen plasma irradiation, but methylation and demethylation may occur simultaneously in air plasma irradiation. The level of expression of epigenetic-related genes (DME, RDM4, etc.) determined the production of enzymes that catalyzed the reaction that regulated DNA demethylation and histones associated with the chromosomal region of the gene. Therefore, plants are capable of increasing their epigenetic gene expression by irradiating with atmospheric oxygen plasma.
Author Contributions
Conceptualization, N.H. and S.A.; methodology, N.H.; investigation, S.A. and S.K.; writing—original draft, S.A.; writing—review and editing, S.A. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
JSPS KAKENHI Grant Number JP19H05611.
Data Availability Statement
Not applicable.
Acknowledgments
JSPS KAKENHI (Grant Number JP19H05611) for supporting this work and Cell Innovator Co., Ltd., Fukuoka, Japan for their advice on gene expression analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dubinov, A.E.; Lazarenko, E.R.; Selemir, V.D. Effect of glow discharge air plasma on grain crops seed. IEEE Trans. Plasma Sci. 2000, 28, 180–183. [Google Scholar] [CrossRef]
- Hayashi, N.; Ono, R.; Uchida, S. Growth Enhancement of Plant by Plasma and UV Light Irradiation to Seeds. J. Photopolym. Sci. Technol. 2015, 28, 445–448. [Google Scholar] [CrossRef]
- Jiafeng, J.; Xin, H.; Ling, L.; Jiangang, L.; Hanliang, S.; Qilai, X.; Renhong, Y.; Yuanhua, D. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Hayashi, N.; Ono, R.; Nakano, R.; Shiratani, M.; Tashiro, K.; Kuhara, S.; Yasuda, K.; Hagiwara, H. DNA microarray analysis of plant seeds irradiated by active oxygen species in oxygen plasma. Plasma Med. 2016, 6, 459–471. [Google Scholar] [CrossRef]
- Nakano, R.; Yamashita, Y.; Kobayashi, A.; Hayashi, N. Gene expression effect of plant seeds by irradiation with low-pressure oxygen plasma. J. IAPS 2018, 26, 91–95. [Google Scholar]
- Koichi, T.; Nobuya, H.; Douyan, W.; Takayuki, O. High-voltage technologies for agriculture and food processing. J. Phys. D Appl. Phys. 2019, 52, 473001. [Google Scholar]
- Maruyama-Nakashita, A.; Ishibashi, Y.; Yamamoto, K.; Zhang, L.; Morikawa-Ichinose, T.; Kim, S.; Hayashi, N. Oxygen plasma modulates glucosinolate levels without affecting lipid contents and composition in Brassica napus seeds. Biosci. Biotechnol. Biochem. 2021, 85, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Hayashi, N. Variation of antioxidative activity and growth enhancement of Brassicaceae induced by low-pressure oxygen plasma. Jpn. J. Appl. Phys. 2015, 54, 06GD03. [Google Scholar] [CrossRef]
- Watanabe, S.; Ono, R.; Hayashi, N.; Tashiro, K.; Kuhara, S.; Inoue, S.; Yasuda, K.; Hagiwara, H. Growth Enhancement and Gene Expression of Arabidopsis thaliana irradiated by active oxygen species. Jpn. J. Appl. Phys. 2016, 55, 07LG10. [Google Scholar] [CrossRef]
- Nakano, R.; Tashiro, K.; Aijima, R.; Hayashi, N. Effect of oxygen plasma irradiation on gene expression in plant seeds induced by active oxygen species. Plasma Med. 2016, 6, 303–313. [Google Scholar] [CrossRef]
- Hayashi, N.; Yagyu, Y. Treatment of protein using oxygen plasma produced by RF discharge. Trans. Mater. Res. Soc. Jpn. 2008, 33, 791–794. [Google Scholar] [CrossRef]
- Yagyu, Y.; Hayashi, N.; Guan, W.; Kawasaki, H. Influence of Atomic and Singlet Molecular Oxygen Generated by RF Plasma on Reduction of Protein. J. Plasma Fusion Res. 2009, 8, 578–581. [Google Scholar]
- Hayashi, N.; Nakahigashi, A.; Liu, H.; Goto, M. Treatment of second order structures of protein using oxygen RF plasma. Jpn. J. Appl. Phys. 2010, 49, 08JH02. [Google Scholar] [CrossRef]
- Hayashi, N.; Kometani, R.; Yoshida, Y. Treatment of dipicolinic acid and inactivation mechanism of thermophile spore using active oxygen. Jpn. J. Appl. Phys. 2013, 52, 11NF03. [Google Scholar] [CrossRef]
- Ono, R.; Tanaka, A.; Uchida, S.; Kitazaki, S.; Itarashiki, T.; Hayashi, N. Effect of Active Oxygen Species in Low Pressure Oxygen Plasma on Antioxidative Substances. Front. Appl. Plasma Technol. 2014, 7, 45–46. [Google Scholar]
- Hayashi, N.; Ono, R.; Yagyu, Y.; Yonesu, A. Application of atmospheric discharge plasma to agricultural and marine products. J. Jpn. Soc. Appl. Electromagn. Mech. 2014, 22, 447–452. [Google Scholar]
- Hayashi, N.; Miyamaru, Y.; Aijima, R.; Yamashita, Y. Activation of p53-mediated apoptosis pathway in HSC3 cancer cell irradiated by atmospheric DBD oxygen plasma. IEEE Trans. Plasma Sci. 2018, 47, 1093–1099. [Google Scholar] [CrossRef]
- Hayashi, N.; Inoue, Y.; Kyumoto, Y.; Kukita, T. Characteristics of differentiation of osteoclast cells irradiated with active species in atmospheric oxygen plasma. Jpn. J. Appl. Phys. 2020, 59, SJJF02. [Google Scholar] [CrossRef]
- Subaedah, S.; Uematsu, H.; Hayashi, N. Activation of EL-4 T-cells by irradiation with atmospheric oxygen plasma. Jpn. J. Appl. Phys. 2020, 59, SJJF03. [Google Scholar] [CrossRef]
- Gordon, G. The chemistry and reactions of ozone in our environment. Prog. Nucl. Energy 1995, 29, 89–96. [Google Scholar] [CrossRef]
- Mitsugi, F.; Abiru, T.; Ikegami, T.; Ebihara, K.; Aoqui, S.; Nagahama, K. Influence of Ozone Generated by Surface Barrier Discharge on Nematode and Plant Growth. IEEE Trans. Plasma Sci. 2016, 44, 3071–3076. [Google Scholar] [CrossRef]
- Ohshiro, S.; Katsuto, M.; Satahira, K.; Iriyama, Y.; Nakamura, K.; Ito, S.; Ihara, T. Fabrication of the Plasma-Chemical Indicator and It’s Application. J. Photopolym. Sci. 2013, 26, 533–538. [Google Scholar] [CrossRef]
- Satahira, K.; Ohshiro, S.; Nakamura, K.; Ito, S.; Ihara, S. Visualization Plasma Diagnosis Indicator Using Discoloration Reaction of Au and Pt Ions. J. Photopolym. Sci. 2015, 28, 435–438. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).