Detection of QTL for High-Temperature Tolerance in Rice Using a High-Density Bin Map
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Material and Field Experiment
2.2. Phenotype Evaluation
2.3. QTL Detection
3. Results
3.1. Performance of HD and SF of the RILs
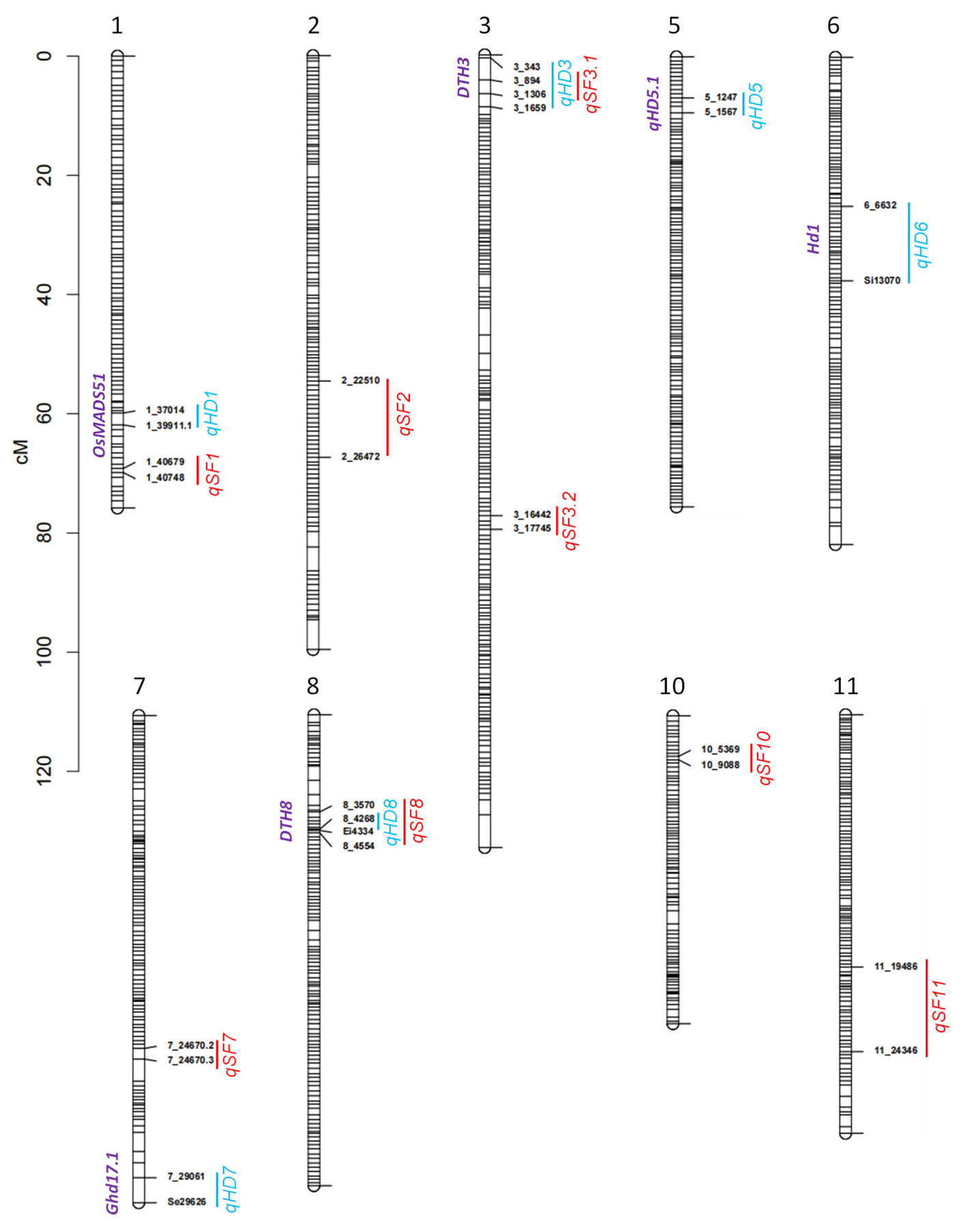
3.2. QTL Detected for HD
3.3. QTL Detected for SF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.-Y.; Yang, C.; Xu, J.; Lu, H.-P.; Liu, J.-X. The hot science in rice research: How rice plants cope with heat stress. Plant Cell Environ. 2022, 46, 1087–1103. [Google Scholar] [CrossRef]
- Ye, C.; Tenorio, F.A.; Argayoso, M.A.; Laza, M.A.; Koh, H.-J.; Redona, E.D.; Jagadish, K.S.V.; Gregorio, G.B. Identifying and confirming quantitative trait loci associated with heat tolerance at flowering state in different rice populations. BMC Genet. 2015, 16, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, P.V.V.; Bheemanahalli, R.; Jagadish, S.V.K. Field crops and the fear of heat stress-Opportunities, challenges and future directions. Field Crop. Res. 2017, 200, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, Q.; Tang, M.; Zhang, X.; Pan, Y.; Yang, X.; Gao, G.; Lv, R.; Tao, W.; Jiang, L.; et al. QTL mapping and identification of candidate genes for heat tolerance at the flowering stage in rice. Front. Genet. 2021, 11, 621871. [Google Scholar] [CrossRef]
- Xiong, D.; Ling, X.; Huang, J.; Peng, S. Meta-analysis and dose-response analysis of high temperature effects on rice yield and quality. Environ. Exp. Bot. 2017, 141, 1–9. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G.; Hu, B.; Wu, J.; Chen, W.; Ren, Z.; Liu, Y.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, 8873. [Google Scholar] [CrossRef]
- Stocher, T.F.; Plattner, G.K.; Tignor, M.M.B.; Allen, S.K.; Boschung, J.; Nauel, A.; Xia, Y.; Bex, V.; Midgley, P. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [Green Version]
- Kan, Y.; Lin, H.-X. Molecular regulation and genetic control of rice thermal response. Crop. J. 2021, 9, 497–505. [Google Scholar] [CrossRef]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.V.K.; Kusilwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seeding growth under heat stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-R.; Yang, W.-T.; Kim, D.-H.; Kim, K.-M. Identification of a novel gene, Osbht, in responese to high temperature tolerance at booting stage in rice. Int. J. Mol. Sci. 2020, 21, 5862. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, F.; Hong, R.; Li, Q.; Gu, M.; Liu, Q. Mapping QTLs for heat tolerance in rice (Oryza sative L.) at heading stage using chromosome segment substitution lines. Agric. Biotechnol. 2017, 6, 15–18. [Google Scholar]
- Wada, T.; Miyahara, K.; Sonoda, J.-Y.; Tsukaguchi, T.; Miyazaki, M.; Tsubone, M.; Ando, T.; Ebana, K.; Yamamoto, T.; Norio, I.; et al. Deteceion of QTLs for white-back and basal-white grains caused by hihg temperature during ripening period in japonica rice. Breed. Sci. 2015, 65, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop. J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Li, X.-M.; Chao, D.-Y.; Wu, Y.; Huang, X.; Chen, K.; Gui, L.-G.; Su, L.; Ye, W.-W.; Chen, H.; Chen, H.-C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.-R.; Zhang, H.; Gao, J.; Shan, J.-X.; Ye, W.-W.; Lin, H.-X. TT2 controls rice thermotolerance through SCT1-dependent alteration of wax biosynthesis. Nat. Plants 2022, 8, 53–57. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.-F.; Shan, J.-X.; Ye, W.-W.; Dong, N.-Q.; Guo, T.; Xiang, Y.-H.; Yang, Y.-B.; Li, Y.-C.; Zhao, H.-Y. A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y.; Tang, H.; Zeng, B.; Tang, X.; Long, Q.; Wu, X.; Cai, Y.; Yuan, L.; Wan, J. Fine mapping of the qHTB1-1QTL, which confers heat tolerance at the booting stage, using an Oryza rufipogon Griff. Introgression line. Theor. Appl. Genet. 2020, 133, 1161–1175. [Google Scholar] [CrossRef]
- Shen, H.; Zhong, X.; Zhao, F.; Wang, Y.; Yan, B.; Li, Q.; Chen, G.; Mao, B.; Wang, J.; Li, Y.; et al. Overexpression of receptor-like kinase ERECTA improves thermotolerance in rice and tomato. Nat. Biotechnol. 2015, 33, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Wei, C.; Liu, X.; Wang, M.; Yu, F.; Xie, Q.; Tu, J. The ring finger ubiquitin E3 ligase OsHTAS enhances heat tolerance by promoting H2O2-induced stomatal closure in rice. Plant Physiol. 2016, 170, 429–443. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Kang, S.; He, L.; Zhao, J.; Zhang, S.; Hu, J.; Zeng, D.; Zhang, G.; Dong, G.; Gao, Z.; et al. The newly identified heat-stress sensitive albino 1 gene affects chloroplast development in rice. Plant Sci. 2018, 267, 168–179. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Fan, Y.-Y.; Wang, K.; Huang, D.-R.; Liu, W.-Z.; Ying, J.-Z.; Zhuang, J.-Y. Rice flowering locus T1 plays an important role in heading date influencing yield traits in rice. Sci. Rep. 2017, 7, 4718. [Google Scholar]
- Chen, J.-Y.; Zhang, H.-W.; Zhang, H.-L.; Ying, J.-Z.; Ma, L.-Y.; Zhuang, J.-Y. Natural variation at qHd1 affects heading date acceleration at high temperatures with pleiotropism for yield traits in rice. BMC Plant Biol. 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Y.; Wang, S.; Fan, Y.; Zhuang, J. Genetic interaction of Hd1 with Ghd7, DTH8 and Hd2 largely determines eco-geographical adaption of rice varieties in southern China. Rice Sci. 2021, 28, 114–118. [Google Scholar]
- Weng, X.; Wang, L.; Wang, J.; Hu, Y.; Du, H.; Xu, C.; Xing, Y.; Li, X.; Xiao, J.; Zhang, Q. Grain number, plant height, and heading date 7 is a central regulator of growth, development, and stress response. Plant Physiol. 2014, 164, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, G.; Chen, T.; Feng, B.; Fu, W.; Yan, J.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 2018, 11, 14. [Google Scholar] [CrossRef]
- Kanyange, L.; Fan, Y.-Y.; Zhang, Z.-H.; Huang, D.-R.; Huang, T.-X.; Zhuang, J.-Y. Genetic association between blast resistance and yield traits in rice detected using a high-density bin map. Agronomy 2022, 12, 1173. [Google Scholar] [CrossRef]
- Meng, L.; Li, H.; Zhang, L.; Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop. J. 2015, 3, 269–283. [Google Scholar] [CrossRef] [Green Version]
- McCouch, S.R. CGSNL (Committee on Gene Symbolization, Nomenclature and Linkage, Rice Genetics Cooperative). Gene nomenclature system for rice. Rice 2008, 1, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Xu, J.; Guo, H.; Jiang, L.; Chen, S.; Yu, C.; Zhou, Z.; Hu, P.; Zhai, H.; Wan, J. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol. 2010, 153, 1747–1758. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.-H.; Wang, P.; Chen, H.-X.; Zhou, H.-J.; Li, Q.-P.; Wang, C.-R.; Ding, Z.-H.; Zhang, Y.-S.; Yu, S.-B.; Xing, Y.-Z.; et al. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol. Plant 2010, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Lee, S.; Kim, H.J.; Nam, H.G.; An, G. OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol. 2007, 145, 1484–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, X.F.; Liu, X.; Zhao, Z.G.; Jiang, L.; Gao, H.; Zhang, Y.H.; Zhang, M.; Chen, L.M.; Liu, S.J.; Zhai, H.Q.; et al. Heading date gene, dth3 controlled late flowering in O.Glaberrima Steud. by down-regulating Ehd1. Plant Cell Rep. 2011, 30, 2243–2254. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Zhan, X.-D.; Lin, Z.-C.; Wu, W.-X.; Yu, P.; Zhang, Y.-X.; Sun, L.-P.; Cao, L.-Y.; Cheng, S.-H. Fine mapping and candidate gene analysis of qHD5, a novel major QTL with pleiotropism for yield-related traits in rice (Oryza sativa L.). Theor. Appl. Genet. 2017, 130, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Liu, H.; Zhou, X.; Li, Q.; Zhang, J.; Lu, L.; Liu, T.; Liu, H.; Zhang, C.; Zhang, Z.; et al. Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Res. 2013, 23, 969–971. [Google Scholar] [CrossRef]
- Dar, M.H.; Bano, D.A.; Waza, S.A.; Zaidi, N.W.; Majid, A.; Shikari, A.B.; Ahangar, M.A.; Hossain, M.; Kumar, A.; Singh, U.S. Abiotic stress tolerance-progress and pathways of sustainable rice production. Sustainability 2021, 13, 2078. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhou, J.; Hu, S.; Chen, H.; Xiang, J.; Zhang, Y.; Zeng, Y.; Shi, Q.; Zhu, D.; et al. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Ye, C.; Argayoso, M.; Redona, E.D.; Sierra, S.N.; Laza, M.; Dilla, C.; Mo, Y.; Thomson, M.J.; Chin, J.; Delavina, C.B.; et al. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 2012, 131, 33–41. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, R.; Wai, H.P.; Xiong, H.; Shen, X.; He, H.; Yan, S. Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice. Physiol. Mol. Biol. Plant 2017, 23, 817–825. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Yu, S.-B.; Li, C.-H.; Mou, T.-M. Identification of QTLs for heat tolerance at flowering stage in rice. Sci. Agric. Sinaca 2008, 41, 315–321, (In Chinese with English Abstract). [Google Scholar]
- Andres, F.; Coupland., G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Qiu, J.; Yong, K.; Fan, J.; Zhang, Q.; Hua, H.; Liu, J.; Wang, Q.; Olsen, K.M.; Han, B.; et al. A quantitative genomics map of rice provides genetic insights and guides breeding. Nat. Genet. 2021, 53, 243–256. [Google Scholar] [CrossRef]


| Trait a | Trial b | Range | Mean ± SD | Skewness | Kurtosis | Parental Mean | |
|---|---|---|---|---|---|---|---|
| D71 | ZH161 | ||||||
| HD (d) | 2019 | 75.9–109.4 | 90.8 ± 8.2 | 0.03 | −1.17 | 92.4 | 89.8 |
| 2020-SD1 | 74.0–110.0 | 90.2 ± 9.0 | 0.09 | −1.21 | 96.0 | 91.0 | |
| 2020-SD2 | 72.0–106.0 | 88.5 ± 9.0 | 0.02 | −1.45 | 91.0 | 85.0 | |
| 2020-SD3 | 73.0–107.0 | 90.3 ± 9.3 | 0.13 | −1.49 | 96.0 | 89.0 | |
| 2020-SD4 | 68.0–107.0 | 87.8 ± 9.4 | 0.04 | −1.19 | 97.0 | 91.0 | |
| SF (%) | 2019 | 55.1–95.1 | 83.9 ± 6.7 | −1.00 | 1.44 | 76.2 | 91.8 |
| 2020-SD1 | 55.9–96.1 | 85.5 ± 6.4 | −1.38 | 2.85 | 62.7 | 95.4 | |
| 2020-SD2 | 63.3–96.1 | 87.7 ± 5.9 | −1.43 | 2.46 | 68.9 | 94.9 | |
| 2020-SD3 | 50.1–97.0 | 84.9 ± 8.5 | −1.30 | 1.94 | 79.1 | 96.1 | |
| 2020-SD4 | 38.3–96.5 | 82.4 ± 11.4 | −1.25 | 1.34 | 60.7 | 92.0 | |
| QTL | Interval a | Trial | LOD | A b | R2 (%) c |
|---|---|---|---|---|---|
| qHD1 | 1_37014–1_39911 | 2019 | 3.4 | −0.6 | 0.6 |
| qHD3 | 3_343–3_1659 | 2019 | 15.6 | 1.5 | 2.9 |
| 2020-SD1 | 10.3 | 1.3 | 1.8 | ||
| 2020-SD2 | 11.3 | 1.5 | 2.6 | ||
| 2020-SD3 | 7.2 | 1.3 | 1.9 | ||
| 2020-SD4 | 8.3 | 1.6 | 2.9 | ||
| qHD5 | 5_1247–5_1567 | 2019 | 3.7 | 0.7 | 0.6 |
| 2020-SD1 | 18.3 | 1.8 | 3.6 | ||
| qHD6 | 6_6632–Si13070 (6_13070) | 2019 | 9.4 | −1.1 | 1.6 |
| 2020-SD1 | 6.9 | −1.0 | 1.2 | ||
| 2020-SD2 | 7.2 | −1.2 | 1.6 | ||
| 2020-SD3 | 8.1 | −1.3 | 2.1 | ||
| 2020-SD4 | 5.4 | −1.3 | 1.9 | ||
| qHD7 | 7_29061–Se29626 (7_29626) | 2019 | 51.3 | 3.1 | 13.3 |
| 2020-SD1 | 32.4 | 2.5 | 7.3 | ||
| 2020-SD2 | 22.3 | 2.2 | 5.7 | ||
| qHD8 | 8_4268–Ei4334 (8_4334) | 2019 | 133.0 | −7.1 | 70.8 |
| 2020-SD1 | 123.3 | −8.0 | 75.6 | ||
| 2020-SD2 | 116.6 | −8.1 | 78.1 | ||
| 2020-SD3 | 108.5 | −8.3 | 83.4 | ||
| 2020-SD4 | 91.1 | −8.2 | 76.5 |
| QTL | Interval a | Trial | Whole Population | Sub1 d | Sub2 e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | Ab | R2 (%) c | LOD | A | R2 (%) | LOD | A | R2 (%) | |||
| qSF1 | 1_40679–1_40748 | 2020-SD4 | 3.4 | 2.1 | 2.9 | - | - | - | - | - | - |
| qSF2 | 2_22510–2_26472 | 2019 | 4.8 | 1.8 | 7.7 | - | - | - | - | - | - |
| 2020-SD1 | 3.5 | 1.4 | 4.8 | - | - | - | - | - | - | ||
| 2020-SD2 | 2.9 | 1.2 | 4.3 | - | - | - | - | - | - | ||
| 2020-SD3 | 2.5 | 1.4 | 2.9 | - | - | - | - | - | - | ||
| 2020-SD4 | 2.5 | 1.7 | 2.1 | - | - | - | - | - | - | ||
| qSF3.1 | 3_894–3_1306 | 2020-SD4 | 6.6 | −3.0 | 6.2 | 5.7 | −5.1 | 17.3 | - | - | - |
| qSF3.2 | 3_16442–3_17745 | 2019 | 5.6 | 1.9 | 9.2 | - | - | - | - | - | - |
| 2020-SD1 | 12.3 | 2.7 | 18.5 | 7.3 | 3.1 | 20.1 | - | - | - | ||
| 2020-SD2 | 8.9 | 2.2 | 14.0 | 7.8 | 3.4 | 22.6 | - | - | - | ||
| 2020-SD3 | 7.7 | 2.6 | 9.5 | 4.5 | 3.4 | 15.5 | - | - | - | ||
| 2020-SD4 | 4.8 | 2.5 | 4.2 | - | - | - | - | - | - | ||
| qSF8 | 8_3570–8_4554 | 2020-SD2 | 5.3 | 1.7 | 8.1 | - | - | - | - | - | - |
| 2020-SD3 | 20.7 | 4.5 | 28.9 | - | - | - | - | - | - | ||
| 2020-SD4 | 30.9 | 7.0 | 34.6 | - | - | - | - | - | - | ||
| qSF11 | 11_19486–11_24346 | 2020-SD1 | 3.6 | 1.4 | 5.0 | - | - | - | - | - | - |
| 2020-SD3 | - | - | - | - | - | - | 3.6 | 1.7 | 13.2 | ||
| 2020-SD4 | - | - | - | - | - | - | 3.5 | 1.7 | 12.4 | ||
| qSF7 | 7_24670–7_24670 | 2020-SD2 | - | - | - | - | - | - | 3.1 | 1.5 | 11.8 |
| qSF10 | 10_5369–10_9088 | 2020-SD1 | - | - | - | 2.6 | −1.8 | 6.7 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Zhang, Z.; Fan, Y.; Tang, S.; Zhuang, J.; Zhu, Y. Detection of QTL for High-Temperature Tolerance in Rice Using a High-Density Bin Map. Agronomy 2023, 13, 1582. https://doi.org/10.3390/agronomy13061582
Huang D, Zhang Z, Fan Y, Tang S, Zhuang J, Zhu Y. Detection of QTL for High-Temperature Tolerance in Rice Using a High-Density Bin Map. Agronomy. 2023; 13(6):1582. https://doi.org/10.3390/agronomy13061582
Chicago/Turabian StyleHuang, Derun, Zhenhua Zhang, Yeyang Fan, Shaoqing Tang, Jieyun Zhuang, and Yujun Zhu. 2023. "Detection of QTL for High-Temperature Tolerance in Rice Using a High-Density Bin Map" Agronomy 13, no. 6: 1582. https://doi.org/10.3390/agronomy13061582
APA StyleHuang, D., Zhang, Z., Fan, Y., Tang, S., Zhuang, J., & Zhu, Y. (2023). Detection of QTL for High-Temperature Tolerance in Rice Using a High-Density Bin Map. Agronomy, 13(6), 1582. https://doi.org/10.3390/agronomy13061582







