Photosynthetic Assimilation of the Guava (Psidium guajava) cv. Paluma under Different Pruning and Fruit Thinning Intensities
Abstract
1. Introduction
2. Materials and Methods
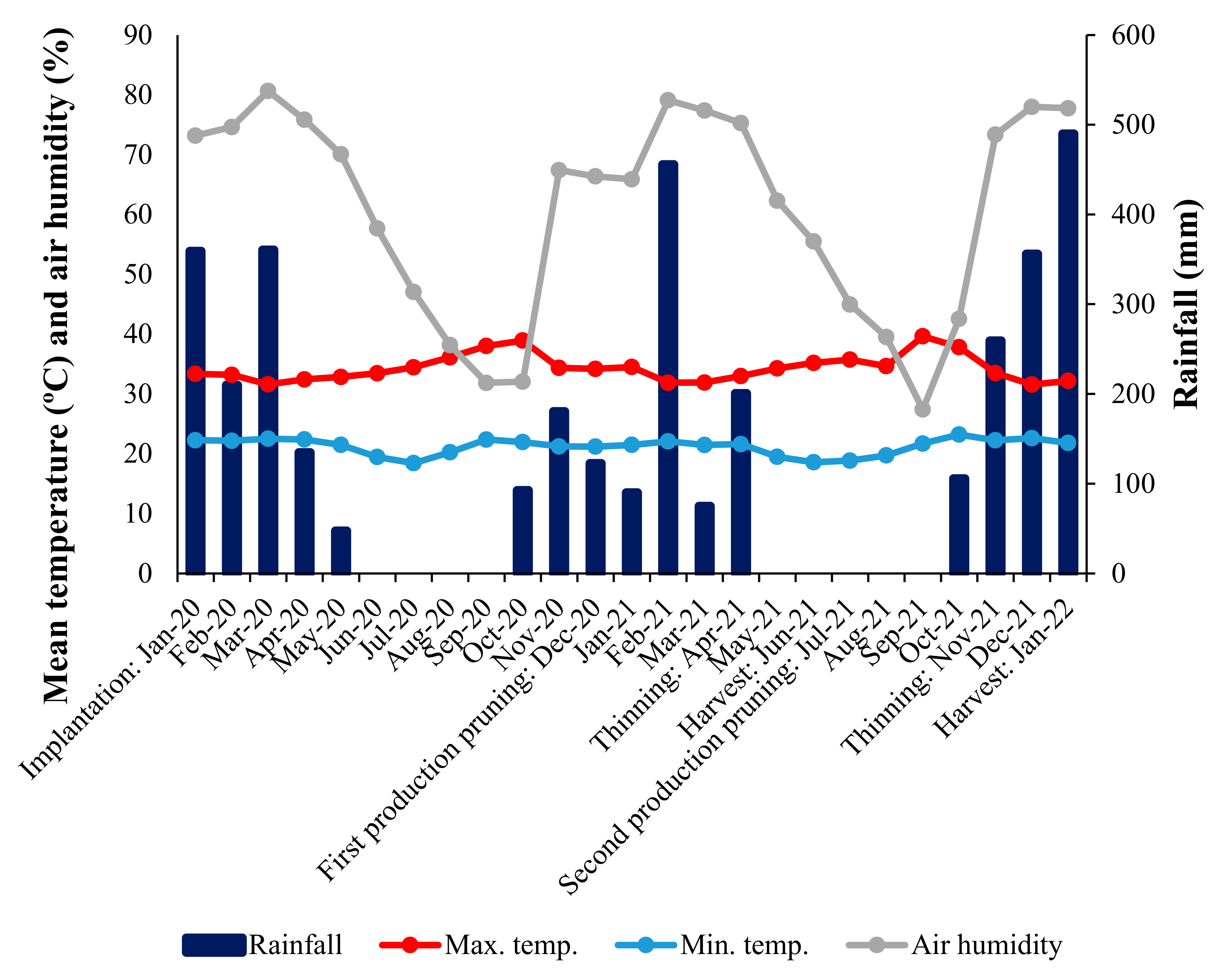
2.1. Cultivation Area and Planting Material
2.2. Experimental Design
2.3. Conduction of the Experiment
2.4. Variables Analyzed
2.5. Statistical Analysis
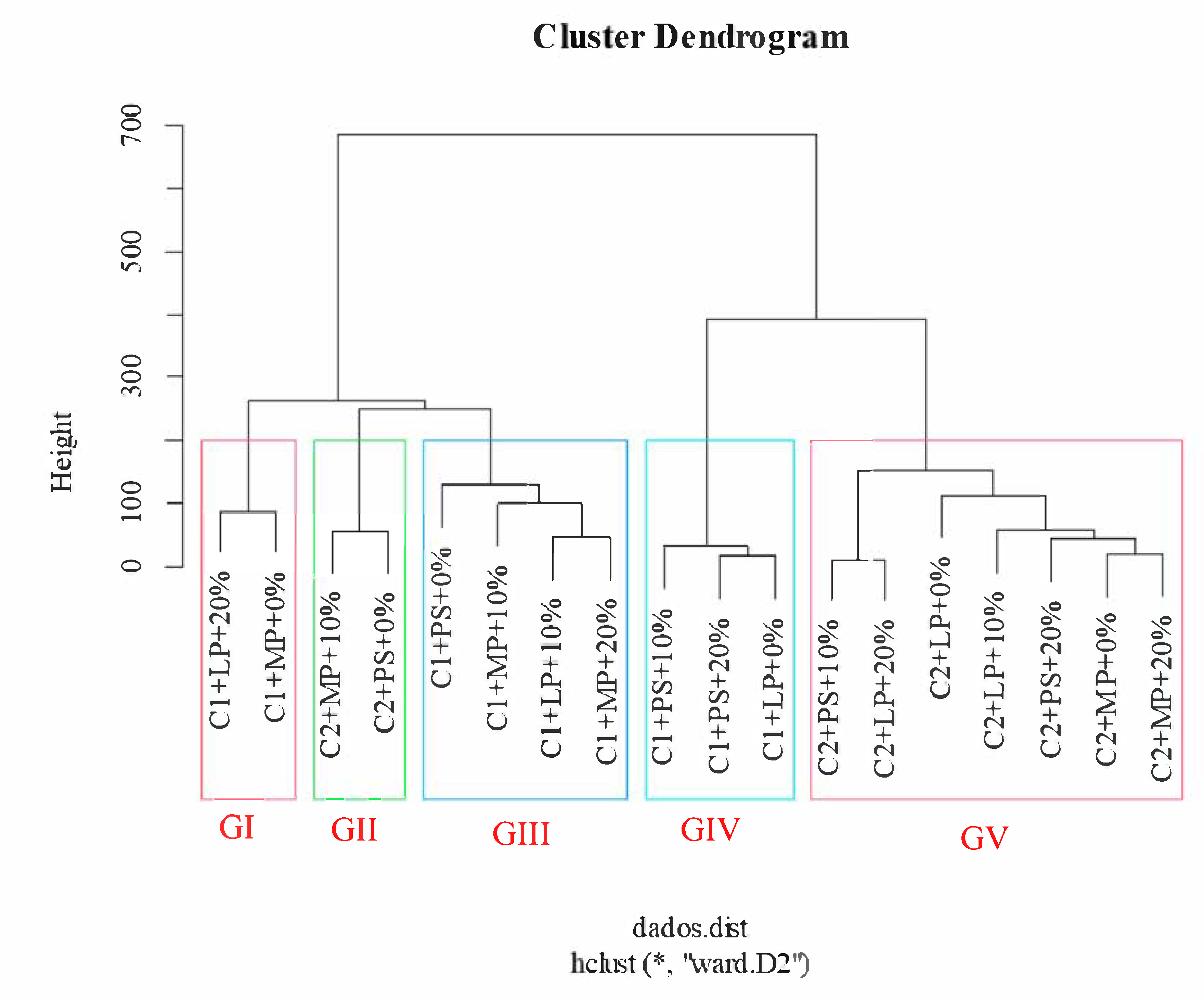
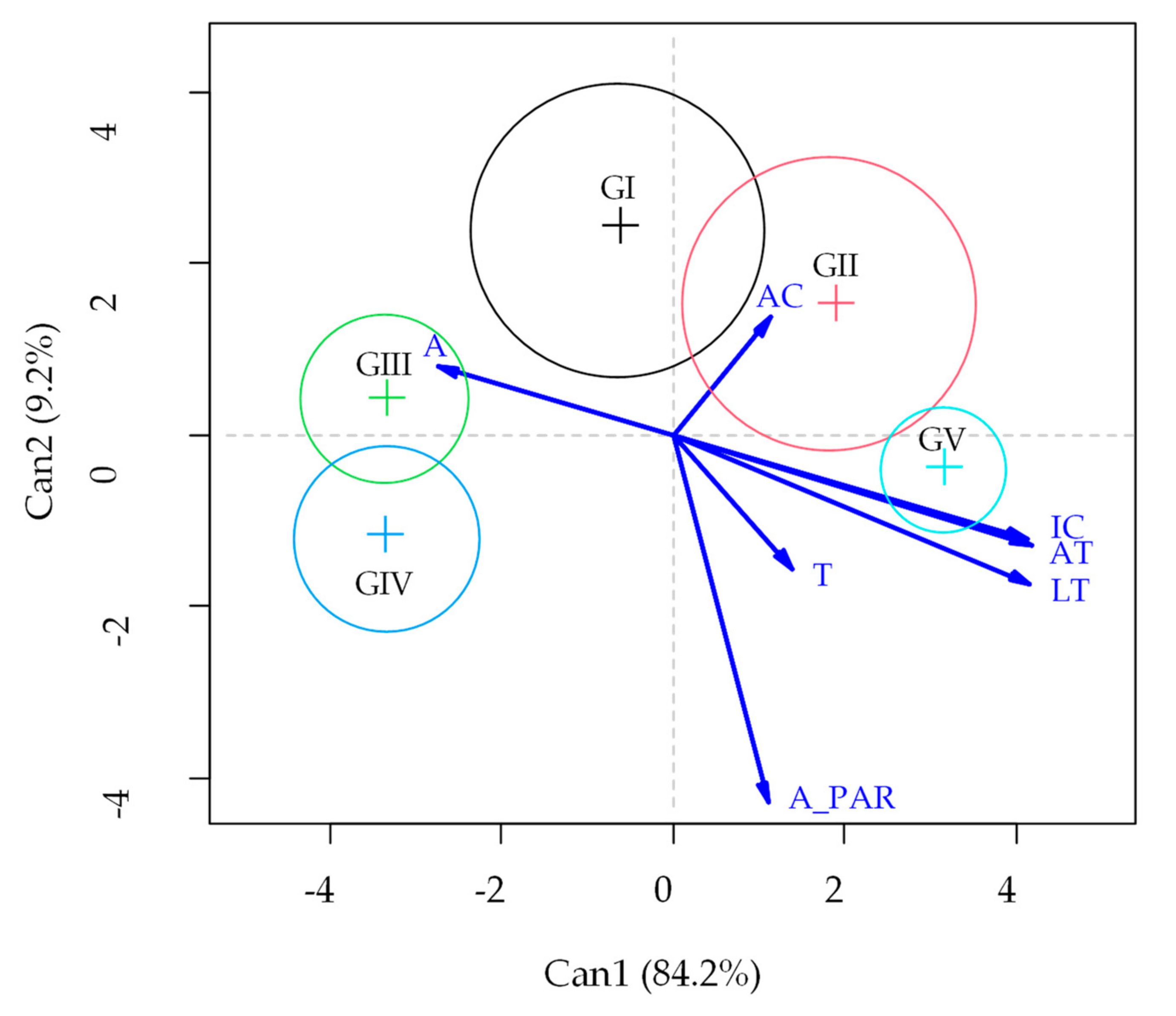
3. Results
4. Discussion
Fruit Pruning and Thinning Improves Gas Exchange in Guava Plants
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IBGE—Instituto Brasileiro de Geografia e Estatística. Tabelas 2017. Produção Agrícola Municipal; Permanentes, L., Ed.; IBGE: Brasília, Brazil, 2017; p. 118. [Google Scholar]
- Morales, R.G.F.; Resende, L.V.; Bordini, I.C.; Galvão, A.G.; Rezende, F.C. Caracterização do tomateiro submetido ao déficit hídrico. Sci. Agrac. 2015, 16, 9–17. [Google Scholar] [CrossRef]
- Oliveira, F.T.; Hafle, O.M.; Mendonça, V.; Moreira, J.N.; Junior, E.B.P.; Rolim, H.O. Respostas de porta-enxertos de goiabeira sob diferentes fontes e proporções de materiais orgânicos. Comun. Sci. 2015, 6, 17–25. [Google Scholar]
- Bonifácio, B.F.; Nobre, R.G.; Sousa, A.D.S.; Gomes, E.M.; Da Silva, E.M.; De Sousa, L.P. Efeitos da adubação potássica e irrigação com águas salinas no crescimento de porta-enxerto de goiabeira. Rev. Ciências Agrárias 2018, 41, 971–980. [Google Scholar]
- Fachi, L.; Garbugio, E.; Ferreira, A.; Machado, R.; Krause, W. Qualidade e correlação dos parâmetros físicos e químicos dos frutos de cultivares de goiaba. Sci. Electron. Arch. 2018, 11, 36–40. [Google Scholar] [CrossRef]
- Moraes, T.M.; Dantas, M.S.M.; de Sousa, C.A.; de Melo, E.A.; Bandeira, M.A.M. Antioxidant and antimicrobial activities of the extracts of Psidium guajava. Rev. Ciências Agrárias 2020, 43, 109–117. [Google Scholar]
- Nascimento, V.E.; Souza, E.L.; Lima, E.O.; Sousa, C.P. Evaluation of antioxidant activity of guava (Psidium guajava L.) seed extract by chemical and electrochemical assays. J. Food Biochem. 2018, 42, e12414. [Google Scholar]
- Vuong, Q.V.; Hirun, S.; Chuen, T.L.K.; Goldsmith, C.D. Fruit and Vegetable Consumption and Health: Benefits and Drawbacks. In Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2019; Volume 12, pp. 367–398, Food Bioconversion. [Google Scholar]
- Costa, A.D.F.S.D.; Costa, A.N.d. Plantio, formação e manejo da cultura. In Tecnologias Para a Produção de Goiaba; Costa, A.D.F.S.D., Costa, A.N.d., Eds.; Incaper: Vitória, Brazil, 2019; pp. 151–195. [Google Scholar]
- Costa, J.G.; Silva, A.V.C.; Araújo, F.L.F.; Araújo, E.F. Viabilidade socioeconômica da cultura da goiaba no município de Cruzeta/RN. Agropecuária Científica No Semi-Árido 2008, 4, 50–55. [Google Scholar]
- Altendorf, S. Mainstreaming a Niche Market. Food Outlook, 1st ed.; FAO: Roma, Italy, 2018; pp. 67–75. [Google Scholar]
- Altendorf, S. Guava World Production, Consumption and International Trade; FAO: Roma, Italy, 2019. [Google Scholar]
- Uesu, L.; Cecchin, D.; Uesu, M.; Ogino, M.; Pereira, C. Análise da viabilidade econômica da produção de goiaba em cachoeiras de macacu–rj. Enci Bios. 2018, 15, 1–19. [Google Scholar] [CrossRef]
- Altendorf, S.; Couto, W.S.; Corrêa, J.C. Cultivation of Goiabeira; Embrapa Informações Tecnológicas: Brasília, Brazil, 2020. [Google Scholar]
- Uesu, Y.O.; Lima, M.B.; Pereira, A.V.A.; Capelini, V.K. Goiaba: O produtor pernambucano e o mercado mundial. Inf. Agropecuário 2015, 36, 7–15. [Google Scholar]
- Silva, B.B.; Moura, M.S.B.; Azevedo, P.V.; Soares, J.M. Medidas de transpiração de um pomar de goiabeiras pelo método do balanço de calor caulinar. Rev. Bras. Agrometeorol. 2002, 10, 19–27. [Google Scholar]
- Barbalho, S.M.; Farinazzi-Machado, F.M.; De Alvares Goulart, R.; Brunnati, A.C.S.; Otoboni, A.M.; Ottoboni, B.J.M.A. Psidium guajava (Guava): A plant of multipurpose medicinal plants. Med. Aromat. Plants 2012, 1, 1–6. [Google Scholar]
- Barbalho, L.C.; Vidal, V.A.; Santana, L.R.; Santana, L.R.; Santos, D.C. Caracterização do sistema de produção de goiaba no Estado da Bahia. Rev. Agropecuária Técnica 2017, 38, 114–119. [Google Scholar]
- Almeida, M.L.L.D. Efeito da Adubação Nitrogenada Antes da poda de Frutificação Sobre Indicadores Fenológicos e de Produção da Goiabeira. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 1999. [Google Scholar]
- Rodríguez, R.; Corrales, I.; Romero, M. Influence of pruning type on the vegetative and reproductive development of guava (Psidium guajava L.) trees. Sci. Hortic. 2010, 126, 189–193. [Google Scholar]
- García, D.; Ortiz, R.A.; Melgarejo, L.M. Influence of thinning and winter pruning on yield and fruit quality of ‘Tardío de Cieza’ apricot. Acta Hortic. 2017, 1161, 235–240. [Google Scholar]
- Alcântara, G.B.; Neto, F.B.; Ledo, A.S. Práticas de manejo na produção da goiabeira ‘Paluma’. Rev. Agric. Neotrop. 2015, 2, 34–40. [Google Scholar]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2018. [Google Scholar]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant. Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. The stress concept in plants: An introduction. Ann. N. Y. Acad. Sci. 1998, 851, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Rissler, H.M.; Lindahl, M.; Vainonen, J.P. Regulation of chlorophyll biosynthesis. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2002; pp. 73–94. [Google Scholar]
- Terashima, I.; Inoue, Y. Chlorophyll fluorescence as a probe for photosynthesis research: An overview of experimental techniques. Photosynth. Res. 2008, 98, 533–557. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Sarhan, F.; Ivanov, A.G. Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Plant Physiol. 1998, 116, 49–59. [Google Scholar]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T.; Ohira, K. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 2003, 44, 1311–1318. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Flexas, J.; Díaz-Espejo, A.; Conesa, M.A.; Coopman, R.A.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Medrano, H.; Ribas-Carbo, M.; et al. Mesophyll conductance to CO2 and Rubisco as targets for improving. Plant Cell Environ. 2016, 39, 965–982. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Syst. 2002, 16, 363–392. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynth. Res. 2005, 82, 389–409. [Google Scholar]
- Flexas, J.; Ribas-Carbó, M.; Bota, J.; Galmés, J.; Henkle, M.; Martínez-Cañellas, S.; Medrano, H. Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol. 2012, 196, 770–781. [Google Scholar] [CrossRef] [PubMed]
- INMET—National Institute of Meteorology of Brazil. Glossário. 2022. Available online: http://www.inmet.gov.br/portal/index.php?r=home/page&page=glossario (accessed on 15 January 2022).
- Prado, M.R.V.; Moraes, M.F.D.; Ramos, F.T.; Santos, C.L.R.D.; Campos, D.V.B.D.; Barros, G.T. Use of buffer methods to estimate the potential acidity of Mato Grosso soils. Cienc. E Agrotecnologia 2020, 44, 1–19. [Google Scholar] [CrossRef]
- Sousa, D.M.G.; Lobato, E. Cerrado: Correção do solo e adubação. In Recomedanção de Adubação Para Solos do Cerrado; Costa Sousa, D.M.G., Lobato, E., Eds.; Embrapa: Brasilia, Brazil, 2004; pp. 118–145. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Published online 2020; European Environment Agency: Copenhagen, Denmark, 2021. [Google Scholar]
- Friendly, M.; Fox, J. Candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. R Package Version 0.6-5. Version (March 2015). Available online: https://cran.r-project.org/web/packages/candisc/index.html (accessed on 9 August 2022).
- Ort, D.R.; Baker, N.R. Consideration of photosynthetic efficiency at low light as a major determinant of crop photosynthetic performance. Plant Physiol. Biochem. 1998, 26, 555–565. [Google Scholar]
- Lopes, N.F.; Lima, M.G.S. Fisiologia da Produção, 1st ed.; UFV: Viçosa, Brazil, 2015; p. 492. [Google Scholar]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosyn. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Mika, A. Physiological responses of fruit trees to pruning. Hortic. Rev. 2011, 8, 337–378. [Google Scholar]
- Serrano, L.A.L.; Marinho, C.S.; Lima, I.D.M.; Martins, M.V.V.; Ronchi, C.P.; Tardin, F.D. Fenologia da goiabeira’Paluma’sob diferentes sistemas de cultivos, épocas e intensidades de poda de frutificação. Bragantia 2008, 67, 701–712. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.T.; Tombesi, S. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Pereira, W.E. Desenvolvimento dos Ramos e Frutos de Seis Variedades de Goiabeira (Psidium guajava L.) no Período Seco do ano. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 1996. [Google Scholar]
- Silva, M.J.R.D.; Tecchio, M.A.; Domiciano, S.; Leonel, S.; Balestrero, R.I. Phenology, yield and fruit quality of ‘Paluma’ guava tree at different pruning times. Cienc. E Agrotecnol. 2016, 40, 317–325. [Google Scholar] [CrossRef]
- Prajapati, R.K.; Singh, V.K.; Upadhayay, A.K. Future of crop diseases and climate change in Bundelkhand Agroclimatic Zone of Madhya Pradesh: A review. Ann. Plant Soil Res. 2018, 20, 86–98. [Google Scholar]
- Urdaneta, T.; Araujo, F.J.; Lugo, L. Estúdio comparativo sobre dos métodos para determinar el potencial hídrico en el cultivo del guayabo (Psidium guajava L.) en la Planicie de Maracaibo. Rev. Fac. Agron. 2003, 20, 1–9. [Google Scholar]
- Monteiro, V.D.F.C.; Gonçalves, E.D.; Moura, P.H.A.; da Silva, L.V.; Martins, F.B.; Norberto, P.M. Phenological stages of the ‘Paluma’guava tree in a region of subtropical climate according to the BBCH scale. Rev. Ciências Agrárias 2021, 16, 1–8. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Gonçalves, B. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hortic. 2018, 1, 226–232. [Google Scholar] [CrossRef]
- Malta, A.O.; da Costa Araújo, R.; Medeiros, J.G.F.; da Costa, N.P.; da Silva, S.I.A. Produção da goiabeira (Psidium guajava L.) em sistema convencional e orgânico. Pesq. Agropec. Pernamb. 2018, 23, 1–20. [Google Scholar] [CrossRef]
- Vega, M.L.B.P. Fitomonitoração e Modelagem de Fotossíntese em Jatobá (Hymenaea courbaril L.) com Redes Neurais Artificiais. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2003. [Google Scholar]
- Wang, B.; Gong, J.; Zhang, Z.; Yang, B.; Liu, M.; Zhu, C.; Yue, K. Nitrogen addition alters photosynthetic carbon fixation, allocation of photoassimilates, and carbon partitioning of Leymus chinensis in a temperate grassland of Inner Mongolia. Agric. For. Meteorol. 2019, 279, 910–923. [Google Scholar] [CrossRef]
| pH | SOM | P | K+ | Ca2+ | Mg2+ | Al3+ | H + Al | T | SB |
|---|---|---|---|---|---|---|---|---|---|
| H2O | g kg−1 | mg dm−3 | -------------------------cmolc dm−3--------------------------- | ||||||
| 5.0 | 7.2 | 1.10 | 0.1 | 0.51 | 0.12 | 0.40 | 1.71 | 2.23 | 0.62 |
| Cu | Fe | Mn | Zn | V | m | Sand | Silt | Clay | |
| --------------mg dm−3--------------- | ------%------ | ---------------g kg−1---------------- | |||||||
| 0.04 | 145.3 | 7.78 | 0.51 | 26.8 | 39.1 | 804 | 35 | 161 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.S.d.; Pereira, G.A.; Fonseca, W.L.; Zuffo, A.M.; da Cunha, J.G.; Soares, N.P.; Morais, E.M.; Nascimento, A.A.S.d.; Santos, D.P.; de Sousa Almeida, M.; et al. Photosynthetic Assimilation of the Guava (Psidium guajava) cv. Paluma under Different Pruning and Fruit Thinning Intensities. Agronomy 2023, 13, 1610. https://doi.org/10.3390/agronomy13061610
Santos ASd, Pereira GA, Fonseca WL, Zuffo AM, da Cunha JG, Soares NP, Morais EM, Nascimento AASd, Santos DP, de Sousa Almeida M, et al. Photosynthetic Assimilation of the Guava (Psidium guajava) cv. Paluma under Different Pruning and Fruit Thinning Intensities. Agronomy. 2023; 13(6):1610. https://doi.org/10.3390/agronomy13061610
Chicago/Turabian StyleSantos, Adaniel Sousa dos, Gustavo Alves Pereira, Wéverson Lima Fonseca, Alan Mario Zuffo, Jenilton Gomes da Cunha, Nemilda Pereira Soares, Estefenson Marques Morais, Antônio Afonso Sousa do Nascimento, Djavan Pinheiro Santos, Murilo de Sousa Almeida, and et al. 2023. "Photosynthetic Assimilation of the Guava (Psidium guajava) cv. Paluma under Different Pruning and Fruit Thinning Intensities" Agronomy 13, no. 6: 1610. https://doi.org/10.3390/agronomy13061610
APA StyleSantos, A. S. d., Pereira, G. A., Fonseca, W. L., Zuffo, A. M., da Cunha, J. G., Soares, N. P., Morais, E. M., Nascimento, A. A. S. d., Santos, D. P., de Sousa Almeida, M., Aguilera, J. G., Morales-Aranibar, L., Salcedo, E. P., Chura, R. M. M., Contreras, W. C., & Alejo, R. C. (2023). Photosynthetic Assimilation of the Guava (Psidium guajava) cv. Paluma under Different Pruning and Fruit Thinning Intensities. Agronomy, 13(6), 1610. https://doi.org/10.3390/agronomy13061610










