Humic Acid Improves the Resilience to Salinity Stress of Drip-Irrigated Mexican Lime Trees in Saline Clay Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Soil and Water Analysis
2.3. Experimental Design
2.4. Studied Parameters
2.4.1. Vegetative Growth
2.4.2. Leaf Analysis
2.4.3. Yield and Fruit Characteristics
2.4.4. Soil Nutrient Contents and Microorganisms
2.5. Statistical Analysis
3. Results
3.1. Soil Nutrient Contents and Microorganisms
3.2. Leaf Nutrient Contents
3.3. Leaf Chlorophyll and Proline Contents
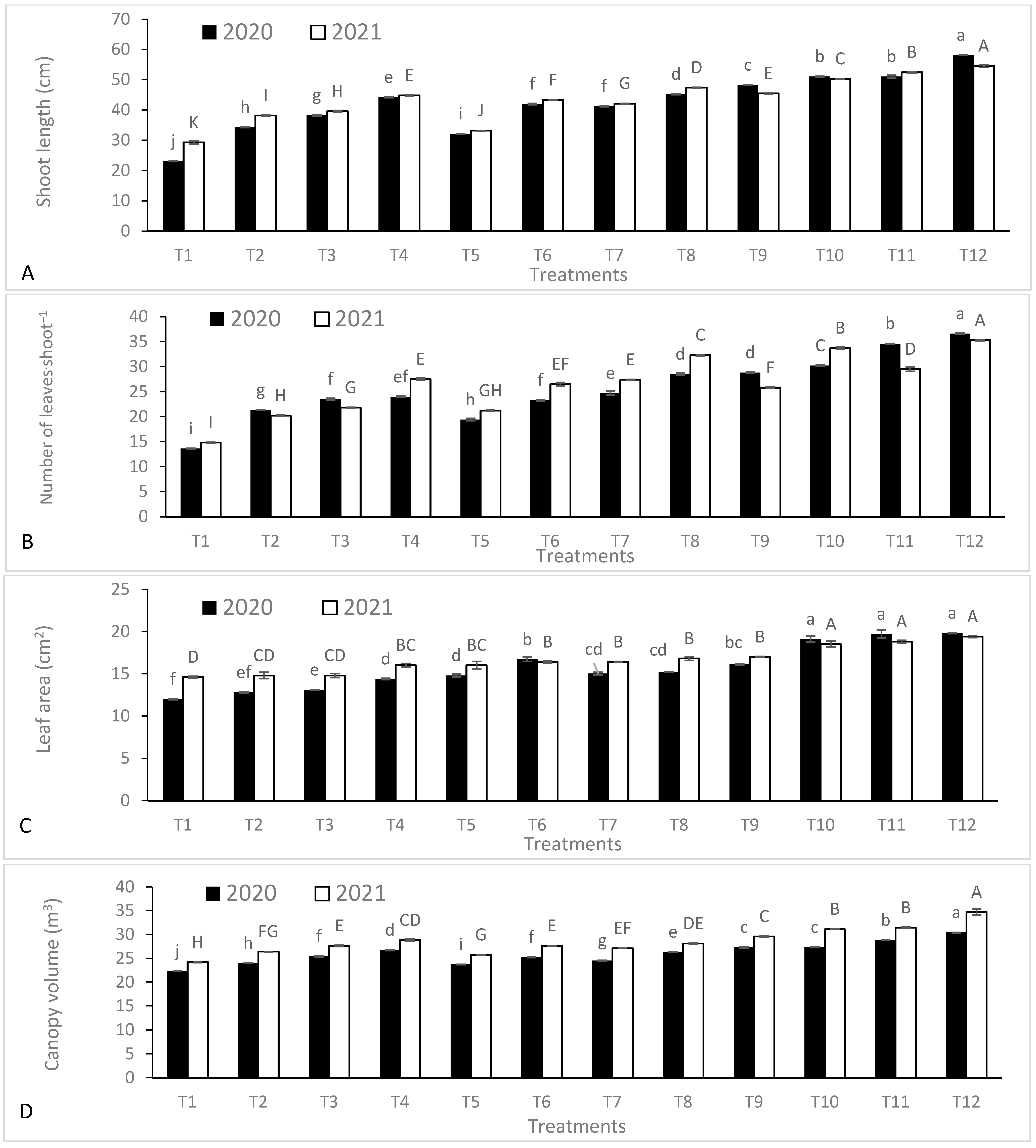
3.4. Vegetative Growth
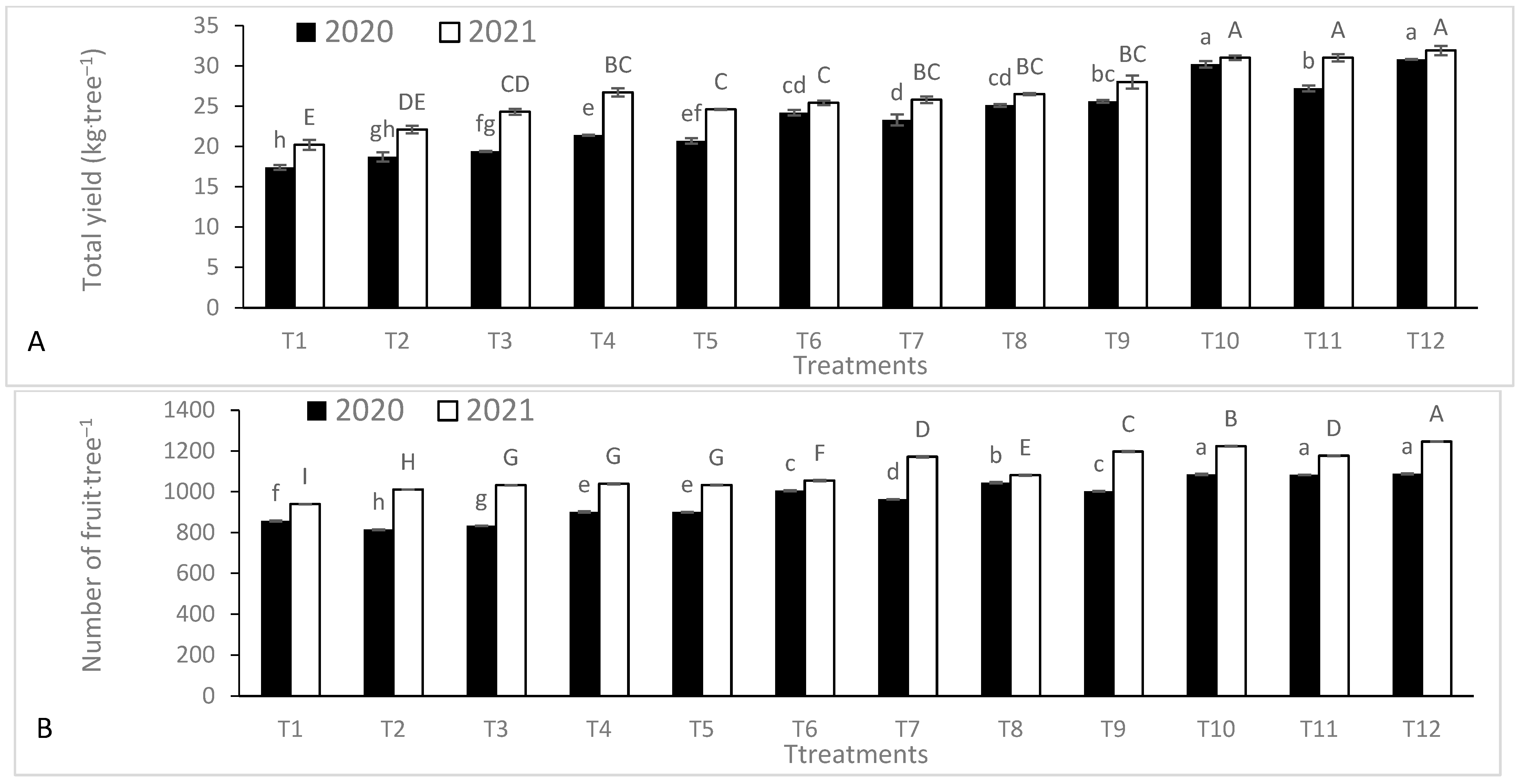
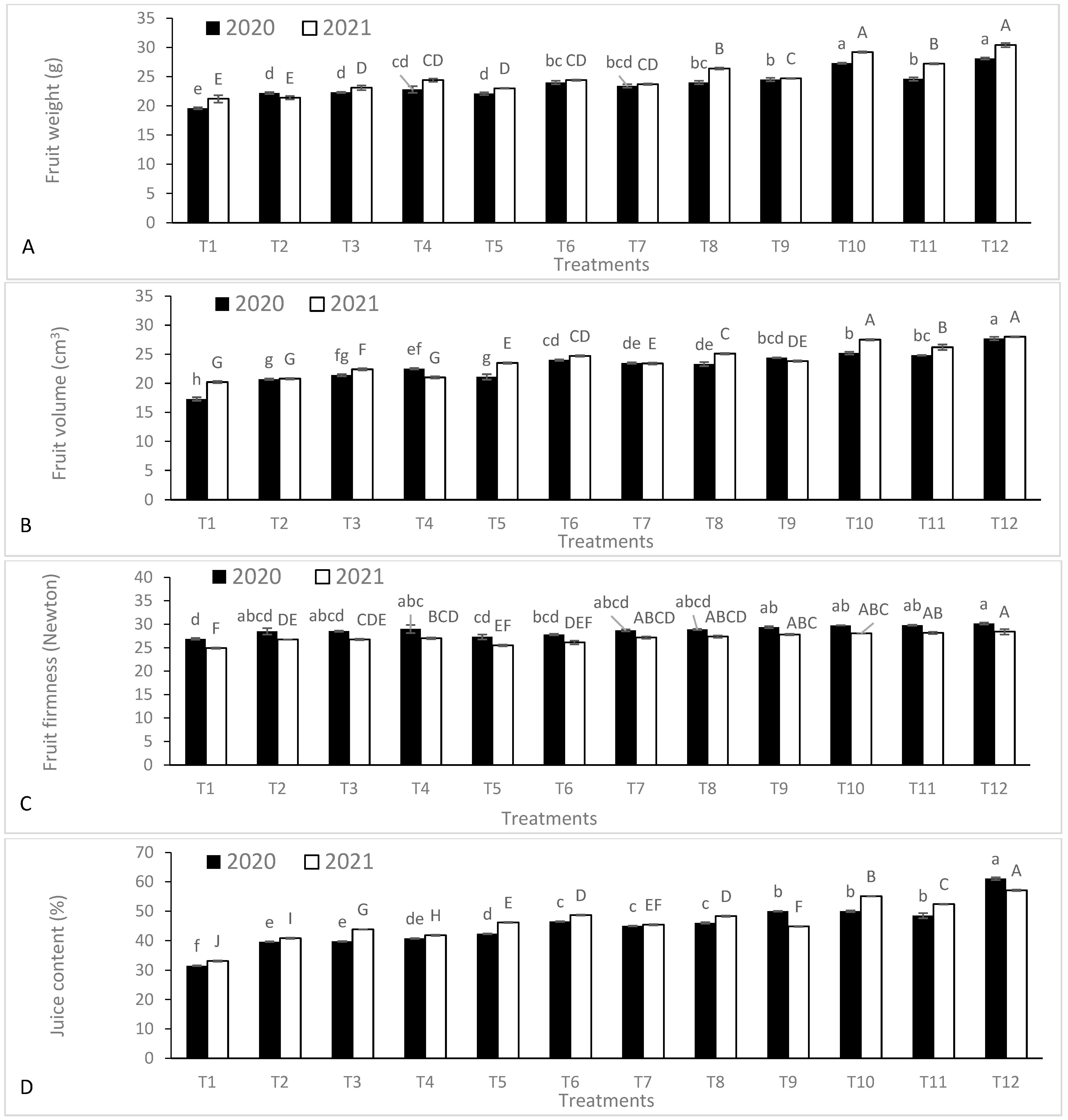
3.5. Yield and Fruit Quality
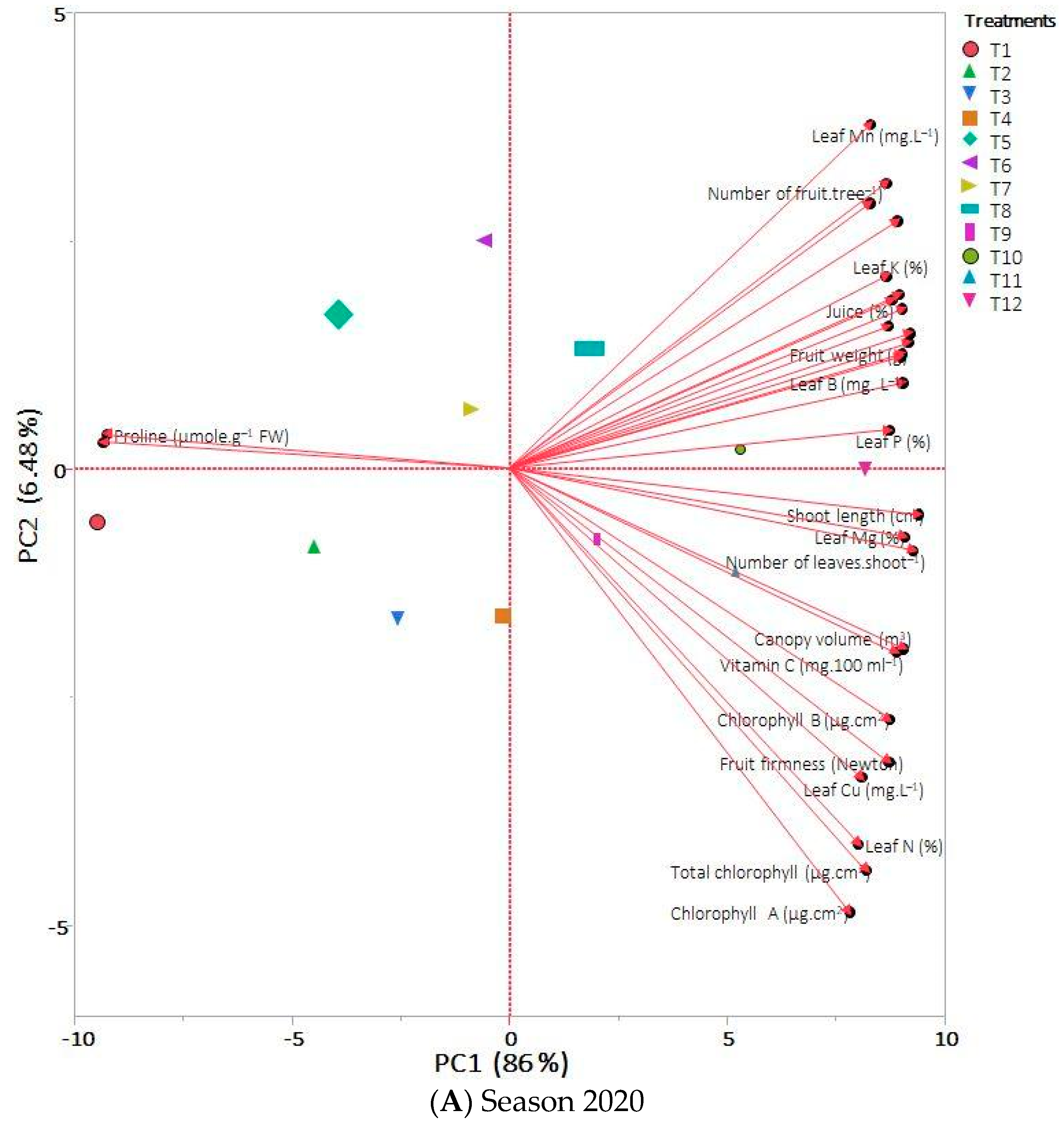
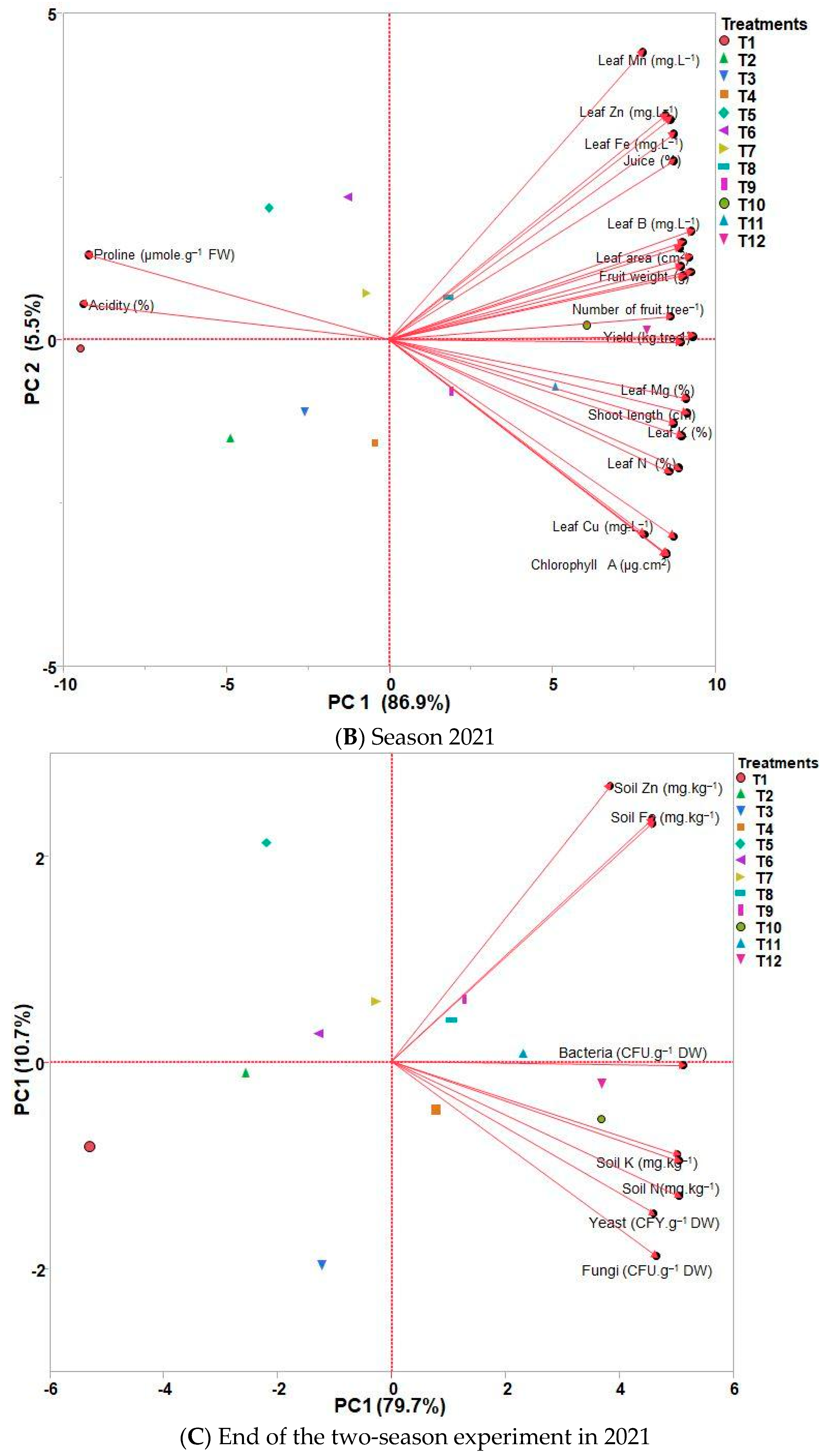
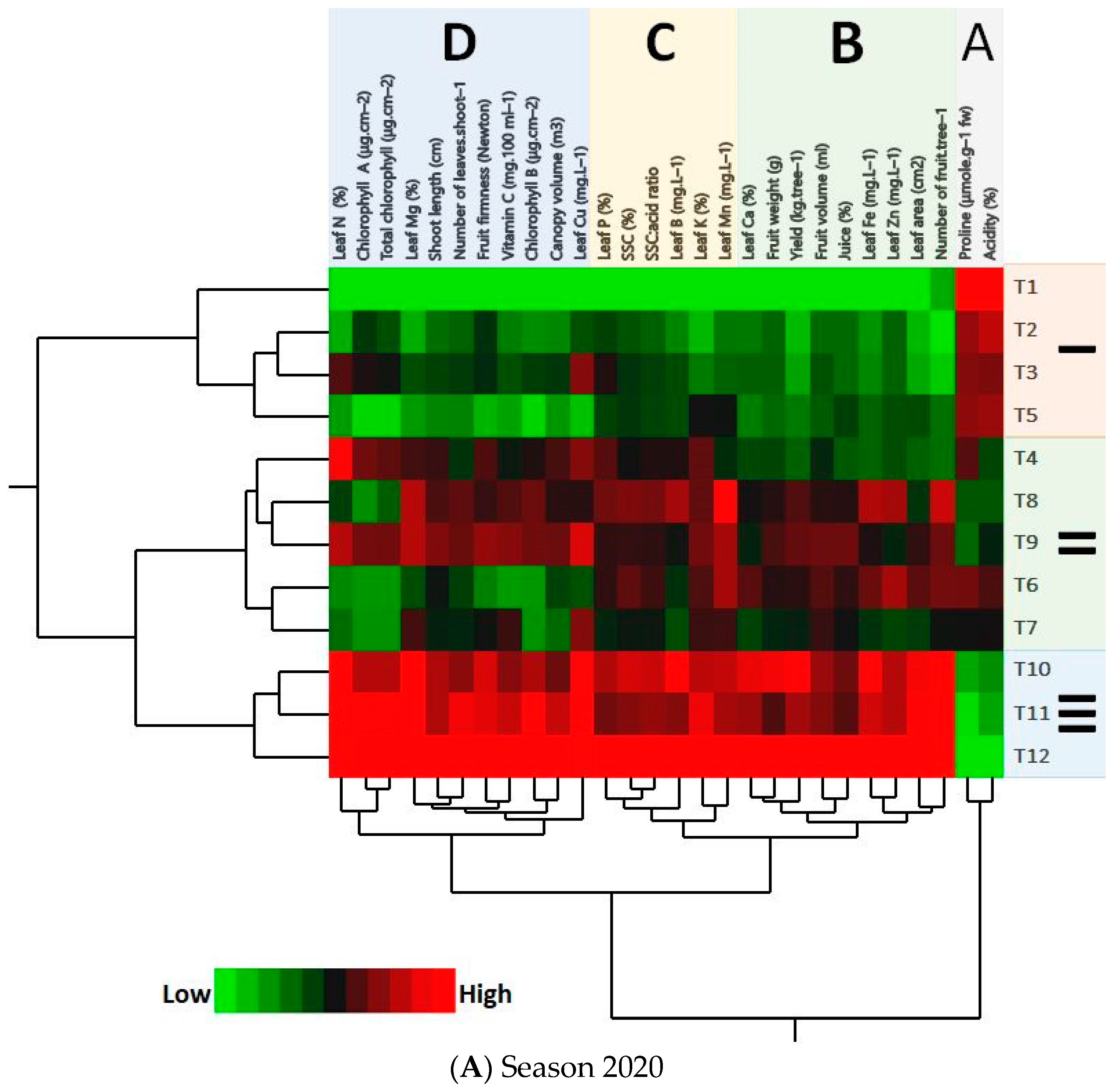
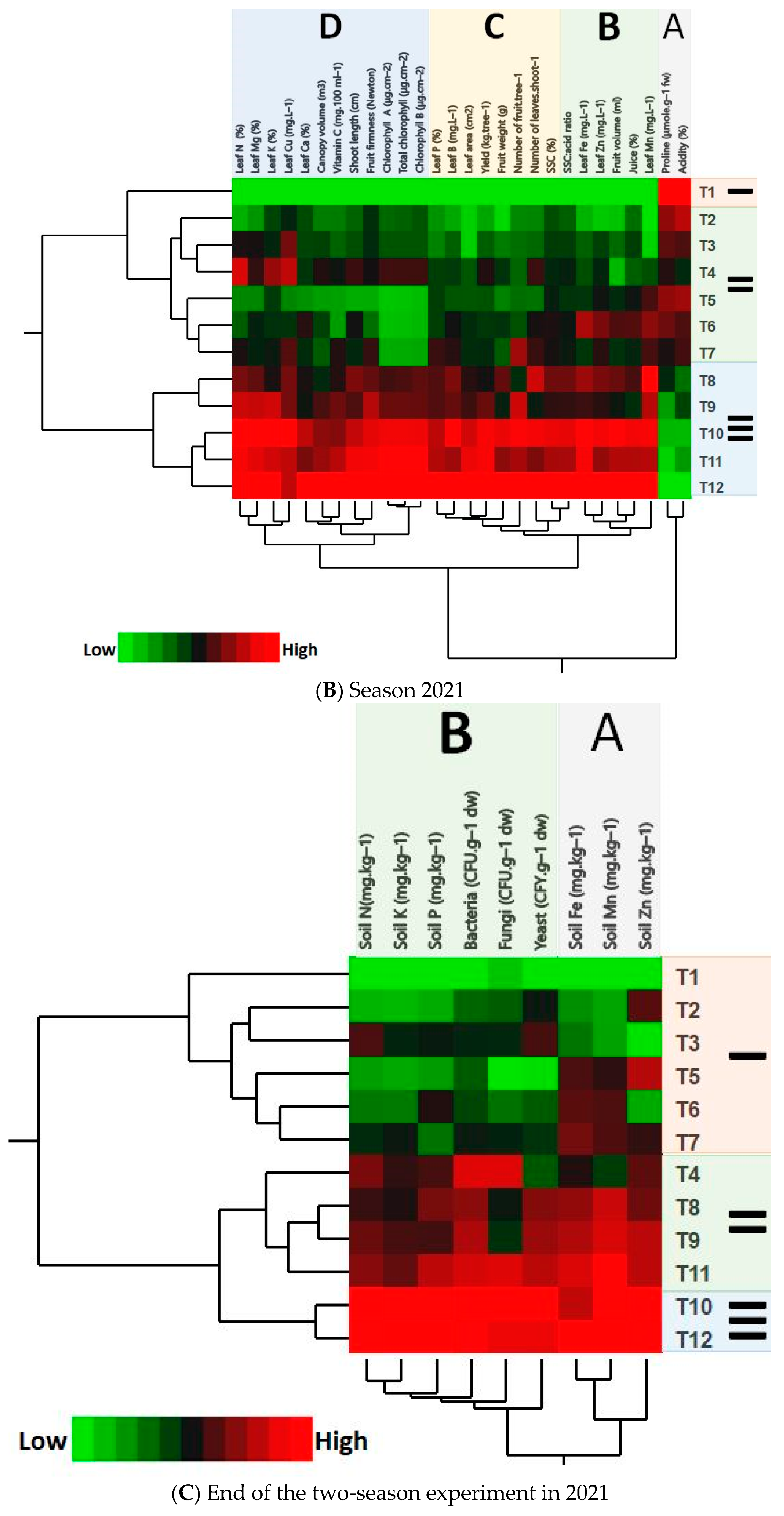
3.6. PCA and HCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mexican Lime. Citrus Variety Collection, College of Natural and Agricultural Sciences, University of California, Riverside, CA, USA. Available online: https://citrusvariety.ucr.edu/citrus/mexican.html (accessed on 5 January 2023).
- Morton, J.F. Mexican lime. In Fruits of Warm Climates, 1st ed.; Morton, J.F., Ed.; Scientific Research: Miami, FL, USA, 1987; pp. 168–172. [Google Scholar]
- Citrus: World Markets and Trade. United States Department of Agriculture (USDA), Foreign Agricultural Services/USDA, Global Market Analysis, July 2022 Report, Washington, DC, USA. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 18 January 2023).
- Lemons and Limes Exporters by Country. World’s Top Exports. Available online: https://www.worldstopexports.com/lemons-exporters-by-country/ (accessed on 18 January 2023).
- Abobatta, W.F. Citrus varieties in Egypt: An impression. Int. Res. J. Appl. Sci. 2019, 1, 63–66. [Google Scholar]
- El-Shereif, A.; Abou Elyazid, D. Tropical and subtropical fruits in Egypt. Chron. Hortic. 2016, 56, 28–31. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistics; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2021; Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 25 April 2023).
- Mitter, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Romero-Romero, J.L.; Inostroza-Blancheteau, C.; Reyes-Diaz, M.; Matte, J.P.; Aquea, F.; Espinoza, C.; Gil, P.M.; Arce-Johnson, P. Increased Drought and salinity tolerance in Citrus aurantifolia (Mexican lemon) plants overexpressing Arabidopsis CBF3 gene. J. Soil Sci. Plant Nutr. 2020, 20, 244–252. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- Shao, H.; Chu, L.; Lu, H.; Qi, W.; Chen, X.; Liu, J.; Kuang, S.; Tang, B.; Wong, V. Towards sustainable agriculture for the salt-affected soil. Land Degrad. Dev. 2019, 30, 574–579. [Google Scholar] [CrossRef]
- Ennab, H.A. Effect of humic acid on growth and productivity of Egyptian lime trees (Citrus aurantifolia Swingle) under salt stress conditions. J. Agric. Res. Kafr El-Sheikh Univ. 2016, 42, 494–505. [Google Scholar]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation induced salinity and sodicity hazards on soil and groundwater: An overview of its causes, impacts and mitigation strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple abiotic stresses occurring with salinity stress in citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Dubey, A.K.; Singh, A.K.; Srivastav, M. Salt stress studies in mango—A review. Agric. Rev. 2007, 28, 75–78. [Google Scholar]
- Hou, Y.; Zeng, W.; Hou, M.; Wang, Z.; Luo, Y.; Lei, G.; Zhou, B.; Huang, J. Responses of the Soil Microbial Community to Salinity Stress in Maize Fields. Biology 2021, 10, 1114. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt stress. In Encyclopedia of Life Science, 2nd ed.; O’Daly, A., Ed.; Wiley, J. & Sons, Ltd.: Chichester, UK, 2007; pp. 1–3. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Dayal, V.; Dubey, A.K.; Awasthi, O.P.; Pandey, R.; Dahuja, A. Growth, lipid peroxidation, antioxidant enzymes and nutrient accumulation in Amrapali mango (Mangifera indica L) grafted on different rootstocks under NaCl stress. Plant Knowl. J. 2014, 3, 15–22. [Google Scholar]
- Moya, J.L.; Tadeo, F.R.; Gomez-Cadenas, A.; Primo-Millo, E.; Talon, M. Transmissible salt tolerance traits identified through reciprocal grafts between sensitive Carrizo and tolerant Cleopatra citrus genotypes. J. Plant Physiol. 2002, 159, 991–998. [Google Scholar] [CrossRef]
- Al-Yassin, A. Adverse effects of salinity on citrus: Review. Int. J. Agric. Biol. 2005, 7, 668–680. [Google Scholar]
- Acosta-Motos, J.R.; Ortuno, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.; Papadakis, I.; Therios, I. Effect of calcium on the ion status and growth performance of citrus rootstocks grown under NaCl stress. Soil Sci. Plant Nutr. 2008, 54, 910–915. [Google Scholar] [CrossRef]
- Murkute, A.A.; Sharma, S.; Singh, S.K. Studies on salt stress tolerance of citrus rootstock genotypes with Arbuscular mycorrhizal fungi. Hort. Sci. 2006, 33, 70–76. [Google Scholar] [CrossRef]
- Agricultural Statistics of Egypt. Water Scarcity in Egypt: The Urgent Need for Regional Cooperation Among the Nile Basin Countries; Report of the Ministry of Water Resources and Irrigation, Government of Egypt: Cairo, Egypt, 2014; p. 5. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Environmental Considerations in Irrigation Development, Irrigation Potential in Africa: A Basin Approach; FAO Land and Water Bulletin # 4 (ISBN 92-5-103966-6); FAO Land and Water Development Division: Rome, Italy, 1997; Available online: https://www.fao.org/3/w4347e/w4347e10.htm#waterlogging%20and%20salinization (accessed on 20 March 2023)ISBN 92-5-103966-6.
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Shafqat, W.; Mazrou, Y.S.A.; Nehela, Y.; Ikram, S.; Bibi, S.; Naqvi, S.A.; Hameed, M.; Jaskani, M.J. Effect of Three Water Regimes on the Physiological and Anatomical Structure of Stem and Leaves of Different Citrus Rootstocks with Distinct Degrees of Tolerance to Drought Stress. Horticulture 2021, 7, 554. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; Pascale, S.D.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Ennab, H.; Alam-Eldein, S.M. Biostimulants Foliar Application to Improve Growth, Yield, and Fruit Quality of ‘Valencia’ Orange Trees under Deficit Irrigation Conditions. J. Amer. Pomol. Soc. 2020, 74, 118–134. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Rhizosphere humic acid interacts with root cell walls to reduce hydraulic conductivity and plant development. Plant Soil. 2010, 336, 313–322. [Google Scholar] [CrossRef]
- Simpson, A.J.; Kingery, W.L.; Hayes, M.H.B.; Spraul, M.; Humpfer, E.; Dvortsak, P.; Kerssebaum, R.; Godejohann, M.; Hofmann, M. Molecular structures and associations of humic substances in the terrestrial environment. Naturwissenschaften 2002, 89, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil. Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Berbara, R.L.L.; García, A.C. Humic substances and plant defense metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment, 2nd ed.; Ahmad, P., Wani, M.R., Eds.; Springer Science + Business Media: New York, NY, USA, 2014; Volume 1, pp. 297–319. [Google Scholar]
- Piccolo, A.; Spiteller, M. Electrospray ionization mass spectrometry of terrestrial humic substances and their size fractions. Anal. Bioanal. Chem. 2003, 377, 1047–1059. [Google Scholar] [CrossRef]
- Varanini, Z.; Pinton, R. Direct versus indirect effects of soil humic substances on plant growth and nutrition. In The rhizosphere, 1st ed.; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; Marcel Dekker: Basel, Belgium, 2001; pp. 141–158. [Google Scholar]
- Ngullie, C.R.; Tank, R.V.; Bhander, D.R. Effect of salicylic acid and humic acid on flowering, fruiting, yield and quality of mango (Mangifera indica L.) cv. Kesar. Adv. Res. J. Crop Improv. 2014, 5, 136–139. [Google Scholar] [CrossRef]
- Gumus, I.; Seker, C. Influence of humic acid applications on soil physicochemical properties. Solid Earth Discuss. 2015, 7, 2481–2500. [Google Scholar]
- Ferrara, G.; Brunetti, G. Effects of the times of application of a soil humic acid on berry quality of table grape (Vitis vinifera L.) cv. Italia. Spanish J. Agric. Res. 2010, 8, 817–822. [Google Scholar] [CrossRef]
- Ali, M.A.; El-Gendy, S.S.; Ahmed, O.A. Minimizing adverse effects of salinity in vineyards. J. Hort. Sci. Ornament. Plants 2013, 5, 12–21. [Google Scholar]
- Canali, S.; Bartolomeo, E.D.; Trinchera, A.; Nisini, L.; Tittarell, F.; Intrigliolo, F.; Roccuzzo, G.; Calabretta, M.L. Effect of different management strategies on soil quality of citrus orchards in southern Italy. Soil Manag. 2009, 25, 34–42. [Google Scholar] [CrossRef]
- El-Khawaga, A.S. Effect of anti-salinity agents on growth and fruiting of different date palm cultivars. Asian J. Crop Sci. 2013, 5, 65–80. [Google Scholar] [CrossRef]
- Abd El-Hamied, S.A. Response of Valencia orange to some natural and synthetic soil conditioners under north Sinai (Egypt) conditions. Int. J. Adv. Res. 2014, 2, 802–810. [Google Scholar]
- Ennab, H.A.; El-Sayed, S.A. Treatments to alleviate adverse effect of saline soil on growth and productivity of Balady mandarin trees (Citrus reticulata Blanco). Alex. J. Agric. Sci. 2016, 61, 491–503. [Google Scholar]
- Chen, Y.; De Nobili, M.; Aviad, T. Stimulatory effects of humic substances on plant growth. In Soil Organic Matter in Sustainable Agriculture, 1st ed.; Magdoff, F.R., Weil, R.R., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 103–129. Available online: http://www.crcpress.com/Magdoff-Weil/p/book/9780367394233 (accessed on 22 February 2023).
- Cavalcante, I.H.; Silva, R.R.; Albano, F.G.; Lima, F.N.; Marques, A.D. Foliar spray of humic substances on seedling production of papaya (Pawpaw). J. Agron. 2011, 10, 118–122. [Google Scholar] [CrossRef]
- Abd El-Razek, E.; Abd-allah, A.; Saleh, M. Yield and fruit quality of Florida prince peach trees as affected by foliar and soil applications of humic acid. J. App. Sci. Res. 2012, 8, 5724–5729. [Google Scholar]
- Hagagg, L.F.; Shahin, M.F.M.; Mustafa, N.S.; Merwad, M.A.; Khalil, F.H. Influence of using humic acid during full bloom and fruit set stages on productivity and fruit quality of ‘Kalamata’ olive trees. J. Appl. Sci. Res. 2013, 9, 2287–2292. [Google Scholar]
- Abbas, T.; Ahmad, S.; Ashraf, M.; Shahid, M.A.; Yasin, M.; Balal, R.M.; Pervez, M.A.; Abbas, S. Effect of humic and application at different growth stages of Kinnow mandarin (Citrus reticulate Blanco) on the basis of physio-biochemical and reproductive responses. Academia J. Biotech. 2013, 1, 14–20. [Google Scholar]
- Pablo Morales, J.P.; William, M.S. Effect of substrates, boron and humic acid on the growth of papaya transplant. In Proceedings of the Florida State Horticultural Society, Lake Alfred, FL, USA, 8–10 June 2003; Volume 116, pp. 28–30. [Google Scholar]
- Chen, Y.; Avaid, T. Effect of Humic Substances on Plant Growth; MacCarthy, P., Clapp, C.E., Malcolm, R.L.P., Bloom, R., Eds.; American Society of Agronomy: Madison, WI, USA, 1990; Volume 18, pp. 161–186. [Google Scholar]
- Mosa, W.F.A.; Abd EL-Megeed, N.A.; Paszt, L.S. The effect of the foliar application of potassium, calcium, boron and humic acid on vegetative growth, fruit set, leaf mineral, yield and fruit quality of ‘Anna’ apple trees. Amer J. Exp. Agric. 2015, 8, 224–234. [Google Scholar] [CrossRef]
- Hidayatullah, K.A.; Mouladad, M.N.; Shah, S.A. Effect of humic acid on fruit yield attributes, yield and leaf nutrient accumulation of apple trees under calcareous soil. Ind. J. Sci. Techn. 2018, 11, 15. [Google Scholar] [CrossRef]
- El-Kosary, S.; El-Shenawy, I.E.; Radwan, S.I. Effect of microelements, amino and humic acids on growth, flowering and fruiting of some mango cultivars. J. Hort. Sci. Ornament. Plants 2011, 3, 152–161. [Google Scholar]
- Fathy, M.A.; Gabr, M.A.; El-Shall, S.A. Effect of humic acid treatments on Canino apricot growth, yield and fruit quality. New York Sci. J. 2010, 3, 109–115. [Google Scholar]
- Eissa, F.M.; Fathi, M.A.; El-Shall, S.A. Response of peach and apricot seedlings to humic acid treatments under salinity condition. J. Agric. Sci. Mansoura Univ. 2007, 32, 3605–3620. [Google Scholar] [CrossRef]
- Worldweatheronline. Baltim, Kafr El-Sheikh, Egypt Historical Weather. 2022. Available online: https://www.worldweatheronline.com/baltim-weather-averages/kafr-ash-shaykh/eg.aspx (accessed on 25 February 2023).
- Kebede, A.A.; Olani, D.D.; Getahun, T.; Damtew, Y.T. Heavy metal Content and physico chemical Properties of soil around solid waste disposal sites. Amer. J. Sci. Indust. Res. 2016, 7, 129–136. [Google Scholar]
- Thomas, G.W.; Hargrove, W.L. The chemistry of soil Acidity. In Soil Acidity and Liming, 2nd ed.; Adams, F., Ed.; American Society of Agronomy: Madison, WI, USA, 1984; Volume 12, pp. 3–49. [Google Scholar]
- Jackson, N.L. Soil Chemical Analysis; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1967; Chapter 16; 210p. [Google Scholar]
- Mostafazadeh-Fard, B.; Khoshravesh, M.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on soil chemical components underneathtrickle irrigation. J. Irrig. Drain. Eng. 2012, 138, 1075–1081. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis Part 2: CHEMICAL and Microbiological Properties, 2nd ed.; American Society of Agronomy & Soil Sciences Society of America: Madison, WI, USA, 1982; Volume 9, p. 1159. [Google Scholar]
- Wilde, S.A.; Corey, R.B.; Layer, J.G.; Voigt, G.K. Soil and Plant Analysis for Tree Culture; Mohan Primlani, Oxford, IBH, Publishing Co.: New Delhi, India, 1985; pp. 1–142. [Google Scholar]
- Chapman, H.D.; Pratt, F.P. Methods of Analysis for Soils, Plants and Waters, 1st ed.; University of California, Division of Agricultural Sciences: Davis, CA, USA, 1961; p. 309. [Google Scholar]
- Turrell, F.M. Tables of Surfaces and Volumes of Spheres and of Prolate and Oblate Spheroids and Spheroidal Coefficients; University of California Press: Berkeley, CA, USA, 1946. [Google Scholar]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N, N dimethyl formamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Evenhuis, B.; Dewaard, P.W. Nitrogen Determination; Department of Agriculture Research, Royal Tropical Institute: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Jones, J.B.; Wolf, B.; Mills, H.A. Plant Analysis Handbook. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, GA, USA, 1991; p. 212. [Google Scholar]
- Tendon, H.L.S. Analysis of Soils, Plants, Waters and Fertilizers; Fertilizer Development and Consultation Organization: New Delhi, India, 2005. [Google Scholar]
- Chang, K.L.; Bray, R.H. Determination of calcium and magnesium in soil and plant material. Soil Sci. 1951, 72, 449–458. [Google Scholar] [CrossRef]
- Rashid, A. Mapping Zinc Fertility of Soil Using Indicator Plant and Soil Analysis. Ph.D. Thesis, University of Hawaii, Manoa, HI, USA, 1986. [Google Scholar]
- Prabhu, M.; Partiban, S.; Kumar, A.R.; Usharani, B.; Vijayasamundeeswari, A. Regulation of flowering in acid lime (Citrus aurantifolia Swingle). Indian J. Agric. Res. 2017, 51, 384–387. [Google Scholar] [CrossRef]
- Gomaa, A.M. Productivity and fruit quality of lime (Citrus aurantifolia L.) as affected by GA3 and NAA foliar spray. HortScience J. Suez Canal Univ. 2020, 9, 63–71. [Google Scholar] [CrossRef]
- Al-Yahyai, R.; Al-Said, F.; Opara, L. Fruit growth characteristics of four pomegranate cultivars from northern Oman. Fruits 2009, 64, 335–341. [Google Scholar] [CrossRef]
- Watkins, C.; Harman, J. Use of penetrometer to measure flesh firmness of fruit. Orchard. N. Z. 1981, 54, 14–16. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemist: Washington, DC, USA, 2000; pp. 16–20. [Google Scholar]
- Iqbal, M.N.; Ashraf, A. Antagonism in rhizobacteria: Application for biocontrol of soil borne plant pathogens. PSM Microbiol. 2017, 2, 78–79. [Google Scholar]
- Sherpa, M.T.; Sharma, L.; Bag, N.; Das, S. Isolation, characterization, and evaluation of native Rhizobacterial consortia developed from the rhizosphere of rice grown in organic state Sikkim, India, and their effect on plant growth. Front. Microbiol. 2021, 12, 713660. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Senapati, A.; Kumar, U.; Sharma, L. Antagonistic and plant-growth promoting novel Bacillus species from long-term organic farming soils from Sikkim, India. 3 Biotech. 2019, 9, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, T.A.; Taha, N.; El-Beltagi, H.S.; El-Ramady, H. Combined Application of Trichoderma harzianum and Paclobutrazol to Control Root Rot Disease Caused by Rhizoctonia solani of Tomato Seedlings. Agronomy 2022, 12, 3186. [Google Scholar] [CrossRef]
- Parkinson, D.; Gray, J.R.G.; Williams, S.T. Methods for Studying the Ecology of Soil Microorganisms; Oxford Blackwell Scientific Publications: Hoboken, NJ, USA, 1971; 116p. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State University Press: Ames, IA, USA, 1990. [Google Scholar]
- Jolliffe, I. Principal Component Analysis; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Michie, M.G. Use of the Bray-Curtis similarity measure in cluster analysis of foraminiferal data. J. Int. Assoc. Math. Geol. 1982, 14, 661–667. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuno, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- El-Galad, M.A.; Sayed, D.A.; El-Shal, R.M. Effect of humic acid and compost applied alone or in combination with sulphur on soil fertility and faba bean productivity under saline soil conditions. J. Soil Sci. and Agric. Eng. Mansoura Univ. 2013, 4, 1139–1157. [Google Scholar]
- Abd EL-Rahman, M.M.A.; Khodair, O.A.; Hamed, M.H. Impact of organic, bio fertilization and humic acid on growth and fruiting of Flame seedless grapevines under sandy soil conditions. J. Plant Prod. Mansoura Univ. 2021, 12, 171–177. [Google Scholar] [CrossRef]
- Tenshia, J.S.V.; Singaram, P. Influence of humic acid application on availability and uptake in tomato. Madrass Agric. J. 2005, 92, 670–676. [Google Scholar]
- Khattak, R.A.; Haroon, K.; Muhammad, D. Mechanism(s) of humic acid induced beneficial effects in salt-affected soils. Sci. Res. Essays 2013, 8, 932–939. [Google Scholar]
- Ibrahim, M.G.; Bassiony, S.S. Alleviating the adverse effects of soil salinity on growth and productivity of "Roby seedless" grapevines (Vitis vinifera L.) using some soil amendments. Menoufia J. Plant Prod. 2019, 4, 375–393. [Google Scholar] [CrossRef]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic acids: Structural properties and multiple functionalities for novel technological developments. Mater. Sci. Eng. 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef]
- Yang, F.; Tang, C.; Antonietti, M. Natural and artificial humic substances to manage minerals, ions, water, and soil microorganisms. Chem. Soc. Rev. 2021, 50, 6221–6239. [Google Scholar] [CrossRef]
- Wu, S.; Li, R.; Peng, S.; Liu, Q.; Zhu, X. Effect of humic acid on transformation of soil heavy metals. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012089. [Google Scholar] [CrossRef]
- Fahramand, M.; Moradi, H.; Noori, M.; Sobhkhizi, A.; Adibian, M.; Abdollahi, S.; Rigi, K. Influence of humic acid on increase yield of plants and soil properties. Int. J. Farm. All. Sci. 2014, 3, 339–341. [Google Scholar]
- Khaled, A.; Shaban, M.; Abd El-Kader, G.; Khalil, Z.M. Effect of soil amendments on soil fertility and sesame crop productivity under newly reclaimed soil conditions. J. Appl. Sci. Res. 2012, 8, 1568–1575. [Google Scholar]
- Mohamed, W.H. Effects of humic acid and calcium forms on dry weight and nutrient uptake of maize plant under saline condition. Aust. J. Basic Appl. Sci. 2012, 6, 597–604. [Google Scholar]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Abdelly, C.; Lakhdar, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar]
- Okba, S.K.; Mazrou, Y.; Mikhael, G.B.; Farag, M.E.H.; Alam-Eldein, S.M. Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’ Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt. Horticulturae 2022, 8, 564. [Google Scholar] [CrossRef]
- Andrade, G.; Mihara, K.L.; Linderman, R.G.; Bethlenfalvay, G.J. Soil aggregation status and rhizobacteria in the mycorrhizosphere. Plant Soil 1998, 202, 89–96. [Google Scholar] [CrossRef]
- Dobereiner, J. Influence of environmental factors on the occurrence of S. lipoferum in soil roots. Environmental role of N2-fixing blue-green-algae and a symbiotic bacteria. Ecol. Bull. 1978, 26, 343–352. [Google Scholar]
- Khan, A.G. Mycorrhizoremediation--an enhanced form of phytoremediation. J. Zhejiang Univ. Sci. B. 2006, 7, 503–514. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2021, 3, 100094. [Google Scholar] [CrossRef]
- El-Mohamedy, R.S.R.; Ahmed, M.A. Effect of biofertilizers and humic acid on control of dry root rots disease and improvement yield quality of mandarin (Citrus reticulate Blanco). Res. J. Agric. Biol. Sci. 2009, 5, 127–137. [Google Scholar]
- Youssef, A.R.M.; Emam, H.S.; Saleh, M.M.S. Olive seedlings growth as affected by humic acid and amino acids, macro and trace elements applications. Agric. Biol. J. N. Am. 2011, 2, 1101–1107. [Google Scholar]
- El-Ghamry, A.M.; Abd El-Hai, K.M.; Ghoneem, K.M. Amino and humic acids promote growth, yield and disease resistance of faba bean cultivated in clayey soil. Aust. J. Basic Appl. Sci. 2009, 3, 731–739. [Google Scholar]
- Hassanein, R.A.; El Khawas, S.A.; Khafaga, H.S.; Abd El-Nabe, A.S.; Abd Elrady, A.S. Amelioration of drought stress on physiological performance of pearl millet (Pennisetum americanum) plant grown under saline condition using potassium humate and silicon source. Egypt J. Exp. Biol. 2017, 13, 57–68. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Scient. Hort. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Van Tol de Castro, T.A.; Berbara, R.L.L.; Tavares, O.C.H.; da Graça Mello, D.F.; Pereira, E.G.; de Souza, C.d.C.B.; Espinosa, L.M.; García, A.C. Humic acids induce a eustress state via photosynthesis and nitrogen metabolism leading to a root growth improvement in rice plants. Plant Physiol. Biochem. 2021, 162, 171–184. [Google Scholar] [CrossRef]
- Cimrin, K.M.; Turkmen, O.; Turan, M.; Tuncer, B. Phosphorus and humic acid application alleviate salinity stress of pepper seedlings. Afr. J. Biotechnol. 2010, 9, 5845–5851. [Google Scholar]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaro, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
- Gaines, I.; Yilmaz, A. Comparison of five humic acids. Fuel 1983, 62, 373–379. [Google Scholar]
- Khan, S.A.; Kha, S.U.; Qayyum, A.; Gurmani, A.R.; Khan, A.; Khan, S.M.; Ahmed, W.; Mehmood, A.; Amin, A.Z. Integration of humic acid with nitrogen wields an auxiliary impact on physiological traits, growth and yield of maize (Zea mays L.) verities. Appl. Ecol. Environ. Res. 2019, 17, 6783–6799. [Google Scholar] [CrossRef]
- Vaughan, D.; Ord, B.G. Uptake and incorporation of 14C-labelled soil organic matter by roots of Pisum sativum L. J. Expt. Bot. 1981, 32, 679–687. [Google Scholar] [CrossRef]
- Can, W.; Kafi, M.; Babalar, M.; Xia, Y. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant Nutr. 2008, 31, 2155–2167. [Google Scholar]
- Li, H.; Kong, F.; Tang, T.; Luo, Y.; Gao, H.; Xu, J.; Xing, G.; Li, L. Physiological and Transcriptomic Analyses Revealed That Humic Acids Improve Low-Temperature Stress Tolerance in Zucchini (Cucurbita pepo L.) Seedlings. Plants 2023, 12, 548. [Google Scholar] [CrossRef]
- Arslan, G.; Pehlivan, E. Uptake of Cr3+ from aqueous solution by lignite-based humic acids. Bioresour. Technol. 2008, 99, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front. Plant Sci. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol Techn. Agric. 2014, 1, 3. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Hokmabadi, H.; Arzani, K.; Grierson, P.F. Growth, chemical composition, and carbon isotope discrimination of pistachio (Pistacia vera L.) rootstock seedlings in response to salinity. Aust. J. Agric. Res. 2005, 56, 135–144. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Wang, L.; Li, M.; Lang, D.; Zhang, X. Silicon alleviates salt and drought stress of Glycyrrhiza uralensis seedling by altering antioxidant metabolism and osmotic adjustment. J. Plant Res. 2017, 130, 611–624. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effect of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]
- Boehme, M.; Schevtschenko, J.; Pinker, I. Iron supply of cucumbers in substrate culture with humate. Acta Hort. 2005, 41, 329–335. [Google Scholar] [CrossRef]
- Farahat, A.A.; Alsayed, A.A.; El-Beltagi, H.S.; Mahfoud, N.M. Impact of organic and inorganic fertilizers on nematode reproduction and biochemical alterations on tomato. Not. Sci. Biol. 2012, 4, 48–55. [Google Scholar] [CrossRef]
- Turkmen, O.; Dursun, A.; Turan, M.; Erdinc, C. Calcium and humic acid affect seed germination, growth, and nutrient content of tomato (Lycopersicon esculentum L.) seedlings under saline soil conditions. Acta Agr. Scand. 2004, 54, 168–174. [Google Scholar] [CrossRef]
- El-Hoseiny, H.M.; Helaly, M.N.; Elsheery, N.I.; Alam-Eldein, S.M. Humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. HortScience 2020, 55, 1026–1037. [Google Scholar] [CrossRef]
- Aydin, A.; Kant, C.; Turan, M. Humic acid application alleviate salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agr. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Haghighi, M.; Kafi, M.; Fang, P. Photosynthetic activity and N metabolism of lettuce as affected by humic acid. Intl. J. Veg. Sci. 2012, 18, 182–189. [Google Scholar] [CrossRef]
- Rajpar, I.; Bhatti, M.B.; Zia-ul-Hassan, A.N.; Tunio, S.D. Humic acid improves growth, yield and oil content of Brassica compestris L. Pak. J. Agric. Agric. Eng. Vet. Sci. 2011, 27, 125–133. [Google Scholar]
- Dobbss, L.B.; Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Peres, L.E.P.; Azevedo, M.; Spaccini, R.; Piccolo, A.; Facxanha, A.R. Bioactivity of chemically transformed humic matter from vermicompost on plant root growth. J. Agr. Food Chem. 2010, 58, 3681–3688. [Google Scholar] [CrossRef]
- Schmidt, W.; Santi, S.; Pinton, R.; Varanini, Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil J. 2007, 300, 259–267. [Google Scholar] [CrossRef]
- Abobatta, W.F. Influence of magnetic iron and k-humate on productivity of Valencia orange trees (Citrus Sinensis L.) under salinity conditions. Int. J. Sci. Res. Agric. Sci. 2015, 2, 108–119. [Google Scholar]
- Sharaf, M.M.; Bakry, K.A.; EL- Gioushy, S.F. The influence of some bio and organic nutritive addenda on growth, productivity, fruit quality and nutritional status of Washington navel orange trees. Egypt. J. Appl. Sci. 2011, 26, 253–269. [Google Scholar]
- Ahmed, S.; Abbas, T.; Anwar, R.; Khan, M.M.; Bilal, M.R.; Pervez, M.A. Effect of humic acid on some morpho-physiological and bio-chemical attributes of Kinnow mandarin (Citrus reticulata Blanco). HortScience 2009, 44, 945. [Google Scholar]
- El-Shazly, S.M.; Mostafa, N.S. Enhancement yield, fruit quality and nutritional status of Washington navel orange trees by application of biostimulants. J. Appl. Sci. Res. 2013, 9, 5030–5034. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant. Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef]
- Mora, V.; Baigorri, R.; Bacaicoa, E.; Zamarreno, A.M.; García-Mina, J.M. The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber. Environ. Exp. Bot. 2012, 76, 24–32. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Santos, M.P.; Dobbss, L.B.; Olivares, F.L.; Canellas, L.P.; Binzel, M.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 2010, 231, 1025–1036. [Google Scholar] [CrossRef]
- Jindo, K.; Canellas, L.P.; Albacete, A.; Figueiredo dos Santos, L.; Frinhani Rocha, R.L.; Carvalho Baia, D.; Oliveira Aguiar Canellas, N.; Goron, T.L.; Olivares, F.L. Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition. Agronomy 2020, 10, 640. [Google Scholar] [CrossRef]
- Hirschi, K.D. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol. 2004, 136, 2438–2442. [Google Scholar] [CrossRef]
- Hao, X.; Papadopoulos, A.P. Effects of calcium and magnesium on growth, fruit yield and quality in a fall greenhouse tomato crop grown on rockwool. Can. J. Plant Sci. 2003, 83, 903–912. [Google Scholar]
- Rubio, J.S.; Garcia-Sanchez, F.; Rubio, F.; Martinez, V. Yield, blossom-end rot incidence, and fruit quality in pepper plants under moderate salinity are affected by K+ and Ca2+ fertilization. Sci. Hortic. 2009, 119, 79–87. [Google Scholar] [CrossRef]
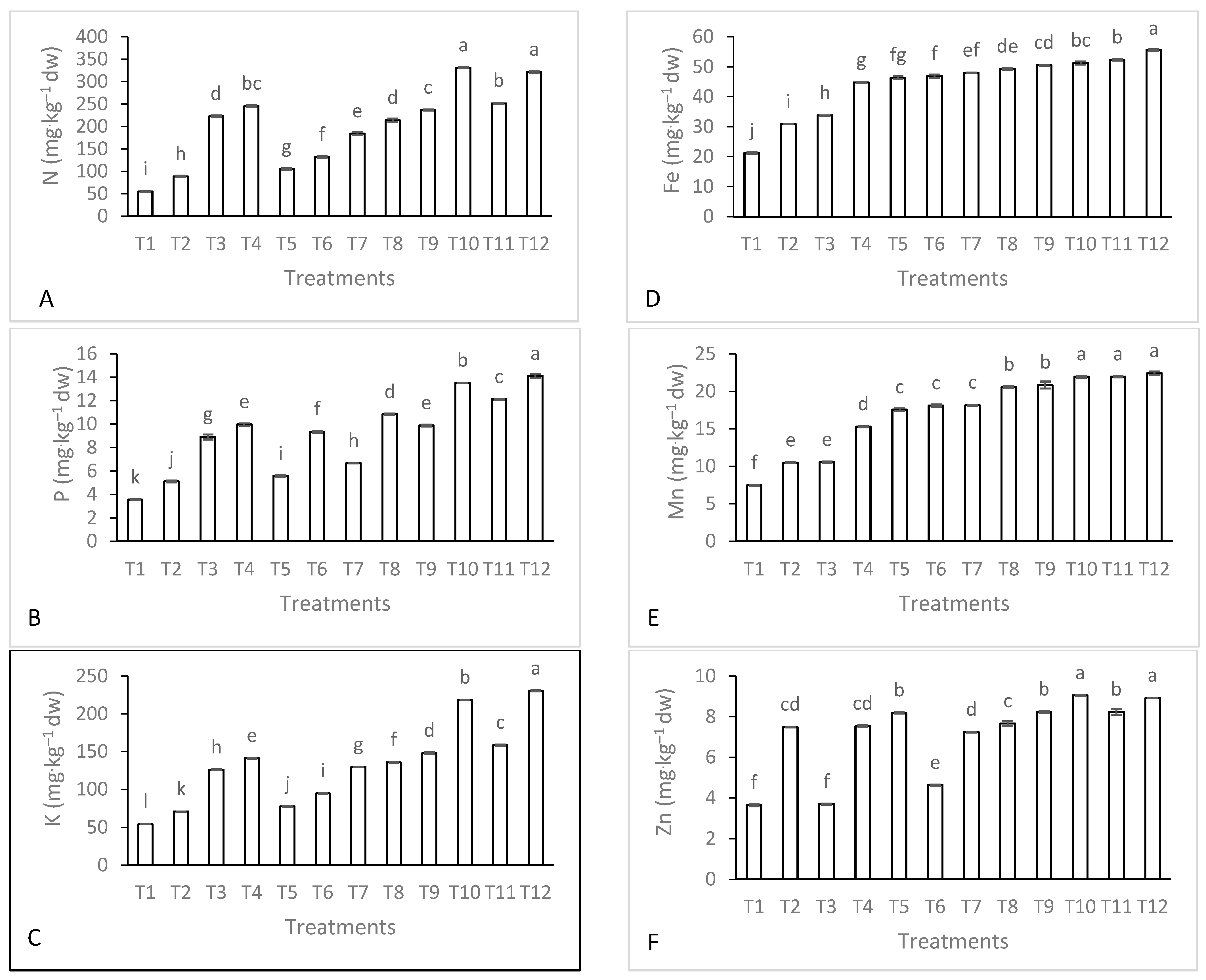

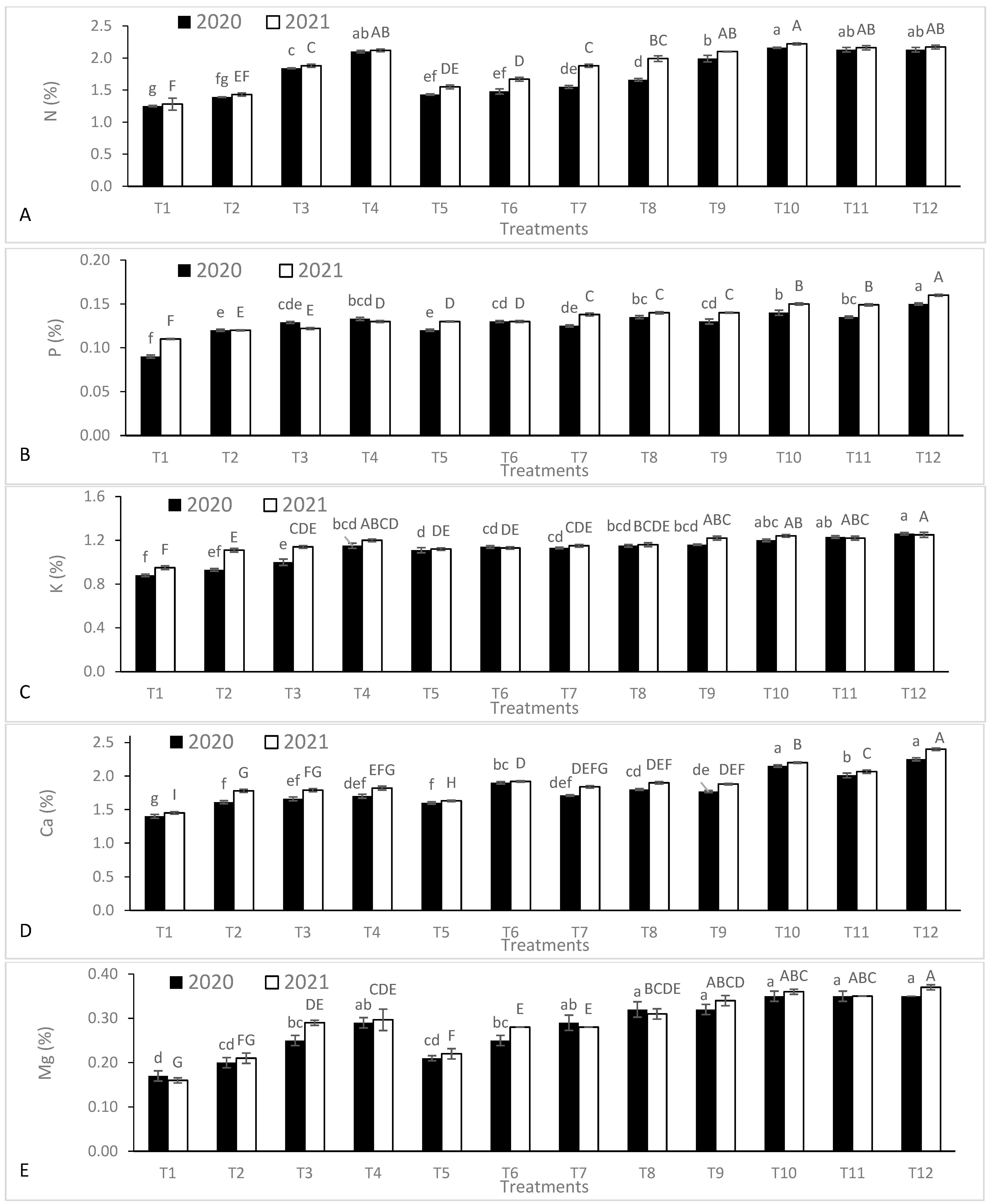
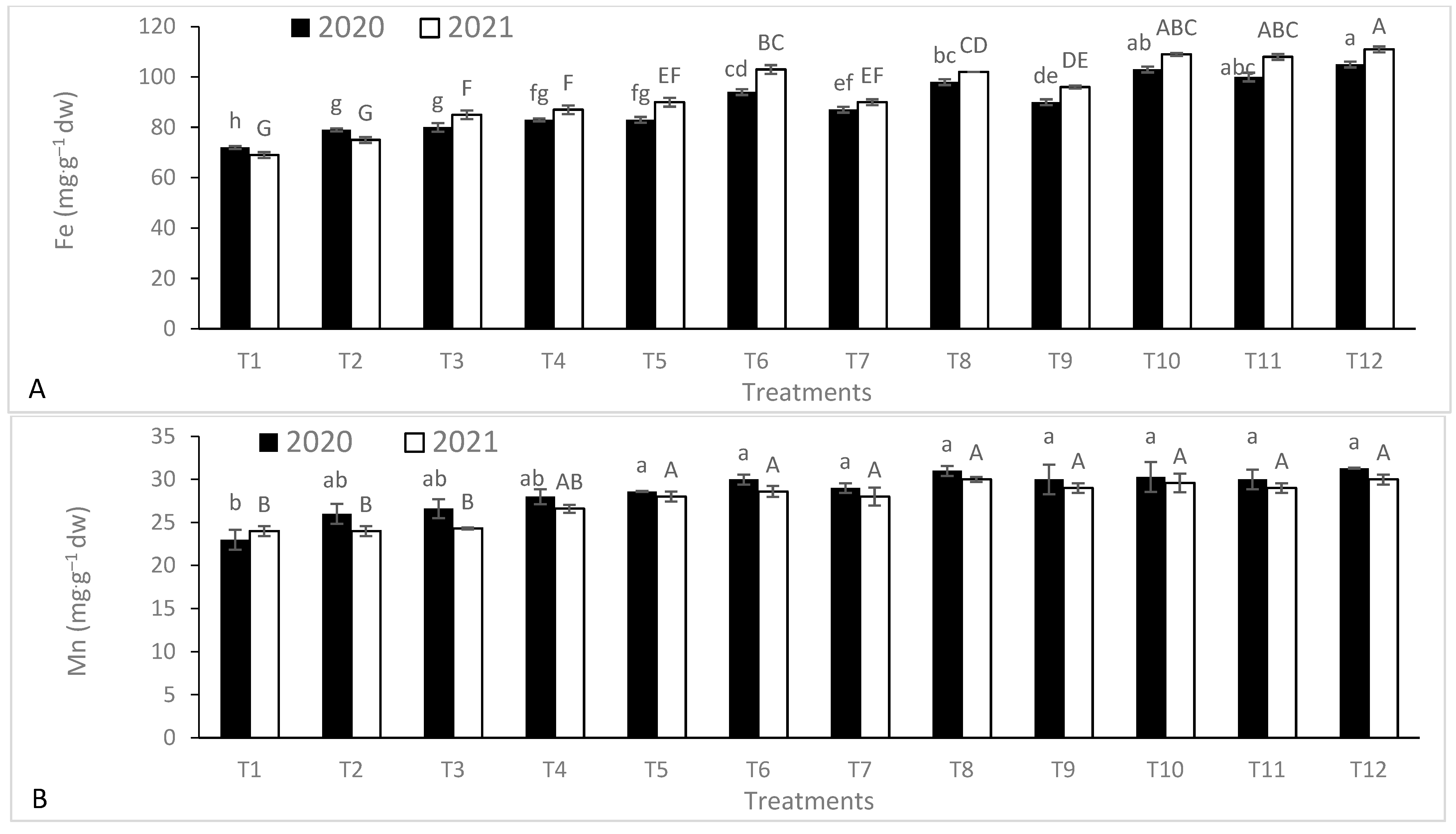
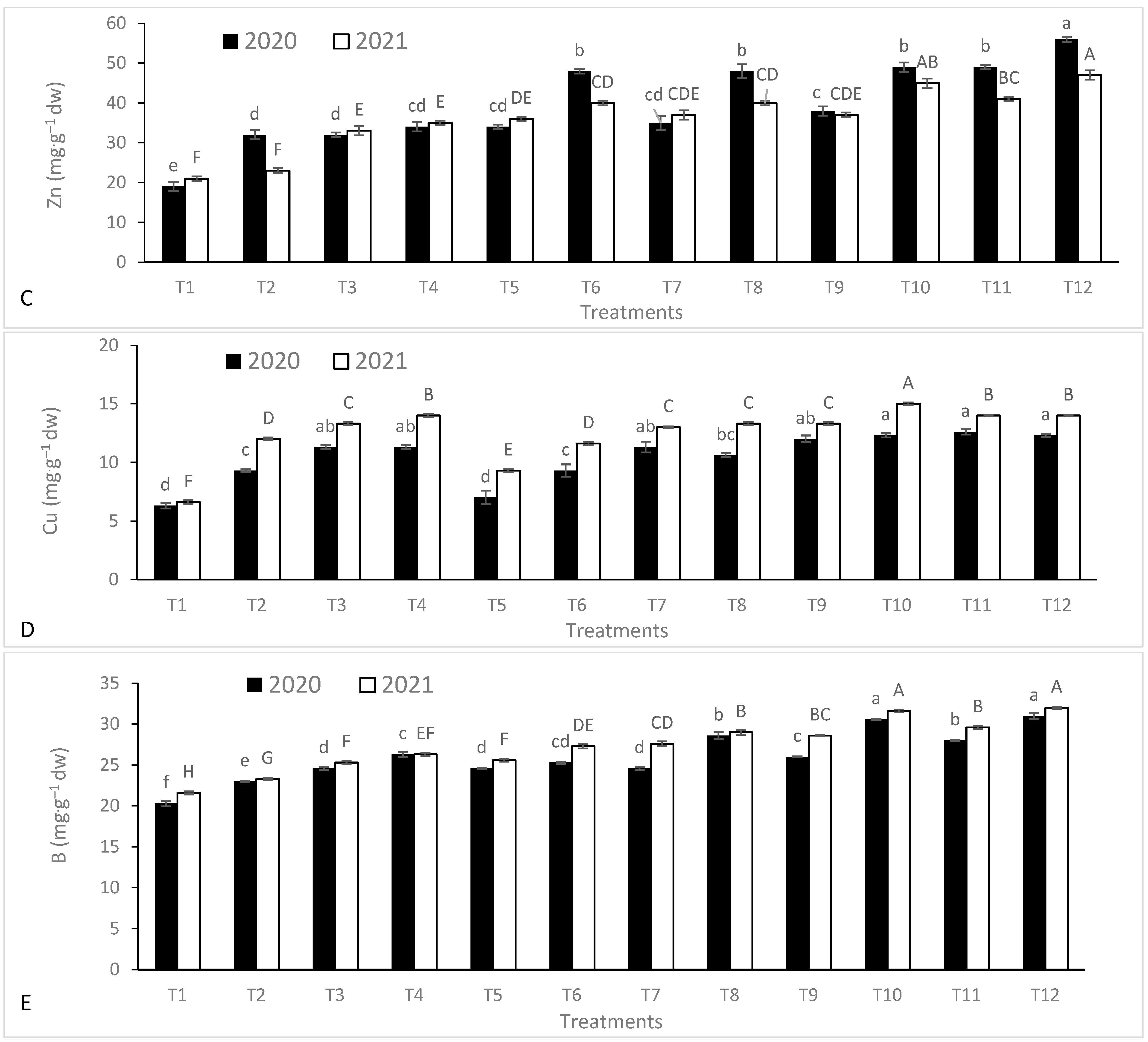


| Season | Temperature (°C) | Humidity (%) | Rainfall (mm·month−1) | Wind Speed (km·h−1) | Cloud (%) | Sun (h·month−1) | UV Index | |
|---|---|---|---|---|---|---|---|---|
| October | 2019 | 26 | 67 | 37.7 | 12.1 | 14 | 341 | 6 |
| 2020 | 27 | 63 | 16.0 | 11.6 | 12 | 325 | 6 | |
| November | 2019 | 23 | 61 | 1.6 | 12.1 | 9 | 343 | 6 |
| 2020 | 21 | 63 | 52.1 | 11.1 | 40 | 147 | 5 | |
| December | 2019 | 18 | 62 | 41.8 | 14.3 | 23 | 278 | 5 |
| 2020 | 18 | 63 | 17.4 | 10.8 | 26 | 242 | 4 | |
| January | 2020 | 14 | 71 | 65.4 | 14.8 | 45 | 154 | 4 |
| 2021 | 17 | 66 | 25.3 | 13.9 | 28 | 249 | 5 | |
| February | 2020 | 15 | 71 | 31.8 | 13.4 | 36 | 152 | 4 |
| 2021 | 17 | 69 | 45.4 | 13.2 | 32 | 191 | 4 | |
| March | 2020 | 18 | 66 | 40.0 | 15.5 | 29 | 246 | 6 |
| 2021 | 18 | 63 | 3.9 | 15.1 | 21 | 276 | 5 | |
| April | 2020 | 21 | 64 | 1.7 | 14.6 | 16 | 294 | 6 |
| 2021 | 22 | 55 | 0.4 | 16.2 | 12 | 306 | 7 | |
| May | 2020 | 26 | 56 | 0.3 | 15.2 | 10 | 341 | 6 |
| 2021 | 28 | 54 | 0.1 | 13.7 | 5 | 364 | 8 | |
| June | 2020 | 28 | 61 | 0.0 | 14.4 | 6 | 353 | 7 |
| 2021 | 28 | 59 | 0.0 | 13.5 | 2 | 360 | 8 | |
| July | 2020 | 30 | 68 | 0.1 | 14.2 | 5 | 365 | 8 |
| 2021 | 31 | 61 | 0.0 | 14.4 | 3 | 370 | 8 | |
| August | 2020 | 30 | 68 | 0.0 | 14.2 | 3 | 367 | 8 |
| 2021 | 32 | 60 | 0.0 | 12.9 | 1 | 372 | 8 | |
| September | 2020 | 30 | 68 | 0.0 | 13.1 | 5 | 352 | 7 |
| 2021 | 29 | 62 | 0.1 | 14.2 | 7 | 352 | 7 | |
| Parameter | Soil (Depth = 0–60 cm) | Water |
|---|---|---|
| Texture | Clay | |
| Sand (%) | 24.1 | |
| Silt (%) | 18.0 | |
| Clay (%) | 57.9 | |
| Organic matter (%) | 1.09 | |
| N (mg·kg−1) | 54.18 | |
| P (mg·kg−1) | 3.56 | |
| K (mg·kg−1) | 54.36 | |
| Fe (mg·kg−1) | 20.31 | |
| Mn (mg·kg−1) | 7.80 | |
| Zn (mg·kg−1) | 3.55 | |
| Ca2+ (meq·L−1) | 14.10 | 5.57 |
| Mg2+ (meq·L−1) | 4.58 | 1.77 |
| Na+ (meq·L−1) | 18.25 | 12.04 |
| Sodium adsorption ratio (SAR) | 8.44 | 6.28 |
| K+ (meq·L−1) | 4.61 | 0.63 |
| CO32− (meq·L−1) | 0.03 | 0.00 |
| HCO3− (meq·L−1) | 3.91 | 4.66 |
| Cl− (meq·L−1) | 17.82 | 12.80 |
| SO42− (meq·L−1) | 19.78 | 2.55 |
| EC (ds·m−1) [1:5 extract] | 3.80 | 1.78 |
| pH (1:2.5 extract) | 8.20 | 7.35 |
| Month | Dripper Discharge Amount (L·h−1) | Number of Drippers Per Tree | Irrigation Period (h·day−1) | Daily Water Quantity (L·tree−1) | Monthly Water Quantity (L·tree−1) |
|---|---|---|---|---|---|
| October | 4 | 8 | 1 h, 48 m | 58.02 | 1798.62 |
| November | 4 | 8 | 1 h, 43 m | 56.46 | 1693.80 |
| December | 4 | 8 | 1 h, 4 m | 34.31 | 1063.61 |
| January | 4 | 8 | 1 h, 2 m | 32.66 | 1012.46 |
| February | 4 | 8 | 1 h, 4 m | 34.39 | 962.92 |
| March | 4 | 8 | 1 h, 29 m | 47.30 | 1466.30 |
| April | 4 | 8 | 1 h, 48 m | 59.21 | 1776.30 |
| May | 4 | 8 | 1 h, 48 m | 59.36 | 1840.16 |
| June | 4 | 8 | 1 h, 52 m | 60.95 | 1828.50 |
| July | 4 | 8 | 1 h, 52 m | 60.67 | 1880.77 |
| August | 4 | 8 | 1 h, 52 m | 60.82 | 1885.42 |
| September | 4 | 8 | 1 h, 52 m | 60.61 | 1818.30 |
| Annual water quantity (m3·tree−1) = 19.027 m3 | |||||
| Annual water quantity (m3·hectare−1) = 7610.86 m3 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ennab, H.A.; Mohamed, A.H.; El-Hoseiny, H.M.; Omar, A.A.; Hassan, I.F.; Gaballah, M.S.; Khalil, S.E.; Mira, A.M.; Abd El-Khalek, A.F.; Alam-Eldein, S.M. Humic Acid Improves the Resilience to Salinity Stress of Drip-Irrigated Mexican Lime Trees in Saline Clay Soils. Agronomy 2023, 13, 1680. https://doi.org/10.3390/agronomy13071680
Ennab HA, Mohamed AH, El-Hoseiny HM, Omar AA, Hassan IF, Gaballah MS, Khalil SE, Mira AM, Abd El-Khalek AF, Alam-Eldein SM. Humic Acid Improves the Resilience to Salinity Stress of Drip-Irrigated Mexican Lime Trees in Saline Clay Soils. Agronomy. 2023; 13(7):1680. https://doi.org/10.3390/agronomy13071680
Chicago/Turabian StyleEnnab, Hassan A., Azza H. Mohamed, Hanan M. El-Hoseiny, Ahmad A. Omar, Islam F. Hassan, Maybelle S. Gaballah, Soha E. Khalil, Amany M. Mira, Ahmed F. Abd El-Khalek, and Shamel M. Alam-Eldein. 2023. "Humic Acid Improves the Resilience to Salinity Stress of Drip-Irrigated Mexican Lime Trees in Saline Clay Soils" Agronomy 13, no. 7: 1680. https://doi.org/10.3390/agronomy13071680
APA StyleEnnab, H. A., Mohamed, A. H., El-Hoseiny, H. M., Omar, A. A., Hassan, I. F., Gaballah, M. S., Khalil, S. E., Mira, A. M., Abd El-Khalek, A. F., & Alam-Eldein, S. M. (2023). Humic Acid Improves the Resilience to Salinity Stress of Drip-Irrigated Mexican Lime Trees in Saline Clay Soils. Agronomy, 13(7), 1680. https://doi.org/10.3390/agronomy13071680











