Research Progress in Crop Root Biology and Nitrogen Uptake and Use, with Emphasis on Cereal Crops
Abstract
1. Introduction
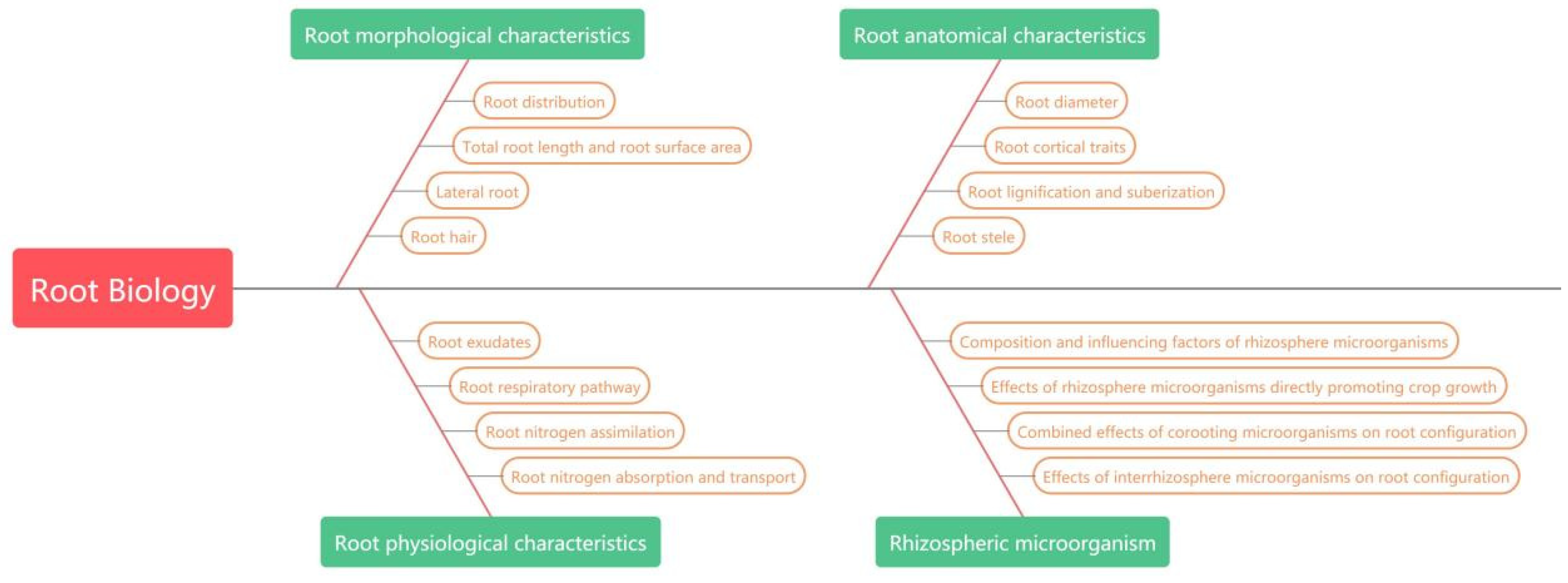
2. Root Morphology and Nitrogen Absorption and Utilization
2.1. Root Distribution
2.2. Root Length and Root Surface Area
2.3. Lateral Root and Root Hair
3. Root Anatomy and Nitrogen Uptake and Utilization
3.1. Root Diameter
3.2. Root Cortex
3.3. Lignification and Embolization of Roots
3.4. Root Column
4. Physiological and Biochemical Characteristics of the Root System
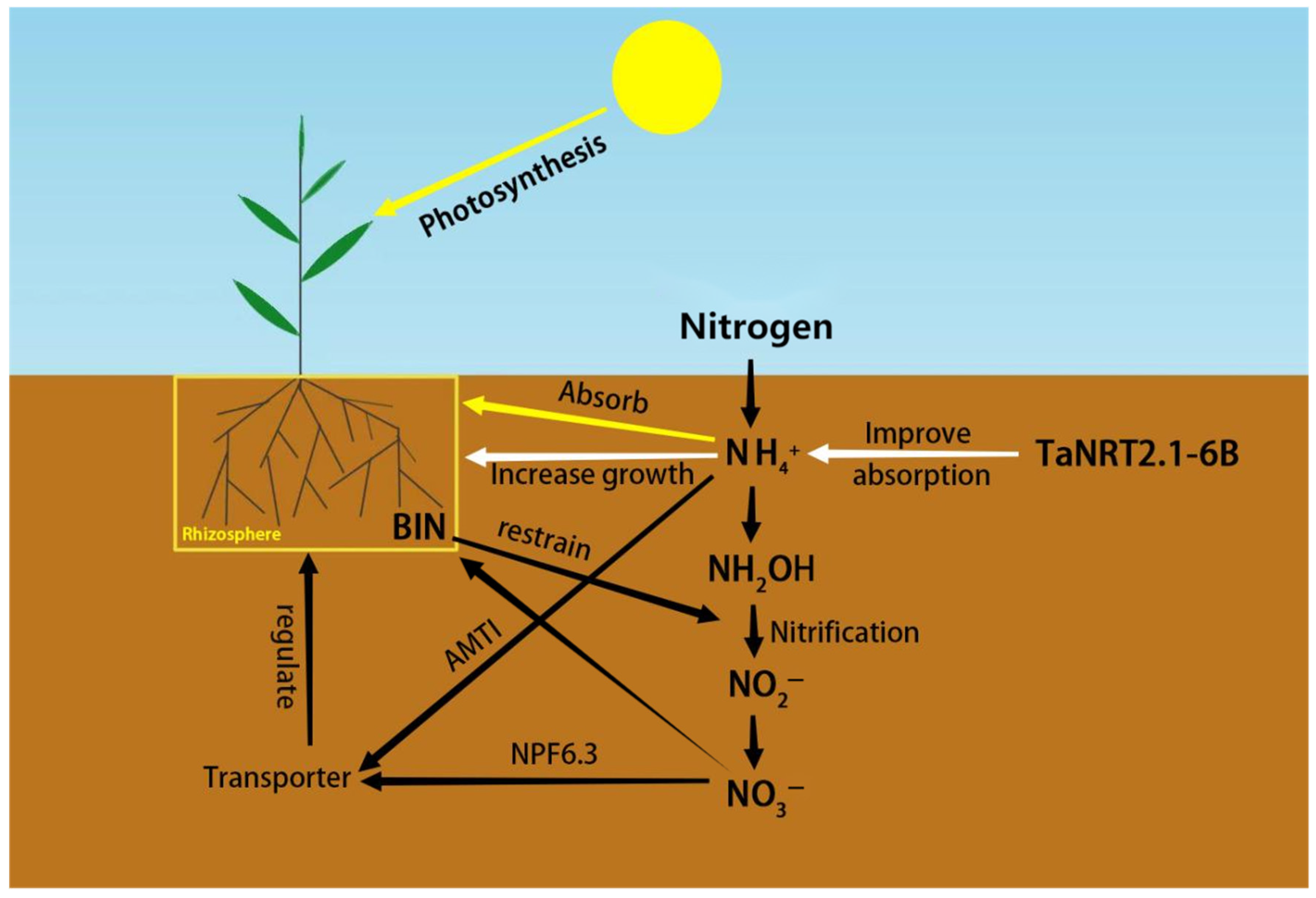
4.1. Nitrogen Uptake and Transport in Roots
4.2. Root Nitrogen Assimilation
4.3. Root Respiratory Metabolic Pathway
4.4. Root Secretion
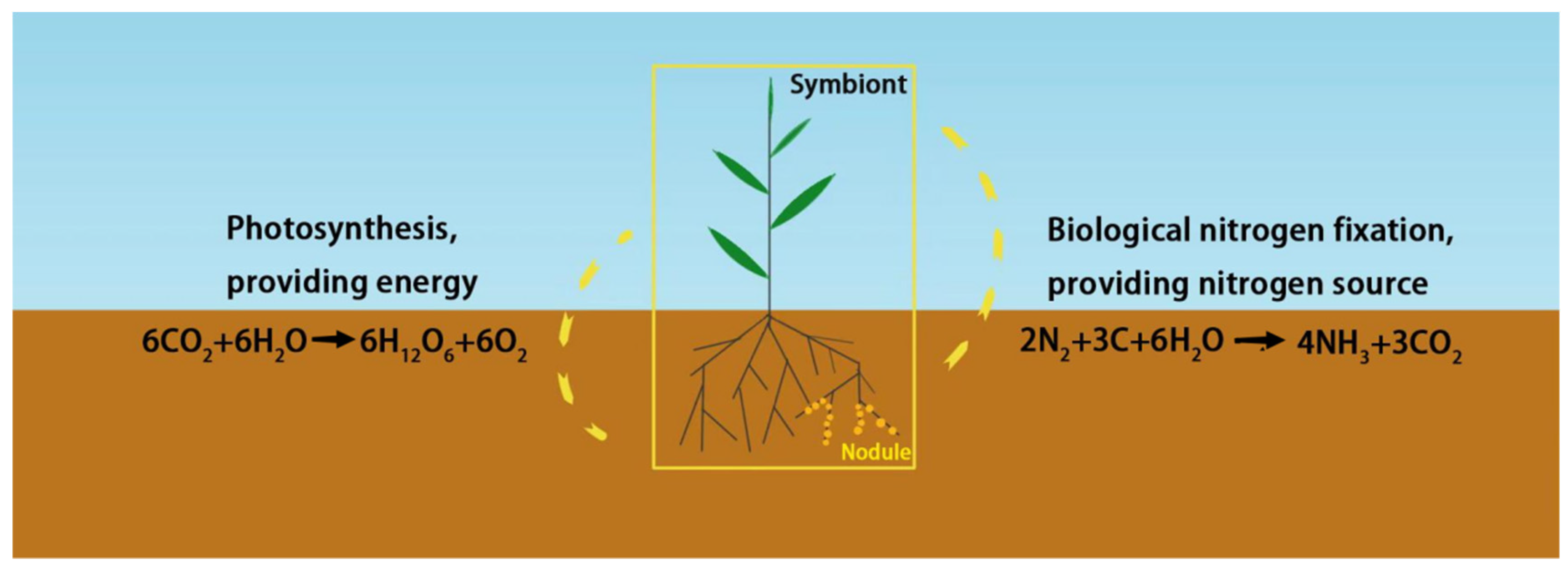
5. Rhizosphere Microorganisms and Nitrogen Uptake and Utilization
5.1. Effects of Interrhizosphere Microorganisms on Root Configuration
5.2. Combined Effects of Corooting Microorganisms on Root Configuration
5.3. Effects of Rhizosphere Microorganisms Directly Promoting Crop Growth
5.4. Composition and Influencing Factors of Rhizosphere Microorganisms
6. Summary and Prospects
- (1)
- The ideal root architecture of rice should be constructed. The ideal root configuration guarantees the growth of a healthy aboveground plant and is an important characteristic for breeding high-yield and high-quality rice varieties. However, due to the lag in root research and the diversity of paddy fields in different regions, the definition of ideal plant is very different. The construction of an ideal root configuration via breeding and cultivation regulations is an important component of rice root research in the future.
- (2)
- The functions and growth-promoting mechanisms of root microorganisms at the community level should be studied. Although the research on plant growth-promoting bacteria dates back to 300 BC and biobiotics have been widely used in the form of biofertilizers, their growth-promoting effects on plants vary, depending on crop varieties or soil environments. This variance results from a failure to fully understand the dynamic construction of the microbial community and its function at the community level. Future microbial comparative genomics studies may help us systematically understand the functional composition of root microorganisms on an unprecedented scale and elucidate the effects of microbe–microbial interactions on the construction and function of root microbial communities.
- (3)
- Some microorganisms have been applied to production practice; however, most beneficial microorganisms are still in the research stage, mainly due to the complex and variable environment. It is reported that the inoculation of Brazilian nitrogen fixing spirillum Ab-V5 under greenhouse conditions significantly increased the dry weight, volume, and biomass of maize and wheat roots; however, under field conditions, the strains had no significant impact on plant growth [136]. Therefore, when preparing microbial agents, the experiments on natural environments should be increased to improve the stability of microbial inoculants in different environments. PGPR, AMF, and rhizobia can play a synergistic role in promoting growth, and the limitations of single strains or single types of bacteria can be broken during microbial agent preparation to realize the application of a comprehensive microbiome system [137]. In conclusion, the regulation of rhizosphere microorganisms on plant root configuration has broad application prospects in production practice, and the multiomics association research method can fully elucidate the mechanism of the role of microorganisms and provide research direction for the selection of strains.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S. Recent Advances on Nitrogen Use Efficiency in Rice. Agronomy 2021, 4, 753. [Google Scholar] [CrossRef]
- Ryan, P.R.; Delhaize, E.; Watt, M.; Richardson, A.E. Plant roots: Understanding structure and function in an ocean of complexity. Ann. Bot. 2016, 118, 555–559. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R.; Lobet, G.; Dinneny, J.R. Environmental Control of Root System Biology. Annu. Rev. Plant Biol. 2016, 67, 619–642. [Google Scholar] [CrossRef]
- Shi, X.; Hu, K.; Batchelor, W.D.; Liang, H.; Wu, Y.; Wang, Q.; Fu, J.; Cui, X.; Zhou, F. Exploring optimal nitrogen management strategies to mitigate nitrogen losses from paddy soil in the middle reaches of the Yangtze River. Agric. Water Manag. 2019, 228, 105877. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between Plant Roots, Rhizosphere Microorganisms, and Nitrogen and Its Special Focus on Rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Liu, L.; Song, N.; Qi, X.; Cui, K. Research Advances on the Relationship between Root Characteristics and Nitrogen Uptake and Utilization Eficiency in Rice. Crops 2022, 1, 11–19. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, C.; Kong, X.; Hou, D.; Gu, J.; Liu, L.; Wang, Z.; Yang, J. Progressive integrative crop managements increase grain yield, nitrogen use efficiency and irrigation water productivity in rice. Field Crops Res. 2018, 215, 1–11. [Google Scholar] [CrossRef]
- Ranjan, R.; Yadav, R. Targeting nitrogen use efficiency for sustained production of cereal crops. J. Plant Nutr. 2019, 9, 1086–1113. [Google Scholar] [CrossRef]
- Li, Z.; Henawy, A.R.; Halema, A.A.; Fan, Q.; Duanmu, D.; Huang, R. A Wild Rice Rhizobacterium Burkholderia cepacia BRDJ Enhances Nitrogen Use Efficiency in Rice. Int. J. Mol. Sci. 2022, 18, 10769. [Google Scholar] [CrossRef]
- Myrold, D.D. Transformations of nitrogen. In Principles and Applications of Soil Microbiology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 385–421. [Google Scholar] [CrossRef]
- Smith, J.L. Cycling of nitrogen through microbial activity. In Soil Biology: Effects on Soil Quality; CRC Press: Boca Raton, FL, USA, 2018; pp. 91–120. [Google Scholar] [CrossRef]
- Nestler, J.; Wissuwa, M. Superior Root Hair Formation Confers Root Efficiency in Some, But Not All, Rice Genotypes upon P Deficiency. Front. Plant Sci. 2016, 7, 1935. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Lin, F.; Li, Y.; Sun, X.; Wang, Z.; Yan, J.; Yuan, T. Com bined ApplicationofHum icAcid andNitrogen FertilizerAfects SoilPhysicaland Chem icalProperties. Chin. Agric. Sci. Bull. 2018, 34, 80–85. [Google Scholar]
- Ding, L.-J.; Cui, H.-L.; Nie, S.-A.; Long, X.-E.; Duan, G.-L.; Zhu, Y.-G. Microbiomes inhabiting rice roots and rhizosphere. FEMS Microbiol. Ecol. 2019, 95, fiz40. [Google Scholar] [CrossRef]
- Chen, D.; Saeed, M.; Ali, M.N.H.A.; Raheel, M.; Ashraf, W.; Hassan, Z.; Hassan, M.Z.; Farooq, U.; Hakim, M.F.; Rao, M.J.; et al. Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal Fungi Combined Application Reveals Enhanced Soil Fertility and Rice Production. Agronomy 2023, 13, 550. [Google Scholar] [CrossRef]
- Zhiyong, Z.; Baomin, F.; Chao, S.; Xiaoxian, Z.; Qingwen, Z.; Bing, Y. Advances in Root System Architecture: Functionality, Plasticity, and Research Methods. J. Resour. Ecol. 2022, 14, 15–24. [Google Scholar] [CrossRef]
- Ji, C.; Li, J.; Jiang, C.; Zhang, L.; Shi, L.; Xu, F.; Cai, H. Zinc and nitrogen synergistic act on root-to-shoot translocation and preferential distribution in rice. J. Adv. Res. 2022, 35, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Kitomi, Y.; Hanzawa, E.; Kuya, N.; Inoue, H.; Hara, N.; Kawai, S.; Kanno, N.; Endo, M.; Sugimoto, K.; Yamazaki, T. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc. Natl. Acad. Sci. USA 2020, 35, 21242–21250. [Google Scholar] [CrossRef]
- Chen, C.; Gong, H.Q.; Zhang, J.Z.; Gao, H.J. Correlation between root morphology and nitrogen uptake of rice. J. Plant Nutr. Fertil. 2017, 23, 333–341. [Google Scholar] [CrossRef]
- Chen, M.; Chen, G.; Di, D.; Kronzucker, H.J.; Shi, W. Higher nitrogen use efficiency (NUE) in hybrid “super rice” links to improved morphological and physiological traits in seedling roots. J. Plant Physiol. 2020, 251, 153191. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, X.; Xie, J.; Deng, G.; Tu, T.; Guan, X.; Yang, Z.; Huang, S.; Chen, X.; Qiu, C.; et al. Reducing nitrogen application with dense planting increases nitrogen use efficiency by maintaining root growth in a double-rice cropping system. Crops J. 2021, 9, 805–815. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, P.; Mo, Z.; Kong, L.; Tian, H.; Duan, M.; Li, L.; Wu, L.; Wang, Z.; Tang, X.; et al. Deep Placement of Nitrogen Fertilizer Affects Grain Yield, Nitrogen Recovery Efficiency, and Root Characteristics in Direct-Seeded Rice in South China. J. Plant Growth Regul. 2020, 40, 379–387. [Google Scholar] [CrossRef]
- Saini, D.K.; Chopra, Y.; Pal, N.; Chahal, A.; Srivastava, P.; Gupta, P.K. Meta-QTLs, ortho-MQTLs and candidate genes for nitrogen use efficiency and root system architecture in bread wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2021, 27, 2245–2267. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.U.; Bian, S.; Liu, Z.; Wang, L.; Wang, Y.; Xu, W.; Yu, Z. Drip irrigation incorporating water conservation measures: Effects on soil water–nitrogen utilization, root traits and grain production of spring maize in semi-arid areas. J. Integr. Agric. 2021, 12, 3127–3142. [Google Scholar]
- Zhang, X.; Yang, Y.; Fu, Q.; Hu, H.; Zhu, J.; Liu, M. Comparing effects of ammonium and nitrate nitrogen on arsenic accumulation in brown rice and its dynamics in soil-plant system. J. Soils Sediments 2021, 21, 2650–2658. [Google Scholar] [CrossRef]
- Liu, B.; Wu, J.; Yang, S.; Schiefelbein, J.; Gan, Y. Nitrate regulation of lateral root and root hair development in plants. J. Exp. Bot. 2020, 15, 4405–4414. [Google Scholar] [CrossRef]
- Carminati, A.; Passioura, J.B.; Zarebanadkouki, M.; Ahmed, M.A.; Ryan, P.R.; Watt, M.; Delhaize, E. Root hairs enable high transpiration rates in drying soils. New Phytol. 2017, 3, 771–781. [Google Scholar] [CrossRef]
- Bowles, T.M.; Atallah, S.S.; Campbell, E.E.; Gaudin, A.C.M.; Wieder, W.R.; Grandy, A.S. Addressing agricultural nitrogen losses in a changing climate. Nat. Sustain. 2018, 8, 399–408. [Google Scholar] [CrossRef]
- Zhang, C.; Simpson, R.J.; Kim, C.M.; Warthmann, N.; Delhaize, E.; Dolan, L.; Byrne, M.E.; Wu, Y.; Ryan, P.R. Do longer root hairs improve phosphorus uptake? Testing the hypothesis with transgenic Brachypodium distachyon lines overexpressing endogenous RSL genes. New Phytol. 2018, 4, 1654–1666. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.-Q.; Chang, Y.-P.; Zhang, B.; Zhao, Q.-Z.; Zhao, W.-L. The basic helix-loop-helix transcription factor OsBLR1 regulates leaf angle in rice via brassinosteroid signalling. Plant Mol. Biol. 2020, 102, 589–602. [Google Scholar] [CrossRef]
- Lou, Q.; Chen, L.; Mei, H.; Xu, K.; Wei, H.; Feng, F.; Li, T.; Pang, X.; Shi, C.; Luo, L.; et al. Root Transcriptomic Analysis Revealing the Importance of Energy Metabolism to the Development of Deep Roots in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1314. [Google Scholar] [CrossRef] [PubMed]
- Streit, J.; Meinen, C.; Rauber, R. Intercropping effects on root distribution of eight novel winter faba bean genotypes mixed with winter wheat. Field Crops Res. 2019, 235, 1–10. [Google Scholar] [CrossRef]
- Sharma, N.; Sinha, V.B.; Gupta, N.; Rajpal, S.; Kuchi, S.; Sitaramam, V.; Parsad, R.; Raghuram, N. Phenotyping for Nitrogen Use Efficiency: Rice Genotypes Differ in N-Responsive Germination, Oxygen Consumption, Seed Urease Activities, Root Growth, Crop Duration, and Yield at Low N. Front. Plant Sci. 2018, 9, 1452. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.J.; Piao, Z.Z.; Zeng, W.; Li, G.X.; Yang, R.F. Effects of different lateral root densities on growth, development and main agronomic characters of rice. Mol. Plant Breed. 2019, 17, 1624–1630. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Siddique, K.H.M.; Li, F.-M. Trade-Off between Root Efficiency and Root Size Is Associated with Yield Performance of Soybean under Different Water and Phosphorus Levels. Agriculture 2021, 11, 481. [Google Scholar] [CrossRef]
- Ruiz, S.; Koebernick, N.; Duncan, S.; Fletcher, D.M.; Scotson, C.; Boghi, A.; Marin, M.; Bengough, A.G.; George, T.S.; Brown, L.K. Significance of root hairs at the field scale–modelling root water and phosphorus uptake under different field conditions. Plant Soil. 2020, 447, 281–304. [Google Scholar] [CrossRef]
- Ueda, Y.; Sakuraba, Y.; Yanagisawa, S. Environmental control of phosphorus acquisition: A piece of the molecular framework underlying nutritional homeostasis. Plant Cell Physiol. 2021, 4, 573–581. [Google Scholar] [CrossRef]
- Klinsawang, S.; Sumranwanich, T.; Wannaro, A.; Saengwilai, P. Effects of root hair length on potassium acquisition in rice (Oryza sativa L.). Appl. Ecol. Environ. Res. 2018, 16, 1609–1620. [Google Scholar] [CrossRef]
- Ding, S.; Liu, C.; Qian, Q. Research progress on genetic of rice root. China Rice 2019, 5, 24–29. [Google Scholar] [CrossRef]
- Abiko, T.; Miyasaka, S.C. Aerenchyma and barrier to radial oxygen loss are formed in roots of Taro (Colocasia esculenta) propagules under flooded conditions. J. Plant Res. 2020, 133, 49–56. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, Y.; Xiang, J.; Zhang, Y.; Zhu, D.; Chen, H. The nitrogen topdressing mode of indica-japonica and indica hybrid rice are different after side-deep fertilization with machine transplanting. Sci. Rep. 2021, 11, 1494. [Google Scholar] [CrossRef]
- Krouk, G.; Kiba, T. Nitrogen and Phosphorus interactions in plants: From agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Ejiri, M.; Sawazaki, Y.; Shiono, K. Some Accessions of Amazonian Wild Rice (Oryza glumaepatula) Constitutively Form a Barrier to Radial Oxygen Loss along Adventitious Roots under Aerated Conditions. Plants 2020, 9, 880. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.C.; Tam, N.F.Y.; Ye, Z. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J. Environ. Sci. 2019, 75, 296–306. [Google Scholar] [CrossRef]
- Melino, V.J.; Plett, D.C.; Bendre, P.; Thomsen, H.C.; Zeisler-Diehl, V.V.; Schreiber, L.; Kronzucker, H.J. Nitrogen depletion enhances endodermal suberization without restricting transporter-mediated root NO3− influx. J. Plant Physiol. 2021, 257, 153334. [Google Scholar] [CrossRef] [PubMed]
- Ranathunge, K.; Schreiber, L.; Bi, Y.-M.; Rothstein, S.J. Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 2016, 243, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Ma, X.; Zarebanadkouki, M.; Kuzyakov, Y.; Blagodatskaya, E.; Pausch, J.; Razavi, B.S. Spatial patterns of enzyme activities in the rhizosphere: Effects of root hairs and root radius. Soil Biol. Biochem. 2018, 118, 69–78. [Google Scholar] [CrossRef]
- Bowsher, A.W.; Mason, C.M.; Goolsby, E.W.; Donovan, L.A. Data from: Fine root tradeoffs between nitrogen concentration and xylem vessel traits preclude unified whole-plant resource strategies in Helianthus. Ecol. Evol. 2016, 4, 1016–1031. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, J.; Zeng, H.; Liu, M.; Miao, Y.; Wu, H.; Kardol, P. The nutrient absorption–transportation hypothesis: Optimizing structural traits in absorptive roots. New Phytol. 2017, 4, 1569–1572. [Google Scholar] [CrossRef]
- Galindo Castañeda, T.; Brown, K.M.; Lynch, J.P. Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell Environ. 2018, 7, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumari, S.; Kumar, A.; Shahid, R.; Kumar, S.; Kumar, G. Nitro-oxidative stress induces the formation of roots’ cortical aerenchyma in rice under osmotic stress. Physiol Plant. 2021, 2, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Rightsizing root phenotypes for drought resistance. J. Exp. Bot. 2018, 13, 3279–3292. [Google Scholar] [CrossRef] [PubMed]
- Onyango, D.A.; Entila, F.; Egdane, J.; Pacleb, M.; Katimbang, M.L.; Dida, M.M.; Ismail, A.M.; Drame, K.N. Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 2. Root oxidation ability and oxidative stress control. Funct. Plant Biol. 2020, 2, 145–155. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, J.; Li, W.; Gao, W.; Shen, R.; Meng, L. Effects of grafting on root growth, anaerobic respiration enzyme activity and aerenchyma of bitter melon under waterlogging stress. Sci. Hortic. 2020, 261, 108977. [Google Scholar] [CrossRef]
- Mohammed, U.; Caine, R.S.; Atkinson, J.A.; Harrison, E.L.; Wells, D.; Chater, C.C.; Gray, J.E.; Swarup, R.; Murchie, E.H. Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Sci. Rep. 2019, 1, 5584. [Google Scholar] [CrossRef]
- Jia, X.; Wu, G.; Strock, C.; Li, L.; Dong, S.; Zhang, J.; Zhao, B.; Lynch, J.P.; Liu, P. Correction to: Root anatomical phenotypes related to growth under low nitrogen availability in maize (Zea mays L.) hybrids. Plant Soil 2022, 477, 843–844. [Google Scholar] [CrossRef]
- Singhal, V.P.; Mehar, S.K. Effect of limited nutrient availability on the development and relevance of root cortical aerenchyma. Plant Arch. 2020, 20, 1284–1288. [Google Scholar]
- Rajhi, I.; Mhadhbi, H. Mechanisms of aerenchyma formation in maize roots. Afr. J. Agr. Res. 2019, 14, 680–685. [Google Scholar] [CrossRef]
- Schneider, H.M.; Yang, J.T.; Brown, K.M.; Lynch, J.P. Nodal root diameter and node number in maize (Zea mays L.) interact to influence plant growth under nitrogen stress. Plant Direct 2021, 3, e310. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Zhou, B.; Wang, X.; Wang, J.; Zhao, M.; Li, C. Characterization of Root Morphology and Anatomical Structure of Spring Maize under Varying N Application Rates and Their Effects on Yield. Agronomy 2022, 12, 2671. [Google Scholar] [CrossRef]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of Roots to Nitrogen Deficiency Revealed by 3D Quantification and Proteomic Analysis. Plant Physiol. 2018, 179, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, M.; Ding, L.; Chen, Y.; Lu, Z.; Hu, J.; Guo, S. High water uptake ability was associated with root aerenchyma formation in rice: Evidence from local ammonium supply under osmotic stress conditions. Plant Physiol. Biochem. 2020, 150, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H.; Wortmann, C.S. Does occasional tillage undo the ecosystem services gained with no-till? A review. Soil Tillage Res. 2020, 198, 104534. [Google Scholar] [CrossRef]
- Lynch, J.P. Harnessing root architecture to address global challenges. Plant J. 2021, 109, 415–431. [Google Scholar] [CrossRef]
- Chai, X.; Wang, X.; Pi, Y.; Wu, T.; Zhang, X.; Xu, X.; Han, Z.; Wang, Y. Nitrate transporter MdNRT2.4 interacts with rhizosphere bacteria to enhance nitrate uptake in apple rootstocks. J. Exp. Bot. 2022, 73, 6490–6504. [Google Scholar] [CrossRef]
- Tang, W.; Ye, J.; Yao, X.; Zhao, P.; Xuan, W.; Tian, Y.; Zhang, Y.; Xu, S.; An, H.; Chen, G. Genome-wide associated study identifies NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice. Nat. Commun. 2019, 1, 5279. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, J.; Han, Y.; Hua, Y.; Guan, C.; Zhang, Z. Low Nitrogen Enhances Nitrogen Use Efficiency by Triggering NO3− Uptake and Its Long-Distance Translocation. J. Agric. Food Chem. 2019, 67, 6736–6747. [Google Scholar] [CrossRef]
- Li, M.; Wang, T.; Zhang, H.; Liu, S.; Li, W.; Elwafa, S.F.A.; Tian, H. TaNRT2.1-6B is a dual-affinity nitrate transporter contributing to nitrogen uptake in bread wheat under both nitrogen deficiency and sufficiency. Crops J. 2022, 10, 993–1005. [Google Scholar] [CrossRef]
- Zhang, Z.H. The relationship between nitrate transport and utilization in crop and nitrogen utilization efficiency. J. Plant Nutr. 2017, 23, 217–223. [Google Scholar] [CrossRef]
- Yao, X.; Nie, J.; Bai, R.; Sui, X. Amino Acid Transporters in Plants: Identification and Function. Plants 2020, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, K.; Nie, H.; Zeng, Q.; Wu, B.; Qian, J.; Fang, Z. Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield. Rice 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tang, S.; Zhang, S.; Tian, Y.; Qu, H.; Gu, M.; Xu, G. Rice circadian clock regulator Nhd1 controls the expression of the sucrose transporter gene OsSUT1 and impacts carbon–nitrogen balance. J. Exp. Bot. 2023, 5, 1460–1474. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.-X.; Zhang, N.; Hu, B.; Jin, T.; Xu, H.; Qin, Y.; Yan, P.; Zhang, X.; Guo, X.; et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 2019, 37, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Xu, G. Genomic Basis of Rice Adaptation to Soil Nitrogen Status. Chin. Bull. Bot. 2021, 56, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Jiang, Z.; Wang, W.; Xu, R.; Wang, Q.; Zhang, Z.; Li, A.; Liang, Y.; Ou, S. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 2021, 7847, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Asif, I.; Dong, Q.; Wang, Z.; Wang, X.; Gui, H.; Zhang, H.; Pang, N.; Zhang, X.; Song, M. Growth and nitrogen metabolism are associated with nitrogen-use efficiency in cotton genotypes. Plant Physiol. Biochem. 2020, 149, 61–74. [Google Scholar] [CrossRef]
- Guo, H.; Tian, Z.; Sun, S.; Li, Y.; Jiang, D.; Cao, W.; Dai, T. Preanthesis Root Growth and Nitrogen Uptake Improved Wheat Grain Yield and Nitrogen Use Efficiency. Agron. J. 2019, 6, 3048–3056. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Tabuchi-Kobayashi, M.; Funayama, K.; Kojima, S.; Maruyama, K.; Yamamoto, Y.Y.; Nishizawa, T.; Kobayashi, M.; Wakazaki, M.; et al. Cytosolic GLUTAMINE SYNTHETASE1;1 Modulates Metabolism and Chloroplast Development in Roots. Plant Physiol. 2020, 4, 1894–1909. [Google Scholar] [CrossRef]
- Fujita, T.; Beier, M.P.; Tabuchi-Kobayashi, M.; Hayatsu, Y.; Nakamura, H.; Umetsu-Ohashi, T.; Sasaki, K.; Ishiyama, K.; Murozuka, E.; Kojima, M.; et al. Cytosolic Glutamine Synthetase GS1;3 Is Involved in Rice Grain Ripening and Germination. Front. Plant Sci. 2022, 13, 835835. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yuan, Z.; Fu, L.; Zhu, M.; Luo, X.; Xu, W.; Yuan, H.; Zhu, R.; Hu, Z.; Wu, X. Integrative Transcriptomic and Proteomic Analysis Reveals an Alternative Molecular Network of Glutamine Synthetase 2 Corresponding to Nitrogen Deficiency in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2021, 14, 7674. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Marmagne, A.; Park, J.; Fabien, C.; Yim, Y.; Kim, S.; Kim, T.; Lim, P.O.; Masclaux-Daubresse, C.; Gil Nam, H. Concurrent activation of OsAMT1;2 and OsGOGAT1 in rice leads to enhanced nitrogen use efficiency under nitrogen limitation. Plant J. 2020, 103, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar] [CrossRef]
- Dissanayaka, D.; Ghahremani, M.; Siebers, M.; Wasaki, J.; Plaxton, W.C. Recent insights into the metabolic adaptations of phosphorus-deprived plants. J. Exp. Bot. 2021, 2, 199–223. [Google Scholar] [CrossRef]
- Li, S.; Yang, W.; Guo, J.; Li, X.; Lin, J.; Zhu, X. Changes in photosynthesis and respiratory metabolism of maize seedlings growing under low temperature stress may be regulated by arbuscular mycorrhizal fungi. Plant Physiol. Biochem. 2020, 154, 1–10. [Google Scholar] [CrossRef]
- Ma, J.; Rukh, G.; Ruan, Z.; Xie, X.; Ye, Z.; Liu, D. Effects of hypoxia stress on growth, root respiration, and metabolism of Phyllostachys praecox. Life 2022, 6, 808. [Google Scholar] [CrossRef]
- Wang, M.; He, D.; Shen, F.; Huang, J.; Zhang, R.; Liu, W.; Zhu, M.; Zhou, L.; Wang, L.; Zhou, Q. Effects of soil compaction on plant growth, nutrient absorption, and root respiration in soybean seedlings. Environ. Sci. Pollut. Res. Int. 2019, 26, 22835–22845. [Google Scholar] [CrossRef]
- Shi, P.; Qin, Y.; Liu, Q.; Zhu, T.; Li, Z.; Li, P.; Ren, Z.; Liu, Y.; Wang, F. Soil respiration and response of carbon source changes to vegetation restoration in the Loess Plateau, China. Sci. Total. Environ. 2020, 707, 135507. [Google Scholar] [CrossRef]
- Xu, G.; Lu, D.; Wang, H.; Jia, F.; Chen, M. Coupling effect of alternate wetting and drying irrigation and nitrogen rate on organic acid in rice root secretion at heading stage. Zhongguo Shengtai Nongye Xuebao/Chin. J. Eco-Agric. 2018, 4, 516–525. [Google Scholar]
- Li, S.; Chen, Y.; Yu, F.; Zhang, Y.; Liu, K.; Zhuo, X.; Qiu, Y.; Zhang, H.; Gu, J.; Wang, W.; et al. Reducing methane emission by promoting its oxidation in rhizosphere through nitrogen-induced root growth in paddy fields. Plant Soil. 2022, 474, 541–560. [Google Scholar] [CrossRef]
- Xuan, W.; Beeckman, T.; Xu, G. Plant nitrogen nutrition: Sensing and signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yang, J. Nitrogen (N) transformation in paddy rice field: Its effect on N uptake and relation to improved N management. Crop Environ. 2022, 1, 7–14. [Google Scholar] [CrossRef]
- Kelly, C.; Haddix, M.L.; Byrne, P.F.; Cotrufo, M.F.; Schipanski, M.E.; Kallenbach, C.M.; Wallenstein, M.D.; Fonte, S.J. Long-term compost amendment modulates wheat genotype differences in belowground carbon allocation, microbial rhizosphere recruitment and nitrogen acquisition. Soil Biol. Biochem. 2022, 172, 108768. [Google Scholar] [CrossRef]
- Ma, B.; Ma, T.; Xian, W.; Hu, B.; Chu, C. Interplay between ethylene and nitrogen nutrition: How ethylene orchestrates nitrogen responses in plants. J. Integr. Plant Biol. 2023, 65, 399–407. [Google Scholar] [CrossRef]
- Lei, L.; Jingju, X.U. Research Progress on Relationship Between Rice Rhizosphere Environment and Nitrogen Utilization. China Rice. 2021, 5, 33. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Hestrin, R.; Hammer, E.C.; Mueller, C.W.; Lehmann, J. Synergies between mycorrhizal fungi and soil microbial communities increase plant nitrogen acquisition. Commun. Biol. 2019, 2, 233. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Yao, H.; Wang, J.; Dai, F.; Wu, Y.; Chapman, S. Nitrification and urease inhibitors improve rice nitrogen uptake and prevent denitrification in alkaline paddy soil. Appl. Soil Ecol. 2020, 154, 103665. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.N.; Wozniak, K.J.; Porter, S.S.; Friesen, M.L. Rhizobia protect their legume hosts against soil-borne microbial antagonists in a host-genotype-dependent manner. Rhizosphere 2019, 9, 47–55. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhao, Q.; Liao, H. Genetic improvement of legume roots for adaption to acid soils. Crops J. 2023, in press. [Google Scholar] [CrossRef]
- Junta, M.K.; Gupta, A.K.; Mahajan, R. Biological control of hairy root (Rhizobium rhizogenes) in apple nurseries through Rhizobium radiobacter antagonists (strain K-84 and native strain UHFBA-218). Biol. Control 2021, 164, 104762. [Google Scholar] [CrossRef]
- de Souza Campos, P.M.; Borie, F.; Cornejo, P.; Meier, S.; López-Ráez, J.A.; López-Garcia, Á.; Seguel, A. Wheat root trait plasticity, nutrient acquisition and growth responses are dependent on specific arbuscular mycorrhizal fungus and plant genotype interactions. J. Plant Physiol. 2021, 256, 153297. [Google Scholar] [CrossRef]
- Bhantana, P.; Rana, M.S.; Sun, X.-C.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A.; et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Hui, J.; An, X.; Li, Z.; Neuhäuser, B.; Ludewig, U.; Wu, X.; Schulze, W.X.; Chen, F.; Feng, G.; Lambers, H. The mycorrhiza-specific ammonium transporter ZmAMT3; 1 mediates mycorrhiza-dependent nitrogen uptake in maize roots. Plant Cell 2022, 10, 4066–4087. [Google Scholar] [CrossRef]
- Faltine-Gonzalez, D.Z.; Kebschull, J.M. A mosaic of new and old cell types. Science 2022, 377, 1043–1044. [Google Scholar] [CrossRef]
- Chiu, C.H.; Choi, J.; Paszkowski, U. Independent signalling cues underpin arbuscular mycorrhizal symbiosis and large lateral root induction in rice. New Phytol. 2018, 217, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, C.; Li, Y.; Wu, Y.; Fransson, P.; Rillig, M.C.; Zhai, S.; Xie, J.; Tong, Z.; Zhang, Q.; et al. Temporal complementarity between roots and mycorrhizal fungi drives wheat nitrogen use efficiency. New Phytol. 2022, 236, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ramirez, L.F.; Uribe-Velez, D. Phosphorus Solubilizing and Mineralizing Bacillus spp. Contribute to Rice Growth Promotion Using Soil Amended with Rice Straw. Curr. Microbiol. 2021, 78, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Adl, S.M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Environmental and Microbial Biotechnology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 147–203. [Google Scholar] [CrossRef]
- Chu, T.N.; Van Bui, L.; Hoang, M.T.T. Pseudomonas PS01 Isolated from Maize Rhizosphere Alters Root System Architecture and Promotes Plant Growth. Microorganisms 2020, 8, 471. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernández, J.; Pelagio-Flores, R.; López-Bucio, J.; García-Juárez, P.; Macías-Rodríguez, L. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 2017, 254, 2201–2213. [Google Scholar] [CrossRef]
- Kallala, N.; Sehli, W.M.; Jelali, K.; Kais, Z.; Mhadhbi, H. Inoculation with efficient nitrogen fixing and indoleacetic acid producing bacterial microsymbiont enhance tolerance of the model legume Medicago truncatula to iron deficiency. Biomed Res. Int. 2018, 2018, 9134716. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Wu, Y.; Zhu, M.; Yu, W.; Yao, H.; Zhu, Y.; Chu, H. Suppressed N fixation and diazotrophs after four decades of fertilization. Microbiome 2019, 1, 143. [Google Scholar] [CrossRef]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.; Olson, A.; Liseron-Monfils, C.; Bågman, A.; Foret, J.; Abbitt, S.; Tang, M.; Li, B. Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 2018, 7730, 259–264. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar] [CrossRef]
- Yi, X.; Yuan, J.; Zhu, Y.; Yi, X.; Zhao, Q.; Fang, K.; Cao, L. Comparison of the Abundance and Community Structure of N-Cycling Bacteria in Paddy Rhizosphere Soil under Different Rice Cultivation Patterns. Int. J. Mol. Sci. 2018, 12, 3772. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Y.; Yang, J.; Fan, F.; Zhang, W.; Li, J.; Zhou, C.; Shi, G.; Tong, F.; Fan, G. Taxonomical and functional bacterial community selection in the rhizosphere of the rice genotypes with different nitrogen use efficiencies. Plant Soil 2022, 470, 111–125. [Google Scholar] [CrossRef]
- Prasad, A.A.; Babu, S. Compatibility of Azospirillum brasilense and Pseudomonas fluorescens in growth promotion of groundnut (Arachis hypogea L.). An. Acad. Bras. Ciências 2017, 89, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; van der Heijden, M.G. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 1, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Donn, S.; Ryan, P.R.; Mathesius, U.; Devilla, R.; Jones, A.; Watt, M. Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PLoS ONE 2016, 10, e164533. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Dennis, P.G.; Forstner, C.; Raghavendra, A.K.H.; Richardson, A.E.; Watt, M.; Mathesius, U.; Gilliham, M.; Ryan, P.R. The microbiomes on the roots of wheat (Triticum aestivum L.) and rice (Oryza sativa L.) exhibit significant differences in structure between root types and along root axes. Funct. Plant Biol. 2021, 48, 871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-H.; Wang, N.; Yu, M.-K.; Yu, J.-G.; Xue, L.-H. Rhizosphere and Straw Return Interactively Shape Rhizosphere Bacterial Community Composition and Nitrogen Cycling in Paddy Soil. Front. Microbiol. 2022, 13, 945927. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, H.-F.; Li, H.-L.; An, X.-H.; Song, L.-Q.; You, C.-X.; Zhao, L.-L.; Tian, Y.; Wang, X.-F. MdMYB10 affects nitrogen uptake and reallocation by regulating the nitrate transporter MdNRT2.4-1 in red-fleshed apple. Hortic. Res. 2022, 9, uhac016. [Google Scholar] [CrossRef]
- Wei, X.; Fu, T.; He, G.; Cen, R.; Huang, C.; Yang, M.; Zhang, W.; He, T. Plant types shape soil microbial composition, diversity, function, and co-occurrence patterns in cultivated land of a karst area. Land Degrad Dev. 2023, 4, 1097–1109. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef]
- Wang, J.; Liao, L.; Ye, Z.; Liu, H.; Zhang, C.; Zhang, L.; Liu, G.; Wang, G. Different bacterial co-occurrence patterns and community assembly between rhizosphere and bulk soils under N addition in the plant-soil system. Plant Soil 2022, 471, 697–713. [Google Scholar] [CrossRef]
- Li, Y.; Pan, F.; Yao, H. Response of symbiotic and asymbiotic nitrogen-fixing microorganisms to nitrogen fertilizer application. J. Soils Sediments 2019, 19, 1948–1958. [Google Scholar] [CrossRef]
- Hungria, M.; Ribeiro, R.A.; Nogueira, M.A. Draft Genome Sequences of Azospirillum brasilense Strains Ab-V5 and Ab-V6, Commercially Used in Inoculants for Grasses and Legumes in Brazil. Genome Announc. 2018, 6, e318–e393. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span J. Agric Res. 2018, 16. [Google Scholar] [CrossRef]
| Root Trait | Correlations with Nitrogen Uptake and Utilization Efficiency | Reference |
|---|---|---|
| Root distribution | The quality of roots shorter than 10 cm is positively correlated with crop yield and nitrogen absorption and utilization efficiency. | [18,19] |
| Total root length and root surface area | ① Crop root length and root surface area are generally positively correlated with nitrogen absorption and utilization efficiency. ② Varieties with high root sap flow and vigor under low-nitrogen conditions still have high nitrogen absorption and utilization efficiency under short root length and low surface area conditions. ③ The root length and root surface area are negatively correlated with nitrogen use efficiency when the roots are grown in redundancy. | [20,21,22,23,24] |
| Lateral root | Lateral root growth and nitrogen absorption interact and are positively correlated within a certain range. | [25,26,27] |
| Root hair | Root hair growth is positively correlated with nitrogen uptake efficiency. | [28,29,30] |
| Root Trait | Correlations with Nitrogen Uptake and Utilization Efficiency | Reference |
|---|---|---|
| Root diameter | Properly increase the proportion of coarse branched roots, increase the root length and root surface area to shape a good root configuration, and improve the nitrogen absorption and utilization efficiency of crops. | [41] |
| Root cortical traits | The formation of root aerenchyma enhances the nitrogen absorption and utilization efficiency of plants, improves the adaptability to stress, and is conducive to dry-matter accumulation. | [42,43,44] |
| Root lignification and suberization | Improve the resistance of crops to adverse environments, while lower root lignification and corkification levels have higher nitrogen absorption efficiency and are negatively correlated with external NH4+ levels. | [45,46,47,48] |
| Root stele | The transport capacity of root system is positively correlated with the diameter of root stele. | [49,50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Sun, C.; Cai, S.; Liu, F.; Xie, H.; Xiong, Q. Research Progress in Crop Root Biology and Nitrogen Uptake and Use, with Emphasis on Cereal Crops. Agronomy 2023, 13, 1678. https://doi.org/10.3390/agronomy13071678
Wang R, Sun C, Cai S, Liu F, Xie H, Xiong Q. Research Progress in Crop Root Biology and Nitrogen Uptake and Use, with Emphasis on Cereal Crops. Agronomy. 2023; 13(7):1678. https://doi.org/10.3390/agronomy13071678
Chicago/Turabian StyleWang, Runnan, Changhui Sun, Shuo Cai, Fangping Liu, Hengwang Xie, and Qiangqiang Xiong. 2023. "Research Progress in Crop Root Biology and Nitrogen Uptake and Use, with Emphasis on Cereal Crops" Agronomy 13, no. 7: 1678. https://doi.org/10.3390/agronomy13071678
APA StyleWang, R., Sun, C., Cai, S., Liu, F., Xie, H., & Xiong, Q. (2023). Research Progress in Crop Root Biology and Nitrogen Uptake and Use, with Emphasis on Cereal Crops. Agronomy, 13(7), 1678. https://doi.org/10.3390/agronomy13071678





