Transcriptome Revealed the Effect of Shading on the Photosynthetic Pigment and Photosynthesis of Overwintering Tea Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Shading Treatment
2.2. Determination of the Physiological Biochemistry Indexes of the Tea Plant
2.2.1. Determination of Fv/Fm
2.2.2. Determination of Chlorophyll and Carotenoid Content
2.2.3. Determination of Photosynthetic Parameters
2.2.4. Determination of Soluble Sugar (SS) Content
2.3. RNA Extraction and Library Construction
2.4. Transcriptome Analysis
2.5. Identification of Differentially Expressed Genes (DEGs)
2.6. Annotation of Gene Function
2.7. Quantitative Real-Time PCR (qRT-PCR) Verification
2.8. Statistical Analysis
3. Results
3.1. Effects of Different Shading Rates on the Chlorophyll and Carotenoid Contents of Overwintering Tea Leaves
3.2. Effects of Different Shading Rates on Photosynthesis and Sugar Accumulation of Overwintering Tea Leaves
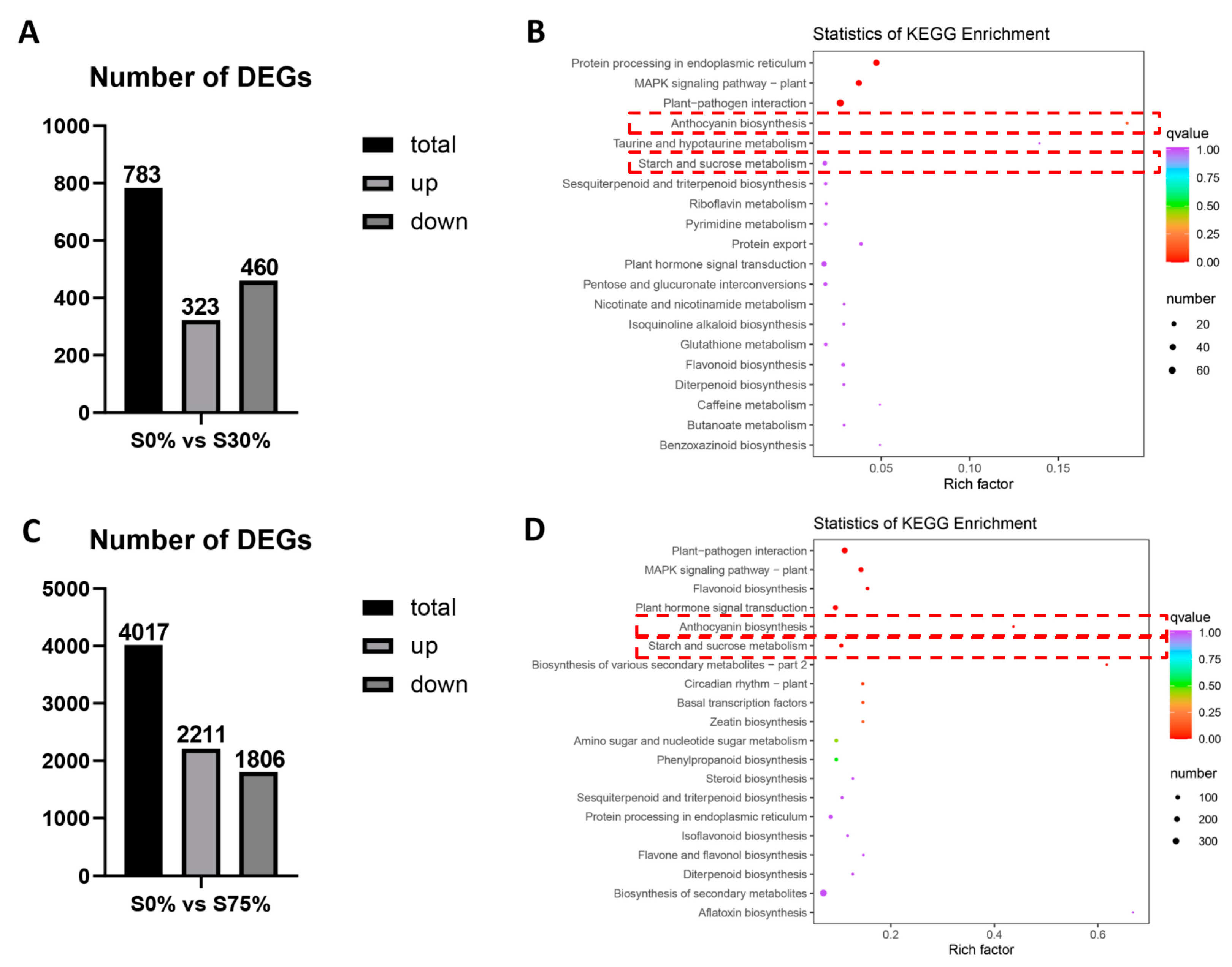
3.3. RNA-Seq Quality, DEGs Identification, and Functional Annotation
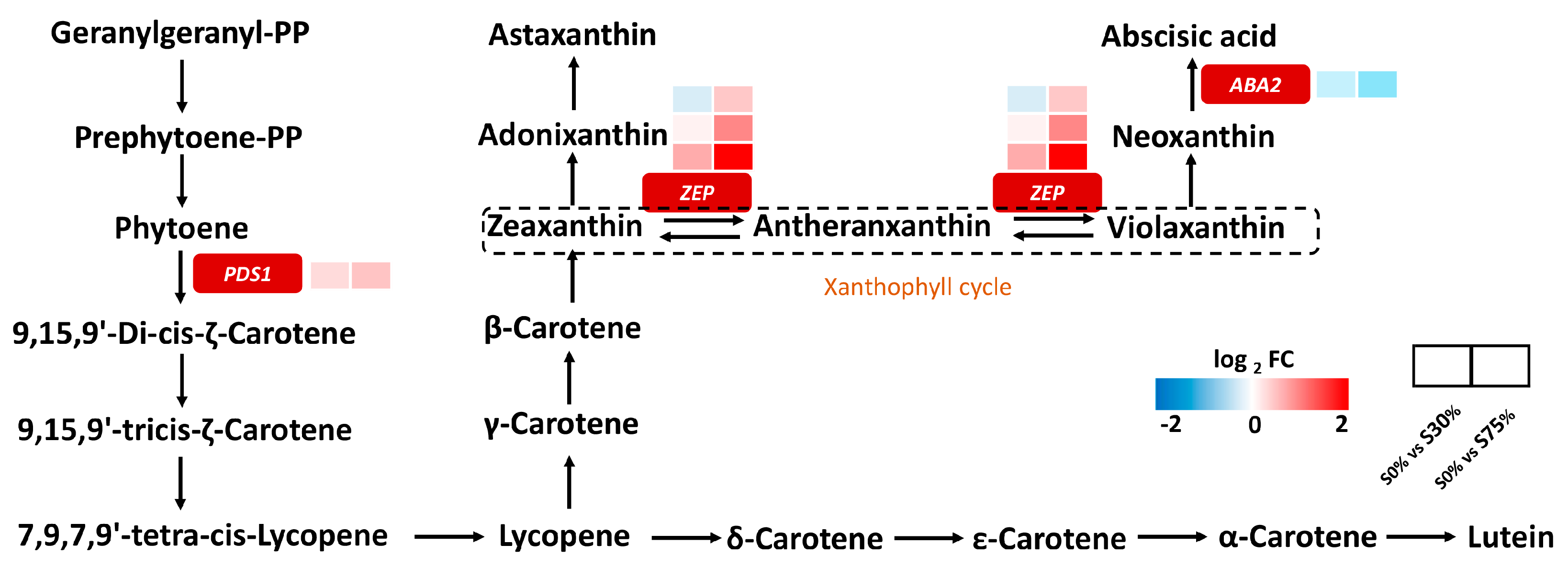
3.4. Effects of Different Shading Rates on the Gene Expression of Chlorophyll and Carotenoid Metabolism
3.5. Effects of Different Shading Rates on Photosynthesis and Related Gene Expression of Overwintering Tea Plants
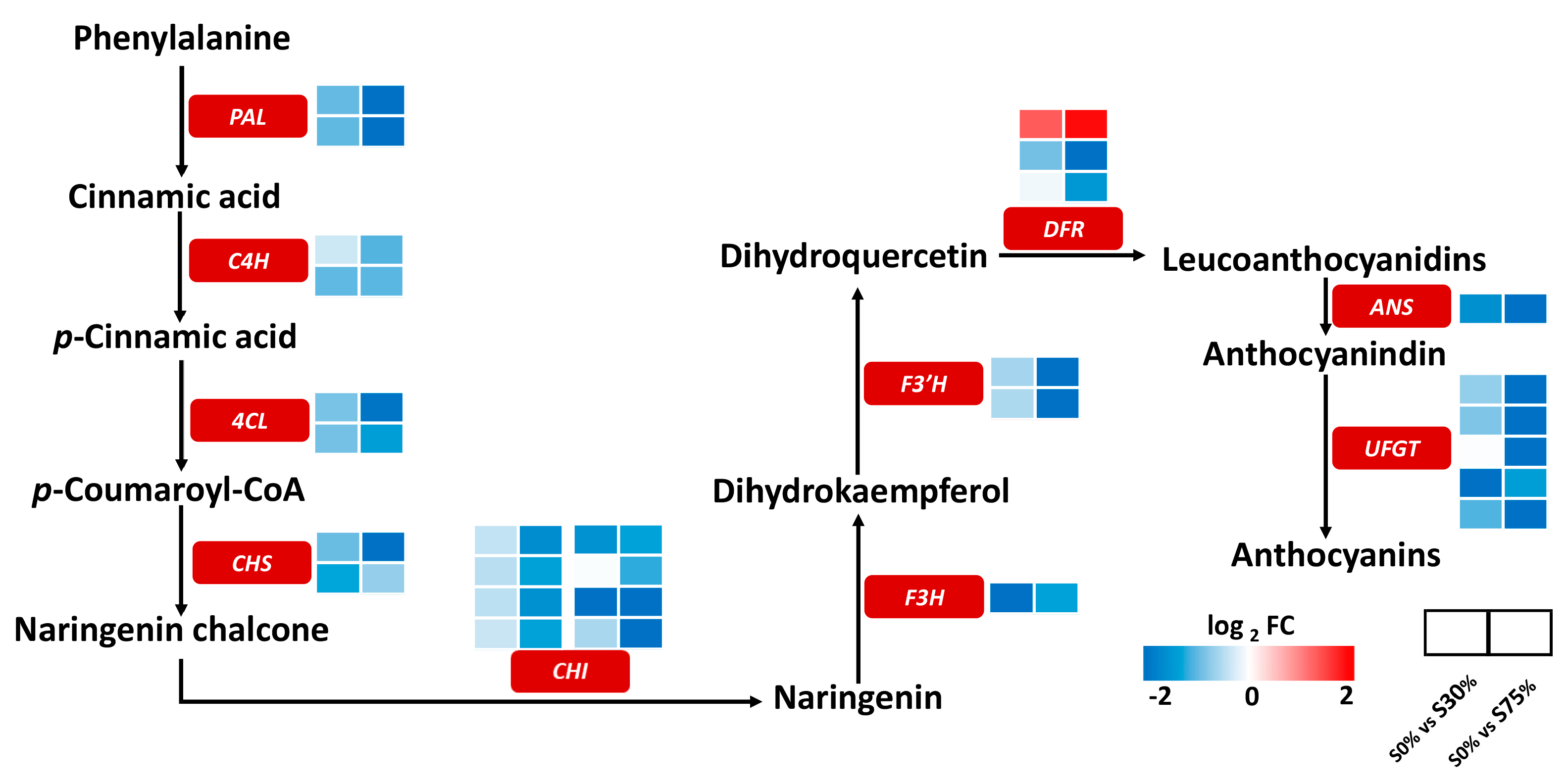
3.6. Effects of Different Shading Rates on Gene Expression of Anthocyanin Synthesis
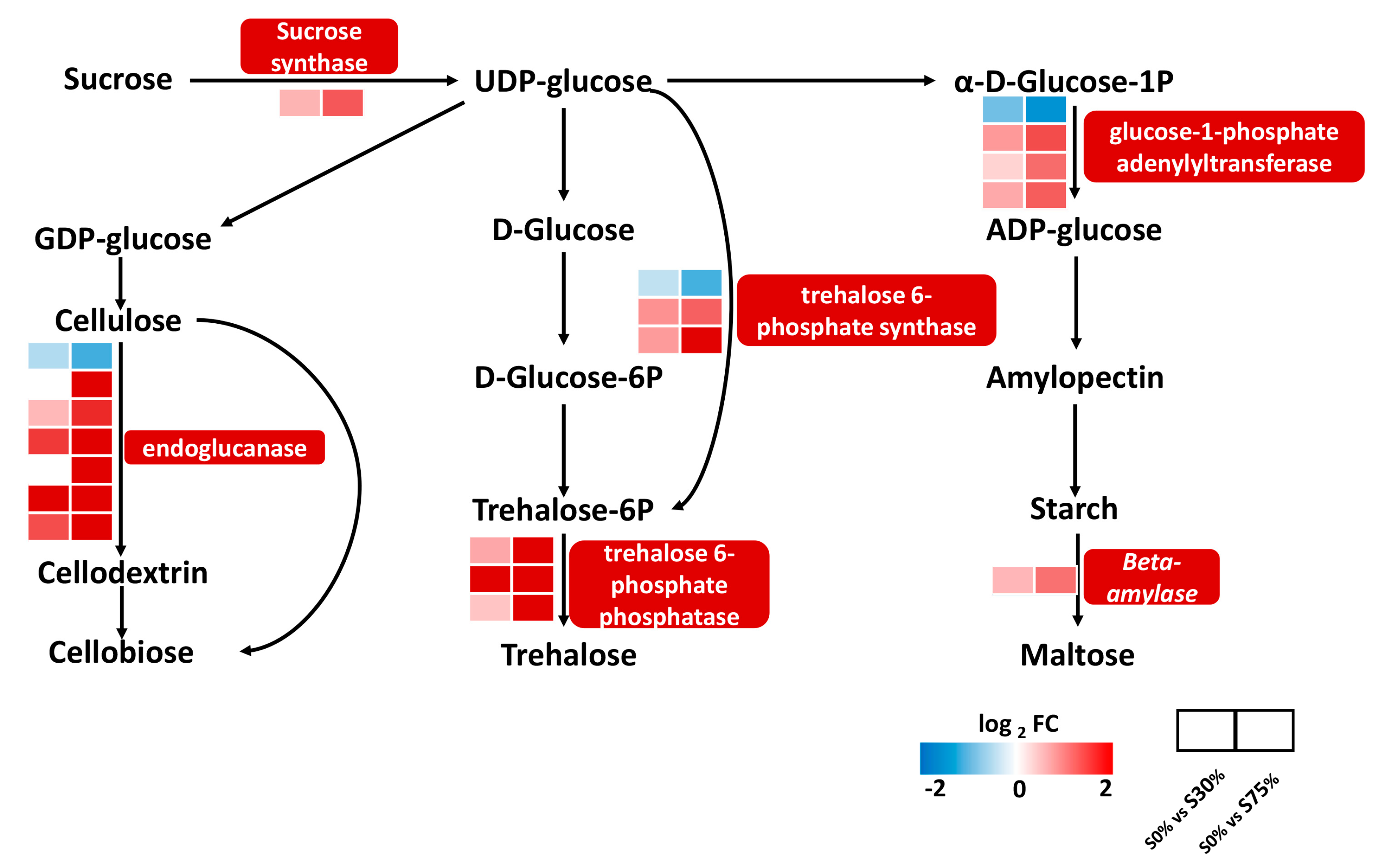
3.7. Effects of Different Shading Rates on the Gene Expression of Starch and Sucrose Metabolism
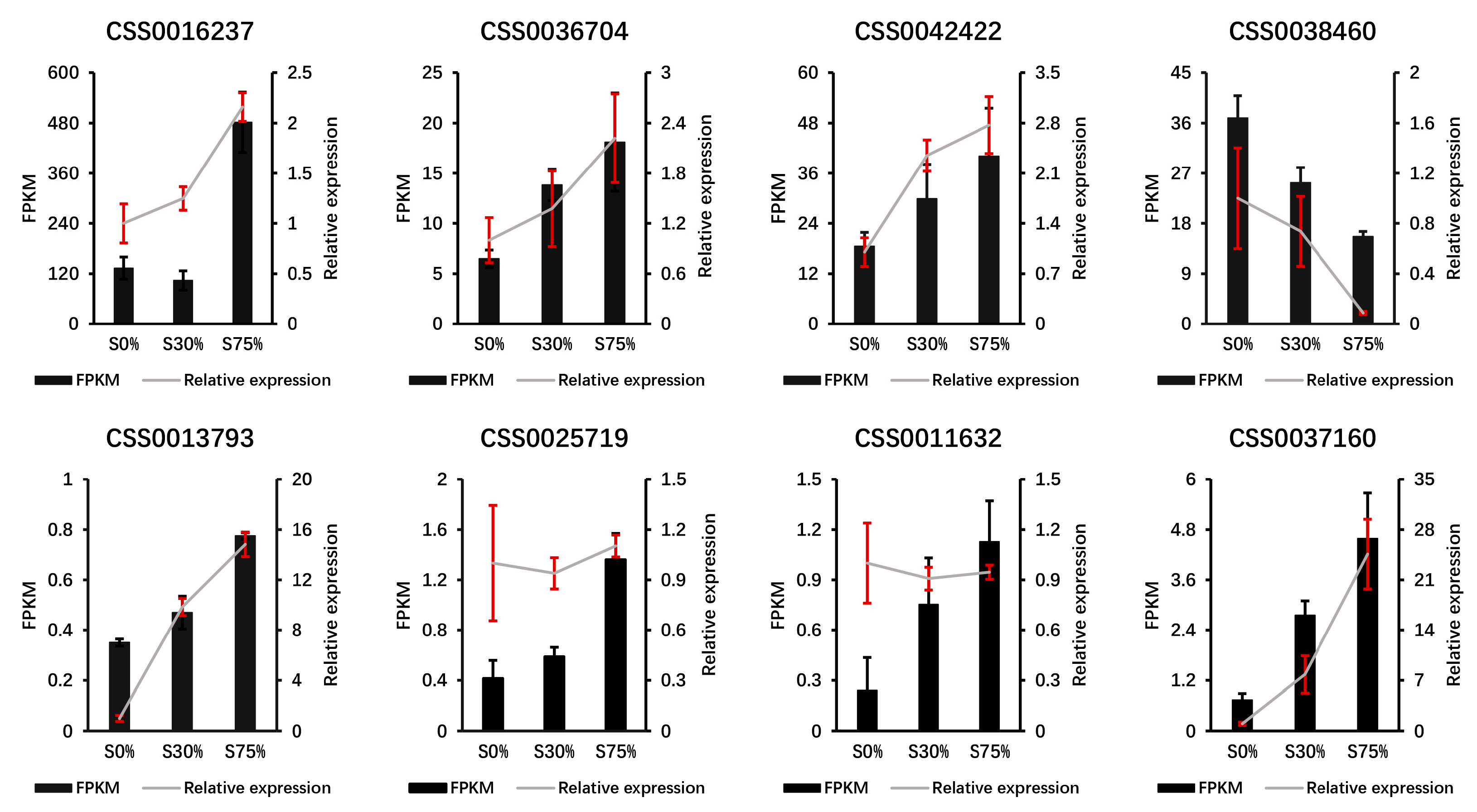
3.8. qRT-PCR Verification
4. Discussion
4.1. Shading Maintained the Green Color of Overwintering Tea Leaves by Regulating the Metabolism of Chlorophyll, Carotenoid, and Anthocyanin
4.2. Shading Maintained Photosynthesis of Overwintering Tea Leaves by Regulating Photosynthetic Proteins
4.3. Shading Maintains the Soluble Sugar Content in Overwintering Tea Leaves by Regulating Starch and Carbohydrate Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.Z.; Li, T.; Teng, R.M.; Han, M.H.; Zhuang, J. Low temperature effects on carotenoids biosynthesis in the leaves of green and albino tea plant (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2021, 285, 110164. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Ou, L.; Ji, D.; Jin, L. Response to the Cold Stress Signaling of the Tea Plant (Camellia sinensis) Elicited by Chitosan Oligosaccharide. Agronomy 2020, 10, 915. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, J.; Xiang, Z.; Xi, P.; Zhao, D. Physiological Changes and Differential Gene Expression of Tea Plants (Camellia sinensis (L.) Kuntze var. niaowangensis Q.H. Chen) Under Cold Stress. DNA Cell Biol. 2021, 40, 906–920. [Google Scholar] [PubMed]
- Li, X.; Ahammed, G.J.; Li, Z.X.; Zhang, L.; Wei, J.P.; Yan, P.; Zhang, L.P.; Han, W.Y. Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci. Hortic. 2017, 230, 155–160. [Google Scholar] [CrossRef]
- Oh, S.; Koh, S. Photosystem II photochemical efficiency and photosynthetic capacity in leaves of tea plant (Camellia sinensis L.) under winter stress in the field. Hortic. Environ. Biotechnol. 2014, 55, 363–371. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.; Dong, F.; Li, J.; Zeng, L.; Tang, J.; Gu, D. Mechanism Underlying the Shading-Induced Chlorophyll Accumulation in Tea Leaves. Front. Plant Sci. 2021, 12, 779819. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Fu, X.; Chen, J.; Li, J.; Dai, G.; Tang, J.; Yang, Z. Mechanism underlying the carotenoid accumulation in shaded tea leaves. Food Chem. X 2022, 14, 100323. [Google Scholar] [CrossRef]
- Bourque, D.P.; Naylor, A.W. Large Effects of Small Water Deficits on Chlorophyll Accumulation and Ribonucleic Acid Synthesis in Etiolated Leaves of Jack Bean (Canavalia ensiformis [L.] DC.). Plant Physiol. 1971, 47, 591–594. [Google Scholar] [CrossRef]
- Lopez-Figueroa, F.; Perez, R.; Niell, F.X. Effects of red and far-red light pulses on the chlorophyll and biliprotein accumulation in the red alga Corallina elongata. J. Photochem. Photobiol. B Biol. 1989, 4, 185–193. [Google Scholar] [CrossRef]
- Huang, W.; Ma, H.Y.; Huang, Y.; Li, Y.; Wang, G.L.; Jiang, Q.; Wang, F.; Xiong, A.S. Comparative proteomic analysis provides novel insights into chlorophyll biosynthesis in celery under temperature stress. Physiol. Plant 2017, 161, 468–485. [Google Scholar] [CrossRef]
- Jiao, B.; Meng, Q.; Lv, W. Roles of stay-green (SGR) homologs during chlorophyll degradation in green plants. Bot. Stud. 2020, 61, 25. [Google Scholar] [CrossRef]
- An, J.; Wei, X.; Huo, H. Transcriptome analysis reveals the accelerated expression of genes related to photosynthesis and chlorophyll biosynthesis contribution to shade-tolerant in Phoebe bournei. BMC Plant Biol. 2022, 22, 268. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Yan, H.; Cai, K.; Li, H.; Wu, Z.; Wu, J.; Yang, X.; Jiang, H.; Wang, Q.; et al. Integrated metabolomic and transcriptomic analyses reveal different metabolite biosynthesis profiles of Juglans mandshurica in shade. Front. Plant Sci. 2022, 13, 991874. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, K.; Tanaka, M.; Kurata, R.; Kai, Y. Comparative transcriptome analysis implied a ZEP paralog was a key gene involved in carotenoid accumulation in yellow-fleshed sweetpotato. Sci. Rep. 2020, 10, 20607. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ye, K.; Xu, Y.; Zhao, Y.; Zhao, D. Effect of Shading on the Morphological, Physiological, and Biochemical Characteristics as Well as the Transcriptome of Matcha Green Tea. Int. J. Mol. Sci. 2022, 23, 14169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Cai, K.; Zhang, Q.; Jiang, L.; Li, H.; Lv, Y.; Qu, G.; Zhao, X. Comparative Transcriptomic and Metabolic Analyses Reveal the Coordinated Mechanisms in Pinus koraiensis under Different Light Stress Conditions. Int. J. Mol. Sci. 2022, 23, 9556. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants [Review]. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Liu, Z.; Zhao, Z.; Zhao, J.; Wang, Z.; Zhou, G.; Liu, P.; Liu, M. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba Mill. peel coloration. Food Chem. 2020, 312, 125903. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.S. Nature’s Swiss Army Knife: The Diverse Protective Roles of Anthocyanins in Leaves. J. Biomed. Biotechnol. 2004, 2004, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, D.; Zhou, L.; Zhang, X.; Liao, J.; Duan, Y.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; et al. Transcriptomic and metabolomic profiling of Camellia sinensis L. cv. ‘Suchazao’ exposed to temperature stresses reveals modification in protein synthesis and photosynthetic and anthocyanin biosynthetic pathways. Tree Physiol. 2019, 39, 1583–1599. [Google Scholar] [CrossRef]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Singh, M.S.; Sharma, R. Sunlight-induced anthocyanin pigmentation in maize vegetative tissues. J. Exp. Bot. 1999, 50, 1619–1625. [Google Scholar] [CrossRef]
- Narbona, E.; Jaca, J.; Del Valle, J.C.; Valladares, F.; Buide, M.L. Whole-plant reddening in Silene germana is due to anthocyanin accumulation in response to visible light. Plant Biol. 2018, 20, 968–977. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, G.; Zhang, W.; Goltsev, V.; Sun, S.; Wang, J.; Li, P.; Ma, F. Anthocyanin concentration depends on the counterbalance between its synthesis and degradation in plum fruit at high temperature. Sci. Rep. 2017, 7, 7684. [Google Scholar] [CrossRef]
- Guo, X.; Shakeel, M.; Wang, D.; Qu, C.; Yang, S.; Ahmad, S.; Song, Z. Metabolome and transcriptome profiling unveil the mechanisms of light-induced anthocyanin synthesis in rabbiteye blueberry (vaccinium ashei: Reade). BMC Plant Biol. 2022, 22, 223. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Xie, Z. Transcriptomic profiling of purple broccoli reveals light-induced anthocyanin biosynthetic signaling and structural genes. PeerJ 2020, 8, e8870. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kouřil, R.; Nosek, L.; Bartoš, J.; Boekema, E.J.; Ilík, P. Evolutionary loss of light-harvesting proteins Lhcb6 and Lhcb3 in major land plant groups--break-up of current dogma. New Phytol. 2016, 210, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, W.; Yang, C.; Yao, J.; Xiao, W.; Xin, Y.; Qiu, J.; Hu, W.; Yao, H.; Ying, W.; et al. Comparative proteomic analysis reveals alterations in development and photosynthesis-related proteins in diploid and triploid rice. BMC Plant Biol. 2016, 16, 199. [Google Scholar] [CrossRef]
- Lambert, D.H.; Bryant, D.A.; Stirewalt, V.L.; Dubbs, J.M.; Stevens, S.E., Jr.; Porter, R.D. Gene map for the Cyanophora paradoxa cyanelle genome. J. Bacteriol. 1985, 164, 659–664. [Google Scholar] [CrossRef]
- Ye, J.H.; Lv, Y.Q.; Liu, S.R.; Jin, J.; Wang, Y.F.; Wei, C.L.; Zhao, S.Q. Effects of Light Intensity and Spectral Composition on the Transcriptome Profiles of Leaves in Shade Grown Tea Plants (Camellia sinensis L.) and Regulatory Network of Flavonoid Biosynthesis. Molecules 2021, 26, 5836. [Google Scholar] [CrossRef]
- Gerotto, C.; Franchin, C.; Arrigoni, G.; Morosinotto, T. In Vivo Identification of Photosystem II Light Harvesting Complexes Interacting with PHOTOSYSTEM II SUBUNIT S. Plant Physiol. 2015, 168, 1747–1761. [Google Scholar] [CrossRef]
- Yadav, K.N.; Semchonok, D.A.; Nosek, L.; Kouřil, R.; Fucile, G.; Boekema, E.J.; Eichacker, L.A. Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 12–20. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Jiang, L.; Yu, X.; Qi, X.; Yu, Q.; Deng, S.; Bai, B.; Li, N.; Zhang, A.; Zhu, C.; Liu, B.; et al. Multigene engineering of starch biosynthesis in maize endosperm increases the total starch content and the proportion of amylose. Transgenic Res. 2013, 22, 1133–1142. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Piro, L.; Flütsch, S.; Santelia, D. Arabidopsis Sucrose Synthase 3 (SUS3) regulates starch accumulation in guard cells at the end of day. Plant Signal. Behav. 2023, 18, 2171614. [Google Scholar] [CrossRef] [PubMed]
- Fulton, D.C.; Stettler, M.; Mettler, T.; Vaughan, C.K.; Li, J.; Francisco, P.; Gil, M.; Reinhold, H.; Eicke, S.; Messerli, G.; et al. Beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. Plant Cell 2008, 20, 1040–1058. [Google Scholar] [CrossRef]
- Monroe, J.D.; Breault, J.S.; Pope, L.E.; Torres, C.E.; Gebrejesus, T.B.; Berndsen, C.E.; Storm, A.R. Arabidopsis β-Amylase2 Is a K(+)-Requiring, Catalytic Tetramer with Sigmoidal Kinetics. Plant Physiol. 2017, 175, 1525–1535. [Google Scholar] [CrossRef]
- Liang, G.; He, H.; Nai, G.; Feng, L.; Li, Y.; Zhou, Q.; Ma, Z.; Yue, Y.; Chen, B.; Mao, J. Genome-wide identification of BAM genes in grapevine (Vitis vinifera L.) and ectopic expression of VvBAM1 modulating soluble sugar levels to improve low-temperature tolerance in tomato. BMC Plant Biol. 2021, 21, 156. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17r–27r. [Google Scholar] [CrossRef]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef]




| Treatments | Shaderate (%) | PAR (μmol m−2 s−1) |
|---|---|---|
| S0% | 0% | 1073.33 ± 10.08 a |
| S30% | 30% | 444.67 ± 10.21 b |
| S75% | 75% | 155.33 ± 5.91 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Shen, Y.; Wang, Y.; Shen, J.; Wang, H.; Ding, S.; Xu, Y.; Mao, Y.; Chen, H.; Song, Y.; et al. Transcriptome Revealed the Effect of Shading on the Photosynthetic Pigment and Photosynthesis of Overwintering Tea Leaves. Agronomy 2023, 13, 1701. https://doi.org/10.3390/agronomy13071701
Han X, Shen Y, Wang Y, Shen J, Wang H, Ding S, Xu Y, Mao Y, Chen H, Song Y, et al. Transcriptome Revealed the Effect of Shading on the Photosynthetic Pigment and Photosynthesis of Overwintering Tea Leaves. Agronomy. 2023; 13(7):1701. https://doi.org/10.3390/agronomy13071701
Chicago/Turabian StyleHan, Xiao, Yaozong Shen, Yu Wang, Jiazhi Shen, Hui Wang, Shibo Ding, Yang Xu, Yilin Mao, Hao Chen, Yujie Song, and et al. 2023. "Transcriptome Revealed the Effect of Shading on the Photosynthetic Pigment and Photosynthesis of Overwintering Tea Leaves" Agronomy 13, no. 7: 1701. https://doi.org/10.3390/agronomy13071701
APA StyleHan, X., Shen, Y., Wang, Y., Shen, J., Wang, H., Ding, S., Xu, Y., Mao, Y., Chen, H., Song, Y., Ding, Z., & Fan, K. (2023). Transcriptome Revealed the Effect of Shading on the Photosynthetic Pigment and Photosynthesis of Overwintering Tea Leaves. Agronomy, 13(7), 1701. https://doi.org/10.3390/agronomy13071701





