Analysis of Phenotypic and Physiological Characteristics of Plant Height Difference in Alfalfa
Abstract
:1. Introduction
2. Materials and Methods
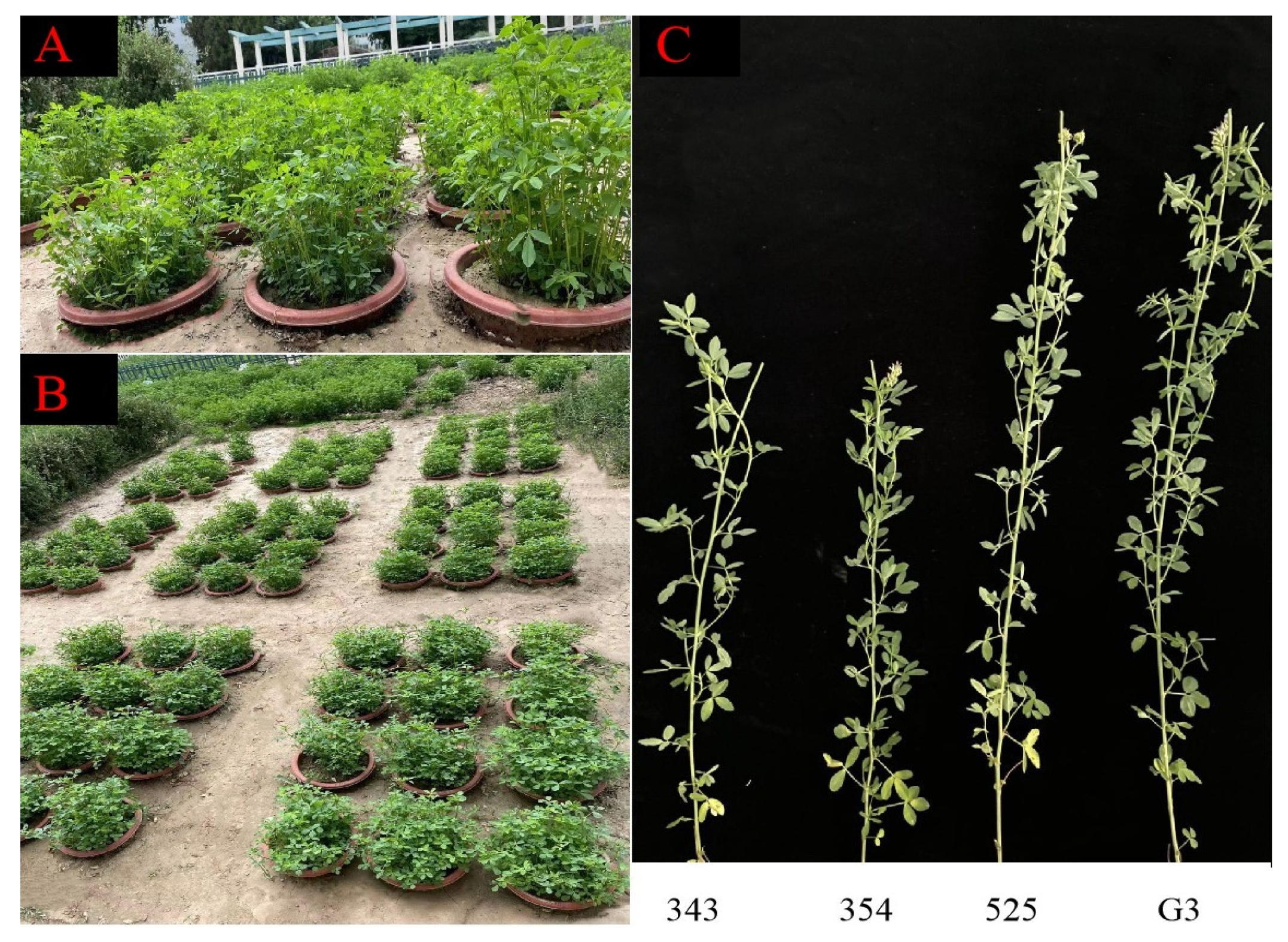
2.1. Plant Material
2.2. Growth Conditions and Treatments
2.3. Measurement Indexes
2.3.1. Phenotypic Trait Indicators
- (i)
- Leaf area (LA):
- (ii)
- Leaf shape index (LI):
2.3.2. Determination of Photosynthetic Parameters
2.3.3. Determination of Photosynthetic Products
2.3.4. Determination of Endogenous Hormone Content
2.4. Statistical Analyses
- (iii)
- Path analysis:
Decision coefficient: R2 = 2Pijriy-P2iy
3. Results
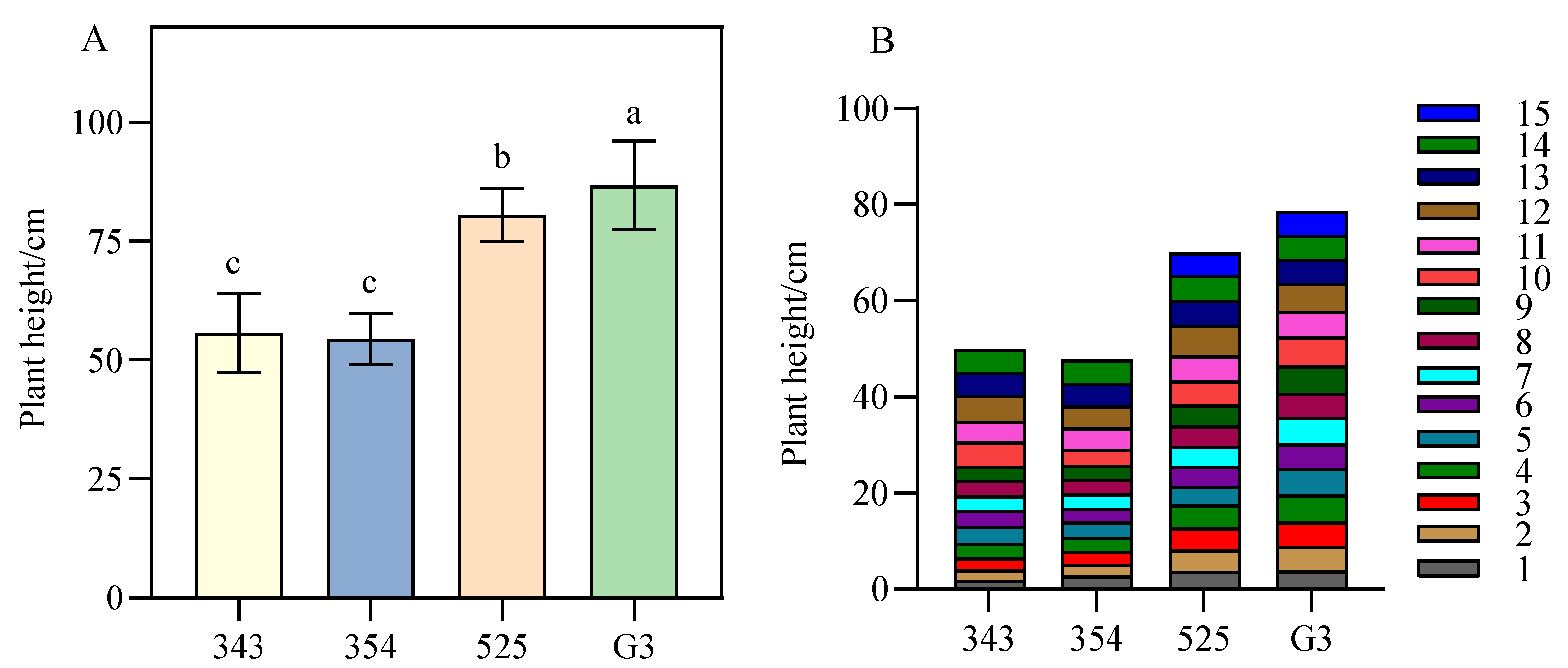
3.1. Differences in Plant Height between Tall- and Short-Stalk Alfalfa Materials
3.2. Differences in Phenotypic Traits between Tall- and Short-Stalk Alfalfa Materials
3.3. Differences in Photosynthetic Physiological Characteristics between Tall- and Short-Stalk Alfalfa Materials
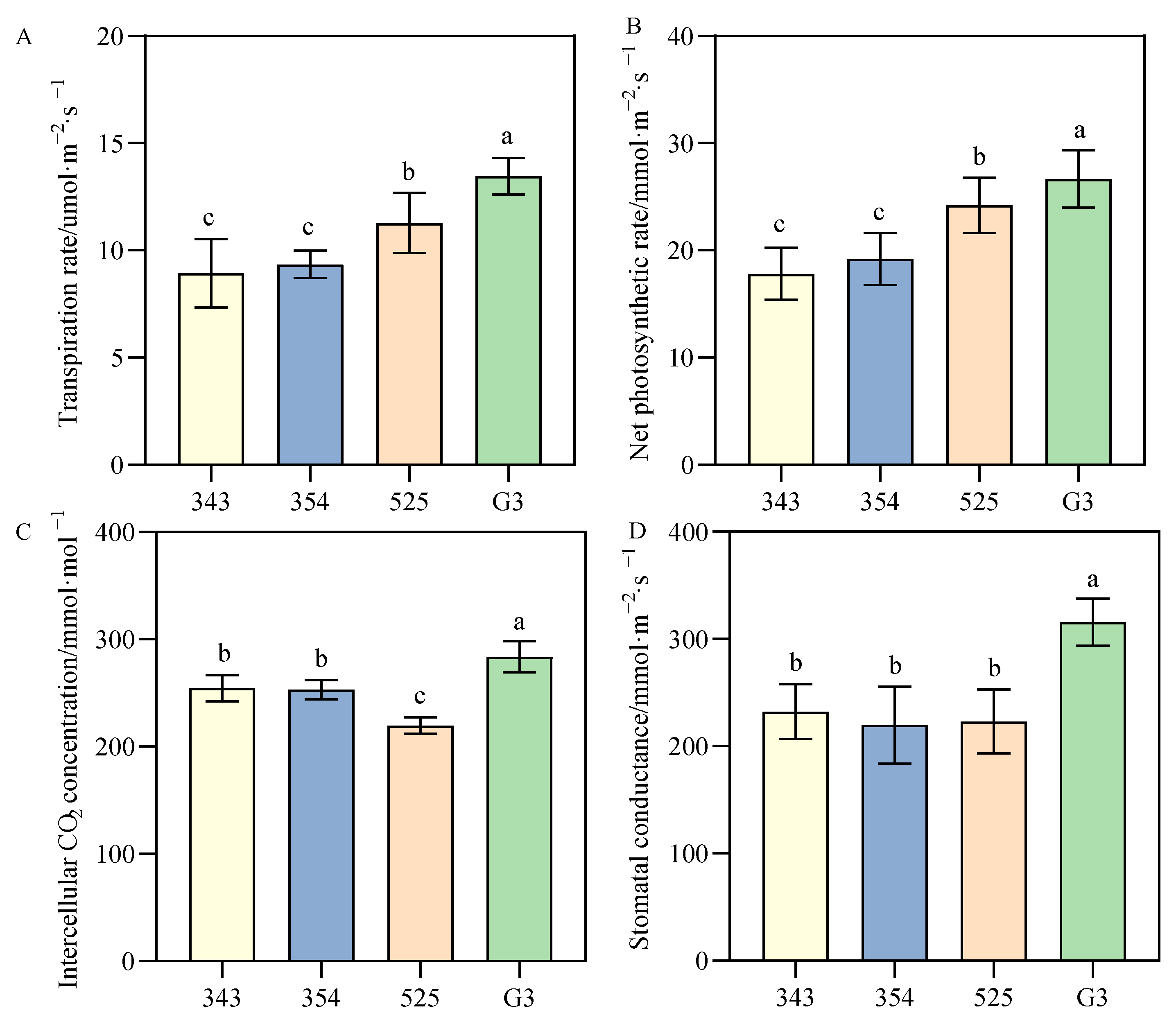
3.3.1. Differences in Photosynthetic Parameters between Tall- and Short-Stalk Alfalfa Materials
3.3.2. Differences in Photosynthetic Product Content between Tall- and Short-Stalk Alfalfa Materials
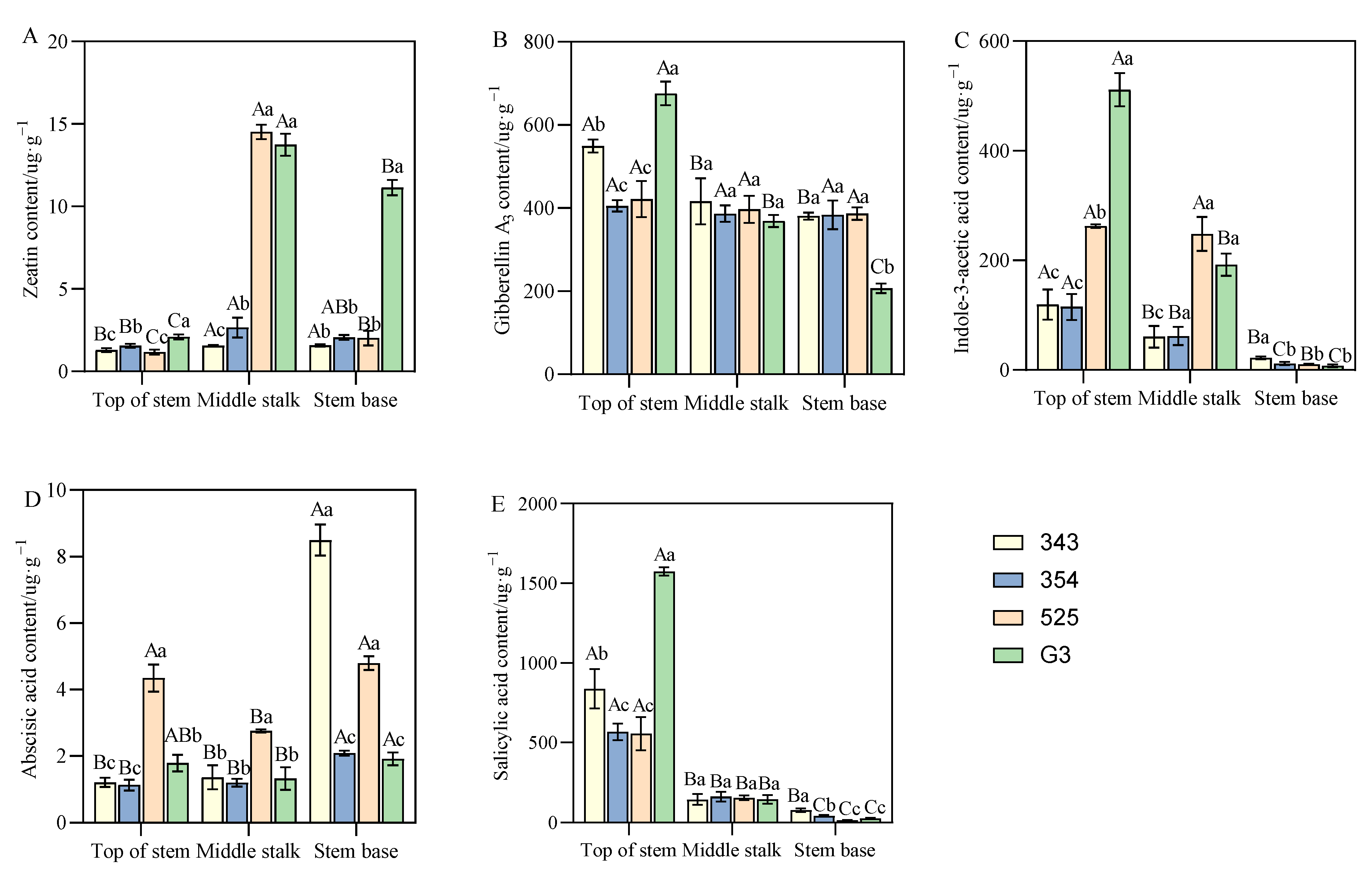
3.4. Differences in Endogenous Hormone Content between Tall- and Short-Stalk Alfalfa Materials
3.5. Correlation Analysis of Alfalfa Plant Height with Phenotypic and Physiological Indicators
3.5.1. Correlation Analysis of Alfalfa Plant Height and Phenotypic Indexes
3.5.2. Correlation Analysis of Alfalfa Plant Height with Light and Physiological Indicators
3.5.3. Correlation Analysis between Alfalfa Plant Height and Endogenous Hormone Content
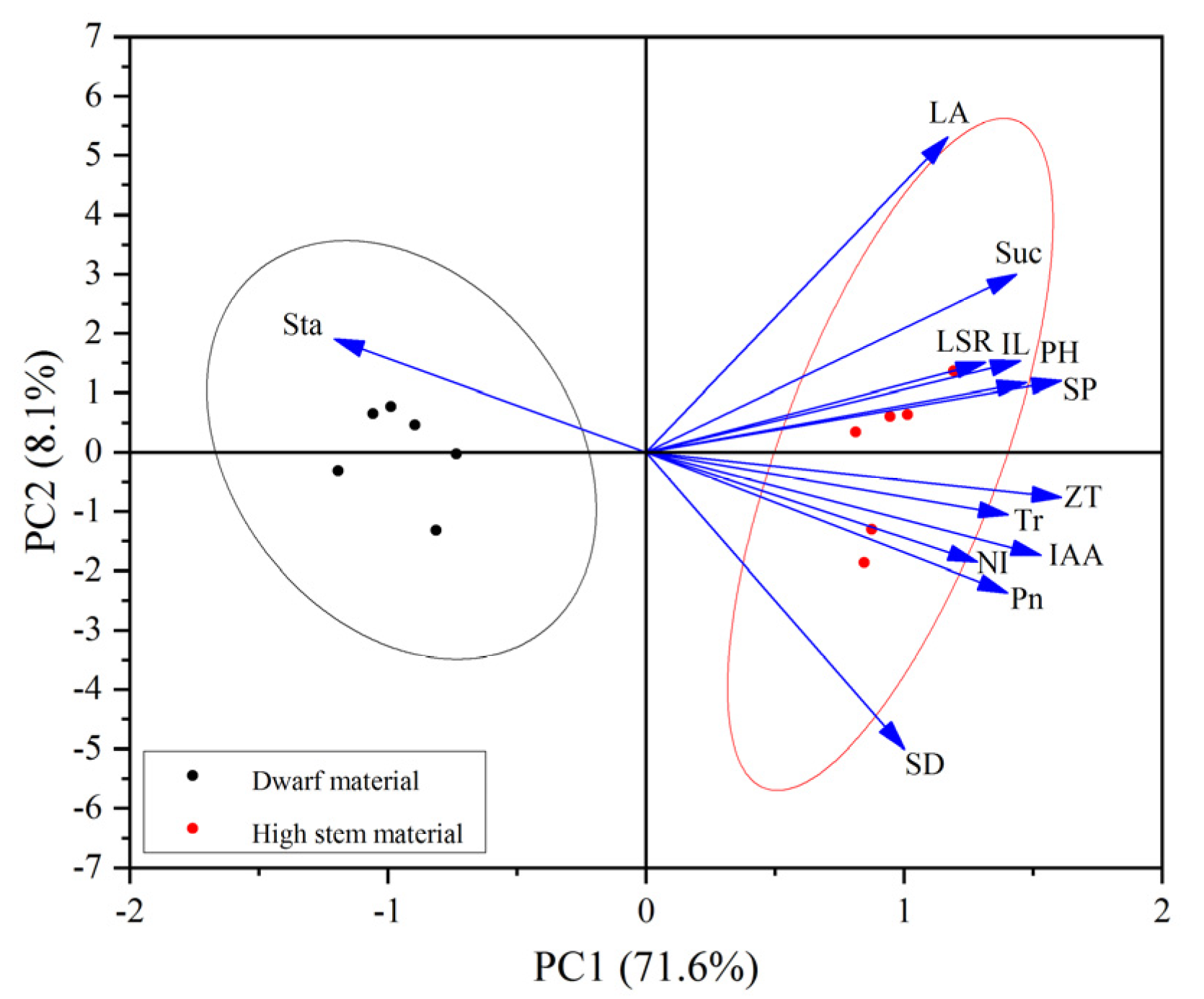
3.6. Principal Component Analysis of Alfalfa Plant Height and Phenotypic Traits and Physiological Indicators
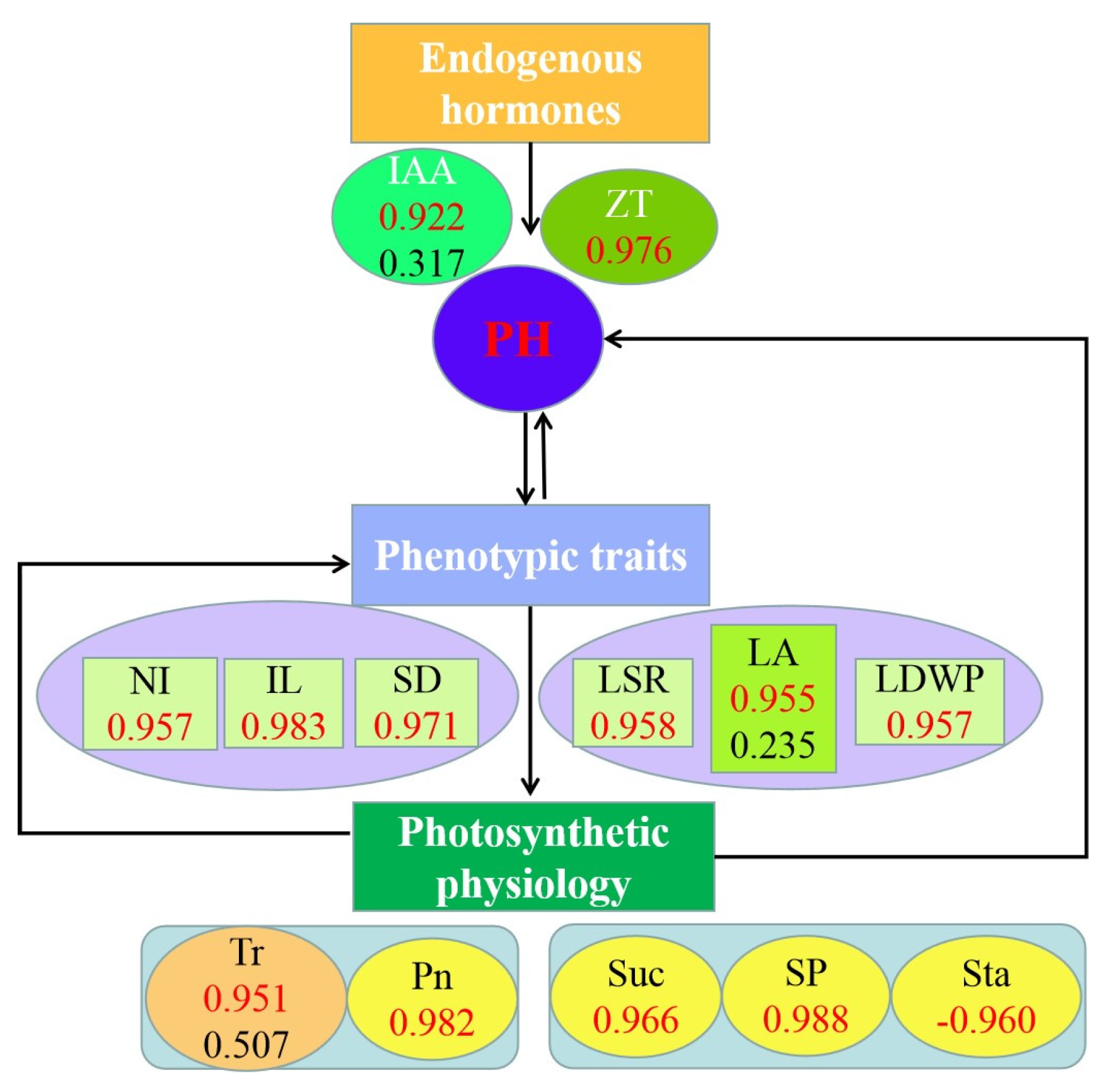
3.7. The Role of Phenotypic Traits and Physiological Indicators on Plant Height in Alfalfa
3.8. Comprehensive Analysis of Plant Height, Phenotypic Traits and Physiological Indicators of Alfalfa
4. Discussion
4.1. Phenotypic Trait Indicators Affecting Plant Height of Alfalfa
4.2. Photosynthetic Physiological Indicators Affecting Alfalfa Plant Height
4.3. Endogenous Hormones Affecting Plant Height of Alfalfa
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schnurr, J.A.; Jung, H.G.; Samac, D.A. A comparative study of alfalfa and Medicago truncatula stem traits: Morphology, chemical composition, and ruminal digestibility. Crop Sci. 2007, 47, 1672–1680. [Google Scholar] [CrossRef] [Green Version]
- Annicchiarico, P.; Piano, E. Use of artificial environments to reproduce and exploit genotype x location interaction for lucerne in northern Italy. Theor. Appl. Genet. 2005, 110, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Hernandez, T.; Zhou, C.; Wang, Z.Y. Alfalfa (Medicago sativa L.). Methods Mol. Biol. 2015, 1223, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Zeng, Y.; Yang, Y.Z.; Huang, L.L.; Tang, B.; Zhang, H.; Hao, F.; Liu, W.; Li, Y.H.; Liu, Y.B.; et al. Allele-aware chromosome-level genome assembly and efficient transgene-free genome editing for the autotetraploid cultivated alfalfa. Nat. Commun. 2020, 11, 2494. [Google Scholar] [CrossRef]
- Long, R.C.; Zhang, F.; Zhang, Z.W.; Li, M.N.; Chen, L.; Wang, X.; Liu, W.W.; Zhang, T.J.; Yu, L.X.; He, F.; et al. Genome assembly of alfalfa cultivar zhongmu-4 and identification of SNPs associated with agronomic traits. Genom. Proteom. Bioinform. 2022, 20, 14–28. [Google Scholar] [CrossRef]
- Shen, C.; Du, H.L.; Chen, Z.; Lu, H.W.; Zhu, F.G.; Chen, H.; Meng, X.Z.; Liu, Q.W.; Liu, P.; Zheng, L.H.; et al. The Chromosome-Level Genome Sequence of the Autotetraploid Alfalfa and Resequencing of Core Germplasms Provide Genomic Resources for Alfalfa Research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef]
- Ku, L.X.; Zhang, L.K.; Tian, Z.Q.; Guo, S.L.; Su, H.H.; Ren, Z.Z.; Wang, Z.Y.; Li, G.H.; Wang, X.B.; Zhu, Y.G.; et al. Dissection of the genetic architecture underlying the plant density response by mapping plant height-related traits in maize (Zea mays L.). Mol. Genet. Genom. 2015, 4, 1223–1233. [Google Scholar] [CrossRef]
- Wang, W.X.; Guo, W.J.; Le, L.; Yu, J.; Wu, Y.; Li, D.W.; Wang, Y.F.; Wang, H.; Lu, X.D.; Qiao, H.; et al. Integration of high-throughput phenotyping, GWAS, and predictive models reveals the genetic architecture of plant height in maize. Mol. Plant 2023, 16, 354–373. [Google Scholar] [CrossRef]
- Zhang, T.J.; Yang, Q.C.; Kang, J.M.; Sun, Y.; Guo, W.S. Advances in Genetic Basic Research of Alfalfa Breeding for yield. Chin. J. Grassl. 2011, 33, 102–106. [Google Scholar]
- Yin, D. Biomass dynamics of every stubble of spring-sowed alfalfa in the loess plateau of LongDong. Agric. Res. Arid. Areas 2009, 37, 178–183. [Google Scholar]
- Wang, H.S.; Gu, L.J.; Zhang, X.G.; Liu, M.L.; Jiang, H.Y.; Cai, R.H.; Zhao, Y.; Cheng, B.J. Global transcriptome and weighted gene co-expression network analyses reveal hybrid-specific modules and candidate genes related to plant height development in maize. Plant Mol. Biol. 2018, 98, 187–203. [Google Scholar] [CrossRef]
- He, H.F.; Yan, C.L.; Wu, N.; Liu, J.L.; Jia, Y.H. Effects of different nitrogen levels on photosynthetic characteristics and drought resistance of switch-grass (Panicum virgatum). Acta Prataculturae Sin. 2021, 30, 107–115. [Google Scholar] [CrossRef]
- Cai, X.X.; Zhang, S.; Wang, H.; Lv, R.L.; Li, X.H.; Zhou, Q. The present research situation and application prospects of rice plant type genes. Mol. Plant Breed. 2017, 15, 2809–2814. [Google Scholar] [CrossRef]
- Chen, X.; Lu, S.C.; Wang, Y.F.; Zhang, X.; Lu, B.; Luo, L.Q.; Xi, D.D.; Shen, J.B.; Ma, H.; Ming, F. Os NAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice. Plant J. 2015, 82, 302–314. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, J.; Lu, W.J.; Deng, D.X. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Fendrych, M.; Leung, J.; Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of Arabidopsis hypocotyls. Elife 2016, 5, e19048. [Google Scholar] [CrossRef]
- Kutschera, U.; Niklas, K.J. The epidermal-growth-control theory of stem elongation: An old and a new perspective. J. Plant Physiol. 2007, 164, 1395–1409. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Barbez, E.; Kleine-Vehn, J.; Estevez, J.M. Auxin and cellular elongation. Plant Physiol. 2016, 170, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, P.D.; Zhang, X.B.; Li, X.F.; Yan, X.H.; Fu, D.H.; Wu, G. The genetic and molecular basis of crop height based on a rice model. Planta 2018, 247, 1–26. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, Y.Y.; Zhang, H.M.; Lin, X.C.; Xia, R.; Song, L.L.; Wu, A.M. MicroRNAs play important roles in regulating the rapid growth of the Phyllostachys edulis culm internode. New Phytol. 2021, 231, 2215–2230. [Google Scholar] [CrossRef]
- Wang, F.X.; Yu, Z.P.; Zhang, M.L.; Wang, M.L.; Lu, X.D.; Liu, X.; Li, Y.B.; Zhang, X.S.; Tan, B.C.; Li, C.L.; et al. ZmTE1 promotes plant height by regulating intercalary meristem formation and internode cell elongation in maize. Plant Biotechnol. J. 2022, 20, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Fan, Y.G.; Yan, H.F.; Zhou, H.W.; Zhou, Z.F.; Weng, M.L.; Huang, X.; Lakshmanan, P.; Li, Y.; Qiu, L.H.; et al. Enhanced Activity of Genes Associated With Photosynthesis, Phytohormone Metabolism and Cell Wall Synthesis Is Involved in Gibberellin-Mediated Sugarcane Internode Growth. Front. Genet. 2020, 11, 570094. [Google Scholar] [CrossRef] [PubMed]
- Amanda, S.L.; Natalie, N.G.; Upendra, K.D.; Esther, C.; Isabel, L.D.; Miguel, A.B.; Julin, N.M. The role of a class III gibberellin 2-oxidase in tomato internode elongation. Plant J. 2019, 97, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.J.; Yu, X.; Wang, X.Z.; Zhang, F.F.; Ma, C.H. Differentially expressed genes related to plant height and yield in two alfalfa cultivars based on RNA-seq. PeerJ 2022, 10, e14096. [Google Scholar] [CrossRef]
- Zhang, H.H.; Shi, S.L.; Wu, B.; Li, Z.L.; Li, X.L. A study of yield interactions in mixed sowings of alfalfa and three perennial grasses. Acta Prataculturae Sin. 2022, 31, 159–170. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Bach Knudsen, K.E. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, M.L.; Zhang, R.; Niu, K.J.; Li, Y.Z.; Zhang, J.Q.; Ma, H.L. The relationship between endogenous hormone content and apomixis rate of wild Kentucky bluegrass in Gansu province. J. Grassl. Ind. 2020, 29, 99–111. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, J.; Li, X.; Jing, F.; Zhang, H.; Ma, R. Photosynthetic Physiological Basis of Forage Mass Stability in a Progeny of Rhizome-Rooted ‘Qingshui’ Medicago sativa L. Agronomy 2023, 13, 685. [Google Scholar] [CrossRef]
- Tsuda, K.; Abraham-Juarez, M.J.; Maeno, A.; Dong, Z.; Aromdee, D.; Meeley, R.; Shiroishi, T.; Nonomura, K.I.; Hake, S. KNOTTED1 cofactors, BLH12 and BLH14,regulate internode patterning and vein anastomosis in maize. Plant Cell 2017, 29, 1105–1118. [Google Scholar] [CrossRef] [Green Version]
- Kovi, M.R.; Zhang, Y.S.; Yu, S.B.; Yang, G.Y.; Yan, W.H.; Xing, Y.Z. Candidacy of a chitin-inducible gibberellin-responsive gene for a major locus affecting plant height in rice that is closely linked to green revolution gene sd1. Theor. Appl. Genet. 2011, 123, 705–714. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Nomura, T.; Xu, Y.H.; Zhang, Y.Y.; Peng, Y.; Mao, B.Z.; Hanada, A.; Zhou, H.C.; Wang, R.X.; Li, P.J.; et al. ELONGATED UPPERMOST INTERNODE encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell 2006, 18, 442–456. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.J.; Cheng, T.T.; Tong, C.C.; Wang, X. Study on leaf characteristics and yield effects of alfalfa with different nitrogen efficiency. Acta Agrestia Sin. 2021, 29, 1941–1949. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Xu, F.; Zhang, Y.Q.; Hai, G.; Yang, C.Y.; Wu, Z.; Wang, B.; Yu, Z. Comparison and correlation of agronomic characteristics and fermentation quality of different types of hybrid corn. Acta Agrestia Sin. 2019, 27, 250–256. [Google Scholar] [CrossRef]
- Zhang, J.S.; Jia, Y.H.; Sun, P.; Liu, C.; Wang, H.; Luo, S.W.; Shi, S.B. Effects of uniform pattern and N application rate on colony, photosynthesis and dry matter accumulation of winter wheat. J. China Agric. Univ. 2021, 26, 12–24. [Google Scholar] [CrossRef]
- Yu, G.R.; Wang, Q.F. Ecophysiological of Plant Photosynthesis, Transpiration, and Water Use; Science Press: Beijing, China, 2010. [Google Scholar]
- Lin, K.Q.; Han, Z.L.; Song, Q.F.; Liang, S.H.; Zhou, Y.F. Photosynthetic physiological features and leaf functional traits of 15 tea cultivars (lines). Southwest China J. Agric. Sci. 2021, 34, 2370–2377. [Google Scholar] [CrossRef]
- Lan, J.H.; Chu, D. Study on the genetic basis of plant height and ear height in maize(Zea mays L.) by QTL dissection. Hereditas 2005, 27, 925–934. [Google Scholar]
- Li, L.L.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B.; Yang, J.S. Characteristics of Accumulation, transportation and distribution of assimilates in summer maize varieties with different plant heights. Acta Agrestia Sin. 2012, 38, 1080–1087. [Google Scholar] [CrossRef]
- Lu, X.L.; Yu, Z.E.; Zhang, Y.L.; Shi, Y. Light energy utilization and distribution characteristics of 13C assimilates in different spike-type wheat varieties and their response to supplementary irrigation. Chin. J. Appl. Ecol. 2019, 30, 3745–3752. [Google Scholar] [CrossRef]
- Xu, M.L.; Ding, L.P.; Luo, J.; Xu, T.T.; Wu, H.Z. Research progress of photosynthetic mechanisms of super high yield-grain-yield rice. Front. Plant Sci. 2021, 25, 385–392. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhang, L.; Liu, S.L.; Cao, Y.J.; Wei, W.W.; Liu, C.G.; Wang, Y.J.; Bian, S.F.; Wang, L.C. Grain Filling Characteristics of Maize Hybrids Differing Maturities. Sci. Agric. Sin. 2014, 47, 3557–3565. [Google Scholar] [CrossRef]
- Richardson, A.D.; Carbone, M.S.; Keenan, T.F.; Czimczik, C.I.; Hollinger, D.Y.; Murakami, P.; Schaberg, P.G.; Xu, X.M. Seasonal dynamics and age of stemwood nonstructural carbohydrates in temperate forest trees. New Phytol. 2013, 197, 850–861. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Z.; Li, G.L.; Zhang, S.Y. The Preliminary analysis on relationship between carbon and nitrogen metabolism and plant height in castor. Chin. J. Oil Crop Sci. 2006, 28, 342–346. [Google Scholar]
- Zheng, Y.; Hong, M.X.; Fan, Y.Q.; Wang, X.S.; Li, D.H. Physiological and biochemical performance of rice semi dwarf mutant B1-24. Chin. J. Trop. Crop. 2009, 30, 1057–1061. [Google Scholar]
- Kebrom, T.H.; McKinley, B.; Mullet, J.E. Dynamics of gene expression during development and expansion of vegetative stem internodes of bioenergy sorghum. Biotechnol. Biofuels 2017, 10, 159. [Google Scholar] [CrossRef]
- Chen, Y.J. Transcriptomic and Metabolomic Integrative Analysis to Identify Key Genes Invoved in the Regulation Castor Plant Height Development; Zhejiang University: Hangzhou, China, 2021; pp. 55–56. [Google Scholar] [CrossRef]
- Ljung, K.; Bhalerao, R.P.; Sandberg, G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001, 28, 465–474. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Robertson-Hoyt, J.; Yun, J.; Benavente, L.M.; Xie, D.Y.; Dolezal, k.; Schlereth, A.; Jürgens, G.; Alonso, J.M. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 2008, 133, 177–191. [Google Scholar] [CrossRef] [Green Version]
- Ni, D.; Xu, Z.H. Auxin biosynthesis, metabolism, receptors and polar transport. Commun. Plant Physiol. 2001, 346–352. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Su, Y.H.; Liu, Y.B.; Bai, B.; Zhang, X.S. Establishment of embryonic shoot–root axis is involved in auxin and cytokinin response during Arabidopsis somatic embryogenesis. Front. Plant Sci. 2015, 5, 792. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.J.; Wang, L.; Sun, W.; Zhang, Y.; Zhou, C.; Su, Y.H.; Li, W.; Sun, T.T.; Zhao, X.Y.; Li, X.G.; et al. Pattern of auxin and cytokinin responses for shoot meristem induction results from the regulation of cytokinin biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol. 2013, 161, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Ioio, R.D.; Nakamura, K.; Moubayidin, L.; Perilli, S.; Taniguchi, M.; Morita, M.T.; Aoyama, T.; Costantino, P.; Sabatini, S. A genetic frame-work for the control of cell division and differentiation in the root meristem. Science 2008, 322, 1380–1384. [Google Scholar] [CrossRef] [Green Version]




| Time(min) | 1 | 3 | 5 | 8 | 12 | 14 |
|---|---|---|---|---|---|---|
| Methanol (%) | 45 | 55 | 65 | 75 | 10 | 10 |
| 0.1% Phosphate (%) | 55 | 45 | 35 | 25 | 90 | 90 |
| Correlation | PH | NI | SD | IL | LSR | LA | LDWP | |
|---|---|---|---|---|---|---|---|---|
| Tr | 0.951 * | 0.986 ** | 0.851 | 0.988 ** | 0.824 | 0.849 | 0.986 ** | |
| Pn | 0.982 ** | 0.957 * | 0.926 * | 0.982 ** | 0.903 * | 0.883 | 0.957 * | |
| Ci | 0.104 | 0.384 | −0.134 | 0.287 | −0.172 | 0.003 | 0.384 | |
| Gs | 0.672 | 0.857 | 0.481 | 0.797 | 0.449 | 0.592 | 0.857 | |
| SP | Upper blade | 0.988 ** | 0.904 * | 0.996 ** | 0.943 * | 0.991 ** | 0.966 * | 0.904 * |
| Middle stem | 0.477 | 0.661 | 0.261 | 0.614 | 0.210 | 0.270 | 0.661 | |
| Middle blade | 0.441 | 0.626 | 0.224 | 0.580 | 0.172 | 0.226 | 0.626 | |
| Suc | Upper blade | 0.966 * | 0.945 * | 0.942 * | 0.954 * | 0.940 * | 0.992 ** | 0.945 * |
| Middle stem | −0.790 | −0.610 | −0.909* | −0.673 | −0.930 * | −0.881 | −0.610 | |
| Middle blade | −0.319 | −0.463 | −0.249 | −0.387 | −0.266 | −0.506 | −0.463 | |
| Sta | Upper blade | 0.438 | 0.674 | 0.211 | 0.597 | 0.171 | 0.323 | 0.674 |
| Middle stem | −0.960 * | −0.934 * | −0.897 | −0.962 * | −0.870 | −0.833 | −0.934 * | |
| Middle blade | −0.727 | −0.724 | −0.738 | −0.710 | −0.758 | −0.888 | −0.724 | |
| SS | Upper blade | 0.883 | 0.710 | 0.967 * | 0.781 | 0.972 * | 0.879 | 0.710 |
| Middle stem | −0.850 | −0.965 * | −0.711 | −0.929* | −0.687 | −0.803 | −0.965 * | |
| Middle blade | 0.418 | 0.139 | 0.618 | 0.243 | 0.645 | 0.472 | 0.139 | |
| Correlation | PH | NI | SD | IL | LSR | LA | LDWP | |
|---|---|---|---|---|---|---|---|---|
| ZT | Upper stem | 0.432 | 0.642 | 0.206 | 0.583 * | 0.157 | 0.248 | 0.642 |
| Middle stem | 0.976 * | 0.873 | 0.991 ** | 0.923 | 0.982 ** | 0.927 * | 0.873 | |
| Basal stem | 0.707 | 0.873 | 0.517 | 0.825 | 0.479 | 0.585 | 0.873 | |
| GA3 | Upper stem | 0.461 | 0.684 | 0.283 | 0.596 | 0.266 | 0.488 | 0.684 |
| Middle stem | −0.603 | −0.679 | −0.447 | −0.680 | −0.396 | −0.350 | −0.679 | |
| Basal stem | −0.677 | −0.857 | −0.482 | −0.801 | −0.446 | −0.570 | −0.857 | |
| IAA | Upper stem | −0.268 | 0.023 | −0.482 | −0.086 | −0.509 | −0.318 | 0.023 |
| Middle stem | 0.922 * | 0.771 | 0.985 ** | 0.835 | 0.987 ** | 0.911 * | 0.771 | |
| Basal stem | −0.724 | −0.667 | −0.668 | −0.718 | −0.630 | −0.497 | −0.667 | |
| ABA | Upper stem | 0.612 | 0.360 | 0.782 | 0.455 | 0.804 | 0.667 | 0.360 |
| Middle stem | 0.473 | 0.207 | 0.670 | 0.302 | 0.701 | 0.563 | 0.207 | |
| Basal stem | −0.392 | −0.418 | −0.274 | −0.442 | −0.222 | −0.103 | −0.418 | |
| SA | Upper stem | 0.570 | 0.784 | 0.373 | 0.708 | 0.345 | 0.523 | 0.784 |
| Middle stem | −0.289 | −0.454 | −0.203 | −0.372 | −0.216 | −0.464 | −0.454 | |
| Basal stem | −0.776 | −0.623 | −0.807 | −0.703 | −0.787 | −0.620 | −0.623 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, F.; Shi, S.; A, Y.; Guan, J.; Lu, B.; Wu, B.; Wang, W.; Ma, R.; Nan, P. Analysis of Phenotypic and Physiological Characteristics of Plant Height Difference in Alfalfa. Agronomy 2023, 13, 1744. https://doi.org/10.3390/agronomy13071744
Jing F, Shi S, A Y, Guan J, Lu B, Wu B, Wang W, Ma R, Nan P. Analysis of Phenotypic and Physiological Characteristics of Plant Height Difference in Alfalfa. Agronomy. 2023; 13(7):1744. https://doi.org/10.3390/agronomy13071744
Chicago/Turabian StyleJing, Fang, Shangli Shi, Yun A, Jian Guan, Baofu Lu, Bei Wu, Wenjuan Wang, Ruihong Ma, and Pan Nan. 2023. "Analysis of Phenotypic and Physiological Characteristics of Plant Height Difference in Alfalfa" Agronomy 13, no. 7: 1744. https://doi.org/10.3390/agronomy13071744
APA StyleJing, F., Shi, S., A, Y., Guan, J., Lu, B., Wu, B., Wang, W., Ma, R., & Nan, P. (2023). Analysis of Phenotypic and Physiological Characteristics of Plant Height Difference in Alfalfa. Agronomy, 13(7), 1744. https://doi.org/10.3390/agronomy13071744




