Abstract
Bamboo is nutritionally significant across the world because the shoots are high in calories and nutritional fiber but low in cholesterol. However, recent research has shown that bamboo shoots also contain a substantial quantity of heavy metals, including arsenic (As). Therefore, we explored whether the co-application of iron oxide nanoparticles (IONPs) and selenium nanoparticles (Se-NPs) would attenuate As toxicity in bamboo plants (Pleioblastus pygmaeus). A greenhouse experiment was performed to investigate plant responses to arsenic toxicity. Bamboo plants exposed to four levels of As (0, 10, 20, and 40 mg L−1) were foliar-sprayed with 60 mg L−1 of Se-NPs and 60 mg L−1 of IONPs alone and in combination. The data indicated that different As concentrations (10, 20, and 40 mg L−1) caused membrane damage and reactive oxide species (ROS) production in bamboo cells, characterized by H2O2, O2•−, MDA, and EL increasing by up to 47%, 54%, 57%, and 65%, respectively, in comparison with a control. The co-application of 60 mg L−1 of Se-NPs + IONP markedly improved the antioxidant enzyme activities (by 75% in SOD, 27% in POD, 52% in CAT, 37% in GR, and 38% in PAL), total flavonoid content (42%), phenolic content (36%), proline (44%), nitric oxide (59%), putrescine (Put) (85%), spermidine (Spd) (53%), relative water content (RWC) (36%), photosynthetic characteristics (27%) in net photosynthesis (Pn) (24% in the intercellular CO2 concentration (Ci), 39% in stomatal conductance (Gs), and 31% in chlorophyll pigments), and ultimately biomass indices and growth. The co-application of Se-NPs + IONPs with 10 and 20 mg L−1 of As raised the TI by 14% and 9% in the shoot and by 18% and 14% in the root, respectively. IONPs and Se-NPs reduced ROS, cell membrane lipoperoxidation, and electrolyte leakage, all contributing to the decrease in oxidative stress by limiting As uptake and translocation. In sum, Se-NPs and IONPs improved bamboo endurance, yet the most effective approach for increasing bamboo’s ability to recover from As toxicity was the concurrent use of 60 mg L−1 of Se-NPs and 60 mg L−1 of IONPs. Our IONP and Se-NP data from single and combined applications offer novel knowledge in improving the tolerance mechanism against As exposure in Pleioblastus pygmaeus.
1. Introduction
Today, soil pollution from heavy metal(oid)s (HMs) has become an important issue. It could restrict plant growth and reduce crop production and pose a potential risk by entering the food web and threatening human health [1]. Indeed, even a single HM present in the soil has the potential to contribute to the significant widespread contamination of underground water supplies [2]. For instance, consuming drinking water containing more than 10 μg L−1 of As is toxic, and arsenic contamination could harm the health of up to 200 million individuals worldwide [3]. Anthropogenic activities such as metal smelting, mining, utilizing fossil fuels, and using disproportionate numbers of insecticides, herbicides, and pesticides in agriculture may result in the accumulation of arsenic (As), a non-essential (toxic) metalloid [4]. In plants, As can bind to proteins and enzyme complexes, thus disrupting cell biochemistry because of the overproduction of reactive oxygen species (ROS), leading to oxidative stress [5] and affecting respiration, photosynthesis, and transpiration, along with damage to carbohydrates and DNA [6]. This might limit plant reproductive capacity and lower the strength of the photosynthetic apparatus [7,8]. Morphologically, As leads to necrosis, leaf senescence, defoliation, reduction in leaf number, and chlorosis. This hinders development by reducing plant biomass and yield and inhibiting cell proliferation and root extension [3].
Researchers have recently proposed incorporating metal-based nanoparticles (NPs) as a feasible way of reducing environmental pollutants [9]. Even though there have been particular concerns arising from NP synthesis techniques, cellular penetration strategies, and ecotoxicological effects, their robust structure, compact size, high surface area, and high potential for adsorption, in general, make NPs valuable candidates for removing HMs from plants [10,11]. NPs can enter plants through the stomata opening (10 nanometers in diameter) and induce several mechanistic modifications, such as a reduction in ROS in cells [12,13,14,15].
One of the crucial trace elements in plants, selenium (Se), is a nutrient that can both enhance plant development and strengthen the immune systems of individuals [16,17]. Several research studies have proven that Se can help plant growth under different metal toxicity levels [18,19]. Selenium, by activating selenoenzymes, can increase antioxidant capacity, which can induce ROS scavenging, resulting in a preserved membrane in the plant cell [1]. Iron (Fe) is another essential micronutrient that can affect plants’ different physiological mechanisms and biochemical processes. Despite Fe being known to be an abundant element, Fe concentrations are generally insufficient for plant needs [20,21]. Because of improved photosystem II efficacy, catalysis of enzymatic reactions, RNA synthesis, DNA transcription, and auxin activity in plants, more substantial plant development has been observed using Fe supplementation [22]. Fe NPs impact plant cells at the molecular level, enhancing nutritional assimilation and absorption [23]. Furthermore, iron oxide nanoparticles (IONPs) increase agricultural production in many plant species by reducing metal and drought stress [24,25]. Because Fe-containing minerals are poorly soluble, applying Fe in NP form externally can be a powerful way to address Fe shortages in plants and improve the plant’s ability to withstand HM stress [21].
Bamboo (Bambusoideae) is a fast-growing plant with the highest biomass that grows in a large area of Chinese forestland (more than 6 million ha) and the world’s forestland (approx. 31.5 million ha) [26,27]. Bamboo is an economical source of livelihood for local people in China and South Asia and is of particular importance [28]. The shoots are high-calorie, low-fat, and have high dietary fiber and can be used as a food source worldwide [29]. Additionally, given its unique qualities, such as high biomass and rapid vegetative development, bamboo can be used as a phytoremediation plant to remove HMs from polluted regions [30]. Therefore, using bamboo species as an eco-friendly option to clean up urban areas might be a practical concept. Bamboo species are also used commercially for gardening and landscape design [31]. Pleioblastus pygmaeus, with an average height of 30–50 cm, has been used specifically for this aim [32]. Numerous Chinese provinces, including Jiangsu, use Pleioblastus pygmaeus. On the other hand, results taken from sampled plants in various Chinese local marketplaces have shown that bamboo shoots have a remarkably high As concentration [33]. Therefore, investigating bio-nutrient and economic variables to detect and curb pollution levels from the ecosystem and, ultimately, the food web is crucial. To the best of our knowledge, this is the first research using foliar-applied Se-NPs and IONPs either alone or in combination to decrease As toxicity in bamboo plants, although the utilization of Fe and Se in ameliorating HM stress has previously been documented. Thus, in the current work, we aimed to investigate the impact of Se-NPs and IONPs in single and combination forms on bamboo plants under As toxicity, emphasizing associated mechanisms in As detoxification. We suggest that IONPs and Se-NPs can increase bamboo tolerance through antioxidant machinery and polyamine activity, thus decreasing bioavailability and, subsequently, As toxicity.
2. Materials and Methods
2.1. Growth Conditions
The current research was performed in a controlled greenhouse with a relative humidity of 69–79% and a photoperiod of 16 h/8 h in light/dark. Perlite and coco peat were used as a growth medium at a ratio of 1:2 in 3 L pots. One-year-old bamboo plants (Pleioblastus pygmaeus) were provided from the greenhouse belonging to the Bamboo Research Institute, Nanjing Forestry University, located in Jiangsu Province, China, and cultivated in the pots for 60 days. Each pot had five bamboo plants. Four concentrations of As, including 0 (control), 10, 20, and 40 mg L−1, were applied via irrigation in a total solution volume of 250 mL for each pot (in four replicates) throughout the course of the trial. The experimental design is provided in Table 1.

Table 1.
The experimental design.
Se-NPs (Sigma-Aldrich, St. Louis, MO, USA) with spherical shapes and in a powder form (10–40 nm dimensions and 99.9% purity) were supplied by Nanjing Jiancheng Company, Nanjing, China. Iron oxide nanoparticles (IONPs) 15–25 nm in size, 4.67 g/cm3 density, and 99% purity were also purchased from Nanjing Jiancheng Company, Nanjing, China) Se-NPs (30 mg) were solvated in water (500 mL) and sprayed onto the leaves of each bamboo plant 20 days after the trial’s start. This was repeated after 40 days (total: 60 mg L−1 Se-NPs). This was similar to IONPs. For co-application treatment, the first spray of 15 mg of Se-NPs + 15 mg IONPs was solvated in 500 mL water and then sprayed onto each bamboo plant’s leaves 20 days after the trial’s start. This was repeated after 40 days (total: 60 mg L−1 Se-NPs + IONPs). Each pot of plants was nourished with 400 mL of nutrient solution at 5-day intervals. The measurement of morphological and photosynthetic parameters was conducted in the greenhouse. Then, the leaves, stem, and roots were separately sampled and transferred to the lab. The samples were maintained in the refrigerator (Haier) for the determination of enzymatic activities and biochemistry measurements.
2.2. As Content in Bamboo Organs (Roots, Stems, and Leaves)
In total, 6 mL of nitric acid (HNO3- 64%) was added to 0.5 g of dry leaf samples and then incubated at 28 °C overnight (o/n). Then, the treatments were moved to an oven (China Energy) at 95 °C so that all the NO2 evaporated. The As concentration in bamboo organs was determined using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 4500 series, USA) [34].
2.3. Calculation of the Bioaccumulation Factor, Translocation Factor, and Tolerance Index
The bioaccumulation factor (BAF), translocation factor (TF), and tolerance index (TI) were calculated as indicators of phytoextraction efficiency using the method of Souri and Karimi [35] to show the phytoremediation potential of bamboo plants. The BAF, TF, and TI values were calculated by using the following formulae:
BAF = (the content of As in root/stem and leaves)/(content of As in the medium)
TF = (the content of As in the stem and leaves)/(content of As in the root)
TI of Shoot = (As in shoot dry weight)/(shoot dry weight of control)
TI of root = (As in root dry weight)/(root dry weight of control)
2.4. Determination of Dry Weight of Shoots and Roots
After the experiment, treated bamboo shoots and roots were washed and cleaned. The water content was removed by keeping samples in a vacuum-drying oven (DZF-6090) (Xiamen Tob New Energy Technology, Xiamen, China) at 118° C for 28 min. Then, the parameters were fixed at 78 °C for 48 h to reach constant weight. Shoot and root samples were weighed for all four replicates and displayed as the shoot and root dry weight.
2.5. Antioxidant Enzyme Activities
In total, 0.6 g of bamboo leaves were crushed in a mortar and pestle using liquid nitrogen (LN) to prepare samples for the antioxidant enzyme activity determinations. The obtained leaf powders were mixed with 4 mg of pH 7.6 phosphate buffer. The mixture was centrifuged at 3500–4500× g at 6 °C for 18 min to obtain the final supernatant.
Superoxide dismutase (SOD) activity was measured by using the photoreduction of nitro blue tetrazolium (NBT) via the Senthilkumar [36] protocol. The peroxidase activity (POD), catalase (CAT), and glutathione reductase (GR) activities were measured using the protocol of Liu [37], Aebi [38], and Foyer and Halliwell with some modifications [39]. Phenylalanine ammonia-lyase (PAL) activity was obtained using the protocol of Berner [40]. The enzymatic activities were expressed as U mg−1 of protein.
2.6. Total Phenolics and Flavonoid Content
Total flavonoid content (TFC) was determined based on [41] by plotting the curve using a quercetin standard. The total phenolic content (TPC) was measured with a reference protocol [42], using gallic acid (GA) as a standard and Folin–Ciocalteu as a reagent.
2.7. Hydrogen Peroxide Malondialdehyde, Superoxide Radical, and Electrolyte Leakage
Hydrogen peroxide (H2O2) content was determined as per Velikova [43]. The superoxide radical (O2•−) content was obtained using the Li method [44]. Malondialdehyde (MDA) content, as a lipid peroxidation biomarker, was estimated using Rao and Sresty’s protocol [45]. Electrolyte leakage (EL) was determined using the Valentovic method [46]. EL was obtained via the difference between primary electrical conductivity (EC1) and final conductivity (EC2). The following formula was used to calculate the EC percentage:
EL (%) = (EC1/EC2) × 100
2.8. Nitric Oxide and Relative Water Content
The nitric oxide content was determined using the detection kit following the manufacturer’s instructions accordingly, which Solar Bio Life Science, Beijing, China provided. RWC was obtained by measuring the fresh weight (FW) and saturation weight (SW) (in leaves immersed in distilled water o/n), as well as the dry weight (DW) (at 70 °C o/n). Relative water content (RWC) was obtained according to the following formula [47]:
RWC (%) = (FW − DW)/(SW − DW) ×100
2.9. Proline Content and Spermidine and Putrescine
Proline content was obtained using the Bates protocol [48]. The spermidine (Spd) and putrescine (Put) contents were analyzed based on the levels of H2O2 produced through polyamine oxidation. The spermidine (Spd) and putrescine (Put) contents were estimated based on the method of Zhao et al. [49].
2.10. Gaseous Exchange and Photosynthetic Pigments
A portable gas exchange device was used to measure the intercellular CO2 concentration (Ci), net photosynthesis (Pn), and stomatal conductance (Gs) on larger leaves at 9:30 in the morning at 26 °C [50]. The total photosynthetic pigments were calculated using the methodology of [51] by adding 80% acetone to a 100 mg leaf extract and centrifuging the mixture for 10 min at 8000 rpm. A spectrophotometer (Hitachi U-2001, Tokyo, Japan) was used to quantify total chlorophyll absorbance at 630, 647, and 664 nm wavelengths.
2.11. Statistics
The study was implemented in a completely randomized design (CRD) with four replicates. A 2-way factorial design was used to analyze variance in the R package. For the computation of the mean differences between treatments, Duncan’s test was used at a level of p < 0.05.
3. Results
3.1. Impact of Se-NPs and IONPs on As Content in Bamboo Plant Organs
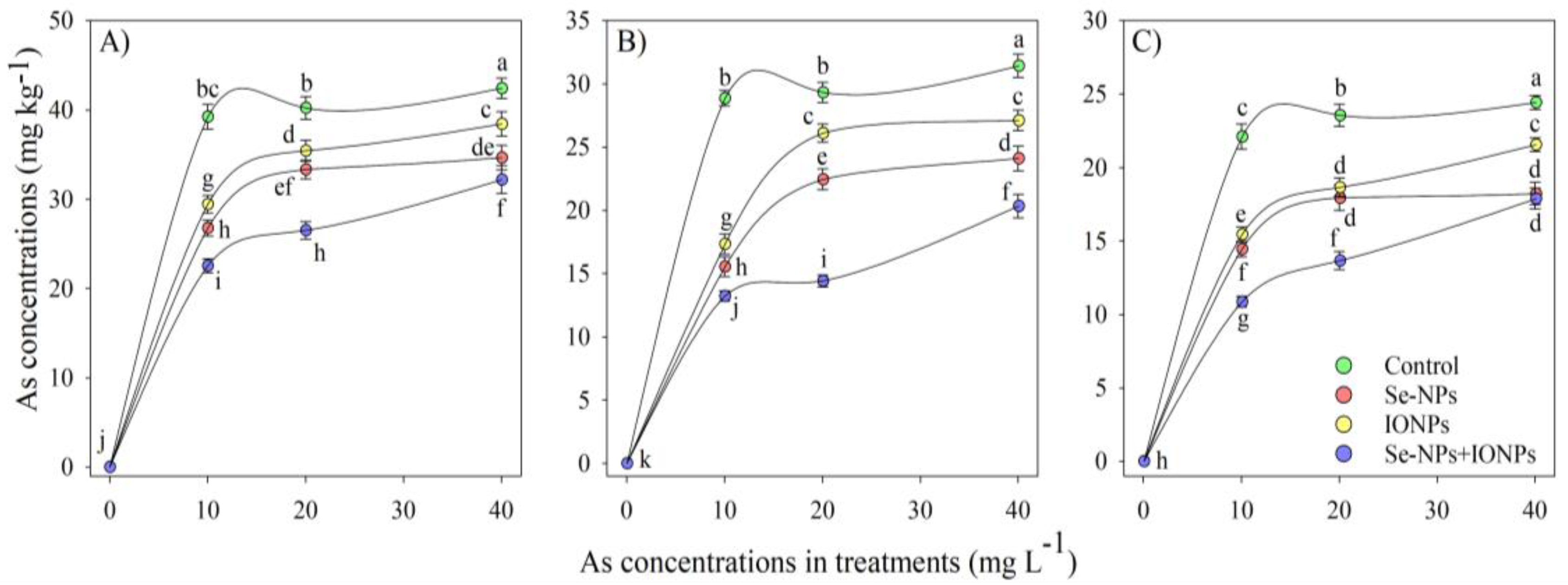
The presence of HMs in plant organs is a significant sign of a plant’s stress level. Our findings revealed a substantial disparity of As content between the bamboo plant leaves, stems, and roots (p < 0.001). The highest reduction in As content was found using the combined Se-NP + IONP group, which showed that As (10 and 20 mg L−1) decreased by 33% and 15% in leaves, 28% and 21% in the stem, and 26% and 13% in roots when compared with the control treatments, respectively (Figure 1). On the other hand, Se-NPs and IONPs (60 mg L−1) alone significantly reduced As levels in bamboo organs. Yet, the combination form of Se-NPs + IONPs showed the most critical impact on As decreases, demonstrating the effectiveness of combining nanoparticles rather than using a single version to reduce As absorption by plants.
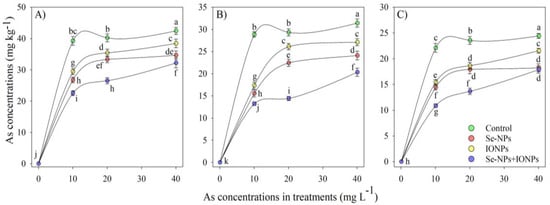
Figure 1.
Arsenic concentrations in bamboo organs: (A) root, (B) stem, (C) leaves. The data display the mean ± standard error (n = 4). The treatments comprise single and combined Se-NPs and IONPs with four different As concentrations. Se-NPs and IONPs: 60 mg L−1. The lowercase letters show significant differences between the treatments (Duncan, p < 0.05).
3.2. Impact of Se-NPs and IONPs on Bioaccumulation Factor, Translocation Factor, and Tolerance Index (TI) in Bamboo Exposed to As Toxicity
To show the efficacy of Se-NPs and IONPs in lowering As toxicity, the phytoextraction potency of bamboo was measured using the bioaccumulation factor (BAF) of the leaves, stems, and roots; the translocation factor (TF) of the stems and leaves; and the TI of the shoots and roots. There was a significant difference in the BAFs between the bamboo treatments (p < 0.001). The BAFs in the leaves, stems, and roots of bamboo subjected to As decreased after applying NPs; thus, Se-NPs and IONPs inhibited As uptake in plant organs. The lowest BAF was related to the co-application of Se-NPs + IONPs with 10, 20, and 40 mg L−1 of As, which led to 50%, 54%, and 42% reductions with 10 mg L−1; 42%, 50%, and 37% reductions with 20 mg L−1; and 26%, 35%, and 24% reductions with 40 mg L−1 As as compared with the controls in leaves, stems and roots, respectively. On the other hand, the capacity of As ions to absorb and adhere to the Se-NP and IONP surfaces caused the decrease in the As-BAF to diminish the TF in the plants. The lowest TF percentage was related to the co-application of Se-NPs + IONPs with 10, 20, and 40 mg L−1 of As, resulting in 20%, 26%, and 14% decreases in the stems and 19%, 11%, and 3% reductions in the leaves, respectively. Thus, the bamboo tolerance index (TI) was enhanced. There was also a significant difference between treatments for the TI of the shoot/root samples (p < 0.001). The findings showed that, in contrast to the control treatment, introducing Se-NPs and IONPs raised the TIs by 22%, 18%, and 26% in the stem and 23%, 21%, and 27% in the root (Table 2).

Table 2.
Changes in the bioaccumulation factor (BAF) of roots, stems, and leaves; the translocation factor (TF) of stems and leaves; and the tolerance index (TI) of shoots and roots in response to Se-NPs and IONPs, whether independently or in combination, with 10, 20, and 40 mg L−1 of As relative to the control. The data display the mean ± standard error for four replicates. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
3.3. Impact of Se-NPs and IONPs on Root and Shoot Dry Weight in Bamboo Exposed to As Toxicity
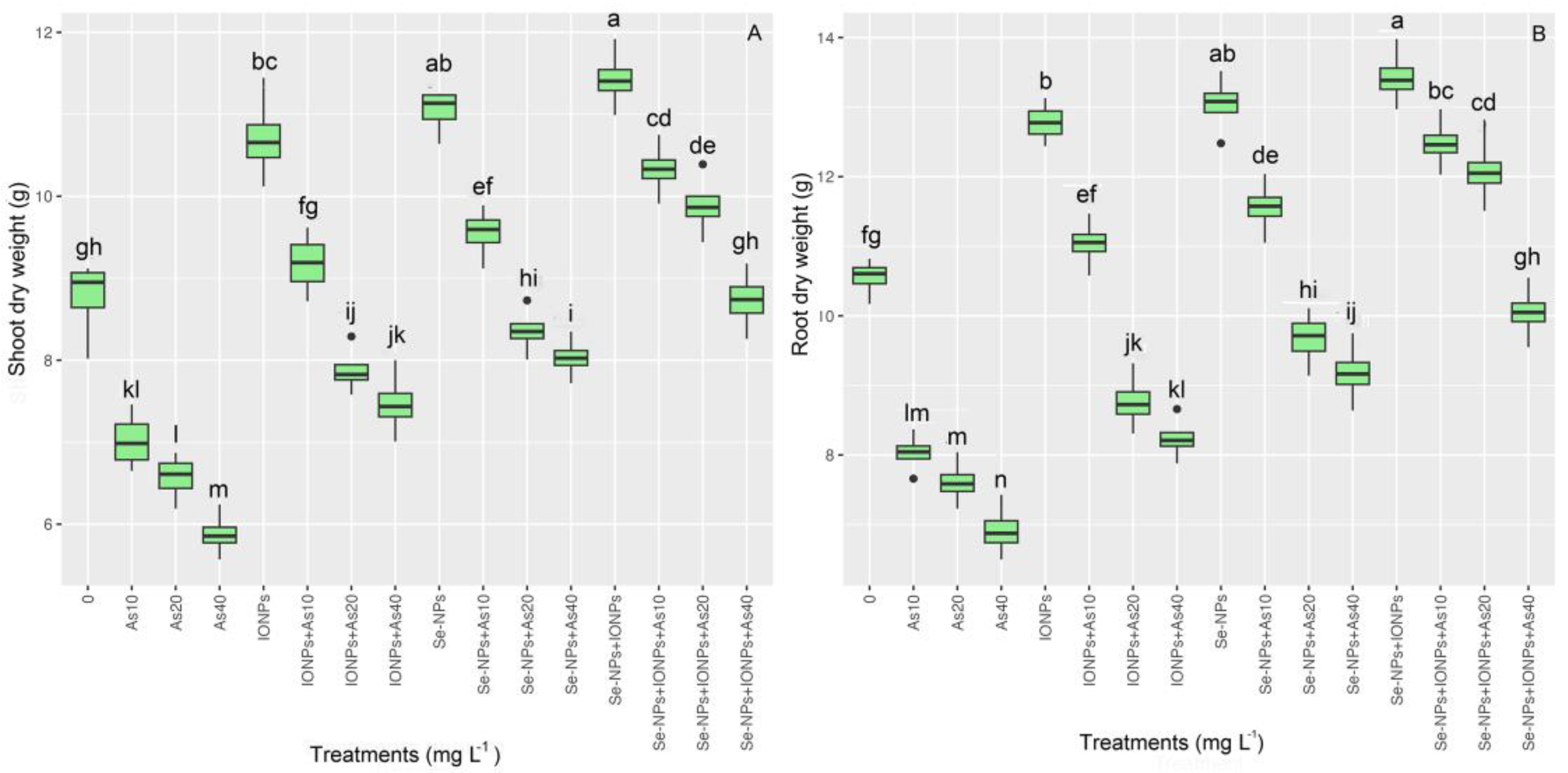
The data showed that applying NPs both independently and in combination improved plant biomass under As toxicity. The different As concentrations substantially reduced the plants’ shoot and root dry weights (SHDWs and RDWs). The SHDWs and RDWs significantly differed between groups (p < 0.001). It was found that SHDWs increased by 26%, 22%, and 18%, while RDWs increased by 27%, 23%, and 21%, respectively, for 10, 20, and 40 mg L−1 of As. In addition, the results showed that both NPs significantly promoted plant biomass under As toxicity compared with the controls. Thus, the highest SHDWs and RDWs were related to the co-application of Se-NPs + IONPs with 10 and 20 mg L−1 of As, with 14% and 9% increases in the SHDWs and 18% and 14% increases in RDWs (Figure 2) (Table 3).
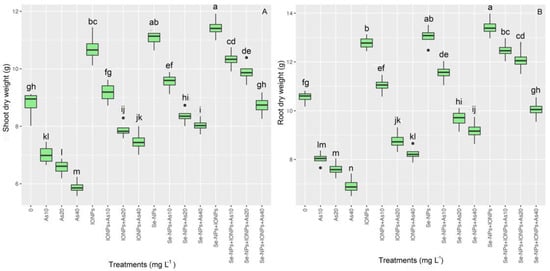
Figure 2.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on the SHDWs (A) and RDWs (B). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).

Table 3.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on Pleioblastus pygmaea L. SHDWs and SHDWs relative to the control; ↓ indicates a decrease, and ↑ indicates an increase.
3.4. Effect of Se-NPs and IONPs on Antioxidant Enzyme Activities in Bamboo Exposed to As Toxicity
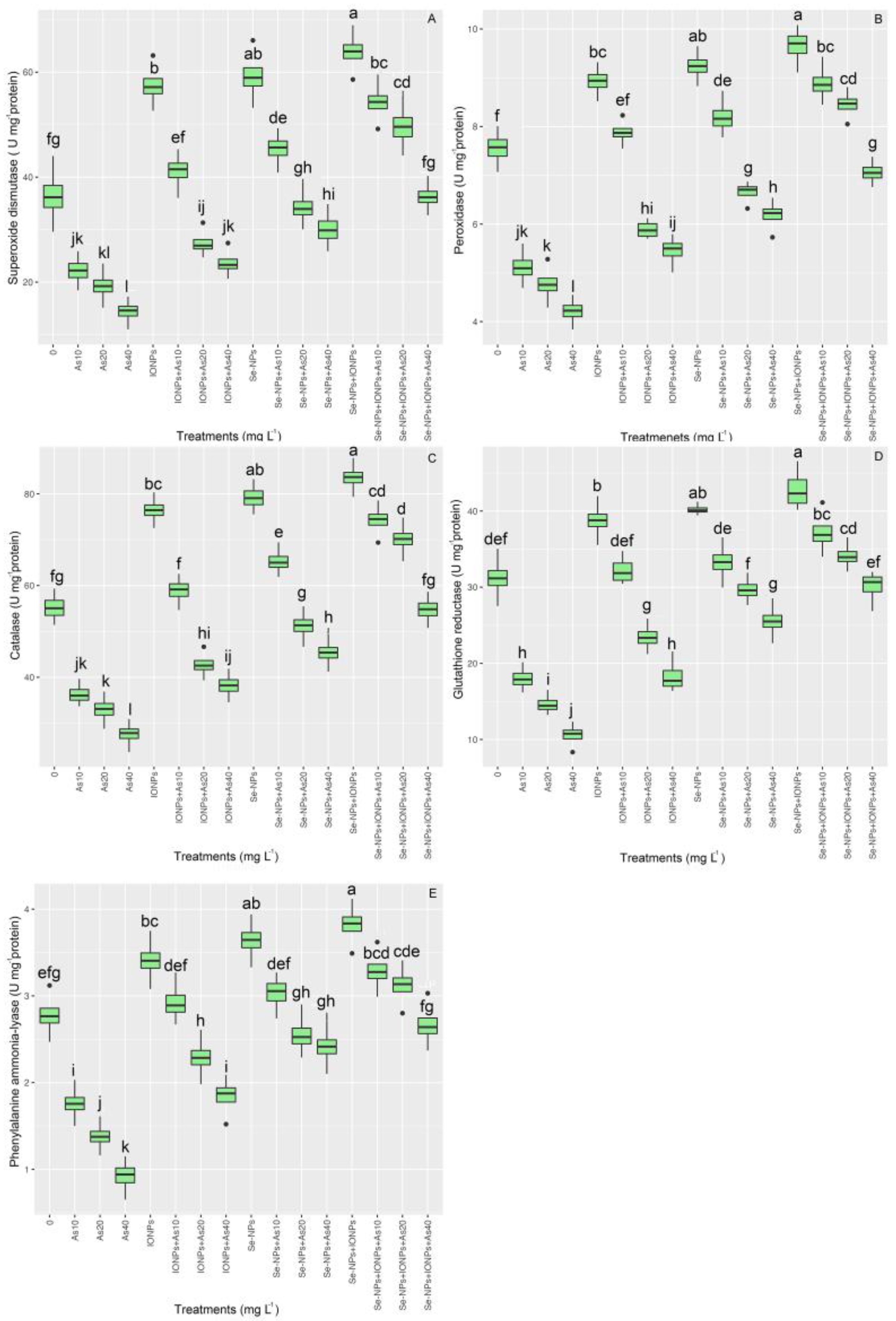
The antioxidant activity varied significantly between the treatments (p < 0.001). The co-application of Se-NPs + IONPs, with increases of 48% in SOD, 17% in POD, 51% in CAT, 19% in GR, and 18% in PAL in comparison with the controls, was found to have the highest antioxidant activity in bamboo plants under As toxicity. In addition, Se-NPs and IONPs alone increased antioxidant activity; however, the highest increase was in the group with 10 mg L−1 of As, with 24% and 12% increases in SOD; 8% and 4% in POD; 18% and 6% in CAT; 6% and 3% in GR; and 8% and 5% in PAL, respectively. On the other hand, As significantly reduced antioxidant activity in bamboo species, as expected. The results showed the lowest antioxidant activity was in the treatments exposed to 40 mg L−1 of As, with a 60% decrement in SOD; 44% in POD; 50% in CAT; 66% in GR; and 66% in PAL activity, as compared with the control groups (Figure 3).
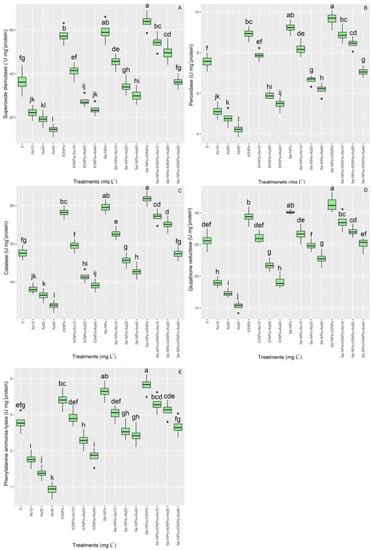
Figure 3.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on antioxidant enzyme activity: (A) superoxide dismutase (SOD); (B) peroxidase (POD); (C) catalase (CAT); (D) glutathione reductase (GR); and (E) phenylalanine ammonia-lyase (PAL). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
3.5. Impact of Se-NPs and IONPs on Flavonoid Content and Phenolics in Bamboo under As Toxicity
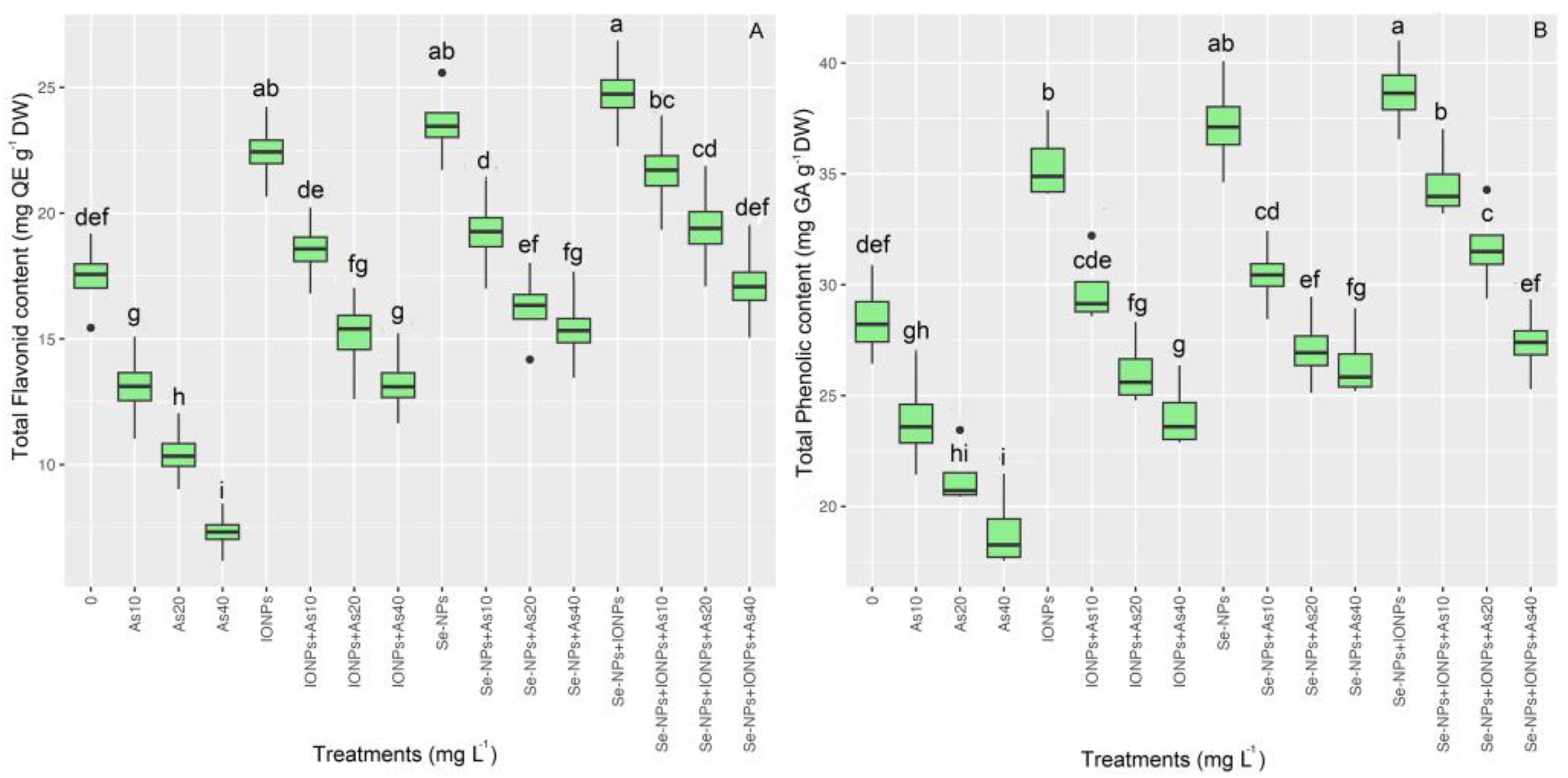
The two non-enzymatic antioxidant factors, i.e., the total flavonoid content (TFC) and total phenolic content (TPC), are indicators of secondary metabolism in plants under stress. We found a significant difference in the TFC and TPC (p < 0.001). Se-NPs and IONPs alone and combined elevated TFC and TFC in bamboo plants under different concentrations of As (10, 20, and 40 mg L−1). However, the highest TFC and TPC were in the co-application of Se-NPs and IONPs exposed to 10 mg L−1 of As, with a 24% increase in TFC and a 21% increase in TPC, compared with the respective controls. On the other hand, the lowest TFC and TPC were found using 40, 20, and 10 mg L−1 As, with 58%, 40%, and 24% decrements in TFC and 33%, 25%, and 16% decrements in TPC, respectively. Therefore, we propose that Se-NPs and IONPs can enhance TFC and TPC under normal and toxic As conditions (Figure 4).
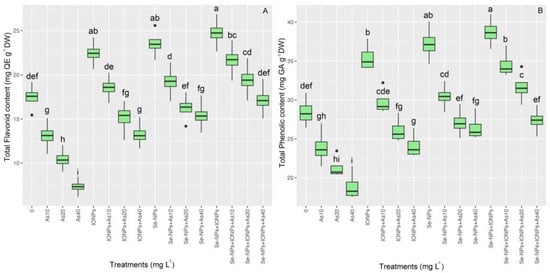
Figure 4.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on TFC (A) and TPC (B). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
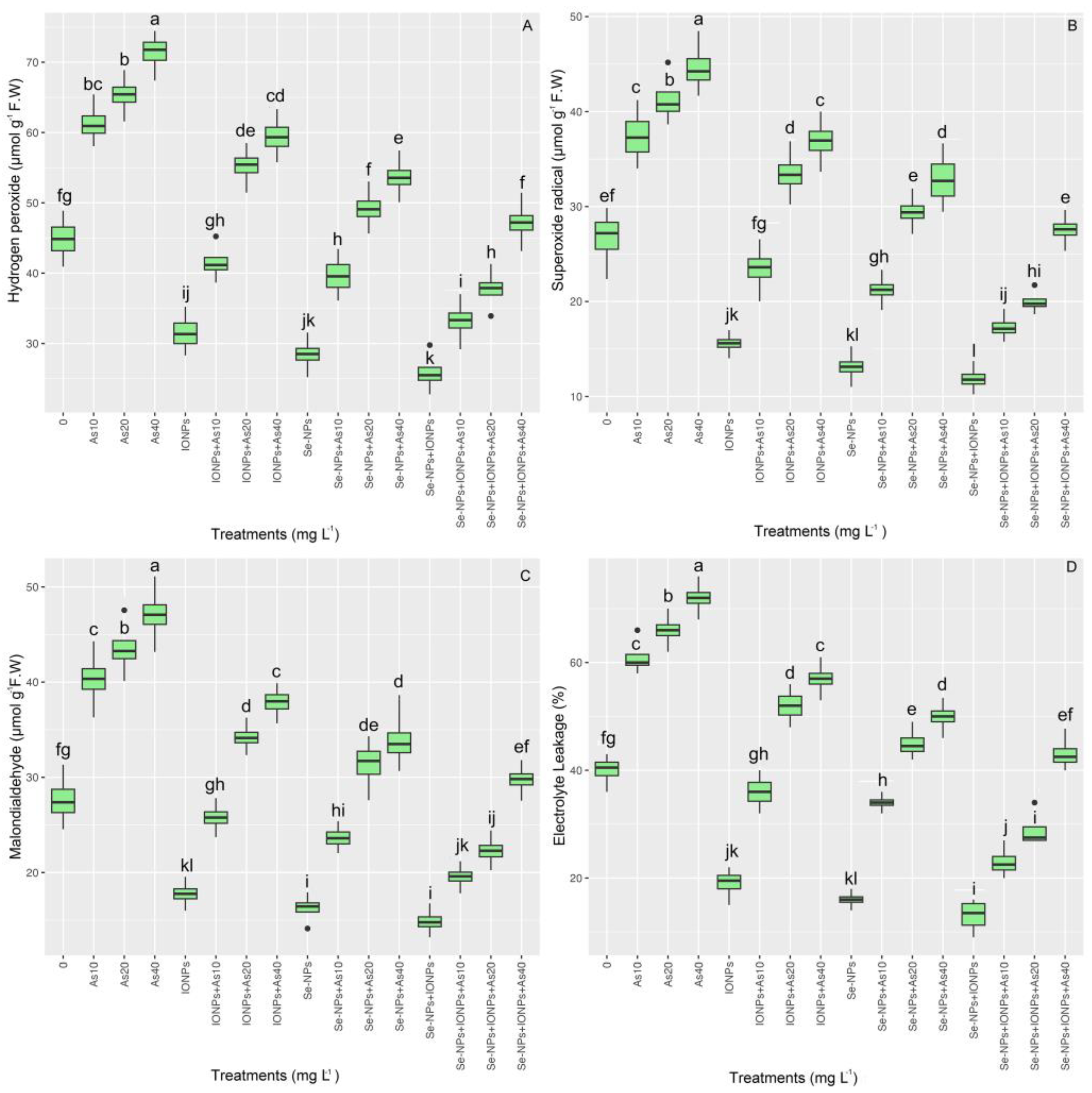
3.6. Impact of Se-NPs and IONPs on Hydrogen Peroxide, Malondialdehyde, Superoxide Radicals, and Electrolyte Leakage in Bamboo Exposed to As Toxicity
ROS compounds such as hydrogen peroxide (H2O2), superoxide radical (O2•−), and malondialdehyde (MDA), as well as electrolyte leakage (EL), are all oxidative stress markers in plants. In this research, bamboo was significantly less susceptible to oxidative stress, with evident reductions in H2O2, O2•−, MDA, and the percentage of EL content when NPs were administered. The data show that there was a considerable change (p < 0.001) in the concentrations of H2O2, O2•−, MDA, and EL among treatments (Figure 5). H2O2 content increased by 29%, 40%, and 48%; O2•− increased by 29%, 55%, and 57%; MDA content increased by 57%, 69%, and 82%; and EL increased by 46%, 65%, and 80% relative to the controls at concentrations of 10, 20, and 40 mg L−1, respectively. ROS production and lipoperoxidation were considerably reduced with the use of NPs. Hence, the co-application of Se-NPs + IONPs subjected to 10 mg L−1 of As led to the most considerable decrease, with corresponding reductions of 42% in H2O2, 52% in O2•−, 52% in MDA content, and 60% in the percentage of EL compared with their respective controls.
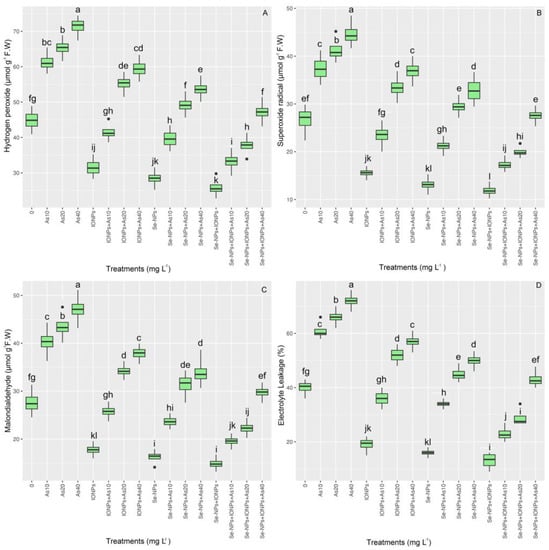
Figure 5.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on H2O2 (A), O2•− (B), MDA (C), and EL (D). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
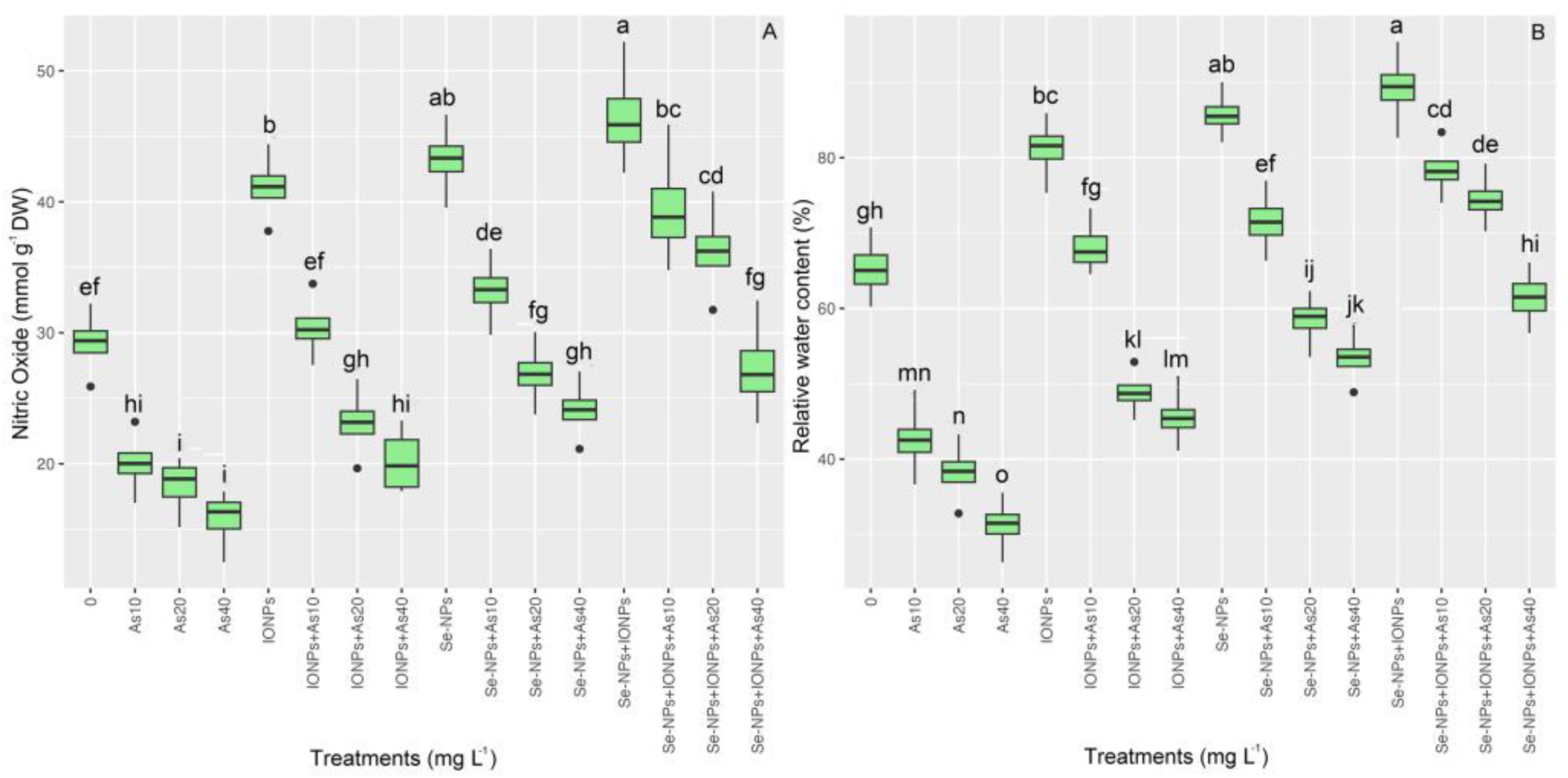
3.7. Impact of Se-NPs and IONPs on Nitric Oxide and Water Content in Bamboo Exposed to As Toxicity
The results of the nitric oxide analysis of bamboo plants revealed that there was substantial variation between the different treatments (p < 0.001). Nitric oxide levels in plants under varying As levels (10, 20, and 40 mg L−1) increased considerably after NP application. The utilization of Se-NPs and IONPs in a combined form led to the highest levels of nitric oxide concentration, rising by 60%. On the other hand, single Se-NPs and single IONPs led to 47% and 40% nitric oxide increments in comparison with the control treatments, respectively (Figure 6). Our findings showed that, whereas the addition of 60 mg L−1 each of Se-NPs and IONPs increased the RWC in plants, varying amounts of As dramatically decreased the RWC. The co-application of 60 mg L−1 of Se-NPs + IONPs with 10 and 20 mg L−1 of As caused 18% and 12% increases in the RWC, respectively, as compared with the treatment used on the controls (Figure 6). By strengthening antioxidant activity and bamboo’s nitric oxide production, we argue that Se + IO nano-sized particles can significantly preserve the RWC.
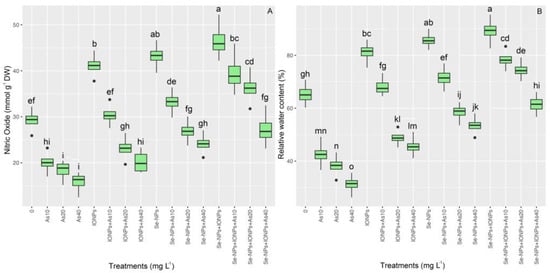
Figure 6.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on nitric oxide (A) and relative water content (RWC) (B). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
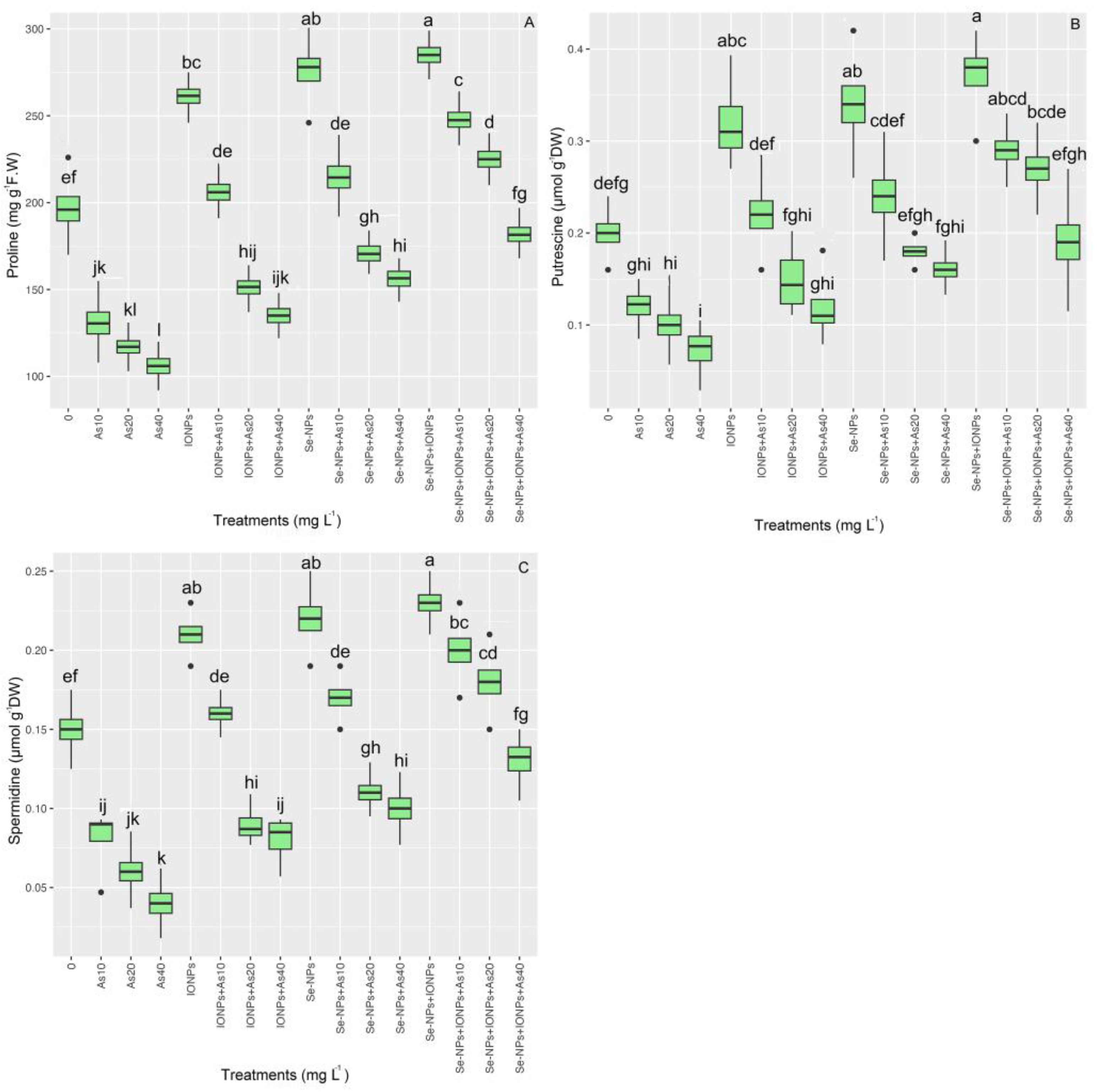
3.8. Impact of Se-NPs and IONPs on Proline, Spermidine (Spd), and Putrescine (Put) in Bamboo under As Toxicity
The data showed a significant variation in proline content across various treatments (p < 0.001). The proline content rose with 60 mg L−1 of each NP in single and combination forms, increasing by 39% for Se-NPs, 32% for IONPs, and 44% for Se-NPs + IONPs compared with the control. The co-application of NPs with 10 and 20 mg L−1 of As is associated with 25% and 14% increases in proline accumulation, respectively.
The putrescine (Put) and spermidine (Spd) levels varied significantly between the treatments (p < 0.001). The data demonstrated that combining Se-NPs and IONPs increased Put and Spd in bamboo plants. As a result, the co-application of Se-NPs + IONPs with 10 and 20 mg L−1 of As yielded the highest amounts, with corresponding Put and Spd increases of 33%, 23%, 26%, and 13%, respectively, compared with the control. The data showed that Se-NPs and IONPs alone could increase Put and Spd contents. The highest Put and Spd contents were found in the treatment with 60 mg L−1 of Se-NPs, with 66% and 40% increases in Put and Spd. Single-treatment IONPs provided 57% and 40% increases in Put and Spd compared with the control groups (Figure 7).
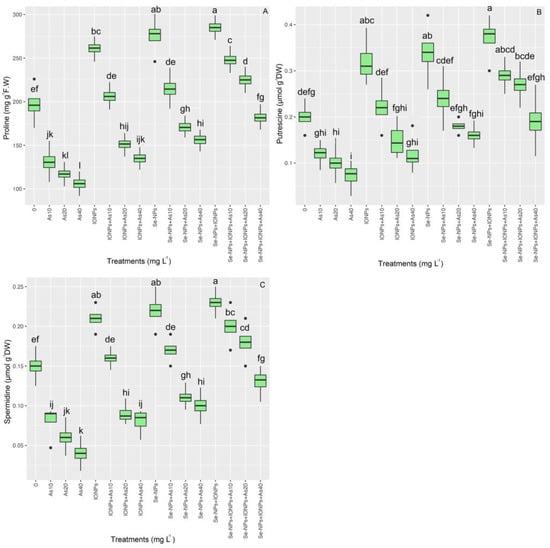
Figure 7.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on proline content (A), putrescine (Put) (B), and spermidine (Spd) (C). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
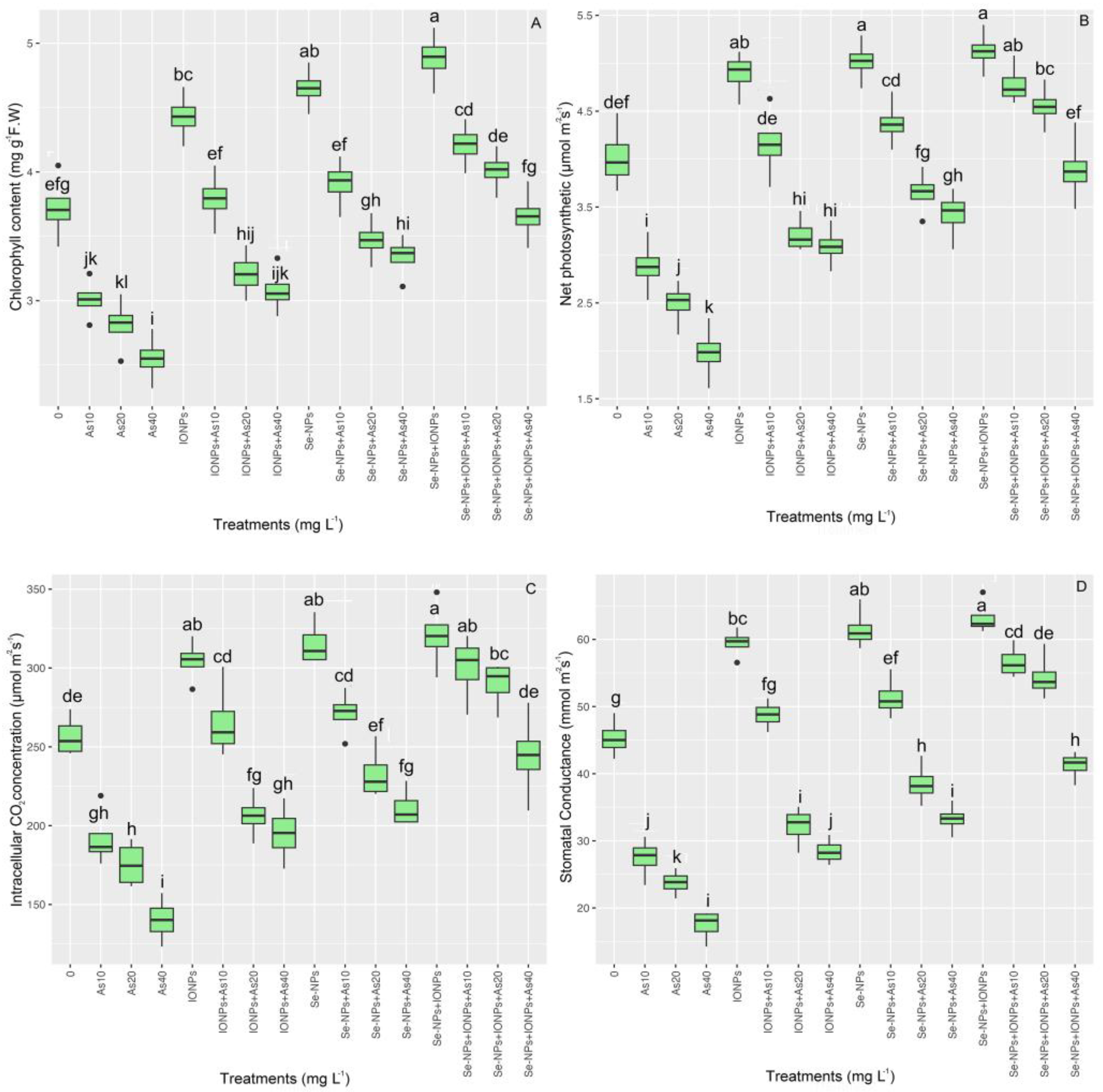
3.9. Effect of Se-NPs and IONPs on Gas Exchange and Photosynthetic Pigments in Bamboo Exposed to As Toxicity
The pattern suggests that the bamboo plant’s photosynthetic rate increased as a result of NPs under As (p < 0.001). The highest net photosynthesis (Pn) content, total pigment, intercellular CO2 concentration (Ci), and stomatal conductance (Gs) was found in the co-application of Se-NP + IONP group with 10 and 20 mg L−1 of As, with 13% and 7% increases in pigment concentration, 19% and 13% increases in net photosynthesis (Pn), 10% and 14% increases in intercellular CO2 concentration (Ci), and 23% and 19% increases in stomatal conductance (Gs), respectively. However, the co-application of Se-NPs + IONPs showed a rising tendency in the treatments under 40 mg L−1 of As. The two NPs (Se-NPs and IONPs) in single and combined forms significantly increased pigment intactness and gas exchange properties under different levels of As (10, 20, and 40 mg L−1) compared with their control treatments (Figure 8).
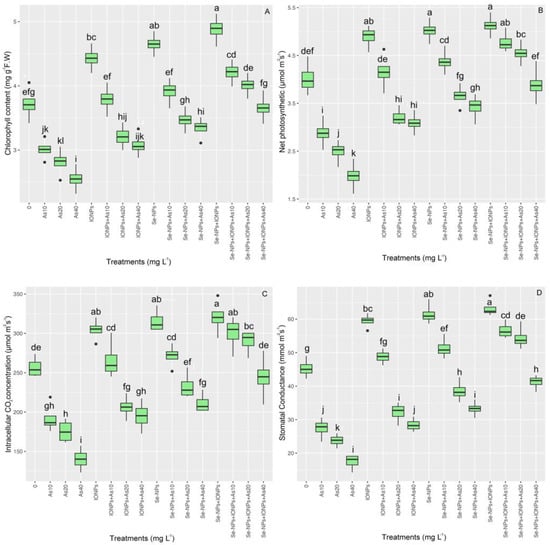
Figure 8.
The effect of Se-NPs and IONPs alone and in combination with varying As concentrations on the total photosynthetic pigment (A), net photosynthesis (Pn) (B), intercellular CO2 concentration (Ci) (C), and stomatal conductance (Gs) (D). Boxes represent the lower and upper quartiles (25–75%). The whiskers show the min–max values. Lines in the boxes indicate median values. The lowercase letters show significant differences between the treatments using Duncan’s test (p < 0.05).
4. Discussion
Arsenic is a naturally occurring metalloid element with a large lithospheric distribution. Even tiny particles in agricultural soil and drinking water can harm human health [52]. However, NPs with unique surface area features can absorb and translocate As in the soil [53]. Selenium and iron oxide stimulate plant development through nano-sized particles [16,17,18,19,20,21]. These two micronutrients positively impact the enhancement of root length, which can help further boost the absorbance of nutrients from the soil. A related phenomenon resulting in improved plant growth was previously reported for pepper (Capsicum annuum L.) using Se and Arachishypogaea via IONPs [54,55]. Nanoparticles may exchange and absorb metal ions on the surface of the leaves and roots of plants, increasing their tolerance to HM stress [56]. In addition, metal-based NPs can release metal ions into plant cells [57]. A study on the impacts of silicon (Si) NPs showed that Si-NPs could adsorb metal ions, which limits Cd accumulation in leaves [27]. In this study, our results showed beneficial effects at multiple levels in plants treated with NPs. Our data demonstrated that foliar-spraying Se and IONPs in bamboo under As toxicity reduced the bioaccumulation of As in plant organs (leaves, stems, and roots). This can be attributed to the role of NPs in the absorption of metal ions and changes in metal distribution rates in leaves, and this has also been reported regarding the utilization of titanium dioxide NPs on Coriandrum sativum L. [58] and Se-NPs [59] and IONPs on wheat (Triticum aestivum) [55]. However, our results showed that the concentration of As in bamboo roots was higher than in bamboo stems and shoots, and it might be inferred that the two NPs applied inhibit the translocation of As from roots to shoots by enhancing the absorption of metal ions in roots, therefore maximizing plant tolerance to As toxicity. We hypothesize that the NPs, after penetrating the plant through the stomata of the bamboo leaves, are transferred to the bamboo rhizome junction and root surface and inhibit arsenic uptake by bamboo roots. Kerui Guo reported a similar mechanism when spraying nano-materials on cucumber leaves [60]. Thus, the reduction in As uptake by NPs in roots can be related to the high surface area of NPs and their potential to adsorb metal ions [61]. Van der Waals forces are also reported to be involved in the physical adsorption of metal ions onto the adsorbent [62]. Therefore, the NPs reduce the translocation of As to aerial parts by limiting arsenic uptake via roots. It was also reported that by modifying gene expression and rising antioxidant capacity in plants, NPs can also limit metal translocation from roots to shoots [63]. The excess of HMs in plants leads to plant oxidative stress by generating ROS compounds [64,65]. This leads to osmotic shock in plants along with lipoperoxidation in membranes and proteins, cell wall thickness, nuclear/DNA injury, water potential, transpiration limitation, and ultimately plant growth inhibition [66]. Our results showed that while different levels of As increased oxidative stress in plants with increasing ROS (H2O2 and O2•−), MDA, and electrolyte leakage, the NP application reduced oxidative stress under different As exposure levels in comparison with control treatments.
The plants contain various defensive mechanisms to scavenge ROS, including increased antioxidant activity [67]. For instance, antioxidants can alleviate the toxicity of H2O2 by splitting molecules into oxygen and water [66]. In this study, we measured antioxidant indices, including SOD, CAT, POD, GR, and PAL. Our results demonstrated that Se-NPs and IONPs in single and combined forms in bamboo under As toxicity led to antioxidant activity increments. This significantly decreased MDA content, H2O2, O2•−, and EL in bamboo under As toxicity. This can be related to the reduction in HM accumulation in tissues by NPs which can reduce oxidative stress resulting in increased antioxidant capacity in plants under stress. With increasing plant nutrient ion availability, we also suggest that NPs may provide some essential co-factors involved in antioxidant molecule synthesis, which can help improve antioxidant activity. The increasing antioxidant capacity caused by NPs has been reported in several studies [68,69]. For instance, Mozafariyan et al. (2014) [54] applied Se-NPs to pepper under Cd toxicity, while IONPs were tested on wheat [55]. Thus, the reported improvement in plant signaling led to the activation of antioxidant activity, which may be influenced by NPs [70].
The antioxidant activity of plants and phenolic substances (flavonoids, phenolic acids) are closely related. They have aromatic rings and OH groups, and because of their redox characteristics, substantial plant antioxidant production can be driven [71]. Phenolic metabolites modulate oxidative stress by oxidizing ROS compounds when the plants are exposed to HMs [34]. Our data show that the Se-NP and IONP application increases TPC and TFC in plants under different levels of As. ROS compounds could be scavenged using both NPs, which can be applied with the help of stimulation from antioxidant activities, which has also been reported in recent studies [59,72].
Proline production can assist in regulating growth in stressed plants [73]. This may be crucial for osmotic stress, membrane stability, and scavenging ROS molecules [74]. Our findings demonstrated that Se-NPs and IONPs might increase proline accumulation in plants under As toxicity, which may be related to the NPs’ function in the proline biosynthesis gene expression network. This phenomenon has been reported in previous research using ZnO-NPs to alleviate Cd and Cu toxicity [75,76,77,78]. On the other hand, the measurement of the RWC in leaves is a critical index to show HM stress in plants [79]. Hence, it was employed to assess the plants’ water status as one of the principal physiological reactions against stress [80]. Based on our data, Se-NPs can enhance water content, optimizing metabolic processes in plant cells and promoting development [81]. Also, we observed that IONPs minimized the toxicity of HMs by preventing further reduction in Fe in the leaves by maintaining the leaf structure, which can help manage plant water content [82]. Our results revealed that, while As at various levels reduces the RWC in bamboo plants, NP applications in both single and combined forms favor RWC indices. This was also reported by Se-NPs on Coriandrum sativum plants under Cd toxicity [83].
Nitric oxide, as a multifunction and gaseous molecule, enhances water content by inducing cell division and cell wall extensibility and impacting leaf area expansion, weight, and length [79]. The improved nitric oxide levels caused by the NPs in bamboo plants under As toxicity were observed in our work. We also found that one of the most significant factors contributing to the rise in the RWC of leaves under As toxicity was the increased nitric oxide concentrations shown in our previous work [84]. According to reports, Se-NPs can help protect chloroplast enzymes by increasing the overall antioxidant ability. Moreover, the catalytic core of selenoproteins like GPX uses Se to eliminate free radicals and prevent photosynthetic pigment degradation [85,86].
Polyamines have a pivotal role in the growth and reproduction of plants, regulating plant cell functions under HM stress [87]. They also regulate gene expressions responsible for HM detoxification via vacuole compartmentalization [88]. Spermidine (Spd) and spermine (Spm) are the primary PAs, along with putrescine (Put), which is a diamine found within plant cells. They are involved in an array of processes, including membrane stability and plant abiotic stress tolerance [87]. Our data demonstrated that the NPs significantly increase putrescine (Put) and spermidine (Spd) in plants under As toxicity. This explains the crucial significance of IONPs and Se-NPs in limiting the toxicity of As in bamboo plants. Because of the disintegration of plant defense mechanisms under HM stress, ROS generation readily causes damage to photosynthesis and limits plant development [55]. Nevertheless, NPs can boost the photosynthesis rate in plants. Many studies have reported that NPs can improve the functions of the photosynthetic apparatus and the integrity of chlorophyll pigments under HM stress [89,90,91,92]. For instance, Zn NPs activate carbonic anhydrase (CA) and Rubisco. These enzymes are responsible for certain defense gene expressions, which can improve chemical energy distribution in the photosynthetic machinery [93]. By boosting antioxidant capacity, Se-NPs have been reported to protect certain chloroplast enzymes [85,94] that increase light absorption through chloroplasts [95,96]. Iron, a trace nutrient in plants, is involved in photosynthetic reactions, which play a pivotal role in balancing photosystem I and II compartments [97]. Our results showed that Se-NPs and IONPs together improved gas exchange parameters and chlorophyll content in bamboo plants under toxic As conditions. Several investigations have supported our hypothesis that Se and iron NPs with higher antioxidant potential chelate ROS molecules, thereby enhancing the efficiency of plant photosynthesis [59,98]. Foliar sprays containing Se can raise the nutritional value of essential phytochemicals, promoting plant development [99]. On the other hand, Fe is known to stimulate cell division in plants, leading cells to expand [100]. Accordingly, the foliar spray of Se-NPs and IONPs improved bamboo biomass (the dry weight of shoots and roots) under different As concentrations, which improved bamboo photosynthesis and development under As toxicity. Se and Iron NPs’ roles in plant growth promotion have been reported in several studies [55,97,101,102,103,104,105,106].
5. Conclusions
We proved that nanoparticles (Se-NPs and IO-NPs) might be effective foliar spray agents. Our data showed that different As concentrations (10, 20, and 40 mg L−1) limit plant photosynthesis and growth in bamboo by increasing the oxidative stress caused by the generation of free radicals in the cells. The external application of 60 mg L−1 of Se-NPs and IONPs alone or in combination promotes plant growth under As toxicity with rising levels of antioxidants (SOD, POD, CAT, GR, PAL) and non-antioxidants (TFC and TPC); the accumulation of proline; and the enhancement of spermidine (Spd) and putrescine (Put). The NPs were able to reduce lipoperoxidation and electrolyte leakage, scavenge ROS molecules (H2O2 and O2•−), and protect plant cells. Optimizing chlorophyll pigment intactness and gas exchange improved the photosynthetic rate and, in turn, plant biomass in bamboo plants exposed to different levels of As. Furthermore, we discovered that NPs enhance nitric oxide concentrations and the RWC, which is essential in strengthening plant tolerance against As toxicity. We hypothesize that NPs, by limiting As uptake and translocation, could increase bamboo tolerance under As toxicity. Accordingly, combining 60 mg L−1 of Se-NPs with IONPs significantly strengthened bamboo tolerance to toxic As. However, a more in-depth study is needed to understand the involved mechanisms.
Author Contributions
Conceptualization, A.E., A.G., J.B., Y.D. and G.L.; methodology, Y.L. and N.P.; software, Y.L.; validation, Y.D., J.B. and G.L.; formal analysis, Y.L.; investigation, A.E., Y.D. and G.L.; resources, A.E., Y.D. and G.L.; data curation, A.E., J.B., Y.L. and N.P.; writing—original draft preparation, A.E., A.G., N.P., J.B., Y.D., G.L. and M.Z.; writing—review and editing, A.E., A.G., N.P. and M.Z.; visualization, A.E., Y.D. and J.B.; supervision, A.E., Y.D. and G.L.; project administration, A.E. and Y.D.; funding acquisition, A.E., Y.D. and G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from the Jiangsu Agriculture Science and Technology Innovation Fund (CX (22) 3049).
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
We would like to extend our sincere gratitude and appreciation to Peijian Shi, Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, Jiangsu, China, for helping with the statistical analysis in this manuscript. This work was supported by the RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Khan, M.B.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). Sci. Total. Environ. 2020, 712, 136497. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Smith, E.; Owens, G.; Bhattacharya, P. Managing Arsenic in the Environment: From Soil to Human Health; CSIRO Publishing: Clayton, Australia, 2006. [Google Scholar]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Chauhan, R.; Srivastava, S.; Tripathi, R.D. The journeyof arsenic from soil to grain in rice. Front. Plant Sci. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar HMs uptake, toxicity and detoxification in plants: Acomparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Pandey, C.; Augustine, R.; Panthri, M.; Zia, I.; Bisht, N.C.; Gupta, M. Arsenic affects the production of glucosinolate, thiol and phytochemical compounds: A comparison of two Brassica cultivars. Plant Physiol. Biochem. 2017, 111, 144–154. [Google Scholar] [CrossRef]
- El-Saadony, T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Hassan, M.A. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Foliar application of silicon nanoparticles affected the growth, vitamin C, flavonoid and antioxidant enzyme activities of coriander (Coriandrumsativum L.) plants grown in lead (Pb)-spiked soil. Environ. Sci. Pollut. Res. 2021, 28, 1417–1425. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Wang, C. Treatment of CrVI-Containing Mg(OH)2 Nanowaste. Angew. Chem. Int. Ed. 2008, 47, 5619–5622. [Google Scholar] [CrossRef]
- Rubio, L.; Pyrgiotakis, G.; Beltran-Huarac, Z.Y.; Gaurav, J.; Deloid, G.; Demokritou, P. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol. 2019, 16, 40. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustain-able agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef]
- Emamverdian, A.; Hasanuzzaman, M.; Ding, Y.; Barker, J.; Mokhberdoran, F.; Liu, G. Zinc Oxide Nanoparticles Improve Pleioblastus pygmaeus Plant Tolerance to Arsenic and Mercury by Stimulating Antioxidant Defense and Reducing the Metal Accumulation and Translocation. Front. Plant Sci. 2022, 13, 841501. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Hasanuzzaman, M.; Barker, J.; Liu, G.; Li, Y.; Mokhberdoran, F. Insight into the biochemical and physiological mechanisms of nanoparticles-induced arsenic tolerance in bamboo. Front. Plant Sci. 2023, 14, 1121886. [Google Scholar] [CrossRef] [PubMed]
- Irmak, S. Effects of Selenium Application on Plant Growth and Some Quality Parameters in Peanut (Arachis hypogaea). Pak. J. Biol. Sci. 2017, 20, 92–99. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U.R. Biomedical poten-tial of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic aspects. Int. J. Nano Med. 2021, 16, 249. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wang, X.; Qing, X.; Wang, P.; Zhang, Y.; Zhao, X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019, 173, 314–321. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar. Hakimi. Meybodi, N.; Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, D.B.A. Effect of NaCl and iron oxide nanoparticles on Mentha piperita essential oil composition. Environ. Exp. Bot. 2016, 14, 27–32. [Google Scholar] [CrossRef]
- Askary, M.; Talebi, S.M.; Amini, F.; Bangan, A.D. Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologia 2017, 63, 65–75. [Google Scholar] [CrossRef]
- Sheykhbaglou, R.; Sedghi, M.; Fathi-Achachlouie, B. The Effect of Ferrous Nano-oxide Particles on Physiological Traits and Nutritional Compounds of Soybean (Glycine max L.) Seed. An. Acad. Bras. Ciênc. 2018, 90, 485–494. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.H.; Ahmad, A. Brassino steroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu nanoparticles to improve seed germination quality in black gram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc bio fortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- Ahmad, Z.; Upadhyay, A.; Ding, Y.; Emamverdian, A.; Shahzad, A. Bamboo: Origin, Habitat, Distributions and Global Prospective. In Biotechnological Advances in Bamboo; Ahmad, Z., Ding, Y., Shahzad, A., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokberdoran, F.; Ahmad, Z.; Xie, Y. Determination of HMs tolerance threshold in a bamboo species (Arundinaria pygmaea) as treated with silicon dioxide nanoparticles. Glob. Ecol. Conserv. 2020, 24, e0130. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Ranaei, F.; Ahmad, Z. Application of Bamboo Plants in Nine Aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef]
- Bal, L.M.; Singhal, P.; Satya, S.; Naik, S.N.; Kar, A. Bamboo shoot preservation for enhancing its business potential and local economy: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 804–814. [Google Scholar] [CrossRef]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo—An untapped plant resource for the phytoremediation of HMs contaminated soils. Chemosphere 2019, 246, 125750. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhou, Q.X.; Sun, T.; Ma, L.Q.; Wang, S. Growth responses of three ornamental plants to Cd and Pb stress and their metal accumulation characteristics. J. Hazard. Mater. 2008, 151, 261–267. [Google Scholar] [CrossRef]
- Huang, W.; Olson, E.; Wang, S.H.; Shi, P. The growth and mortality of Pleioblastus pygmaeus under different light availability. Glob. Ecol. 2020, 24, e01262. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, M.; Wang, H.; Taneike, Y.; Zhang, X. Arsenic speciation in moso bamboo shoot—A terrestrial plant that contains organoarsenic species. Sci. Total Environ. 2006, 371, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Khosropour, E.; Attarod, P.; Shirvany, A.; Pypker, T.G.; Bayramzadeh, V.; Hakimi, L.; Moeinaddini, M. Response of Plat-anus orientalis leaves to urban pollution by HMss. J. For. Res. 2019, 30, 1437–1445. [Google Scholar] [CrossRef]
- Souri, Z.; Karimi, N. Enhanced Phytoextraction by as Hyperaccumulator Isatis cappadocica Spiked with Sodium Nitro-prusside. Soil. Sediment. Contam. Int. J. 2017, 26, 457–468. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of superoxide dismutase (SOD). In Plant-Microbe Interactions; Springer: Berlin/Heidelberg, Germany, 2021; pp. 117–118.49. [Google Scholar]
- Liu, N.; Lin, Z.; Guan, L.; Gaughan, G.; Lin, G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS ONE 2014, 9, e87588. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acidmetabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Berner, M.; Krug, D.; Bihlmaier, C.; Vente, A.; Müller, R.; Bechthold, A. Genes and enzymes involved in caffeic acid bio-synthesisin the actinomycete Saccharothrix espanaensis. J. Bacteriol. 2006, 188, 2666–2673. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug. Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenoliccontent and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protectiverole of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Li, C.; Bai, T.; Ma, F.; Han, M. Hypoxia tolerance and adaptation of anaerobic respiration to hypoxia stress in two Malus species. Sci. Hortic. 2010, 124, 274–279. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef]
- Valentovic, P.; Luxova, M.; Kolarovic, L.; Gasparikova, O. Effect of osmotic stress on compatible solutes content, mem-brane stability and water relations in two maize cultivars. Plant Soil Environ. 2006, 52, 186–191. [Google Scholar] [CrossRef]
- Dhopte, A.M.; Manuel, L.M. Principles and techniques for plant scientists. In Updesh Purohit for Agrobios, 1st ed.; Agrobios: Jodhpur, India, 2002; Volume 81, p. 373. [Google Scholar]
- Bates, L.S.; Walden, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, G.; Li, S. Polyamines and plant stress resistance. J. South Agric. 2004, 35, 443–447. [Google Scholar]
- Holá, D.; Benešová, M.; Honnerová, J.; Hnilicka, F.; Rothová, O.; Kocová, M.; Hniličková, H. The evaluation of photo-synthetic parameters in maize inbred lines subjected to water deficiency: Can these parameters be used for the prediction of performance of hybrid progeny? Photosynthetica 2010, 48, 545–558. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphe-noloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectivenessof nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective Role of Selenium on Pepper Exposed to Cadmium Stress During Reproductive Stage. Biol. Trace Elem. Res. 2014, 160, 97–107. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia Ur Rehman, M.; Waris, A.A. Zinc and iron oxide na-noparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Mihailovic, V.; Katanic Stankovic, J.S.; Selakovic, D.; Rosic, G. An Overview of the Beneficial Role of Antioxidants in the Treatment of Nanoparticle-Induced Toxicities. Oxid. Med. Cell. Longev. 2021, 15, 7244677. [Google Scholar] [CrossRef] [PubMed]
- Sardar, R.; Ahmed, S.; Yasin, N.A. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ. Pollut. 2022, 292 Pt A, 118373. [Google Scholar] [CrossRef]
- Babashpour-Asl, M.; Farajzadeh-Memari-Tabrizi, E.; Yousefpour-Dokhanieh, A. Foliar-applied selenium nanoparticles alleviate cadmium stress through changes in physio-biochemical status and essential oil profile of coriander (Coriandrum sativum L.) leaves. Environ. Sci. Pollut. Res. 2022, 29, 80021–80031. [Google Scholar] [CrossRef]
- Guo, K.; Hu, A.; Wang, K.; Wang, L.; Fu, D.; Hao, Y.; Wang, Y.; Ali, A.; Adeel, M.; Rui, Y.; et al. Effects of spraying nano-materials on the absorption of metal(loid)s in cucumber. IET Nanobiotechnol. 2019, 13, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Mobasherpour, I.; Salahi, E.; Pazoukib, M. Removal of nickel (II) from aqueous solutions by using nano-crystalline calcium hydroxyapatite. J. Saudi Chem. Soc. 2011, 15, 105–112. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional andnanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Zia ur Rehman, M.; Riaz, M.; Adrees, M.; Hussain, A.; Zahir, Z.A.; Rinklebe, J. Effects of nanoparticles on trace element uptake and toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2021, 221, 112437. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Castroverde, C.D.M.; Kalaji, H.M.; Albaqami, M.; Aftab, T. Molecular mechanisms of nitric oxide (NO) signaling and reactive oxygen species (ROS) homeostasis during abiotic stresses in plants. Int. J. Mol. Sci. 2021, 22, 9656. [Google Scholar] [CrossRef]
- Gechev, T.; Petrov, V. Reactive oxygen species and abiotic stress in plants. Int. J. Mol. Sci. 2020, 21, 7433. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Khan, S.; Rehman, S.U.; Rehman, Z.U.; Khatoon, A.; Rha, E.S.; Jamil, M. Synergistic Effects of Zinc Oxide Na-noparticles and Bacteria Reduce Heavy Metals Toxicity in Rice (Oryza sativa L.) Plant. Toxics 2021, 9, 113. [Google Scholar] [CrossRef]
- Jiang, D.; Hou, J.; Gao, W.; Tong, X.; Li, M.; Chu, X. Exogenous spermidine alleviates the adverse effects of aluminum toxicity on photosystem II through improved antioxidant system and endogenous polyamine contents. Ecotoxicol. Environ. Saf. 2021, 207, 111265. [Google Scholar] [CrossRef] [PubMed]
- Jurkow, R.; Pokluda, R.; Sękara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Qiu, W.Y.; Sun, L.; Ding, Z.C.; Ya, J.K. Peparation, characterization, and antioxidant capacities of selenium nanoparticles stabilized using polysaccharide-protein complexes from Corbicula fluminea. Food Biosci. 2018, 26, 177–184. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Ahmad, Z.; Xie, Y. The Investigation of TiO2 NPs Effect as a Wastewater Treatment to Mitigate Cd Negative Impact on Bamboo Growth. Sustainability 2021, 13, 3200. [Google Scholar] [CrossRef]
- Ali-Arab, H.; Bahadori, F.; Mirza, M.; Badi, H.N.; Kalate-Jari, S. Variability in essential oil composition and phenolic acid profile of Thymus daenensis Celak. populations from Iran. Ind. Crops Prod. 2022, 178, 114345. [Google Scholar] [CrossRef]
- Memari-Tabrizi, E.F.; Yousefpour-Dokhanieh, A.; Babashpour-Asl, M. Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.). Plant Physiol. Biochem. 2021, 165, 71–79. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effect of foliarspray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J. Agric. Sci. Technol. 2016, 18, 1013–1025. Available online: http://jast.modares.ac.ir/article-23-5061-en.html (accessed on 1 January 2020).
- Bandurska, H. Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injuries? II. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol. Plant. 2001, 23, 483–490. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles and 24-epibrassinolide alleviates Cu toxicity in tomato by regulating ROS scavenging, stomatal movement and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Helaly, M.N.; El-Metwally, M.A.; El-Hoseiny, H.; Omar, S.A.; El-Sheery, N.I. Effect of nanoparticles on biological con-tamination of in vitro culturesand organogenic regeneration of banana. Aust. J. Crop. Sci. 2014, 8, 612–624. [Google Scholar]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticleson crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41; Hayat, S., Pichtel, J., Faizan, M., Fariduddin, Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 83–99. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol. 2018, 186, 999–1016. [Google Scholar] [CrossRef]
- Merwad, A.R.M.; Desok, E.S.M.; Rady, M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Shirani Bidabadi, S.; Sabbatini, P.; Vander Weide, J. Iron oxide (IO) nanoparticles alleviate PEG-simulated drought stress in grape (Vitis vinifera L.) plants by regulating leaf antioxidants. Sci. Hortic. 2023, 312, 111847. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Sha, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Barker, J.; Liu, G.; Li, Y.; Mokhberdoran, F. Sodium Nitroprusside Improves Bamboo Resistance under Mn and Cr Toxicity with Stimulation of Antioxidants Activity, Relative Water Content, and Metal Trans-location and Accumulation. Int. J. Mol. Sci. 2023, 24, 1942. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Hashem, A.M.; Batool, M.; Sherif, A.; Nishawy, E.; Ayaad, M.; Hassan, H.M.; Elrewainy, I.M.; Wang, J.; Kuai, J.; et al. Comparative efficacy of bio-selenium nanoparticles and sodium selenite on mor-pho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L. J. Nanobiotechnology 2022, 20, 163. [Google Scholar] [CrossRef]
- Moloi, M.J.; Khoza, B.M. The Effect of Selenium Foliar Application on the Physiological Responses of Edamame under Different Water Treatments. Agronomy 2022, 12, 2400. [Google Scholar] [CrossRef]
- Malik, A.; Yadav, P.; Singh, S. Role of polyamines in HMs stressed plants. Plant Physiol. Rep. 2022, 27, 680–694. [Google Scholar] [CrossRef]
- Spormann, S.; Soares, C.; Teixeira, J.; Fidalgo, F. Polyamines as key regulatory players in plants under metal stress—A way for an enhanced tolerance. Ann. Appl. Biol. 2021, 178, 209–226. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Ijaz, M.; Ali, S.; Rizwan, M.; Ijaz, U.; Hameed, A.; Ahmad, U.; Wang, Y.; Sun, G.; et al. Current trends and future prospective in nanoremediation of HMs contaminated soils: A way forward towards sustainable agriculture. Ecotoxicol. Environ. Saf. 2021, 227, 112888. [Google Scholar] [CrossRef]
- Khalid, M.F.; Iqbal Khan, R.; Jawaid, M.Z.; Shafqat, W.; Hussain, S.; Ahmed, T.; Rizwan, M.; Ercisli, S.; Pop, O.L.; Alina Marc, R. Nanoparticles: The Plant Saviour under Abiotic Stresses. Nanomaterials 2022, 12, 3915. [Google Scholar] [CrossRef] [PubMed]
- Reddy Pullagurala, V.L.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense systemin plants. In Nanotechnology and Plant Sciences; Siddiqui, M., Al-Whaibi, M., Mo-hammad, F., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, Common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Smirnoff, N. Chapter 4—Vitamin C: The metabolism and functions of ascorbic acid in plants. In Advances in Botanical Research; Rébeillé, F., Douce, R., Eds.; Academic Press: Cambridge, UK, 2011; pp. 107–177. [Google Scholar]
- Ze, Y.; Liu, C.; Wang, L.; Hong, M.; Hong, F. The regulation of TiO2 nanoparticles on the expression of light-harvesting complex II and photosynthesis of chloroplasts of Arabidopsis thaliana. Biol. Trace Elem. Res. 2011, 143, 1131–1141. [Google Scholar] [CrossRef]
- Rai, P.; Samarth Sharma, S.; Tripathi, S.; Prakash, V.; Tiwari, K.; Suri, S.; Sharma, S. Nanoiron: Uptake, translocation and accumulation in plant systems. Plant Nano Biol. 2022, 2, 100017. [Google Scholar] [CrossRef]
- Sheykhbaglou, R.; Sedghi, M.; Shishevan, M.T.; Sharifi, R.S. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef]
- Moussa, H.R.; El-Fatah, A.; Ahmed, M. Protective role of selenium on development and physiological responses of Viciafaba. Int. J. Veg. Sci. 2010, 16, 174–183. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Bano, I.; Skalickova, S.; Sajjad, H.; Skladanka, J.; Horky, P. Uses of Selenium Nanoparticles in the Plant Production. Agronomy 2021, 11, 2229. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, K.; Liu, Z.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on the subcellular distribution of cadmium and oxidative stress induced by cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2019, 26, 16220–16228. [Google Scholar] [CrossRef]
- Qi, W.Y.; Li, Q.; Chen, H.; Liu, J.; Xin, S.F.; Xu, M.; Wang, S.G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef] [PubMed]
- Bidi, H.; Fallah, H.; Niknejad, Y.; Tari, D.B. Iron Oxide Nanoparticles Alleviate Arsenic Phytotoxicity in Rice by Improving Iron Uptake, Oxidative Stress Tolerance and Diminishing Arsenic Accumulation. Plant Physiol. Biochem. 2021, 163, 348–357. [Google Scholar] [CrossRef]
- Koleva, L.; Umar, A.; Yasin, N.A.; Shah, A.A.; Siddiqui, M.H.; Alamri, S.; Riaz, L.; Raza, A.; Javed, T.; Shabbir, Z. Iron Oxide and Silicon Nanoparticles Modulate Mineral Nutrient Homeostasis and Metabolism in Cadmium-Stressed Phaseolus vulgaris. Front. Plant Sci. 2022, 13, 806781. [Google Scholar] [CrossRef]
- Pehlivan, N.; Gedik, K.; Eltem, R.; Terzi, E. Dynamic interactions of Trichoderma harzianum TS 143 from an old mining site in Turkey for potent metal(oid)s phytoextraction and bioenergy crop farming. J. Hazard. Mater. 2021, 403, 123609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).