Optimization of an Ultrasound-Assisted Extraction Method for the Extraction of Gingerols and Shogaols from Ginger (Zingiber officinale)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. The Plant Matrix
2.3. Ultrasound-Assisted Extraction
2.4. Identification of Gingerols and Shogaols by UHPLC-QToF-MS
2.5. Analysis of the Gingerols and Their Analogues by UHPLC-DAD
2.6. Experimental Design
2.7. Statistical Analysis
3. Results and Discussion
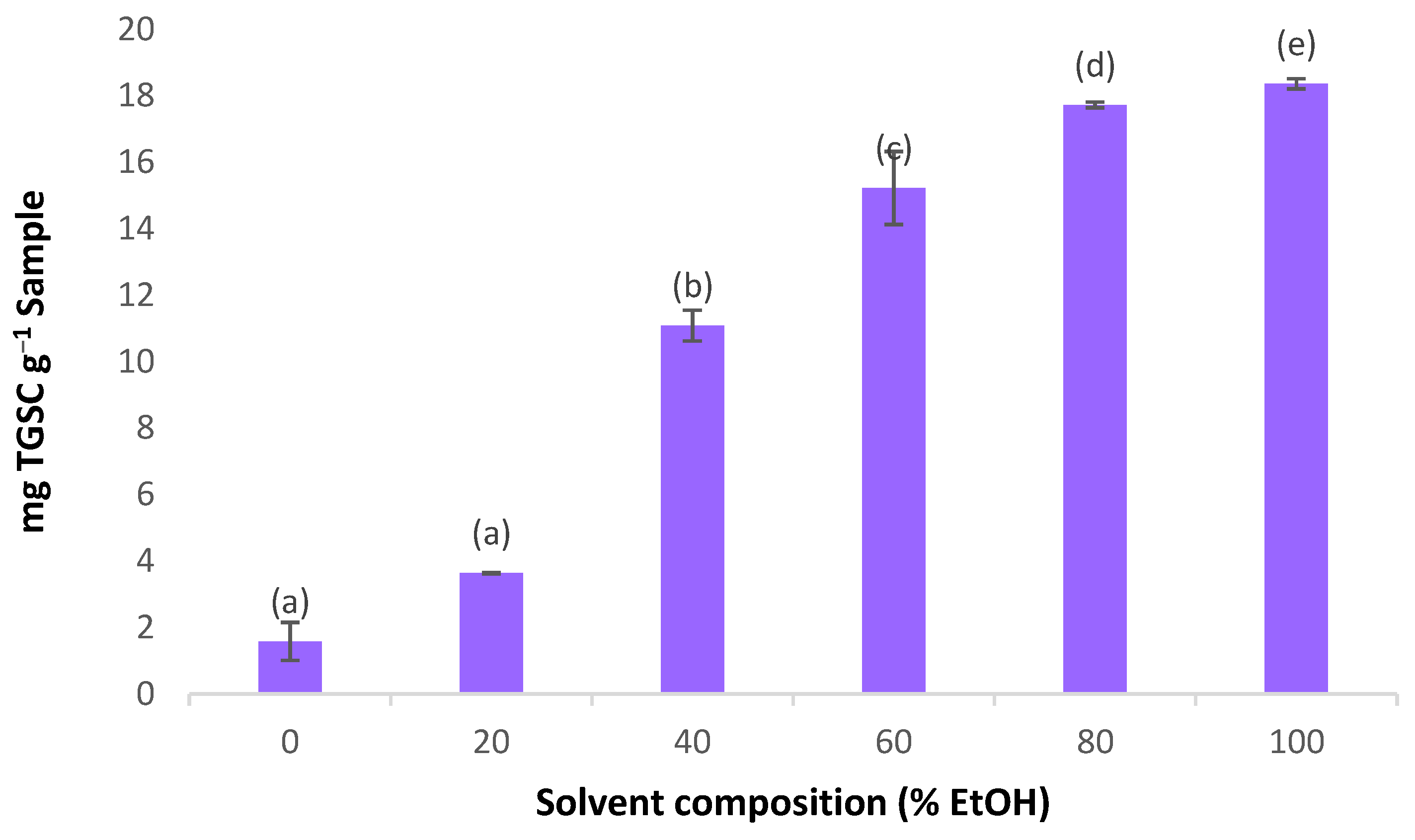
3.1. Determining the Extraction Solvent Optimum Range
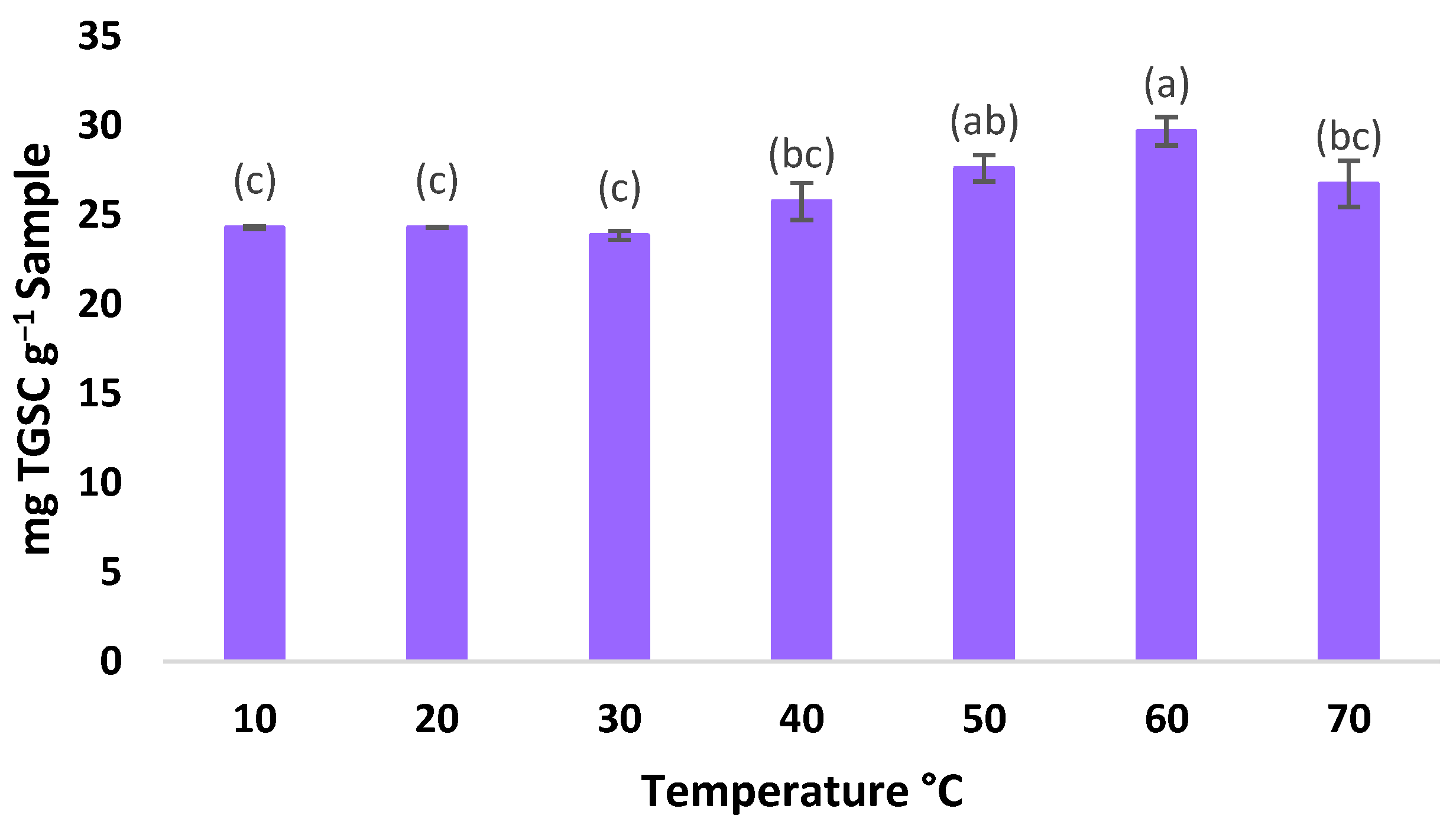
3.2. Determining the Extraction Temperature Range
3.3. Optimization of the Conditions for Ultrasound-Assisted Extraction
1.28358·X5 − 1.88867·X12 + 0.634846·X1·X2 + 0.200774·X1·X3 + 0.467455·X1·X4 −
0.751269·X1·X5 − 0.865545·X22 + 0.139133·X2·X3 − 0.360238·X2·X4 −
0.908232·X2·X5 − 0.401138·X32 + 0.24099·X3·X4 + 0.134113·X3·X5 −
0.0374793·X42 + 0.463491·X4·X5 − 0.0317111·X52
3.4. Optimal Extraction Conditions
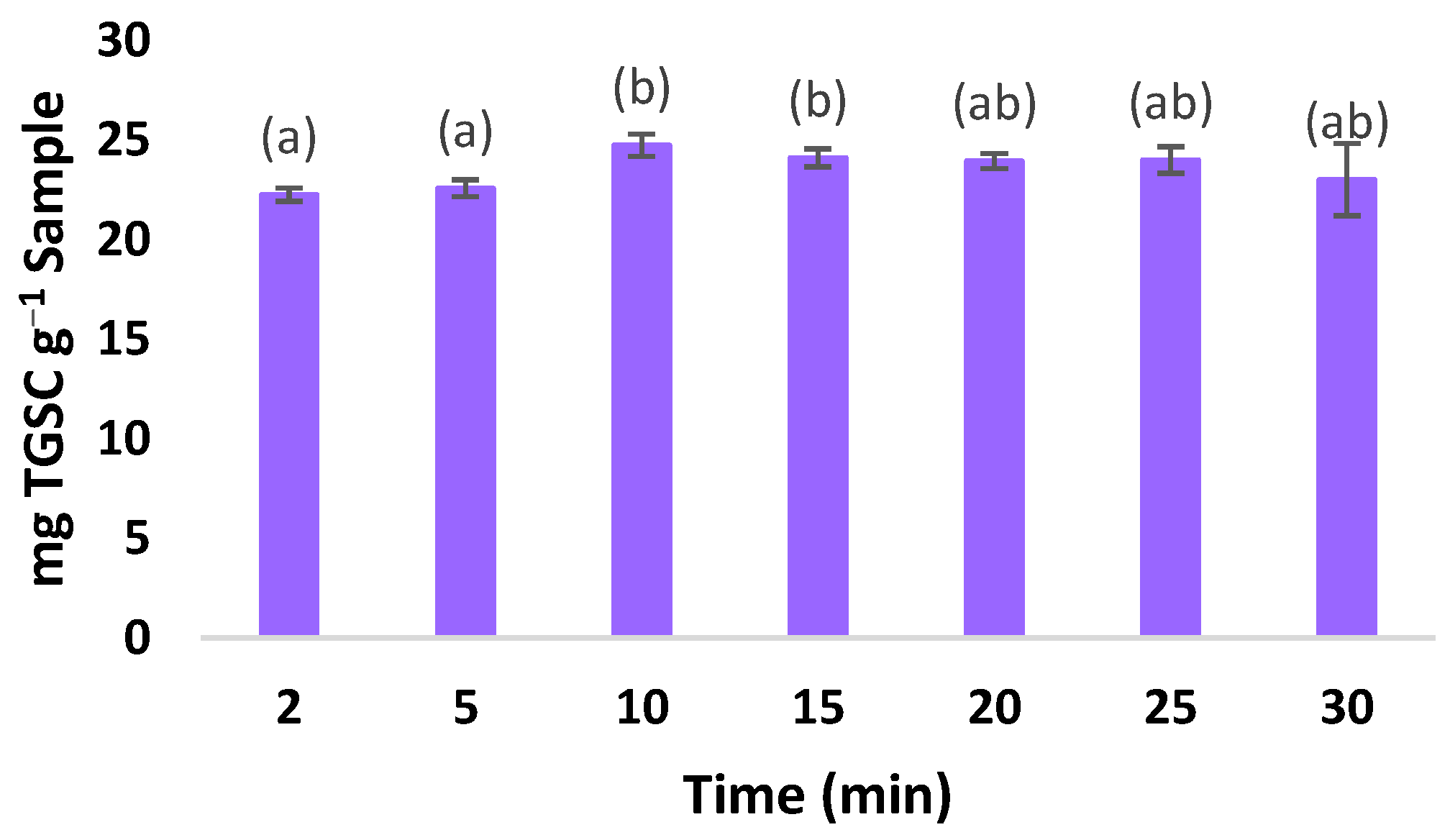
3.5. Determining the Optimal Extraction Time
3.6. Repeatability and Intermediate Precision of the Method
3.7. Implementation of the Optimized Extraction Method to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karna, P.; Chagani, S.; Gundala, S.R.; Rida, P.C.G.; Asif, G.; Sharma, V.; Gupta, M.V.; Aneja, R. Benefits of Whole Ginger Extract in Prostate Cancer. Br. J. Nutr. 2012, 107, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Singletary, K. Ginger: An Overview of Health Benefits. Nutr. Today 2010, 45, 171. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and Shogaols: Important Nutraceutical Principles from Ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Giacosa, A.; Morazzoni, P.; Bombardelli, E.; Riva, A.; Bianchi Porro, G.; Rondanelli, M. Can Nausea and Vomiting Be Treated with Ginger Extract? Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1291–1296. [Google Scholar]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.-A.P.M.; de Oliveira Santos, J.V.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and Therapeutic Potential of Ginger (Zingiber officinale) Extract and [6]-Gingerol in Cancer: A Comprehensive Review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Zhang, G.; Nitteranon, V.; Chan, L.Y.; Parkin, K.L. Glutathione conjugation attenuates biological activities of 6-dehydroshogaol from ginger. Food Chem. 2013, 140, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Mukherjee, K.; Mal, M.; Wahile, A.; Saha, B.P.; Mukherjee, P.K. Determination of 6-Gingerol in Ginger (Zingiber officinale) Using High-Performance Thin-Layer Chromatography. J. Sep. Sci. 2006, 29, 2292–2295. [Google Scholar] [CrossRef]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-Gingerol, 8-Gingerol, 10-Gingerol, and 6-Shogaol and Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. The Stability of Gingerol and Shogaol in Aqueous Solutions. J. Pharm. Sci. 2001, 90, 1658–1664. [Google Scholar] [CrossRef]
- Anisa, N.I.; Azian, N.; Sharizan, M.; Iwai, Y. Temperature Effects on Diffusion Coefficient for 6-Gingerol and 6-Shogaol in Subcritical Water Extraction. J. Phys. Conf. Ser. 2014, 495, 012009. [Google Scholar] [CrossRef]
- Phoungchandang, S.; Nongsang, S.; Sanchai, P. The Development of Ginger Drying Using Tray Drying, Heat Pump–Dehumidified Drying, and Mixed-Mode Solar Drying. Dry. Technol. 2009, 27, 1123–1131. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; White, S.; Brown, P.; Naiker, M. Quantitative Profiling of Gingerol and Its Derivatives in Australian Ginger. J. Food Compos. Anal. 2021, 104, 104190. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Optimization Protocol for the Extraction of 6-Gingerol and 6-Shogaol from Zingiber officinale Var. Rubrum Theilade and Improving Antioxidant and Anticancer Activity Using Response Surface Methodology. BMC Complement. Altern. Med. 2015, 15, 258. [Google Scholar] [CrossRef] [Green Version]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the Chemopreventive Effects of Ginger and Its Phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some Phytochemical, Pharmacological and Toxicological Properties of Ginger (Zingiber officinale Roscoe): A Review of Recent Research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.H.L.; Kim, J.Y. Side-Chain Length Is Important for Shogaols in Protecting Neuronal Cells from β-Amyloid Insult. Bioorg. Med. Chem. Lett. 2004, 14, 1287–1289. [Google Scholar] [CrossRef]
- Jung, M.Y.; Lee, M.K.; Park, H.J.; Oh, E.B.; Shin, J.Y.; Park, J.S.; Jung, S.Y.; Oh, J.H.; Choi, D.S. Heat-induced conversion of gingerols to shogaols in ginger as affected by heat type (dry or moist heat), sample type (fresh or dried), temperature and time. Food Sci. Biotechnol. 2017, 27, 687–693. [Google Scholar] [CrossRef]
- Ho, S.; Su, M. Optimized heat treatment enhances the anti-inflammatory capacity of ginger. Int. J. Food Prop. 2016, 19, 1884–1898. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Q.; Peng, Y.; Qi, L.; Li, P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011, 129, 1785–1792. [Google Scholar] [CrossRef]
- Kalantari, K.; Moniri, M.; Boroumand Moghaddam, A.; Abdul Rahim, R.; Bin Ariff, A.; Izadiyan, Z.; Mohamad, R. A Review of the Biomedical Applications of Zerumbone and the Techniques for Its Extraction from Ginger Rhizomes. Molecules 2017, 22, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasikala, P.; Chandralekha, A.; Chaurasiya, R.S.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Ultrasound-Assisted Extraction and Adsorption of Polyphenols from Ginger Rhizome (Zingiber officinale). Sep. Sci. Technol. 2018, 53, 439–448. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar] [CrossRef]
- Leighton, T.G. The Acoustic Bubble; Academic Press: Cambridge, MA, USA, 1997; ISBN 978-0-12-441921-6. [Google Scholar]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic Enhancement of the Supercritical Extraction from Ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and Opportunities for Ultrasound Assisted Extraction in the Food Industry—A Review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the Use of Ultrasound in the Extraction of Antioxidants from Rosmarinus officinalis for the Food and Pharmaceutical Industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef]
- Lakshmipathy, K.; Thirunavookarasu, N.; Kalathil, N.; Chidanand, D.V.; Rawson, A.; Sunil, C.K. Effect of different thermal and non-thermal pre-treatments on bioactive compounds of aqueous ginger extract obtained using vacuum-assisted conductive drying system. J. Food Process Eng. 2023, 46, e14223. [Google Scholar] [CrossRef]
- Garza-Cadena, C.; Ortega-Rivera, D.M.; Machorro-García, G.; Gonzalez-Zermeño, E.M.; Homma-Dueñas, D.; Plata-Gryl, M.; Castro-Muñoz, R. A comprehensive review on Ginger (Zingiber officinale) as a potential source of nutraceuticals for food formulations: Towards the polishing of gingerol and other present biomolecules. Food Chem. 2023, 413, 135629. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response Surface Methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; García-Alvarado, M.Á.; Palma, M.; Rodríguez-Jimenes, G.d.C.; Barbero, G.F. Optimization of an Ultrasound-Assisted Extraction Method Applied to the Extraction of Flavonoids from Moringa Leaves (Moringa oleífera Lam.). Agronomy 2022, 12, 261. [Google Scholar] [CrossRef]
- Watson, M.; Long, H.; Lu, B. Investigation of Wrinkling Failure Mechanics in Metal Spinning by Box-Behnken Design of Experiments Using Finite Element Method. Int. J. Adv. Manuf. Technol. 2015, 78, 981–995. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Espinosa, M.; Álvarez-Romero, M.; Velasco González de Peredo, A.; Ruiz Rodríguez, A.; Ferreiro-González, M.; Barbero, G.; Palma, M. Capsaicinoid Content in the Pericarp and Placenta of Bolilla Peppers (Capsicum annuum L.) throughout the Ripening of the Fruit at Two Different Stages of Plant Maturation. Agronomy 2023, 13, 435. [Google Scholar] [CrossRef]
- Vázquez Espinosa, M.; Fayos, O.; Velasco González de Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Palma, M.; Garcés-Claver, A.; Barbero, G. Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum Spp.) at Different Ripening Stages. Agronomy 2020, 10, 1337. [Google Scholar] [CrossRef]
- Vázquez Espinosa, M.; Fayos, O.; Velasco González de Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Palma, M.; Garcés-Claver, A.; Barbero, G. Content of Capsaicinoids and Capsiate in “Filius” Pepper Varieties as Affected by Ripening. Plants 2020, 9, 1222. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Rojas, J.; Fayos, O.; Vázquez León, L.A.; Ferreiro-González, M.; Rodriguez-Jimenes, G.; Palma, M.; Garcés-Claver, A.; Barbero, G. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Stipcovich, T.; Barbero, G.F.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Fast analysis of capsaicinoids in Naga Jolokia extracts (Capsicum chinense) by high-performance liquid chromatography using fused core columns. Food Chem. 2018, 239, 217–224. [Google Scholar] [CrossRef]
- Vázquez Espinosa, M.; Velasco González de Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.; Palma, M. Simultaneous Determination by UHPLC-PDA of Major Capsaicinoids and Capsinoids Contents in Peppers. Food Chem. 2021, 356, 129688. [Google Scholar] [CrossRef]
- Lu, M.; Xia, X.; Chou, G.; Liu, D.; Zuberi, A.; Ye, J.; Liu, Z. Variations in the Contents of Gingerols and Chromatographic Fin-gerprints of Ginger Root Extracts Prepared by Different Preparation Methods. J. AOAC Int. 2001, 97, 50–57. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to Extract Bioactive Compounds from Food By-Products of Plant Origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized Liquid Extraction of Bioactive Compounds from Grape Marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-Assisted Extraction of Capsaicinoids from Peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Gopi, S.; Ac, K.; Jude, S. Study on Temperature Dependent Conversion of Active Components of Ginger. Int. J. Pharma. Sci. 2016, 6, 1344–1347. [Google Scholar]
- Varakumar, S.; Umesh, K.V.; Singhal, R.S. Enhanced extraction of oleoresin from ginger (Zingiber officinale) rhizome powder using enzyme-assisted three phase partitioning. Food Chem. 2017, 216, 27–36. [Google Scholar] [CrossRef]
- George, J.M.; Sowbhagya, H.B.; Rastogi, N.K. Effect of high pressure pretreatment on drying kinetics and oleoresin extraction from ginger. Dry. Technol. 2018, 36, 1107–1116. [Google Scholar] [CrossRef]
- Sánchez, M.G.; Sánchez, A.M.; Collado, B.; Malagarie-Cazenave, S.; Olea, N.; Carmena, M.J.; Prieto, J.C.; Díaz-Laviada, I. Expression of the Transient Receptor Potential Vanilloid 1 (TRPV1) in LNCaP and PC-3 Prostate Cancer Cells and in Human Prostate Tissue. Eur. J. Pharmacol. 2005, 515, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.L.; Rafferty, M.F.; Boctor, A.M. Inhibition of Human Neutrophil 5-Lipoxygenase Activity by Gingerdione, Shogaol, Capsaicin and Related Pungent Compounds. Prostaglandins Leukot. Med. 1986, 24, 195–198. [Google Scholar] [CrossRef]
- Kamaruddin, M.S.H.; Chong, G.H.; Mohd Daud, N.; Putra, N.R.; Md Salleh, L.; Suleiman, N. Bioactivities and green advanced extraction technologies of ginger oleoresin extracts: A review. Food Res. Int. 2023, 164, 112283. [Google Scholar] [CrossRef]
- Guo, J.-B.; Fan, Y.; Zhang, W.-J.; Wu, H.; Du, L.-M.; Chang, Y.-X. Extraction of gingerols and shogaols from ginger (Zingi-ber officinale Roscoe) through microwave technique using ionic liquids. J. Food Compos. Anal. 2017, 62, 35–42. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, C.-L.; Zhao, J.; Chen, D.; Li, Q.-H. Optimized Microwave-Assisted Extraction of 6-Gingerol from Zingiber officinale Roscoe and Evaluation of Antioxidant Activity in Vitro. Acta Sci. Pol. Technol. Aliment. 2014, 13, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.; Surh, Y.J. Induction of Apoptosis in HL-60 Cells by Pungent Vanilloids, [6]-Gingerol and [6]-Paradol. Cancer Lett. 1998, 134, 163–168. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized Liquid Extraction as a Green Approach in Food and Herbal Plants Extraction: A Review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of Thermal Processing on Anthocyanin Stability in Foods; Mechanisms and Kinetics of Degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Álvarez, J.A.; Ayuso, J.; Barbero, G.F. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 1: Pressurized Liquid Extraction. Agronomy 2020, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Jung, M.Y. UHPLC-ESI-MS/MS for the Quantification of Eight Major Gingerols and Shogaols in Ginger Products: Effects of Ionization Polarity and Mobile Phase Modifier on the Sensitivity. J. Food Sci. 2016, 81, C2457–C2465. [Google Scholar] [CrossRef] [PubMed]
- Vedashree, M.V.; Naidu, M. Optimization of 6-Gingerol Extraction Assisted by Microwave From Fresh Ginger Using Response Surface Methodology. J. Adv. Chem. 2018, 15, 6173–6185. [Google Scholar] [CrossRef]
- Daharia, A.; Jaiswal, V.; Royal, K.; Sharma, H.; Joginath, A.; Kumar, R.; Saha, P. A Comparative Review on Ginger and Garlic with Their Pharmacological Action. Asian J. Pharm. Res. Dev. 2022, 10, 65–69. [Google Scholar] [CrossRef]
- Lindblad, A.J.; Koppula, S. Ginger for nausea and vomiting of pregnancy. Can. Fam. Physician 2016, 62, 145. [Google Scholar]
- Jafarzadeh, A.; Jafarzadeh, S.; Nemati, M. Therapeutic Potential of Ginger against COVID-19: Is There Enough Evidence? J. Tradit. Chin. Med. Sci. 2021, 8, 267–279. [Google Scholar] [CrossRef]
- Safa, O.; Hassaniazad, M.; Farashahinejad, M.; Davoodian, P.; Dadvand, H.; Hassanipour, S.; Fathalipour, M. Effects of Ginger on Clinical Manifestations and Paraclinical Features of Patients with Severe Acute Respiratory Syndrome Due to COVID-19: A Structured Summary of a Study Protocol for a Randomized Controlled Trial. Trials 2020, 21, 841. [Google Scholar] [CrossRef]
- Rabie, A.M. New Potential Inhibitors of Coronaviral Main Protease (CoV-Mpro): Strychnine Bush, Pineapple, and Ginger Could Be Natural Enemies of COVID-19. Int. J. New Chem. 2022, 9, 433–445. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Zhang, N.; Udenigwe, C.C.; Zhang, Y.; Fu, Y. Preparation, Pungency and Bioactivity of Gingerols from Ginger (Zingiber officinale Roscoe): A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Saeed, Z.; Pervaiz, M.; Ali, F.; Younas, U.; Bashir, R.; Bukhari, S.M.; Mahmood khan, R.R.; Jelani, S.; Sikandar, R. Evaluation of Essential Oil Extracted from Ginger, Cinnamon and Lemon for Therapeutic and Biological Activities. Biocatal. Agric. Biotechnol. 2022, 44, 102470. [Google Scholar] [CrossRef]


| Factor | −1 | 0 | +1 | Unit |
|---|---|---|---|---|
| X1: Ethanol in water | 50 | 75 | 100 | % |
| X2: Temperature | 20 | 40 | 60 | °C |
| X3: Amplitude | 20 | 40 | 60 | % Maximum amplitude |
| X4: Cycle | 0.2 | 0.6 | 1 | s−1 |
| X5: Sample-to-solvent ratio | 0.3 | 0.5 | 0.7 | g:20 mL |
| Run | Factors | Responses | |||||
|---|---|---|---|---|---|---|---|
| Total Gingerol and Shogaol Compounds (mg g−1) | |||||||
| X1 | X2 | X3 | X4 | X5 | Experimental | Predicted | |
| 1 | 0 | 0 | 1 | 1 | 0 | 23.570 | 23.296 |
| 2 | 0 | −1 | 0 | 0 | −1 | 23.532 | 21.798 |
| 3 | 0 | 0 | 0 | 1 | 1 | 22.579 | 22.238 |
| 4 | 0 | 0 | 0 | −1 | −1 | 24.779 | 24.813 |
| 5 | 0 | −1 | 0 | 0 | 1 | 20.043 | 21.047 |
| 6 | 0 | 0 | −1 | 0 | −1 | 21.997 | 23.752 |
| 7 | −1 | 0 | 0 | 0 | 1 | 17.296 | 16.878 |
| 8 | 1 | 0 | 1 | 0 | 0 | 27.366 | 25.206 |
| 9 | 0 | 1 | −1 | 0 | 0 | 22.733 | 22.172 |
| 10 | 1 | 0 | 0 | 0 | −1 | 25.850 | 27.045 |
| 11 | −1 | −1 | 0 | 0 | 0 | 17.471 | 16.400 |
| 12 | 1 | 0 | 0 | 1 | 0 | 25.140 | 25.468 |
| 13 | 0 | −1 | 1 | 0 | 0 | 20.523 | 21.278 |
| 14 | 0 | 0 | 1 | 0 | 1 | 22.847 | 21.912 |
| 15 | 1 | 0 | 0 | 0 | 1 | 21.338 | 22.976 |
| 16 | 0 | 1 | 0 | 0 | −1 | 27.530 | 25.237 |
| 17 | 0 | 1 | 0 | −1 | 0 | 23.745 | 23.403 |
| 18 | −1 | 0 | −1 | 0 | 0 | 17.103 | 16.878 |
| 19 | −1 | 0 | 1 | 0 | 0 | 16.930 | 17.204 |
| 20 | 0 | 0 | −1 | 0 | 1 | 21.758 | 20.917 |
| 21 | 1 | 0 | 0 | −1 | 0 | 26.272 | 24.541 |
| 22 | 0 | −1 | 0 | 1 | 0 | 22.219 | 21.773 |
| 23 | 1 | −1 | 0 | 0 | 0 | 22.295 | 22.731 |
| 24 | 0 | 0 | 1 | 0 | −1 | 22.550 | 24.211 |
| 25 | 1 | 0 | −1 | 0 | 0 | 26.736 | 24.077 |
| 26 | 0 | 0 | 0 | −1 | 1 | 21.868 | 21.319 |
| 27 | 0 | 0 | 1 | −1 | 0 | 22.813 | 22.819 |
| 28 | 0 | 1 | 0 | 1 | 0 | 25.000 | 22.675 |
| 29 | 0 | 0 | 0 | 1 | −1 | 23.636 | 23.878 |
| 30 | −1 | 0 | 0 | −1 | 0 | 18.479 | 17.876 |
| 31 | −1 | 0 | 0 | 1 | 0 | 15.478 | 16.933 |
| 32 | 0 | 1 | 1 | 0 | 0 | 22.503 | 23.178 |
| 33 | −1 | 0 | 0 | 0 | −1 | 18.803 | 17.943 |
| 34 | 0 | 0 | −1 | 1 | 0 | 20.719 | 22.084 |
| 35 | 0 | −1 | −1 | 0 | 0 | 21.310 | 20.828 |
| 36 | 1 | 1 | 0 | 0 | 0 | 22.669 | 25.623 |
| 37 | 0 | 0 | −1 | −1 | 0 | 20.926 | 22.573 |
| 38 | −1 | 1 | 0 | 0 | 0 | 15.306 | 16.753 |
| 39 | 0 | −1 | 0 | −1 | 0 | 19.524 | 21.061 |
| 40 | 0 | 1 | 0 | 0 | 1 | 20.409 | 20.853 |
| 41 | 0 | 0 | 0 | 0 | 0 | 19.112 | 23.131 |
| 42 | 0 | 0 | 0 | 0 | 0 | 24.420 | 23.131 |
| 43 | 0 | 0 | 0 | 0 | 0 | 22.862 | 23.131 |
| 44 | 0 | 0 | 0 | 0 | 0 | 24.492 | 23.131 |
| 45 | 0 | 0 | 0 | 0 | 0 | 23.819 | 23.131 |
| 46 | 0 | 0 | 0 | 0 | 0 | 24.080 | 23.131 |
| Source | Sum of Squares | Degrees of Freedom | Coefficients | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| X1: %EtOH | 231.048 | 1 | 23.131 | 231.048 | 66.08 | 0.000 |
| X2: Temperature | 10.526 | 1 | 3.800 | 10.526 | 3.01 | 0.095 |
| X3: Amplitude | 2.117 | 1 | 0.811 | 2.117 | 0.61 | 0.443 |
| X4: Cycle | 0.000 | 1 | 0.363 | 0.000 | 0.00 | 0.993 |
| X5: Ratio | 26.361 | 1 | −0.003 | 26.361 | 7.54 | 0.011 |
| X1X1 | 31.130 | 1 | −1.283 | 31.130 | 8.90 | 0.006 |
| X1X2 | 1.612 | 1 | −1.888 | 1.612 | 0.46 | 0.503 |
| X1X3 | 0.161 | 1 | 0.634 | 0.161 | 0.05 | 0.831 |
| X1X4 | 0.874 | 1 | 0.200 | 0.874 | 0.25 | 0.621 |
| X1X5 | 2.257 | 1 | 0.467 | 2.257 | 0.65 | 0.429 |
| X2X2 | 6.538 | 1 | −0.751 | 6.538 | 1.87 | 0.183 |
| X2X3 | 0.077 | 1 | −0.865 | 0.077 | 0.02 | 0.882 |
| X2X4 | 0.519 | 1 | 0.139 | 0.519 | 0.15 | 0.703 |
| X2X5 | 3.299 | 1 | −0.360 | 3.299 | 0.94 | 0.340 |
| X3X3 | 1.404 | 1 | −0.908 | 1.404 | 0.40 | 0.532 |
| X3X4 | 0.232 | 1 | −0.401 | 0.232 | 0.07 | 0.798 |
| X3X5 | 0.071 | 1 | 0.240 | 0.071 | 0.02 | 0.887 |
| X4X4 | 0.012 | 1 | 0.134 | 0.012 | 0.00 | 0.953 |
| X4X5 | 0.859 | 1 | −0.037 | 0.859 | 0.25 | 0.624 |
| X5X5 | 0.008 | 1 | 0.463 | 0.008 | 0.00 | 0.960 |
| Error total | 87.418 | 25 | −0.031 | 3.496 | ||
| Total (corr.) | 404.918 | 45 |
| Factor | Optimum Extraction Conditions |
|---|---|
| X1: Ethanol in water (%) | 100 |
| X2: Temperature (°C) | 60 |
| X3: Amplitude (%) | 51.8 |
| X4: Cycle (s−1) | 0.458 |
| X5: Sample-to-solvent ratio (g:20 mL) | 0.302 |
| Ginger | 6-G | 6-S | 8-G | 8-S | 10-G | 10-S | Total |
|---|---|---|---|---|---|---|---|
| Sample 1 | 5.63 ± 0.07 | 1.84 ± 0.00 | 2.09 ± 0.01 | 1.24 ± 0.08 | 0.56 ± 0.09 | 0.73 ± 0.01 | 12.11 ± 0.00 |
| Sample 2 | 3.38 ± 0.01 | 1.20 ± 0.05 | 3.59 ± 0.03 | 0.74 ± 0.01 | 0.22 ± 0.10 | 0.21 ± 0.01 | 9.34 ± 0.04 |
| Sample 3 | 6.18 ± 0.06 | 1.97 ± 0.00 | 3.12 ± 0.00 | 1.53 ± 0.09 | 0.46 ± 0.06 | 4.16 ± 0.04 | 17.44 ± 0.03 |
| Sample 4 | 5.49 ± 0.13 | 2.15 ± 0.02 | 1.57 ± 0.04 | 1.33 ± 0.22 | 0.96 ± 0.26 | 2.09 ± 0.04 | 13.61 ± 0.36 |
| Sample 5 | 7.17 ± 0.17 | 2.26 ± 0.04 | 2.53 ± 0.07 | 1.56 ± 0.26 | 0.63 ± 0.20 | 3.99 ± 0.09 | 18.15 ± 0.65 |
| Sample 6 | 7.25 ± 0.30 | 2.42 ± 0.01 | 1.64 ± 0.00 | 1.54 ± 0.14 | 1.07 ± 0.11 | 4.32 ± 0.20 | 18.24 ± 0.65 |
| Sample 7 | 3.76 ± 0.14 | 4.15 ± 0.38 | 0.91 ± 0.00 | 0.90 ± 0.03 | 0.30 ± 0.09 | 0.28 ± 0.00 | 10.32 ± 0.28 |
| Sample 8 | 4.25 ± 0.02 | 1.40 ± 0.03 | 1.27 ± 0.02 | 0.71 ± 0.04 | 0.50 ± 0.05 | 0.26 ± 0.01 | 8.42 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Gonzalez, M.; Yerena-Prieto, B.J.; Carrera, C.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; García-Alvarado, M.Á.; Palma, M.; Rodríguez-Jimenes, G.d.C.; Barbero, G.F. Optimization of an Ultrasound-Assisted Extraction Method for the Extraction of Gingerols and Shogaols from Ginger (Zingiber officinale). Agronomy 2023, 13, 1787. https://doi.org/10.3390/agronomy13071787
Gonzalez-Gonzalez M, Yerena-Prieto BJ, Carrera C, Vázquez-Espinosa M, González-de-Peredo AV, García-Alvarado MÁ, Palma M, Rodríguez-Jimenes GdC, Barbero GF. Optimization of an Ultrasound-Assisted Extraction Method for the Extraction of Gingerols and Shogaols from Ginger (Zingiber officinale). Agronomy. 2023; 13(7):1787. https://doi.org/10.3390/agronomy13071787
Chicago/Turabian StyleGonzalez-Gonzalez, Monserrat, Beatriz Juliana Yerena-Prieto, Ceferino Carrera, Mercedes Vázquez-Espinosa, Ana Velasco González-de-Peredo, Miguel Ángel García-Alvarado, Miguel Palma, Guadalupe del Carmen Rodríguez-Jimenes, and Gerardo Fernández Barbero. 2023. "Optimization of an Ultrasound-Assisted Extraction Method for the Extraction of Gingerols and Shogaols from Ginger (Zingiber officinale)" Agronomy 13, no. 7: 1787. https://doi.org/10.3390/agronomy13071787
APA StyleGonzalez-Gonzalez, M., Yerena-Prieto, B. J., Carrera, C., Vázquez-Espinosa, M., González-de-Peredo, A. V., García-Alvarado, M. Á., Palma, M., Rodríguez-Jimenes, G. d. C., & Barbero, G. F. (2023). Optimization of an Ultrasound-Assisted Extraction Method for the Extraction of Gingerols and Shogaols from Ginger (Zingiber officinale). Agronomy, 13(7), 1787. https://doi.org/10.3390/agronomy13071787









