Abstract
We applied a genomic in situ hybridization (GISH) to analyze the genomic constitution of and meiotic pairing in interspecific somatic hybrids, and in a wide subset of backcrossing derivatives (BC1–BC5), from three interspecific combinations involving the cultivated potato, Solanum tuberosum (AAAA genome), and three diploid (1 EBN) wild Mexican potato species (genome BB)—S. tarnii, S. pinnatisectum, and S. bulbocastanum. The theoretically expected genomic composition was detected in the somatic hybrids (AAAABB) and in the BC1 progeny (AAAAB), whereas in the subsequent BC2–BC4 generations, the partial loss of alien chromosomes was observed and almost all the BC5 genotypes showed a complete chromosome elimination of wild species. GISH revealed a homoeologous pairing between the chromosomes of the A- and the B-genomes in each of the hybrid progenies. Using GISH, we selected introgression lines with a single chromosome of the wild species in a potato genome background, as well as introgression lines with intergenomic recombinant chromosomes. Moreover, via molecular screening, BC hybrids with diagnostic markers for the R-genes conferring resistance to late blight disease and to the quarantine pest of the potato–Columbia root-knot nematode—were selected. The potential application of the results obtained for the planning of introgressive schemes directed to the breeding of advanced lines with multiple disease and pest resistance is discussed.
1. Introduction
The common potato, Solanum tuberosum L. (2n = 4x = 48, 4 EBN, AAAA genome), is the third most important crop and the most important of the non-cereals; however, its cultivation has been continually challenged by numerous diseases and pests. Interspecific hybridization and alien introgression from the wild potato species of section Petota Dumort. of the genus Solanum L. is an important tool in potato breeding for the transfer of important agronomic traits into the cultivated potato gene pool. Wild diploid B-genome potato species (2n = 2x = 24, 1 EBN, genome BB) native to Mexico have been shown to be a valuable source of resistance to many harmful organisms, such as: Phytophthora infestans (Mont.) de Bary, that causes the most destructive potato disease late blight, viruses (PVY and PLRV), root-knot nematodes (Meloidogyne spp.), different aphid species, and the Colorado potato beetle [1]. According to the taxonomic treatment of Hawkes [1], Mexican diploid B-genome species belong to the series Pinnatisecta (Solanum cardiophyllum Lind., Solanum pinnatisectum Dunal, Solanum tarnii, Hawkes and Hjert., S. × michoacanum (Bitter.) Rydb.) and Bulbocastana (Solanum bulbocastanum Dun.). These wild relatives belong to the tertiary gene pool and are sexually incompatible or very difficult to cross with S. tuberosum using double-bridge crosses ((Solanum acaule × S. bulbocastanum) × Solanum phureja) × S. tuberosum) [2,3,4], or through manipulations of EBN and ploidy levels and the use of the S-locus inhibitor gene (Sli) [5,6,7]. Bridge crosses of the diploid Mexican species S. bulbocastanum with South American potato species followed by subsequent backcrosses with potato cultivars took about 50 years to enrich the genetic diversity of the common potato with the alien introgressed Rpi-blb2 gene, conferring broad-spectrum resistance to late blight pathogens and developing two new cultivars, ‘Bionica’ and ‘Toluca’ [8].
To increase the efficiency of interspecific hybridization for broadening the genetic diversity of S. tuberosum (tbr), protoplast fusion techniques have been frequently used to overcome the interspecific incompatibility between the common potato and the diploid B-genome Mexican potato species: S. bulbocastanum (blb) [9,10,11,12,13,14,15,16,17], S. pinnatisectum (pnt) [12,13,18,19,20,21,22,23,24,25,26,27,28], and S. tarnii (trn) [29]; S. cardiophyllum (cph) [23,30,31] and S. x michoacanum (Bitter.) Rydb. (mch) [32,33]. In most cases, the interspecific somatic hybrids between the potato and diploid B-genome Mexican species had a low level of fertility or were sterile, were characterized by poor agronomic characters, and the degree of their disease and/or pest resistance was lower than in the parental clones of wild species [12,15,17,25,32,34,35]. These undesirable characteristics could be due to somaclonal variation induced during protoplast isolation and fusion and the regeneration of hybrid plants [17,36,37]. Thus, cytogenetic instability as mitotic and meiotic irregularities, aneuploidy, and mixoploidy, were detected using cytological methods in the somatic hybrids tbr(+)blb [12,38,39] and tbr(+)pnt [23]. The elimination of parental alleles and markers and genome structural variation were demonstrated in tbr(+)blb, tbr(+)pnt, and tbr(+)mch somatic hybrids using species-specific RAPD [38,40], RFLP [40], ISSR [15,27], and SSR [17] markers; DArT analysis [33]; and genome sequence analysis [28].
In several cases, genetically stable and fertile genotypes have been selected from the numerous interspecific somatic hybrids (SH) and advanced introgression lines that maintain disease and/or pest resistance in their repeated backcross (BC) progenies:
- -
- SH tbr(+)blb and their BC1–BC5 lines with resistance to root-knot nematodes (Meloidogyne chitwoodi Golden and Meloidogyne fallax Karssen), having the RMc1(blb) gene introgressed from blb [41,42,43];
- -
- SH tbr(+)blb and their BC1–BC2 lines with late blight resistance, that possess the Rb/Rpi-blb1 gene [10,40,44,45] and the Rpi-blb1 and Rpi-blb3 genes introgressed from blb [16];
- -
- SH tbr(+)blb and BC1–BC2 clones with resistance to viruses PVY and PLRV, and resistance to the aphid species from blb [14];
- -
- SH tbr(+)trn and BC1–BC3 clones with extreme resistance to PVY and resistance to different aphid species from trn [21,29,46];
- -
- SH tbr(+)pnt and BC1 hybrids with resistance to PVY and to aphids transferred from pnt [21,46];
- -
- SH tbr(+)pnt and BC1 lines with late blight resistance, having the Rpi-blb2 gene from pnt [27,28];
- -
- SH tbr(+)mch and BC1 clones with late blight resistance introgressed from mch [47].
To monitor alien introgressions into cultivated potato genomes, molecular markers were applied in the BC progenies of the interspecific somatic hybrids between S. tuberosum and the diploid B-genome Mexican species. The data from a segregation analysis of the species-specific RFLP and RAPD markers in the BC1–BC2 populations derived from the tbr(+)blb somatic hybrids indicate that intergenomic recombination may occur in these hybrids [40,41].
Knowledge about genome affinity in parental genomes is important for developing efficient breeding strategies [48]. Data on the affinity between the A-genome of S. tuberosum and the B-genome of the Mexican diploid (1 EBN) species indicate the potential for intergenomic pairing and meiotic recombination in interspecific hybrids. Synteny in the BAC position and orientation and identical oligo-FISH patterns were demonstrated across the homoeological chromosomes of S. tuberosum and S. bulbocastanum [48,49,50,51]. Braz et al. [51] demonstrated that the arm ratio and relative length of individual S. bulbocastanum chromosomes were highly similar to those of the homoeologous potato chromosomes. A majority of the DArT markers derived from S. bulbocastanum and South American potato species were collinear, although some amount of variability between their genomes was detected [52,53]. A genome sequence analysis showed relatedness and gene collinearity between the S. pinnatisectum genome and the A-genome of the potato [28].
Cytogenetic techniques are particularly helpful in monitoring interspecific chromatin transfers and estimating the potential for developing genetically stable alien introgression lines. The B-genome of the Mexican diploid species S. bulbocastanum has been identified in interspecific hybrids through genomic in situ hybridization (GISH). Thus, GISH has been successfully performed for parental genome discrimination in somatic hybrids between S. tuberosum and S. bulbocastanum [16,44], and in sexual hybrids between S. pinnatisectum and S. tuberosum and their progenies [7]. However, there is still little information available about the potential for intergenomic pairing and meiotic recombination between homoeologous chromosomes of the A-genome of S. tuberosum and the B-genome of the diploid (1 EBN) Mexican species, because GISH analysis was previously performed mainly on mitotic preparations of interspecific hybrids [16,44] and because there was only a single systematic study of meiotic chromosome pairing. This includes the recent study of Kikuchi and colleagues [7], who applied GISH to analyze the genomic affinity between the A-genome potato species and S. pinnatisectum in sexual hybrids and their progenies [7], and the work of Ramanna and Hermsen [54], who have extensively studied meiotic pairing behavior in the sexual hybrids between S. bulbocastanum and the South American A-genome potato species, S. acaule and S. phureja, using conventional cytological methods [3,54,55]. Both studies have demonstrated meiotic pairing between homoeologous chromosomes and the potential for alien gene introgression.
We provide here cytogenetic information which was obtained using the GISH method about homoeologous chromosome pairing and alien introgression in the backcrossing progenies derived from the somatic hybrids between S. tuberosum and the diploid Mexican B-genome potato species: S. tarnii, S. pinnatisectum, and S. bulbocastanum. Additional objectives of our research were to select perspective genotypes among the backcrossing derivatives for further pre-breeding programs: introgression lines with alien segment introgressions in recombinant chromosomes, putative monosomic alien addition lines, and hybrid genotypes with diagnostic markers for the R-genes conferring resistance to late blight disease and to quarantine pests of the potato–Columbia root-knot nematode.
The hybrid material analyzed here was characterized in previous research using nSSR and AFLP markers, and was also analyzed for resistance to PVY and aphids, and/or for resistance to late blight [16,21,29,39,46,56,57,58].
2. Materials and Methods
2.1. Plant Materials
The plant material in the present study was represented by 8 parental accessions that were involved in protoplast fusion or backcrossing, 2 selected genotypes of somatic hybrids, and 46 selected backcross derivatives from BC1–BC5 progenies from three interspecific combinations (Figure 1):
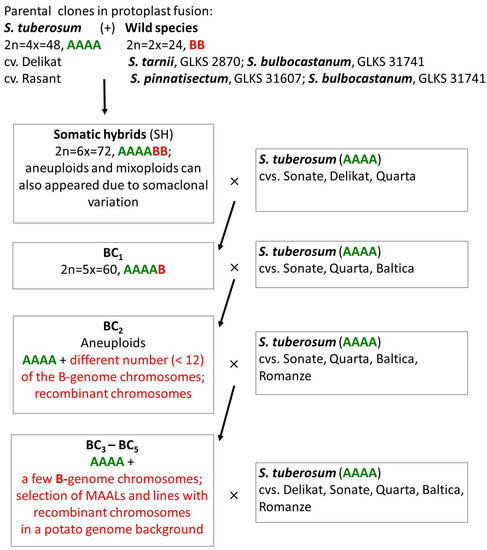
Figure 1.
Breeding scheme for developing introgression lines in three interspecific combinations which are analyzed in the present study. The theoretically expected genome compositions of the somatic hybrids and their backcross derivatives are indicated in brackets. The A-genome of S. tuberosum is marked by green and the B-genome of the wild Mexican diploid species by red.
- (a)
- (tbr+trn)–somatic hybrids were obtained through protoplast fusion between S. tuberosum cv. Delikat and accession GLKS 2870 of the wild Mexican species S. tarnii [29,56]; two fertile somatic hybrids, 838/2 and 838/7, were selected for the present study. BC1–BC5 progenies were produced in sexual crosses between selected hybrid genotypes as female and potato cultivars as a male parent. Somatic hybrids and their backcrossing derivatives have been tested in previous research mainly for resistance to PVY and aphids, as well as to late blight, and have been characterized for agronomic characters [21,29,46,57,58].
- (b)
- (tbr+pnt)–somatic hybrids were obtained using protoplast fusion between S. tuberosum cv. Rasant and accession GLKS 31607 of the wild Mexican species S. pinnatisectum [21,57]. Resistant to PVY and aphids and/or resistance to late blight genotypes were selected in the subsequent BC generations [21,57,58].
- (c)
- (tbr+blb)–somatic hybrids were produced through protoplast fusion between potato cultivars and accession GLKS 31741 of the wild Mexican species S. bulbocastanum and backcross progenies were obtained in subsequent crosses of selected hybrid genotypes with different potato cultivars [16,39,57]. Parental clones, somatic hybrids, and their backcross derivatives were characterized for resistance to late blight and agronomic characters in previous studies [16,57].
GISH was performed for the somatic hybrids and for 37 of 46 backcross derivatives from three interspecific combinations. While GISH was applied for the first time for all the hybrid genotypes from combinations (tbr+trn) and (tbr+pnt) (Figure 1), several hybrids from the third combination, (tbr+blb), were analyzed by GISH previously. Rakosy-Tican et al. [16] performed GISH analysis for four hybrid genotypes from the combination—S. tuberosum, cv. Delikat (+) S. bulbocastanum, GLKS 31741—using preparations of somatic chromosomes. One of the four genotypes (BC2 95/1/4/59) was used in GISH analysis here to study meiotic chromosome behavior. Here we selected an additional seven backcross genotypes from this combination to continue the study of Rakosy-Tican and colleagues [16], and included in our research five new backcross derivatives from another hybrid family (S. tuberosum, cv. Rasant (+) S. bulbocastanum, GLKS 31741) that was not analyzed by GISH before.
Molecular screening was performed for the whole subset: 8 parental accessions, 2 somatic hybrids, and 46 backcross derivatives.
2.2. In Situ Hybridization
The plant material for the genomic in situ hybridization (GISH) assays was grown in a greenhouse. Root tips and flower buds were used for detecting the genome composition of the hybrids. After pretreatment in water with ice for 24 h, the root tips were fixed in Carnoy’s solution (an ethanol: glacial acetic acid = 3:1) and further stored at −20 °C for chromosome counts. The flower buds were fixed directly in Carnoy’s solution and then stored at −20 °C for meiotic analysis in pollen mother cells (PMC). Chromosome slides were prepared after enzymatic treatment of the anthers and root meristems. The enzyme solution contained 4% cellulase (1.14 U/mg) and 1% pectolyase (0.94 U/mg); the time of incubation was 65–120 min.
Genomic DNA was isolated from the young leaves according to the protocol of Bernatzky and Tanksley [59]. For GISH, DNA was isolated from the young leaves of the parental accessions of the cultivated potato S. tuberosum (AAAA genome), cv. Delikat, and from the accessions of the B-genome diploid species: S. tarnii (GLKS 2870) and S. bulbocastanum (PI 498223). The DNA of the A- and the B-genome species were labeled using Nick-Translation Digoxigenin-NT Labeling Kit or BIO-NT Labeling Kit (Jena Bioscience, Jena, Germany, PP-310-DIGX and PP-310-BIO16). The differentially labeled DNA from the A- and B-genomic species were used in the hybridization mix. GISH was performed according to the standard techniques [60] with slight modifications [61].
After GISH, chromosome preparations of some selected introgression lines were re-probed in FISH experiments using an rDNA probe. Selected slides were washed and re-probed using 18S/25S-labeled rDNA in the FISH experiments to identify satellite chromosomes. The 18S/25S rDNA plasmid sequence in the VER17 probe [62] was labeled using DIG-Nick Translation Mix (Roche Diagnostics, Monneheim, Germany 11745816910).
FITS anti-DIG conjugate (FAB fragments) (Roche, Monneheim, Germany 11207741910) and Rhodamine conjugated avidin Red TM-X (Thermo Fisher Scientific, Waltham, MA, USA, S6366) were used in a two-color GISH; FITS anti-DIG conjugate (FAB fragments) was used in FISH.
An AxioImager M2 epifluorescence microscope with an AxioCam, Carl Zeiss, Germany, MRm camera and AxioVision Rel 4.8 software was used to analyze the slides, and create and process the images. In addition, Adobe Photoshop 6.0 was used for image processing.
2.3. Marker-Assisted Selection
Genomic DNA was isolated from the leaf material of the greenhouse-grown plants following the CTAB-extraction method with some modifications [63]. The quality and quantity of the isolated DNA samples were checked using agarose gel electrophoresis (0.8% gel) and a spectrophotometer (Implen NanoPhotometer N60 Touch).
Molecular screening was performed for the whole subset that included 56 genotypes: 8 parental accessions, 2 somatic hybrids, and 46 backcrossing derivatives from three interspecific combinations. The seven markers of the four R-genes conferring resistance to root-knot nematode, RMc1(blb) [43], and resistance to late blight, R8 [64], Rpi-blb1 [65,66], and Rpi-blb3 [67,68], used in this study are shown in Supplementary Table S1. PCR reactions were carried out in a total volume of 20 µL, containing 10 ng DNA template, 1 × PCR reaction buffer (‘Dialat’ [13]), 2 mM MgCl2, 0.4 mM of each dNTP (‘Dialat’ [69]), 0.5 µM of forward/reverse primer (synthesized by ‘Evrogen’ [70]), and 1U Taq polymerase (‘Dialat’). For marker 193I9 the MgCl2 concentration was reduced to 1.5 mM. PCR was carried out according to the standard procedures. The annealing temperatures and cycling conditions corresponded to those indicated by the primer-developers (see references in Supplementary Table S1). All reactions were conducted with at least three replicates.
For CAPS-markers, enzyme RsaI (‘SibEnzyme’ LLC, Novosibirsk, Russia [71]) was used. The restriction was performed in a total volume of 30 µL overnight according to the manufacturer’s protocol.
The PCR products were separated by electrophoresis in 2.0% agarose gels in a TBE buffer, followed by ethidium bromide staining, and visualization in UV light using a GelDoc XR gel system (BIO-RAD, Hercules, CA, USA). The sizes of the amplification products corresponded to those published by the authors (Supplementary Table S1). Diagnostic bands of molecular markers were registered for the hybrids and introgression lines involved in our research.
3. Results
3.1. GISH Analysis of the Genomic Composition and Alien Chromosome Introgression in the Somatic Hybrids and their Backcross Progenies
The genomic composition of the 39 hybrid genotypes from three interspecific combinations of tbr(+)trn, tbr(+)pnt, and tbr(+)blb, were determined using GISH both in mitotic and meiotic cells. Table 1 and Figure 2, Figure 3 and Figure 4 present the content of the B-genomes of the wild diploid Mexican species S. tarnii, S. pinnatisectum, and S. bulbocastanum in the somatic hybrids and backcrossing derivatives.

Table 1.
Genomic composition of somatic hybrids and backcrossing derivatives from three interspecific combinations between S. tuberosum and the wild Mexican diploid B-genome potato species.
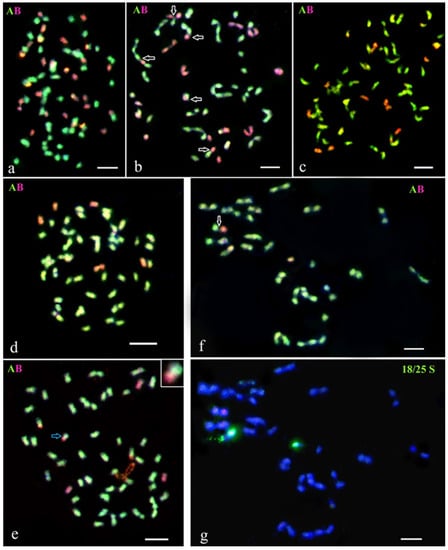
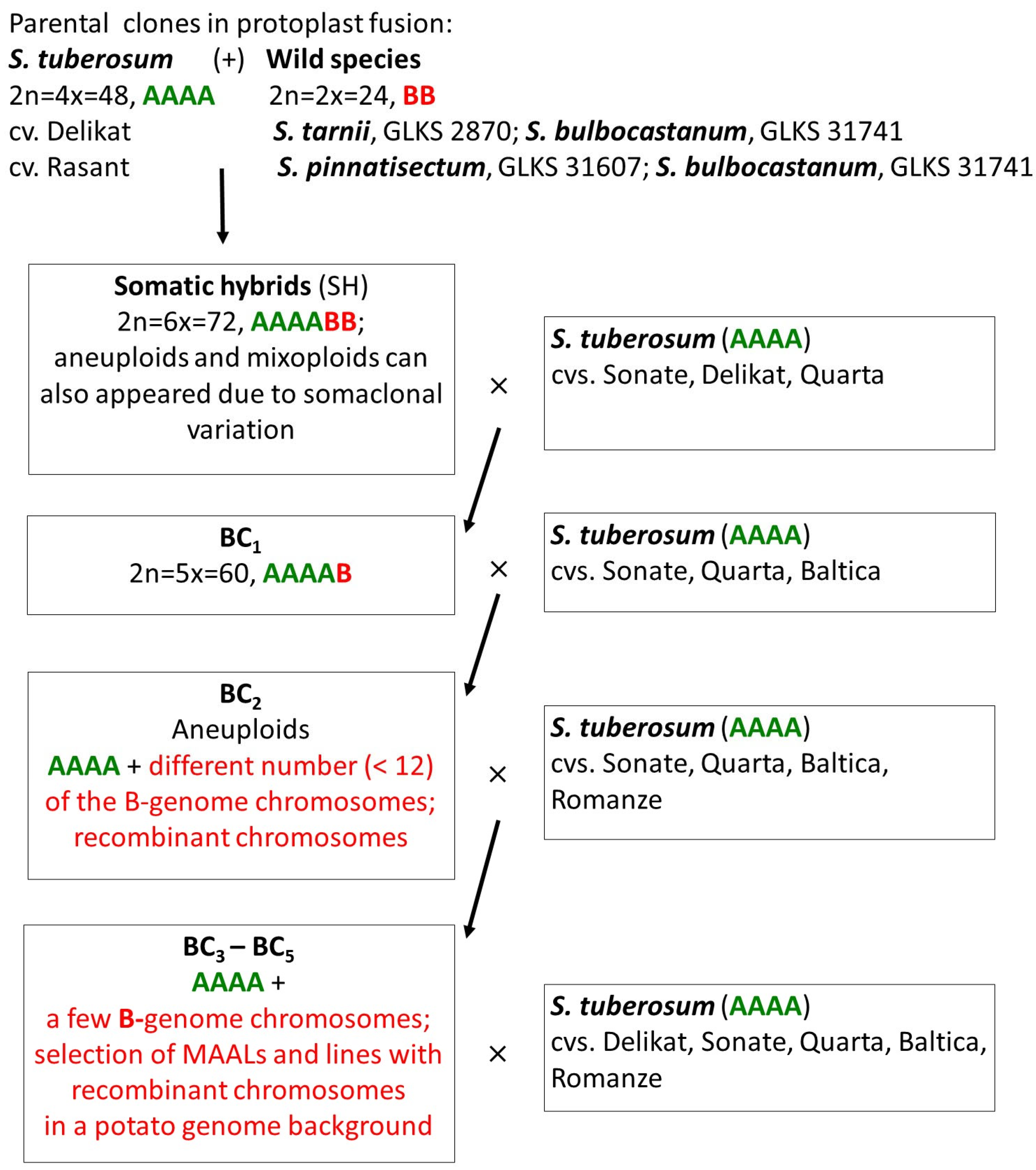
Figure 2.
GISH analysis of the somatic hybrid SH 838/7 from the fusion combination S. tuberosum (+) S. tarnii, and selected lines from its BC progenies. (a) Somatic chromosomes of the fusion hybrid SH 838/7: 48 chromosomes of the A-genome and 24 chromosomes of the B-genome. (b) Homoeologous chromosomal associations at diakinesis in PMC of the SH 838/7: AAAA-B pentavalent, AA-B trivalent, and three A-B bivalents. (c) Somatic chromosomes of the BC1 hybrid 838/7/53: 48 chromosomes of the A-genome and 12 chromosomes of the B-genome. (d) Somatic chromosomes of the BC4 hybrid 838/7/53/3/23/4: 48 chromosomes of the A-genome and 6 chromosomes of the B-genome. (e) Somatic chromosomes of the BC4 hybrid 838/7/53/3/23/6: 48 chromosomes of the A-genome (one of them with the B-genome introgression, blue arrow) and 3 chromosomes of the B-genome. (f,g) MI in PMC of the BC5 hybrid 838/7/53/3/23/4/7: (f) 47 chromosomes of the A-genome and one chromosome of the B-genome, rod A-B bivalent is detected; (g) the same cell after FISH re-probing using 18S/25S rDNA. Blue arrows indicate a recombinant chromosome. White arrows indicate the homoeologous pairing between chromosomes of the A- and the B-genomes. Scale bar = 5 µ.
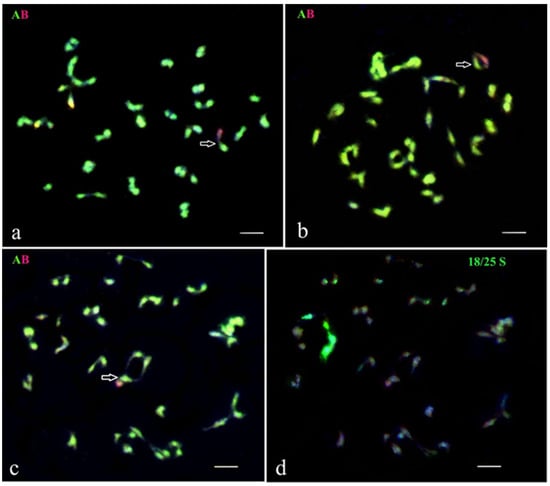
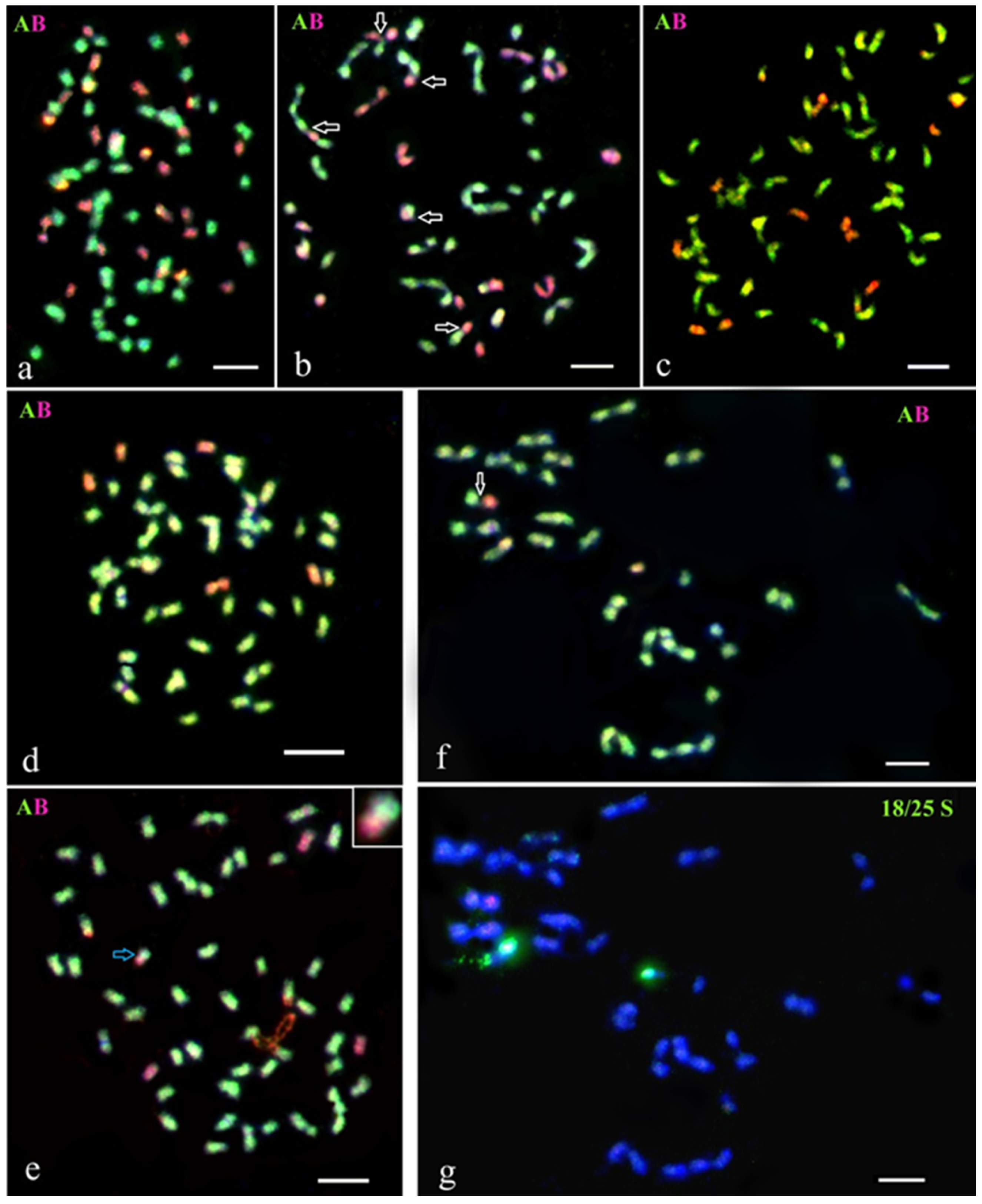
Figure 3.
GISH analysis of the meiotic chromosomes of selected lines from the BC progenies of somatic hybrid SH 2045/2, from the fusion combination of S. tuberosum (+) S. pinnatisectum. Genomic constitution and homoeologous chromosomal associations were recorded at diakinesis in PMCs. (a) BC3 hybrid 2045/2/7/17/7: 48 chromosomes of the A-genome of potato and one chromosome of the B-genome of S. pinnatisectum. (b) BC4 hybrid 2045/2/7/17/7/1: 48 chromosomes of the A-genome and one chromosome of the B-genome; one A-B rod bivalent is detected. (c,d) BC4 hybrid 2045/2/7/17/7/26: (c) 48 chromosomes of the A-genome and one chromosome of the B-genome. (d) The same cell after FISH re-probing using 18S/25S rDNA. White arrows indicate homoeologous pairing between chromosomes of the A- and the B-genomes. Scale bar = 5 µ.
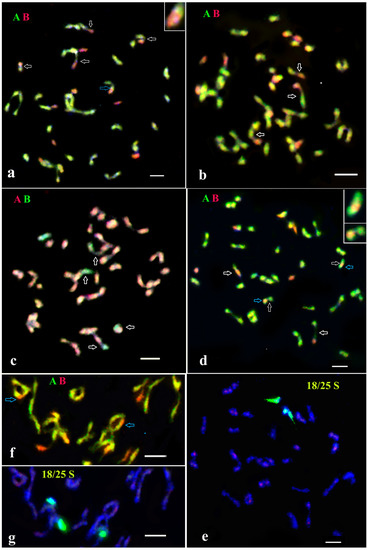
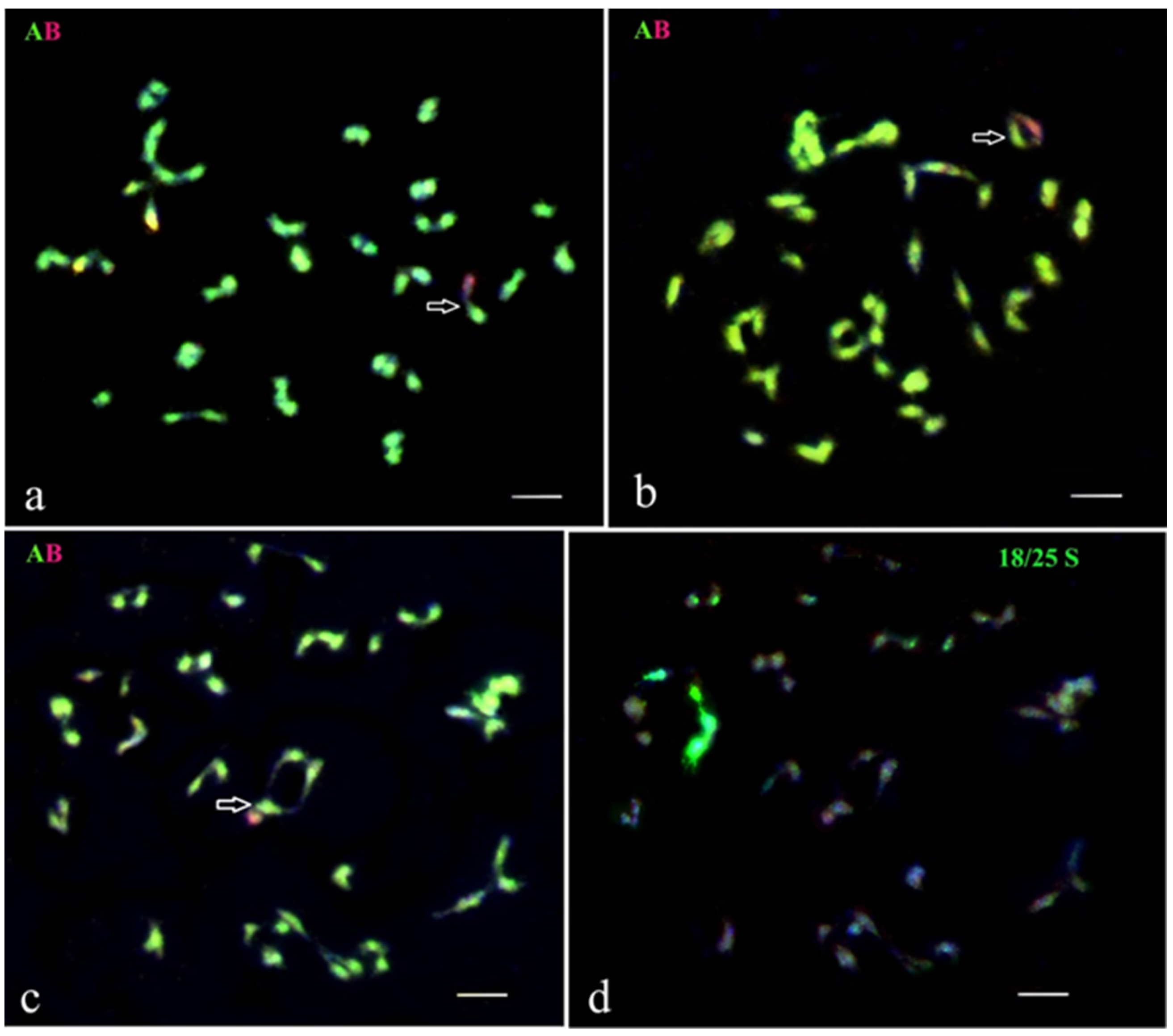
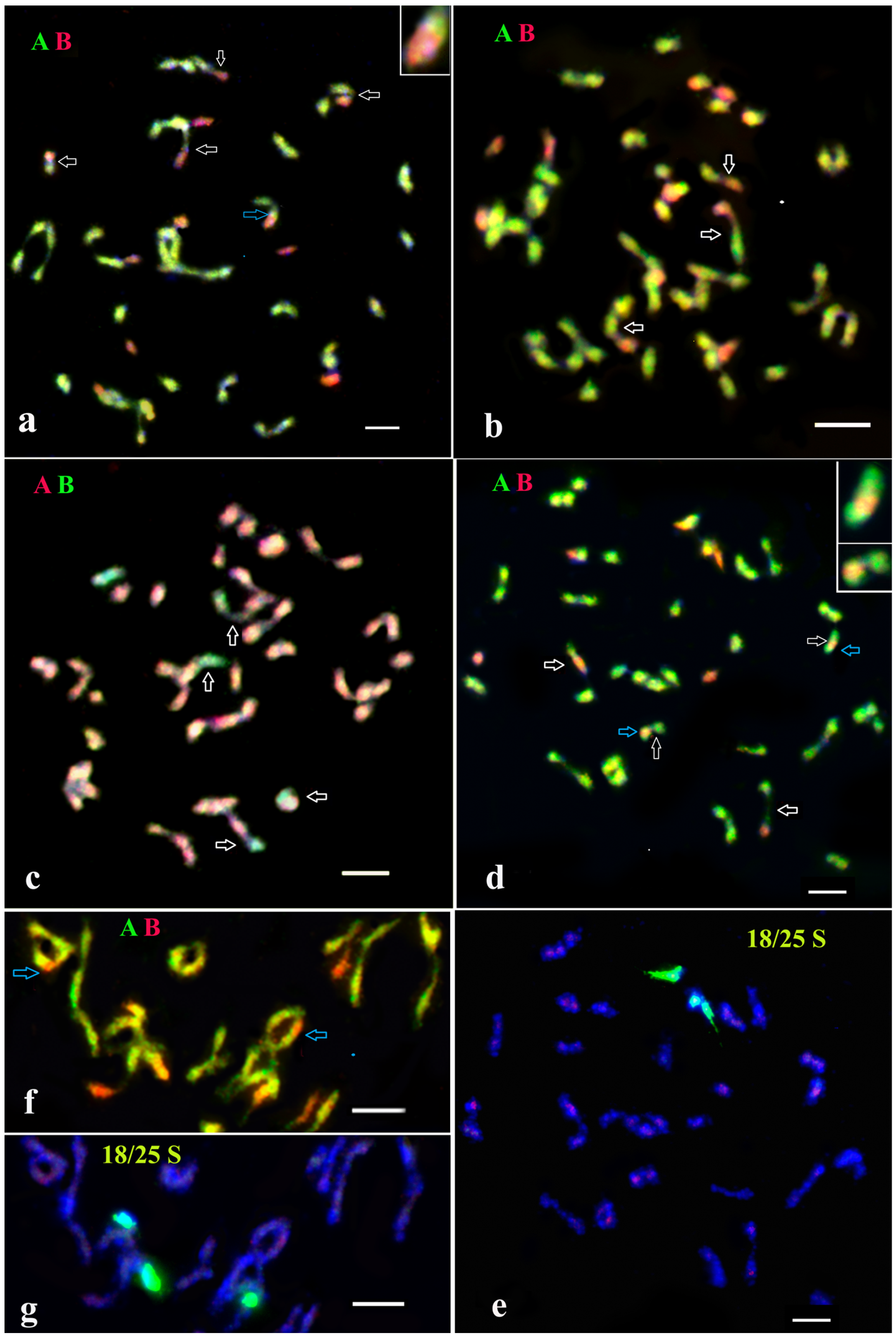
Figure 4.
GISH analysis of selected genotypes from BC1 and BC2 progenies of somatic hybrids from fusion combinations of S. tuberosum (+) S. bulbocastanum (a–e). Genomic constitution and homoeologous chromosomal associations were analyzed at diakinesis in PMCs. (a) BC1 82/4/43: 48 chromosomes of the A-genome and 12 chromosomes of the B-genome, one of them with the A-genome introgression (blue arrow) (this recombinant chromosome of the B-genome is involved in an association with the chromosome of the A-genome). Moreover, four A/B bivalents were also observed. (b) BC1 83/9/44: 48 chromosomes of genome A and 10 chromosomes of genome B; three A-B bivalents were detected. (c) BC2 82/4/46/14: 48 chromosomes of the A-genome and 5 chromosomes of the B-genome; four A-B bivalents were detected. (d,e) BC2 95/1/4/59: (d) 48 chromosomes of the A-genome (two of them with the B-genome introgression, blue arrows) and 5 chromosomes of the B-genome; two A-B bivalents were detected. (e) The same cell after FISH re-probing with 18S/25S rDNA. (f,g) A fragment of the PMC of the BC2 hybrid 95/1/4/59; two chromosomes of the A-genome with introgressions from the B-genome are marked by blue arrows. (g) The same cell after FISH re-probing with 18S/25S rDNA. The red hybridization spots in several DAPI-colored chromosomes (e,g) are the remains of GISH signals.
3.1.1. Genomic Constitution of the Somatic Hybrids, S. tuberosum (+) S. tarnii, and Their Introgression Lines from Backcross Progenies BC1–BC5
In our study, GISH was used for the first time to differentiate the parental chromosomes in the interspecific hybrids between S. tuberosum and S. tarnii. Two somatic hybrids, 838/2 and 838/7, derived from the protoplast fusion of S. tuberosum (2n = 4x = 48, AAAA) and the wild species S. tarnii (2n = 2x = 24, BB), were hexaploid (2n = 6x = 72) and had the theoretically expected AAAABB genomic composition, with 48 chromosomes of potato and 24 chromosomes of S. tarnii (Table 1a, Figure 2a,b). Their BC progenies from two hybrid families were further analyzed using GISH. Correspondingly, the BC1 lines, derived from crosses between the hexaploid somatic hybrids (AAAABB) and the tetraploid parental cultivar Delikat (AAAA), had a 5x (2n = 60) ploidy level and an AAAAB genome constitution, with 48 chromosomes of the A-genome of S. tuberosum and 12 chromosomes of the B-genome of S. tarnii (Table 1a, Figure 2c).
Four BC2 hybrid genotypes selected from the crosses between the pentaploid BC1 hybrids (AAAAB) and the tetraploid cultivar Sonate (AAAA) were aneuploids, indicating the loss of the B-genome chromosomes in the backcrossing. According to the GISH results, these BC2 hybrids have the 48 chromosomes of the A-genome and from two to eight alien chromosomes of the B-genome of S. tarnii (Table 1a).
Introgression lines with 1–3 and 6 alien chromosomes of S. tarnii were detected in the subsequent backcross generations (Table 1a, Figure 2d–f); the genotypes that lost all alien chromosomes of the B-genome were also identified.
Almost all the analyzed BC5 hybrids lost all the chromosomes of the B-genome. Out of seven BC5 hybrids, six had 48 chromosomes of S. tuberosum, and one BC5 genotype (838/7/53/3/23/4/7) had chromosome counts of 2n = 48, with 47 chromosomes of the A-genome of potato and one chromosome of the B-genome from S. tarnii (Table 1a, Figure 2f). A FISH analysis revealed only three hybridization sites of 18S/25S rDNA genes in this genotype, and all three signals were located on three chromosomes of the A-genome of S. tuberosum, indicating the absence of one of four satellite chromosomes of the potato (Figure 2f,g). Hence, the single chromosome of the B-genome in the BC5 genotype, 838/7/53/3/23/4/7 (2n = 48), did not replace one of the missing satellite chromosomes of the potato; it is an alien-addition monosome chromosome transferred from S. tarnii into the genome of S. tuberosum.
3.1.2. Genomic Constitution of the Introgression Lines from Backcross Progenies BC3–BC4 of the Interspecific Somatic Hybrids S. tuberosum (+) S. pinnatisectum
One selected BC3 hybrid had the chromosome count 2n = 49, with 48 chromosomes of the A-genome of S. tuberosum and one extra alien chromosome of the B-genome from S. pinnatisectum (Figure 3). This alien chromosome was transferred into its two BC4 derivatives analyzed here (Table 1b, Figure 3a,b). This monosomic alien chromosome from S. pinnatisectum was not a satellite, as confirmed using FISH, with the 18S/25S rDNA (Figure 3c,d).
3.1.3. Genomic constitution of the Introgression Lines from Backcross Progenies BC1–BC3 of Interspecific Somatic Hybrids S. tuberosum (+) S. bulbocastanum
Three of four BC1 hybrids from the combination tbr(+)blb, which were analyzed using GISH, had an AAAAB genomic composition, with 48 chromosomes of the A-genome and 12 chromosomes of the B-genome of S. bulbocastanum. The other BC1 hybrid (83/9/44) was an aneuploid missing two alien chromosomes (2n = 5x = 58; 48 chromosomes of the A-genome and 10 chromosomes of the B-genome) (Table 1c′, Figure 4a,b).
The white arrows indicate homoeologous pairing between chromosomes of the A- and the B-genomes. The blue arrows indicate recombinant chromosomes. The scale bar = 5 µ.
All eight analyzed BC2 hybrids from the combination blb(+)tbr were aneuploids having 48 chromosomes of the A-genome and from 2 to 5 chromosomes of the B-genome of S. bulbocastanum (Table 1c′,c″; Figure 4c,d). One BC3 genotype (82/4/68/32/27) analyzed here lost all alien chromosomes according to the GISH data (Table 1c′).
3.2. Detection of Alien Chromatin Introgression in Recombinant Chromosomes of Selected BC Genotypes
GISH clearly distinguished between the chromatin of the A- and B-genomes of the parental species, both on the mitotic and meiotic preparations of the somatic hybrids and backcrossing derivatives (Figure 2, Figure 3 and Figure 4). In GISH, the cross-hybridization with genomic DNA probes of both parental species was observed only at the nucleolus organizer region (NOR) that is localized at the short arm of chromosome II in the potato species. Alien introgressed fragments detected with GISH are not always easy to distinguish from cross-hybridization sites, especially at late diakinesis and MI, because the chromosomes of potato species are small and have a similar shape (see review [72]). To exclude misinterpretation of the cross-hybridization sites as introgressed segments in the recombinant chromosomes, we washed the slides after performing GISH and re-probed them in FISH experiments using DIG-labeled 18S/25S rDNA as the probe. It was shown that the cross-hybridization sites observed in GISH were always co-localized with the rDNA hybridization sites detected in FISH, but not with the recombinant chromosomes (Figure 2f,g and Figure 4d–g). Thus, GISH/FISH re-probing was helpful in precisely identifying the introgression of alien chromatin in the recombinant chromosomes of five backcrossing derivatives (Table 1). They included: two BC4 hybrids from the combination trn(+)tbr (Table 1a, Figure 2e) and two BC1 hybrids from the combination blb(+)tbr, with one recombinant chromosome each, and one BC2 hybrid (95/1/4/59) that possessed two chromosomes of the A-genome with introgressions from the B-genome (Table 1c′, Figure 4d,e).
One of the 37 hybrids analyzed here was involved in a previous GISH analysis [16]; this is BC2 95/1/4/59 (2n = 53) from the blb(+)tbr combination. According to our results, it has 48 chromosomes of potato, including 2 recombinant chromosomes and 5 alien chromosomes of S. bulbocastanum. We did not reveal any instability in the chromosome number or in the genomic composition of the microsporocytes of this introgression line. Our results for this genotype do not coincide with those of Rakosy-Tican et al. [16] (2n = 48, 50—mixoploidy; the hybrid had four alien chromosomes and recombinations; chromosome counts were performed in somatic cells). It can be assumed that in this case, during the development of sporogenous tissue in this mixoploid genotype, the genetically stable PMCs were selected.
3.3. Homoeologous Chromosome Pairing Behavior in Hybrid Genotypes from Three Interspecific Combinations Analyzed using In Situ Hybridization
GISH differentiation among the chromatin of the A- and B-genomes allowed identification of the homoeologous genomes involved in each meiotic pairing configuration in the somatic hybrids and introgression lines. Figure 2b,f,g, Figure 3 and Figure 4 present the chromosome behavior during diakinesis and MI for selected hybrid genotypes. Meiotic chromosome pairing was analyzed in 11 hybrid genotypes from three interspecific combinations, tbr(+)trn, tbr(+)pnt, tbr(+)blb, that are representatives of all hybrid generations: somatic hybrids and BC1–BC5. Table 2 summarizes the following meiotic chromosome pairing characters: average frequency of homologous and homoeologous chromosomal associations per cell, type of meiotic configurations, and the number of chromosomal arms involved in homoeologous A/B pairing.

Table 2.
Chromosome pairing in hybrids derived from three interspecific combinations: S. tuberosum (+) S. tarnii, S. tuberosum (+) S. pinnatisectum, and S. tuberosum (+) S. bulbocastanum.
SH (2n = 6x = 72, AAAABB).
According to the GISH results, the most frequent chromosome configurations in hexaploid somatic hybrid 838/7 (2n = 6x = 72, AAAABB genome), were homologous bivalents; on average, 17.8 per cell involving chromosomes of the A-genome and 7.55 involving the B-genome of S. tarnii (Table 2, Figure 2b). Nevertheless, the average frequencies of homologous bivalents were less than the theoretically expected preferential bivalent pairing (24 II AA + 12 II BB) for the distant hybrid with genome constitution AAAABB, because of the appearance homoeologous chromosomal associations. The average frequency of intergenomic chromosomal association in the somatic hybrid was recorded as 4.75 per cell, including 3.45 II A-B + 0.15 III AA-B + 0.05 III A-BB + 0.15 IV AAA-B + 0.05 IV AA-BB + 0.05 V AAAA-B.
Note that among the homoeologous bivalents (II A-B), we detected not only rod, but also ring (closed-type) bivalents with two chiasmata, as well as closed-type associations among the homoeologous quadrivalents (IV AAA-B). Therefore, when summing up all the chromosomal pairs involved in allosyndetic associations, the obtained value of 3.90 on average per cell increased up to 4.75 (Table 2).
The average chromosome configuration also included univalents—6.80 per cell of S. tuberosum and 4.90 of S. tarnii (Table 2).
BC1 hybrids (2n = 5x = 60, AAAAB).
None of the chromosomes of the B-genome formed homologous B-B bivalents in the four analyzed BC1 hybrids; alien chromosomes of the B-genome were present as univalents in all PMCs, which means that these plants possess 12 individual alien chromosomes of the wild species S. bulbocastanum in a univalent state (basic chromosome number x = 12, as in all potato species) (Table 2, Figure 4a,b). At the same time, homoeologous chromosomal associations were detected in each of the four analyzed BC1 hybrids that included A-B bivalents, and occasionally AA-B trivalents and AAA-B quadrivalents. The average frequency of the homoeologous associations varied from 3.05 to 3.63 per cell (Table 2, Figure 4a,b).
BC2 hybrids.
Two selected BC2 hybrids each possessed five alien chromosomes of the wild species S. bulbocastanum and recombinant chromosomes—one in BC2 82/4/46/14 and two in BC2 95/1/4/59 (Table 1c′). At meiosis for these hybrids, the average frequency of homoeologous chromosomal associations per cell was recorded as 2.38 and 2.16 per cell, respectively (Table 2, Figure 4c,d). GISH results showed that the recombinant chromosomes were paired with chromosomes of the A-genome of potato (Figure 4a,d,f).
BC3–BC5 hybrids.
Four selected introgression lines from the BC3–BC5 generations had a tetraploid ploidy level. Each of them possessed one additional alien chromosome of the B-genome; three of them were from the tbr(+)pnt combination and one was from tbr(+)trn combination (Table 2). As expected, the average chromosome configurations were mostly A-A bivalents between homologous S. tuberosum chromosomes, with frequencies of 18.40–21.33. Moreover, t I, III, and IV involving A-genome chromosomes of potato were observed in these introgression lines (Table 2, Figure 2f,g and Figure 3).
The frequency of intergenomic associations between the chromosomes of the A-genome and an additional monosomic chromosome of the B-genome were detected in each of the four introgression lines. The single alien chromosome of S. tarnii in the BC5 hybrid (838/7/53/3/23/4/7) took part in homoeologous pairing, with average frequency of 0.2 per cell (Table 2, Figure 2f,g).
The BC3 and BC4 hybrids from the combination tbr(+)pnt represent the same family. These three putative monosomic alien addition lines bring the same single alien chromosome from S. pinnatisectum (Table 1b, Figure 3) that was not lost during backcrossing, because it took part in homoeologous pairing in the BC3 hybrid 2045/2/7/17/7, with an average frequency of 0.65 per cell (Table 2). Our data demonstrate that this alien chromosome was involved in at least the second cycle of homoeologous pairing and recombination in the BC3 and in the BC4 hybrids.
3.4. Marker-Assisted Selection of the Somatic Hybrids and Introgression Lines
The whole subset of 56 genotypes (backcrossing derivatives, somatic hybrids, and parental accessions) was tested with seven DNA markers of four resistance genes to select the hybrid genotypes with introgressed alien genes.
3.4.1. Molecular Screening with Markers of the RMc1(blb) Gene Conferring Resistance to M. chitwoodi
Two DNA markers, 193I9 and 39E18, closely linked to the RMc1(blb) gene [43] conferring resistance to Columbia root-knot nematode, were used in the molecular screening (Supplementary Tables S1 and S2). Both markers were detected in parental accessions of the wild species S. tarnii, GLKS 2870 and S. bulbocastanum, GLKS 31741. Only one of these markers (193I9) was detected in accession GLKS 31607 of S. pinnatisectum. Both markers 193I9 and 39E18 were absent in the potato cultivars analyzed here that were involved in protoplast fusion and backcrossing (Supplementary Table S2).
Two somatic hybrids, SH 838/2 and SH 838/7 (2n = 6x = 72, AAAABB) from the combination trn(+)tbr, possessed both of these markers, whereas their four BC1 derivatives (2n = 5x = 60, AAAAB) lost them. This might be explained by the heterozygosity of the locus RMc1(blb) in the parental accession of S. tarnii that was segregated in the BC1 progeny. In contrast, in the combination blb(+)tbr, the markers of the RMc1(blb) gene were detected in all 10 BC1 hybrids and in 5 BC2 hybrids (Supplementary Table S2, Supplementary Figure S1).
3.4.2. Molecular Screening with Markers of the Genes Conferring Resistance to Late Blight Diseases
The marker 184-81-RsaI, specific for the R8 gene conferring resistance to late blight, was detected in all three parental accessions of the wild species: S. tarnii, GLKS 2870, S. bulbocastanum, GLKS 31741, and S. pinnatisectum, GLKS 31607. At the same time, it was absent in the potato cultivars analyzed here that were involved in protoplast fusion and backcrossing. Both somatic hybrids SH 838/2 and SH 838/7 (2n = 6x = 72, AAAABB) from the combination trn(+)tbr, and three out of four of their BC1 hybrids (2n = 5x = 60, AAAAB), had this marker, whereas one BC1 genotype (BC1 03.838/2/25) lost it. This might be explained by the heterozygous condition of the R8 gene in the parental accession of S. tarnii. Two of the four BC2 derivatives analyzed here from the combination trn(+)tbr had the R8 gene. In the combination blb(+)tbr, the marker 184-81-RsaI of the R8 gene was detected in all the BC1 hybrids and in three BC2 genotypes. The backcross derivatives from combination tbr(+)pnt lost it (Supplementary Table S2, Supplementary Figure S2).
The parental accession GLKS 31741 of S. bulbocastanum possess four markers of the two Rpi genes conferring broad-spectrum late blight resistance—two markers, 1/1′ and blb1 F/R, specific for the Rpi-blb1 gene, and two markers, Rpi-blb3 and blb3 F/R, specific for the Rpi-blb3 gene (Supplementary Table S2, Supplementary Figures S3–S6). These markers were not detected in the parental accessions of S. tarnii, GLKS 2870, or S. pinnatisectum, GLKS 31607.
All 10 BC1 hybrids from the combination blb(+)tbr had markers of the Rpi-blb1 and Rpi-blb3 genes. Moreover, we detected six BC2 hybrids with Rpi-blb1, two with Rpi-blb3, and one BC3 genotype, 82/4/68/32/27, with the marker Rpi-blb3 (Supplementary Table S2, Supplementary Figures S3–S6).
Thus, 12 genotypes (10 BC1 and 2 BC2) from the combination blb(+)tbr with four resistance genes from S. bulbocastanum, GLKS 31741, were selected using MAS for a further breeding program.
4. Discussion
4.1. Genomic Composition of Interspecific Hybrids and Transmission of Alien B-Genome Chromosomes through Backcrossing
The desirable alien genes of wild species could be introgressed into the recipient genome of crop plants through alien chromosome additions or substitutions, intergenomic translocations, homoeologous pairing, and meiotic recombination. The application of GISH for the offspring of distant hybrids is an efficient approach for the monitoring of alien chromatin introgression into the genome of crop species [73,74], especially for species with small chromosomes and low levels of karyotype differentiation, such as potato.
Previous research has showed that GISH has been applied effectively for the distinction of parental genomes in hybrids of potato and non-tuber-bearing species of the genus Solanum, belonging to the sections Lycopersicon and Etuberosum (see reviews [48,72,75]). Within the section Petota Dumort, which includes numerous tuber-bearing species (potatoes) of the genus Solanum, GISH has been successfully used to differentiate the A-genome of S. tuberosum and the B-genome of Mexican diploids in interspecific somatic and in sexual hybrids and their progenies [7,16,44]. It has also been used to distinguish the B-subgenome in natural Mexican allopolyploid species [61,76] or chromosomes of the B-subgenome in sexual hybrids between the wild Mexican allotetraploid species and the common potato [77].
In the present study, we detected the genomic composition of 39 hybrid genotypes of different generations from three interspecific combinations between the common potato, S. tuberosum (genome AAAA), and the wild diploid (1 EBN) Mexican species: S. tarnii, S. pinnatisectum, and S. bulbocastanum (BB genome). The genomic composition of the hexaploid somatic hybrids from the combinations trn(+)tbr corresponded to what was theoretically expected—AAAABB, as well as the genomic composition of the BC1 hybrids—seven BC1 hybrids from the combinations trn(+)tbr and blb(+)tbr were pentaploids having an AAAAB genomic constitution. As expected, these BC1 hybrids produced aneuploid progeny, indicating the loss of the B-genome chromosomes in the subsequent BC3–BC5 backcrossing. From six to one alien chromosomes were detected in the BC4 introgression lines; six of the seven BC5 genotypes lost chromosomes of the B-genome.
Our results indicate the potential for the selection of monosomic alien addition lines of the B-genome Mexican species in a S. tuberosum genome background. Five putative monosomic addition lines with single alien chromosomes were detected among the BC3-BC5 derivatives analyzed here—two in the combination trn(+)tbr and three in the combination pnt(+)tbr. The single alien chromosome from S. pinnatisectum was not lost during BC3 backcrossing and was transferred to the BC4 hybrids because it took part in intergenomic pairing. Homoeologous chromosomal associations involving a single alien chromosome of S. tarnii were also observed in the BC5 838/7/53/3/23/4/7 (2n = 48) introgression line, indicating the possibility of their transfer during subsequent backcrossing.
Similarly, using GISH, Kikuchi et al. [7] selected three introgression lines with a single chromosome of S. pinnatisectum in the potato genome background among the BC4 progeny of sexual interspecific hybrids. In potato, a set of alien addition and/or substitution lines was established for more distantly related non-tuber-bearing species of the genus Solanum, belonging to the sections Lycopersicon [78,79] and Etuberosum [80], using BC progenies derived from somatic hybrids.
4.2. Meiotic Pairing of A/B Homoeologous Chromosomes, Detection of Recombinant Chromosomes
The results of introgressive breeding in potatoes depend largely on the potential of homoeologous pairing and meiotic recombination, because it predicts the success of desirable alien gene introgression into the S. tuberosum genome through subsequent backcrossing, and helps to develop introgressive hybridization schemes [3,7,48,54,77,81,82,83]. Therefore, information about meiotic chromosome behavior in interspecific hybrids and about the peculiarities of the interaction of the A-genome of S. tuberosum and the B-genome of Mexican diploid species is important for potato introgressive breeding.
To assess the potential of homoeologous meiotic A/B pairing and recombination, a GISH analysis was performed here for the PMCs of 11 hybrid genotypes from three interspecific combinations. Homoeologous meiotic chromosome pairing between chromosomes of the A- and the B-genomes was detected in each of the 11 analyzed introgression lines, even in the somatic hybrid trn(+)tbr (2n = 72, AAAABB), for which we expected to detect preferential bivalent pairing (24 II A + 12 II B). The average frequency of intergenomic chromosomal association in the somatic hybrid was recorded as 4.75 per cell; in the BC1 hybrids it varied from 3.05 to 3.63 per PMC, including II A-B, III AA-B, and IV AAA-B. An analysis of the homoeological chromosome pairing associations indicates that the intergenomic A-B bivalents prevailed in all the 11 analyzed hybrid genotypes, among which associations of the open type (rod bivalents) predominated; closed (ring) A-B bivalents were less common, but they were observed in all the hybrids, except for one line—BC4 2045/2/17/07/1.
Our results are in agreement with a few previous studies of meiotic chromosome behavior in interspecific hybrids, which indicated that chromosomes of the B-genome of wild Mexican potato species can recombine with chromosomes of the A-genome. Thus, intergenomic chromosome pairing at meiosis was demonstrated using GISH in sexual hexaploid interspecific hybrids between S. pinnatisectum and S. tuberosum having four genomes from wild species and two genomes from potato; about 20% of the S. pinnatisectum chromosomes were involved in intergenomic associations, with an average frequency of 6.7 per cell [7]. Regular meiosis was reported in the sexual hybrids between S. bulbocastanum (BB genome) and the wild Mexican species S. verrucosum (AA genome) [81]. A quadrivalent formation was detected in the interspecific sexual hybrid ((Solanum acaule × S. bulbocastanum) × Solanum phureja), that was analyzed using the conventional cytological method [3,54]. A homoeologous pairing was detected using GISH in the sexual hybrids between chromosomes of the B-subgenome of the wild allotetraploid Mexican species Solanum stoloniferum Schltdl. (AABB genome) and chromosomes of the A-genome. The average frequency of intergenomic associations was 0.82 per cell in the hexaploid hybrid (mitotic doubling, genome composition AAAABB), and up to 2.50 in the BC1 hybrid (genome composition AAAAB) [77].
GISH/FISH re-probing was helpful for precisely identifying the introgression of alien chromatin in recombinant chromosomes. Five introgression lines (two BC1, one BC2, and two BC4) having one–two chromosomes with alien segment introgression were identified among the backcrossing derivatives from the trn(+)tbr and blb(+)tbr combinations. These recombinant chromosomes took part in homoeological pairing and, therefore, have a potential to be transferred to the subsequent offspring, where the size of the alien introgressed segments may change as a result of homoeological pairing and crossing-over. Taking into account the number of homoeological chromosomal associations detected in our study, we can assume that the observed number of recombinant chromosomes recognizable using GISH in the five BC genotypes is significantly less than expected. This can possibly be explained by the fact that the homoeological chromosomes were paired by terminal chiasmata and some of the introgressed fragments were out of range of the GISH resolution.
We could suppose a different origin for some of the recombinant chromosomes that arose, not only from homoeological pairing and meiotic recombination, but also from chromosomal rearrangements due to somaclonal variation. Thus, the findings of Rakosy-Tican et al. [16], who detected a single recombinant chromosome at a very early stage of introgression breeding, still in the somatic hybrid blb(+)tbr, which obviously could be the result of chromosomal rearrangements during protoplast isolation and fusion.
In previous GISH studies of backcrossing progenies of interspecific somatic hybrids between the common potato, S. tuberosum, and the non-tuber-bearing species, S. etuberosum, recombinant chromosomes were not detected [84,85], whereas in the closer combination between S. tuberosum and S. pinnatisectum, intergenomic translocations were often observed in the BC2–BC4 progenies of sexual interspecific hybrids [7].
4.3. Marker–Assisted Selection (MAS) of the Somatic Hybrids and the Backcrossing Derivatives
Molecular screening data confirmed the introgression of alien genetic material into the potato genome in the somatic hybrids and their backcrossing derivatives.
MAS forRMc1(blb)geneconferring resistance to Columbia root-knot nematode.
We detected the RMc1(blb) gene encoding resistance to M. chitwoodi, using two DNA markers closely linked to it, in 17 out of the 48 hybrid genotypes, including 10 BC1 and 5 BC2 hybrids from the combination blb(+)tbr and in 2 from SH trn(+)tbr. The utility of these markers for the MAS of resistance to Columbia root-knot nematode genotypes was demonstrated earlier in the nematode resistance tests [43]. A successful program for the introgression of the RMc1(blb) gene from S. bulbocastanum into the cultivated potato gene pool has already been carried out at Washington State University, where advanced breeding clones with root-knot resistance were developed from blb(+)tbr hybrids with subsequent backcross cycles [42].
MAS for genes encoding resistance to late blight.
Previous studies have shown that the parental accessions of three wild diploid Mexican species, trn (GLKS 2870), pnt (GLKS 31607), and blb (GLKS 31741), are involved in somatic hybridization and are highly resistant to late blight, and/or to PVY, and aphids; these resistance traits are transferred to the somatic hybrids and BC progenies [16,21,29,39,56,57,58].
Lokossou et al. [67] reported the presence of the well-studied Rpi genes conferring broad-spectrum late blight resistance, in natural populations of Mexican potato species, including S. bulbocastanum (Rpi-blb1, Rpi-blb2, and Rpi-blb3 genes). The diagnostic fragments of the specific markers of the Rpi-blb1 and Rpi-blb3 genes—BLB1F/R, Blb3F/R [16] and 1/1′, and Rpi-blb3 (the present study)—were detected in the late-blight-resistant parental accession GLKS 31741 of S. bulbocastanum. The primers specific for the Rpi-blb2 gene were not amplified in this accession. Rakosy-Tican and colleagues [16] confirmed the functionality of the detected alleles of the Rpi-blb1 and Rpi-blb3 genes in the parental accession GLKS 31741 of S. bulbocastanum, and in selected genotypes of SH and BC1-BC2, using agro-infiltration with the effectors of the corresponding Avr genes. A similar approach was used to detect the functional alleles of the R3a and R3b late-blight-resistance genes in the same genotypes [16]. Molecular screening and phenotyping of the BC1 and BC2 hybrids from the combination blb, GLKS 31741(+)tbr, cv. Delikat, with specific primers for the Rpi-blb1, Rpi-blb3, R3a, and R3b genes allowed the identification of plants carrying all four genes that were resistant to foliage blight in greenhouse and field trials [16]. Twelve hybrid genotypes from this previous study were included in our subset, which was expanded with additional BC1, BC2, and BC3 hybrids, and was screened with additional gene-specific primers for the Rpi-blb1, Rpi-blb3, and R8 genes (see Supplementary Tables S1 and S2). A comparison of the data from the literature [16] with our results shows that 11 of 12 late-blight-resistant genotypes have a stacking of 5 genes with distinct resistance specificities to late blight that are located on four chromosomes: Rpi-blb1 (VIII), Rpi-blb3 (IV), R3a (XI), R3b (XI), and R8 (IX). In addition, the markers of the RMc1(blb) (XI) gene encoding resistance to Columbia root-knot nematode were detected in each of these 11 genotypes (9 BC1 hybrids having a complete chromosomal set of alien chromosomes and three BC2 genotypes, two of them with 4 and 5 alien chromosomes, according to the GISH data).
Three genes from S. bulbocastanum—RMc1(blb), R3a, and R3b—are located on the same chromosome XI [41,86,87]. They all were transferred from parental accession GLKS 31741 to 11 BC genotypes (9 BC1 and 2 BC2, see Supplementary Table S2). One introgression line, BC2 95/1/4/59 (2n = 53, 48 chromosomes of potato, including 2 recombinant chromosomes and 5 alien chromosomes), had the R3a and R3b genes, but lost the markers tightly linked to the RMc1(blb) gene, which might be explained by recombination.
Particularly interesting is the BC3 82/4/68/32/27 genotype that possesses gene-specific markers of the Rpi-blb3 gene introgressed from blb, GLKS 31741, although it lost all alien chromosomes, according to the GISH results. Based on the data obtained in the present study, it can be assumed that the alien segment with the Rpi-blb3 gene, which is located on chromosome IV of S. bulbocastanum, was introgressed into the A-genome of S. tuberosum by meiotic recombination, and that the size of this alien fragment was too small to be detected with GISH.
MAS of trn(+)tbr and pnt(+)tbr combinations.
The primers specific for the genes Rpi-blb1–Rpi-blb3 were not amplified in the late-blight-resistant parental accession GLKS 2870 of S. tarnii. This parental accession, two SH and five BC hybrids, had the diagnostic fragments of the gene-specific primers for the late-blight- resistant R8 gene.
Lokossou et al. [67] also reported the presence of the Rpi-blb3 gene conferring broad-spectrum late blight resistance, in natural populations of S. pinnatisectum. However, the primers specific for the Rpi-blb3 and Rpi-blb1 genes (the present study) and for Rpi-blb2 were not amplified in the late-blight-resistant parental accession GLKS 31607 of S. pinnatisectum. The novel, major late-blight-resistance locus derived from S. pinnatisectum (GLKS 31607) was mapped onto chromosome VII using DArT markers [88]. However, DNA markers associated with this novel locus and that are well suited for MAS have not yet been developed. Additional studies are required to identify chromosome VII of S. pinnatisectum, which was transferred to the late-blight-resistant hybrid genotypes.
4.4. Strategy for Introgressive Breeding
Knowledge about the level of meiotic chromosome pairing affinity in hybrids is important for developing efficient breeding strategies. In this case, the most significant is the meiosis of the pentaploid (2n = 5x) BC1 hybrids having the AAAAB genomic composition (48 chromosomes of the A-genome and 12 chromosomes of the B-genome), which possesses a complete haploid set of alien chromosomes of a wild species, because no homologous B-B bivalents were found in any PMCs. At the same time, from 3.05 to 3.63 intergenomic A/B associations per PMC were observed in these BC1 hybrids. It is important that breeding programs directed to developing advanced lines with resistance to multiple diseases do not lose recombination potential during subsequent backcrosses, in which alien chromosomes are rapidly lost. Therefore, it can be assumed that several cycles of self-pollination of the BC1 hybrids may accumulate their A/B recombination potential. The subsequent backcrossing of the self-pollinated BC1 hybrids will promote the cytological stability of the introgression lines, having potato chromosomes with alien segment introgressions.
5. Conclusions
Using the GISH and MAS methods, we provided information about the potential of homoeologous chromosome pairing and alien gene introgression in a wide subset of backcrossing derivatives, from three interspecific combinations between S. tuberosum and the diploid Mexican B-genome potato species: S. tarnii, S. pinnatisectum, and S. bulbocastanum.
Hybrids from BC progenies contain different amounts of chromatin from wild species, including monosomic alien addition chromosomes, alien segments that were introgressed through homoeologous pairing and recombination, as well as hybrid genotypes with stacking up to six genes (Rpi-blb1, Rpi-blb3, R3a, R3b, R8, and RMc1(blb)) from S. bulbocastanum.
Selected hybrid genotypes with alien introgressions might be used in potato pre-breeding programs for their resistance to multiple diseases and pests, such as late blight, the quarantine pest of potato, M. chitwoodi, as well as PVY and aphids.
In the present work we demonstrated the efficiency of applying two methods—GISH and MAS—for monitoring the alien introgression of the B-genome diploid (1 EBN) wild Mexican species into the A-genome of potato, S. tuberosum. This is consistent with the recent results of Kikuchi et al. [7] and Rakosy-Tican et al. [16]. At the same time, additional studies will be required to identify the individual alien chromosomes passed on to backcross derivatives from wild Mexican B-genome species.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13071809/s1, Table S1: Primers used in this study for the marker-assisted selection; Table S2: Selection of hybrid genotypes that are potentially resistant to Columbia root-knot nematode (Meloidogyne chitwoodi) and have several genes conferring resistance to late blight (P. infestans), based on molecular DNA markers. Figure S1. The diagnostic marker of the RMc1(blb) gene conferring resistance to Columbia root-knot nematode; Figure S2. Diagnostic bands of the marker 184-81 (indicated by an arrow) for the R8 gene conferring late blight resistance; Figure S3. The diagnostic marker 1/1’ for identification of the Rpi-blb1 gene conferring broad-spectrum late blight resistance; Figure S4. The diagnostic fragment of the blb1 F/R -marker specific for the Rpi-blb1 gene conferring broad-spectrum late blight resistance; Figure S5. The diagnostic bands of the marker Rpi-blb3 specific for the Rpi-blb3 gene conferring broad-spectrum late blight resistance; Figure S6. The diagnostic fragment of the blb3F/R-marker specific for the Rpi-blb3 gene conferring broad-spectrum late blight resistance.
Author Contributions
Conceptualization, T.G.; methodology (GISH), G.P. and T.M.; methodology (MAS), O.A. and T.M.; resources, R.T.; writing—review and editing, T.G.; GISH methods and results, G.P. and T.G.; MAS methods and results, O.A. and T.G.; project administration, T.G. and R.T.; supervision, T.G. and R.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant (20-54-00043-bel-a) from the Russian Foundation of Basic Research (RFBR) and by the German–Russian Bilateral Project 131-KAD. The paper was prepared with assistance provided within the framework of Topic No. FGEM-2022-0004.
Acknowledgments
We appreciate that all the authors contribute to this manuscript and thank all anonymous reviewers for their constructive advice. The authors express their deep gratitude to N. Oskina for her kind help with preparing the manuscript for submission.
Conflicts of Interest
The authors declare there are no conflict of interest.
References
- Hawkes, J.G. The Potato: Evolution, Biodiversity and Genetic Resources; Belhaven Press: London, UK, 1990; p. 259. [Google Scholar]
- Dionne, L.A. Studies on the use of Solanum acaule as a bridge between Solanum tuberosum and species in the series Bulbocastana, Cardiophylla and Pinnatisecta. Euphytica 1963, 12, 263–269. [Google Scholar] [CrossRef]
- Hermsen, J.G.T.; Ramanna, M.S. Double-bridge hybrids of Solanum bulbocastanum and cultivars of Solanum tuberosum. Euphytica 1973, 22, 457–466. [Google Scholar] [CrossRef]
- Hanneman, R.E. The potato germplasm resource. Am. Potato J. 1989, 66, 655–667. [Google Scholar] [CrossRef]
- Sanetomo, R.; Akino, S.; Suzuki, N.; Hosaka, K. Breakdown of a hybridization barrier between Solanum pinnatisectum Dunal and potato using the S locus inhibitor gene (Sli). Euphytica 2014, 197, 119–132. [Google Scholar] [CrossRef]
- Sanetomo, R.; Habe, I.; Hosaka, K. Sexual Introgression of the Late Blight Resistance Gene Rpi-Blb3 from a Mexican wild diploid species Solanum pinnatisectum Dunal into potato varieties. Mol. Breed. 2019, 39, 13. [Google Scholar] [CrossRef]
- Kikuchi, S.; Ishii, H.; Hosaka, K.; Sanetomo, R. Behavior of chromosomes from the mexican wild diploid species Solanum pinnatisectum in the interspecific hybrid with cultivated potato and its backcross progenies. Euphytica 2022, 218, 56. [Google Scholar] [CrossRef]
- Haverkort, A.J.; Struik, P.C.; Visser, R.G.F.; Jacobsen, E. Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res. 2009, 52, 249–264. [Google Scholar] [CrossRef]
- Helgeson, J.P.; Haberlach, G.T.; Pohlman, J.; Austin, S. Somatic fusions of Solanum species. Plant Cell Tissue Organ Cult. 1988, 12, 185–187. [Google Scholar] [CrossRef]
- Helgeson, J.P.; Pohlman, J.D.; Austin, S.; Haberlach, G.T.; Wielgus, S.M.; Ronis, D.; Zambolim, L.; Tooley, P.; McGrath, J.M.; James, R.V.; et al. Somatic hybrids between Solanum bulbocastanum and potato: A new source of resistance to late blight. Theor. Appl. Genet. 1998, 96, 738–742. [Google Scholar] [CrossRef]
- Austin, S.; Pohlman, J.D.; Brown, C.R.; Mojtahedi, H.; Santo, G.S.; Douches, D.S.; Helgeson, J.P. Interspecific somatic hybridization between Solanum tuberosum L. and S. bulbocastanum Dun. as a means of transferring nematode resistance. Am. Potato J. 1993, 70, 485–495. [Google Scholar] [CrossRef]
- Thieme, R.; Darsow, U.; Gavrilenko, T.; Dorokhov, D.; Tiemann, H. Production of somatic hybrids between S. tuberosum L. and late blight resistant Mexican wild potato species. Euphytica 1997, 97, 189–200. [Google Scholar] [CrossRef]
- Greplova, M.; Polzerova, H.; Vlastnikova, H. Electrofusion of protoplasts from Solanum tuberosum, S. bulbocastanum and S. pinnatisectum. Acta Physiol. Plant 2008, 30, 787–796. [Google Scholar] [CrossRef]
- Davis, J.A.; Radcliffe, E.B.; Thill, C.A.; Ragsdale, D.W. Resistance to aphids, late blight and viruses in somatic fusions and crosses of Solanum tuberosum L. and Solanum bulbocastanum Dun. Am. J. Potato Res. 2012, 89, 489–500. [Google Scholar] [CrossRef]
- Iovene, M.; Aversano, R.; Savarese, S.; Caruso, I.; Di Matteo, A.; Cardi, T.; Frusciante, L.; Carputo, D. Interspecific somatic hybrids between Solanum bulbocastanum and S. tuberosum and their haploidization for potato breeding. Biol. Plant. 2012, 56, 1–8. [Google Scholar] [CrossRef]
- Rakosy-Tican, E.; Thieme, R.; König, J.; Nachtigall, M.; Hammann, T.; Denes, T.-E.; Kruppa, K.; Molnar-Lang, M. Introgression of two broad-spectrum late blight resistance genes, Rpi-Blb1 and Rpi-Blb3, from Solanum bulbocastanum Dun. plus race-specific R genes into potato pre-breeding lines. Front Plant Sci. 2020, 11, 699. [Google Scholar] [CrossRef]
- Sedlak, P.; Sedlakova, V.; Vasek, J.; Zeka, D.; Cilova, D.; Melounova, M.; Orsak, M.; Domkarova, J.; Dolezal, P.; Vejl, P. phenotypic, molecular and biochemical evaluation of somatic hybrids between Solanum tuberosum and S. bulbocastanum. Sci. Rep. 2022, 12, 4484. [Google Scholar] [CrossRef]
- Sidorov, V.A.; Zubko, M.K.; Kuchko, A.A.; Komarnitsky, I.K.; Gleba, Y.Y. Somatic hybridization in potato: Use of γ-irradiated protoplasts of Solanum pinnatisectum in genetic reconstruction. Theor. Appl. Genet. 1987, 74, 364–368. [Google Scholar] [CrossRef]
- Ward, A.C.; Phelpstead, J.S.-J.; Gleadle, A.E.; Blackhall, N.W.; Cooper-Bland, S.; Kumar, A.; Powell, W.; Power, J.B.; Davey, M.R. Interspecific somatic hybrids between dihaploid Solanum tuberosum L. and the wild species, S. pinnatisectum Dun. J. Exp. Bot. 1994, 45, 1433–1440. [Google Scholar] [CrossRef]
- Menke, U.; Schilde-Rentschler, L.; Ruoss, B.; Zanke, C.; Hemleben, V.; Ninnemann, H. Somatic hybrids between the cultivated potato Solanum tuberosum L. and the 1EBN wild species Solanum pinnatisectum Dun.: Morphological and molecular characterization. Theor. Appl. Genet. 1996, 92, 617–626. [Google Scholar] [CrossRef]
- Thieme, R.; Schubert, J.; Nachtigall, M.; Hammann, T.; Truberg, B.; Heimbach, U.; Thieme, T. Wild potato species of the series Pinnatisecta—Progress in their utilisation in potato breeding. In Crop Plant Resistance to Biotic and Abiotic Factors: Current Potential and Future Demands, Proceedings of the International Symposium on Plant Protection and Plant Health in Europe, DPG, Berlin/Heidelberg, Germany, 14–16 May 2009; Deutsche Phytomedizinische Gesellschaft eV Verlag: Braunschweig, Germany, 2009; pp. 428–436. [Google Scholar]
- Szczerbakowa, A.; Bołtowicz, D.; Lebecka, R.; Radomski, P.; Wielgat, B. Characteristics of the interspecific somatic hybrids Solanum pinnatisectum (+) S. tuberosum H-8105. Acta Physiol. Plant 2005, 27, 265–273. [Google Scholar] [CrossRef]
- Chen, Q.; Li, H.Y.; Shi, Y.Z.; Beasley, D.; Bizimungu, B.; Goettel, M.S. Development of an effective protoplast fusion system for production of new potatoes with disease and insect resistance using Mexican wild potato species as gene pools. Can. J. Plant Sci. 2008, 88, 611–619. [Google Scholar] [CrossRef]
- Sarkar, D.; Tiwari, J.K.; Sharma, S.; Poonam; Sharma, S.; Gopal, J.; Singh, B.P.; Luthra, S.K.; Pandey, S.K.; Pattanayak, D. Production and characterization of somatic hybrids between Solanum tuberosum L. and S. pinnatisectum Dun. Plant Cell Tissue Organ Cult. 2011, 107, 427–440. [Google Scholar] [CrossRef]
- Polzerova, H.; Patzak, J.; Greplova, M. Early characterization of somatic hybrids from symmetric protoplast electrofusion of Solanum pinnatisectum Dun. and Solanum tuberosum L. Plant Cell Tissue Organ Cult. 2011, 104, 163–170. [Google Scholar] [CrossRef]
- Tiwari, J.; Poonam; Kumar, V.; Sharma, S.; Luthra, S.; Bhardwaj, V. Evaluation of potato somatic hybrids of dihaploid S. tuberosum (+) S. Ppinnatisectum for late blight resistance. Potato J. 2013, 40, 176–179. [Google Scholar]
- Tiwari, J.; Luthra, S.; Devi, S.; Kumar, V.; Ali, N.; Zinta, R.; Chakrabarti, S. Development of advanced back-cross progenies of potato somatic hybrids and linked ISSR markers for late blight resistance with diverse genetic base- First ever produced in Indian potato breeding. Potato J. 2018, 45, 17–27. [Google Scholar]
- Tiwari, J.K.; Rawat, S.; Luthra, S.K.; Zinta, R.; Sahu, S.; Varshney, S.; Kumar, V.; Dalamu, D.; Mandadi, N.; Kumar, M.; et al. Genome sequence analysis provides insights on genomic variation and late blight resistance genes in potato somatic hybrid (parents and progeny). Mol. Biol. Rep. 2021, 48, 623–635. [Google Scholar] [CrossRef]
- Thieme, R.; Rakosy-Tican, E.; Gavrilenko, T.; Antonova, O.; Schubert, J.; Nachtigall, M.; Heimbach, U.; Thieme, T. Novel somatic hybrids (Solanum tuberosum L. + Solanum tarnii) and their fertile BC1 progenies express extreme resistance to Potato Virus Y and late blight. Theor. Appl. Genet. 2008, 116, 691–700. [Google Scholar] [CrossRef]
- Thieme, R.; Rakosy-Tican, E.; Nachtigall, M.; Schubert, J.; Hammann, T.; Antonova, O.; Gavrilenko, T.; Heimbach, U.; Thieme, T. Characterization of the multiple resistance traits of somatic hybrids between Solanum cardiophyllum Lindl. and two commercial potato cultivars. Plant Cell Rep. 2010, 29, 1187–1201. [Google Scholar] [CrossRef]
- Chandel, P.; Tiwari, J.; Ali, N.; Devi, S.; Sharma, S.; Sharma, S.; Luthra, S.; Singh, B. Interspecific potato somatic hybrids between Solanum tuberosum and S. cardiophyllum, potential sources of late blight resistance breeding. Plant Cell Tissue Organ Cult. 2015, 123, 579–589. [Google Scholar] [CrossRef]
- Smyda, P.; Jakuczun, H.; Debski, K.; Sliwka, J.; Thieme, R.; Nachtigall, M.; Wasilewicz-Flis, I.; Zimnoch-Guzowska, E. Development of somatic hybrids Solanum × michoacanum Bitter. (Rydb.) (+) S. tuberosum L. and autofused 4x S. × michoacanum plants as potential sources of late blight resistance for potato breeding. Plant Cell Rep. 2013, 32, 1231–1241. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P.; Sliwka, J.; Wasilewicz-Flis, I.; Jakuczun, H.; Zimnoch-Guzowska, E. Genetic composition of interspecific potato somatic hybrids and autofused 4x plants evaluated by DArT and cytoplasmic DNA Markers. Plant Cell Rep. 2016, 35, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.O.; Nepper, J.P.; Kirk, H.-G.; Tolstrup, K.; Rasmussen, O.S. Combination of resistance to potato late blight in foliage and tubers by intraspecific dihaploid protoplast fusion. Euphytica 1998, 102, 363–370. [Google Scholar] [CrossRef]
- Szczerbakowa, A.; Tarwacka, J.; Oskiera, M.; Jakuczun, H.; Wielgat, B. Somatic hybridization between the diploids of S. × michoacanum and S. tuberosum. Acta Physiol. Plant. 2010, 32, 867–873. [Google Scholar] [CrossRef]
- Orczyk, W.; Przetakiewicz, J.; Nadolska-Orczyk, A. Somatic hybrids of Solanum tuberosum—Application to genetics and breeding. Plant Cell Tissue Organ Cult. 2003, 74, 1–13. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P.; Śliwka, J.; Villano, C.; Janiszewska, M.; Aversano, R.; Bednarek, P.T.; Carputo, D.; Zimnoch-Guzowska, E. Analysis of cytosine methylation in genomic DNA of Solanum × michoacanum (+) S. tuberosum somatic hybrids. Agronomy 2021, 11, 845. [Google Scholar] [CrossRef]
- Masuelli, R.W.; Tanimoto, E.Y.; Brown, C.R.; Comai, L. Irregular meiosis in a somatic hybrid between S. bulbocastanum and S. tuberosum detected by species-specific PCR markers and cytological analysis. Theor. Appl. Genet. 1995, 91, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Rakosy-Tican, E.; Thieme, R.; Nachtigall, M.; Molnar, I.; Denes, T.-E. The recipient potato cultivar influences the genetic makeup of the somatic hybrids between five potato cultivars and one cloned accession of sexually incompatible species Solanum bulbocastanum Dun. Plant Cell Tissue Organ Cult. 2015, 122, 395–407. [Google Scholar] [CrossRef]
- Naess, S.K.; Bradeen, J.M.; Wielgus, S.M.; Haberlach, G.T.; McGrath, J.M.; Helgeson, J.P. Analysis of the introgression of Solanum bulbocastanum DNA into potato breeding lines. Mol. Genet. Genom. 2001, 265, 694–704. [Google Scholar] [CrossRef]
- Brown, C.R.; Yang, C.-P.; Mojtahedi, H.; Santo, G.S.; Masuelli, R. RFLP analysis of resistance to Columbia Root-knot nematode derived from Solanum bulbocastanum in a BC2 population. Theor. Appl. Genet. 1996, 92, 572–576. [Google Scholar] [CrossRef]
- Brown, C.; Mojtahedi, H.; James, S.; Novy, R.; Love, S. Development and evaluation of potato breeding lines with introgressed resistance to Columbia root-knot nematode (Meloidogyne chitwoodi). Am. J. Potato Res. 2006, 83, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Mojtahedi, H.; Kuang, H.; Baker, B.; Brown, C.R. Marker-assisted selection of Columbia root-knot nematode resistance introgressed from Solanum bulbocastanum. Crop Sci. 2007, 47, 2021–2026. [Google Scholar] [CrossRef]
- Iovene, M.; Savarese, S.; Cardi, T.; Frusciante, L.; Scotti, N.; Simon, P.W.; Carputo, D. Nuclear and cytoplasmic genome composition of Solanum bulbocastanum (+) S. tuberosum somatic hybrids. Genome 2007, 50, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bradeen, J.M.; Naess, S.K.; Raasch, J.A.; Wielgus, S.M.; Haberlach, G.T.; Liu, J.; Kuang, H.; Austin-Phillips, S.; Buell, C.R.; et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc. Nat. Acad. Sci. USA 2003, 100, 9128–9133. [Google Scholar] [CrossRef] [PubMed]
- Hühnlein, A. Quantitative Detection of Potato Virus Y and Potato Leaf Roll Virus by Real Time PCR—A Molecular Approach with Numerous Applications in Potato Research. Doctoral Thesis, Julius Kühn-lnstitut, Quedlinburg, Germany, 2016. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P.; Śliwka, J.; Wasilewicz-Flis, I.; Jakuczun, H.; Zimnoch-Guzowska, E. BC1 and F1 progeny from Solanum × Michoacanum (+) S. tuberosum somatic hybrids, autofused 4× S. michoacanum and cultivated potato. Am. J. Potato Res. 2017, 94, 323–333. [Google Scholar] [CrossRef]
- Gaiero, P.; Speranza, P.; de Jong, H. Introgressive hybridization in potato revealed by novel cytogenetic and genomic technologies. Am. J. Potato Res. 2018, 95, 607–621. [Google Scholar] [CrossRef]
- Lou, Q.; Iovene, M.; Spooner, D.M.; Buell, C.R.; Jiang, J. Evolution of chromosome 6 of Solanum species revealed by comparative fluorescence in situ hybridization mapping. Chromosoma 2010, 119, 435–442. [Google Scholar] [CrossRef]
- Szinay, D.; Wijnker, E.; van den Berg, R.; Visser, R.G.F.; de Jong, H.; Bai, Y. Chromosome evolution in Solanum traced by cross-species BAC-FISH. New Phytol. 2012, 195, 688–698. [Google Scholar] [CrossRef]
- Braz, G.T.; He, L.; Zhao, H.; Zhang, T.; Semrau, K.; Rouillard, J.-M.; Torres, G.A.; Jiang, J. Comparative oligo-FISH mapping: An efficient and powerful methodology to reveal karyotypic and chromosomal evolution. Genetics 2018, 208, 513–523. [Google Scholar] [CrossRef]
- Traini, A.; Iorizzo, M.; Mann, H.; Bradeen, J.M.; Carputo, D.; Frusciante, L.; Chiusano, M.L. Genome microscale heterogeneity among wild potatoes revealed by diversity arrays technology marker sequences. Int. J. Genom. 2013, 2013, 257218. [Google Scholar] [CrossRef]
- Iorizzo, M.; Gao, L.; Mann, H.; Traini, A.; Chiusano, M.L.; Kilian, A.; Aversano, R.; Carputo, D.; Bradeen, J.M. A DArT marker-based linkage map for wild potato Solanum bulbocastanum facilitates structural comparisons between Solanum A and B genomes. BMC Genet. 2014, 15, 123. [Google Scholar] [CrossRef]
- Ramanna, M.S.; Hermsen, J.G.T. Somatic chromosome elimination and meiotic chromosome pairing in the triple hybrid 6x-(Solanum acaule × S. bulbocastanum) × 2×-S. phureja. Euphytica 1971, 20, 470–481. [Google Scholar] [CrossRef]
- Hermsen, J.G.T.; Ramanna, M.S. Meiosis in different f-l-hybrids of Solanum acaule Bitt x Solanum bulbocastanum Dun. and its bearing on genome relationship, fertility and breeding behaviour. Euphytica 1969, 18, 27–35. [Google Scholar] [CrossRef]
- Thieme, R.; Darsow, U.; Rakosy-Tican, L.; Kang, Z.; Gavrilenko, T.; Antonova, O.; Heimbach, U.; Thieme, T. Use of somatic hybridisation to transfer resistance to late blight and Potato virus Y (PVY) into cultivated potato. Plant Breed. Seed Sci. 2004, 50, 113–118. [Google Scholar]
- Thieme, R.; Hammann, T.; Nachtigall, M. Genetische Diversität für die Widerstandsfähigkeit gegen Schaderreger bei Kartoffel. In 436 Julius-Kühn-Archiv; Biological Diversity in Agricultural Landscapes: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Thieme, R.; Nachtigall, M.; König, J.; Hühnlein, A.; Lindner, K.; Antonova, O.; Pendinen, G.; Gavrilenko, T. Evaluating the potential of Solanum tarnii as a genetic resource of PVY resistance in potato breeding; EAPR Triennial Conference Paper. In Proceedings of the 20th EAPR Triennial Conference, Versailles, France, 9–14 July 2017. [Google Scholar]
- Bernatzky, R.; Tanksley, S.D. Genetics of actin-related sequences in tomato. Theor. Appl. Genet. 1986, 72, 314–321. [Google Scholar] [CrossRef]
- Leitch, A.R.; Schwarzacher, T.; Jackson, D.; Leitch, I.J. In Situ Hybridization: A Practical Guide; Bios Scientific Publishers: Oxford, UK, 1994. [Google Scholar]
- Pendinen, G.; Gavrilenko, T.; Jiang, J.; Spooner, D.M. Allopolyploid speciation of the mexican tetraploid potato species Solanum stoloniferum and S. hjertingii revealed by genomic in situ hybridization. Genome 2008, 51, 714–720. [Google Scholar] [CrossRef]
- Yakura, K.; Tanifuji, S. Molecular cloning and restriction analysis of EcoRI-fragments of vicia faba rDNA. Plant Cell Physiol. 1983, 24, 1327–1330. [Google Scholar] [CrossRef]
- Gavrilenko, T.; Antonova, O.; Shuvalova, A.; Krylova, E.; Alpatyeva, N.; Spooner, D.M.; Novikova, L. Genetic diversity and origin of cultivated potatoes based on plastid microsatellite polymorphism. Genet. Resour. Crop Evol. 2013, 60, 1997–2015. [Google Scholar] [CrossRef]
- Jo, K.-R.; Arens, M.; Kim, T.-Y.; Jongsma, M.A.; Visser, R.G.F.; Jacobsen, E.; Vossen, J.H. Mapping of the S. demissum late blight resistance gene R8 to a new locus on chromosome IX. Theor. Appl. Genet. 2011, 123, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Colton, L.M.; Groza, H.I.; Wielgus, S.M.; Jiang, J. Marker-assisted selection for the broad-spectrum potato late blight resistance conferred by gene RB derived from a wild potato Species. Crop. Sci. 2006, 46, 589–594. [Google Scholar] [CrossRef]
- Wang, M.; Allefs, S.; van den Berg, R.G.; Vleeshouwers, V.G.A.A.; van der Vossen, E.A.G.; Vosman, B. Allele mining in Solanum: Conserved homologues of Rpi-Blb1 are identified in Solanum stoloniferum. Theor. Appl. Genet. 2008, 116, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Lokossou, A.A.; Rietman, H.; Wang, M.; Krenek, P.; van der Schoot, H.; Henken, B.; Hoekstra, R.; Vleeshouwers, V.G.A.A.; van der Vossen, E.A.G.; Visser, R.G.F.; et al. Diversity, distribution, and evolution of Solanum bulbocastanum late blight resistance genes. Mol. Plant Microbe Interact. 2010, 23, 1206–1216. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Vossen, J.H.; Visser, R.G.F.; Jacobsen, E. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res. 2012, 21, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Dialat Ltd. Available online: http://dialat.ru (accessed on 31 May 2023).
- Evrogen. Available online: https://evrogen.ru/ (accessed on 31 May 2023).
- SibEnzyme. Available online: www.russia.sibenzyme.com (accessed on 31 May 2023).
- Gavrilenko, T. Potato Cytogenetics. In Potato Biology and Biotechnology: Advances and Perspectives; Vruegdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Ross, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 203–216. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, B.S. Nonisotopic in situ hybridization and plant genome mapping: The first 10 years. Genome 1994, 37, 717–725. [Google Scholar] [CrossRef]
- Ramzan, F.; Younis, A.; Lim, K.-B. Application of genomic in situ hybridization in horticultural science. Int. J. Genom. 2017, e7561909. [Google Scholar] [CrossRef]
- Gavrilenko, T. Application of molecular cytogenetics in fundamental and applied research of potato. In Genetics, Genomics and Breeding of Potato; Bradeen, J., Kole, C., Eds.; Science Publishers: New York, NY, USA, 2011; pp. 184–206. [Google Scholar]
- Pendinen, G.; Spooner, D.M.; Jiang, J.; Gavrilenko, T. Genomic in situ hybridization reveals both auto- and allopolyploid origins of different north and central american hexaploid potato (Solanum sect. Petota) species. Genome 2012, 55, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, T.A.; Pendinen, G.I.; Yermishin, A.P. GISH analysis of the introgression of the B subgenome genetic material of wild allotetraploid species Solanum stoloniferum into backcrossing progenies with potato. Agronomy 2022, 12, 787. [Google Scholar] [CrossRef]
- Garriga-Calderé, F.; Huigen, D.J.; Angrisano, A.; Jacobsen, E.; Ramanna, M.S. Transmission of alien tomato chromosomes from BC1 to BC2 progenies derived from backcrossing potato (+) tomato fusion hybrids to potato: The selection of single additions for seven different tomato chromosomes. Theor. Appl. Genet. 1998, 96, 155–163. [Google Scholar] [CrossRef]
- Haider Ali, S.N.; Ramanna, M.S.; Jacobsen, E.; Visser, R.G.F. Establishment of a Complete Series of a monosomic tomato chromosome addition lines in the cultivated potato using RFLP and GISH analyses. Theor. Appl. Genet. 2001, 103, 687–695. [Google Scholar] [CrossRef]
- Dong, F.; Tek, A.L.; Frasca, A.B.L.; McGrath, J.M.; Wielgus, S.M.; Helgeson, J.P.; Jiang, J. Development and Characterization of potato-Solanum brevidens chromosomal addition/substitution lines. Cytogenet. Genome Res. 2005, 109, 368–372. [Google Scholar] [CrossRef]
- Hermsen, J.G.T.; Ramanna, M.S. Barriers to hybridization of Solanum bulbocastanum Dun. and S. verrucosum Schlechtd. and structural hybridity in their Fl Plants. Euphytica 1976, 25, 1–10. [Google Scholar] [CrossRef]
- Ortiz, R.; Mihovilovich, E. Genetics and cytogenetics of the potato. In The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Campos, H., Ortiz, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 219–247. [Google Scholar] [CrossRef]
- Bradshaw, J.E. A brief history of the impact of potato genetics on the breeding of tetraploid potato cultivars for tuber propagation. Potato Res. 2022, 65, 461–501. [Google Scholar] [CrossRef]
- Dong, F.; Novy, R.G.; Helgeson, J.P.; Jiang, J. Cytological Characterization of potato—Solanum etuberosum somatic hybrids and their backcross progenies by genomic in situ hybridization. Genome 1999, 42, 987–992. [Google Scholar] [CrossRef]
- Gavrilenko, T.; Thieme, R.; Heimbach, U.; Thieme, T. Fertile somatic hybrids of Solanum etuberosum (+) dihaploid Solanum tuberosum and their backcrossing progenies: Relationships of genome dosage with tuber development and resistance to potato virus Y. Euphytica 2003, 131, 323–332. [Google Scholar] [CrossRef]
- Huang, S.; van der Vossen, E.A.G.; Kuang, H.; Vleeshouwers, V.G.A.A.; Zhang, N.; Borm, T.J.A.; van Eck, H.J.; Baker, B.; Jacobsen, E.; Visser, R.G.F. Comparative genomics enabled the isolation of the R3a late blight resistance gene in potato. Plant J. 2005, 42, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, S.; Guo, X.; Li, Y.; Yang, Y.; Guo, Z.; Kuang, H.; Rietman, H.; Bergervoet, M.; Vleeshouwers, V.G.G.A.; et al. Cloning and characterization of R3b; members of the R3 superfamily of late blight resistance genes show sequence and functional divergence. Mol. Plant Microbe Interact 2011, 24, 1132–1142. [Google Scholar] [CrossRef]
- Nachtigall, M.; König, J.; Thieme, R. Mapping of a novel, major late blight resistance locus in the diploid (1EBN) Mexican Solanum pinnatisectum Dunal on chromosome VII. Plant Breed. 2018, 137, 433–442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).