Combined Small RNA and Degradome Sequencing Reveals Important Roles of Light-Responsive microRNAs in Wild Potato (Solanum chacoense)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. SRNA and Degradome Library Construction and Sequencing
2.3. Pipeline of Bioinformation Analysis
2.4. Defining Differentially Expressed miRNAs (DEMs) and Cluster Analysis
2.5. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
3. Results
3.1. Identification of Conserved miRNAs and Novel miRNAs in S. chacoense
3.2. Target Prediction and Identification via In Silico and Degradome Approaches
3.3. Identification and Cluster Visualization of Light-Responsive miRNAs
3.4. Function Annotation of Regulatory Networks Mediated by miRNAs Responsive to Light
3.5. Subnetworks Analysis Identifies Important Functional miRNA-Target Interactions
4. Discussion
4.1. Identification of Conserved miRNAs and miRNA Candidates in S. chacoense
4.2. Differentially Expressed miRNAs Involved in Abiotic Stress Response
4.3. miRNAs and Target Transcripts Are Important for Primary and Secondary Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The potato of the future: Opportunities and challenges in sustainable agri-food systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Tetlow, I.; Emes, M. Starch synthesis in amyloplasts purified from developing potato tubers. Plant J. 1997, 11, 1095–1103. [Google Scholar] [CrossRef]
- Tanios, S.; Eyles, A.; Tegg, R.; Wilson, C. Potato tuber greening: A review of predisposing factors, management and future challenges. Am. J. Potato Res. 2018, 95, 248–257. [Google Scholar] [CrossRef]
- Bergenstråhle, A.; Tillberg, E.; Jonsson, L. Regulation of glycoalkaloid accumulation in potato tuber discs. J. Plant Physiol. 1992, 140, 269–275. [Google Scholar] [CrossRef]
- Grunenfelder, L.A.; Knowles, L.O.; Hiller, L.K.; Knowles, N.R. Glycoalkaloid Development during Greening of Fresh Market Potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2006, 54, 5847–5854. [Google Scholar] [CrossRef]
- Okamoto, H.; Ducreux, L.J.; Allwood, J.W.; Hedley, P.E.; Wright, A.; Gururajan, V.; Terry, M.J.; Taylor, M.A. Light regulation of chlorophyll and glycoalkaloid biosynthesis during tuber greening of potato S. tuberosum. Front. Plant Sci. 2020, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, X.; You, Q.; Han, L.; Shi, J.; Yang, J.; Cui, W.; Zhang, H.; Chao, Q.; Zhu, Y. Analysis of DNA methylation in potato tuber in response to light exposure during storage. Plant Physiol. Biochem. 2022, 170, 218–224. [Google Scholar] [CrossRef]
- Itkin, M.; Heinig, U.; Tzfadia, O.; Bhide, A.; Shinde, B.; Cardenas, P.; Bocobza, S.; Unger, T.; Malitsky, S.; Finkers, R. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 2013, 341, 175–179. [Google Scholar] [CrossRef]
- Nützmann, H.W.; Huang, A.; Osbourn, A. Plant metabolic clusters–from genetics to genomics. New Phytol. 2016, 211, 771–789. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, J.; Zhang, J.; Wang, Z.; Ran, A.; Guo, H.; Wang, D.; Zhang, J. Integrated RNA-seq and sRNA-seq analysis reveals miRNA effects on secondary metabolism in Solanum tuberosum L. Mol. Genet. Genom. 2017, 292, 37–52. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masuda, T. Transcriptional regulation of tetrapyrrole biosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1811. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.-G.; Okajima, K. Diverse photoreceptors and light responses in plants. J. Plant Res. 2016, 129, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-L.; Tu, S.-L. Alternative splicing and cross-talk with light signaling. Plant Cell Physiol. 2018, 59, 1104–1110. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.-J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Petermann, J.B.; Morris, S.C. The spectral responses of chlorophyll and glycoalkaloid synthesis in potato tubers (Solanum tuberosum). Plant Sci. 1985, 39, 105–110. [Google Scholar] [CrossRef]
- Percival, G. The influence of light upon glycoalkaloid and chlorophyll accumulation in potato tubers (Solanum tuberosum L.). Plant Sci. 1999, 145, 99–107. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wang, D.; Tao, S.; Ji, Y.; Wu, B. Relation between light qualities and accumulation of steroidal glycoalkaloids as well as signal molecule in cell in potato tubers. Acta Agron. Sin. 2010, 36, 629–635. [Google Scholar] [CrossRef]
- Andersen, T.G.; Barberon, M.; Geldner, N. Suberization—The second life of an endodermal cell. Curr. Opin. Plant Biol. 2015, 28, 9–15. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3, 62. [Google Scholar] [CrossRef]
- Tanios, S.; Thangavel, T.; Eyles, A.; Tegg, R.S.; Nichols, D.S.; Corkrey, R.; Wilson, C.R. Suberin deposition in potato periderm: A novel resistance mechanism against tuber greening. New Phytol. 2020, 225, 1273–1284. [Google Scholar] [CrossRef]
- Cárdenas, P.D.; Sonawane, P.D.; Pollier, J.; Vanden Bossche, R.; Dewangan, V.; Weithorn, E.; Tal, L.; Meir, S.; Rogachev, I.; Malitsky, S. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat. Commun. 2016, 7, 10654. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Retuerta, C.; Suaréz-López, P.; Henriques, R. Under a new light: Regulation of light-dependent pathways by non-coding RNAs. Front. Plant Sci. 2018, 9, 962. [Google Scholar] [CrossRef]
- Islam, W.; Waheed, A.; Idrees, A.; Rashid, J.; Zeng, F. Role of plant microRNAs and their corresponding pathways in fluctuating light conditions. BBA-Mol. Cell Res. 2022, 1870, 119304. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, X.H.; Wu, X.; Wang, Y.; Lu, X.; Sun, H.; Xie, X. Genome-wide identification of microRNAs and their targets in wild type and phyB mutant provides a key link between microRNAs and the phyB-mediated light signaling pathway in rice. Front. Plant Sci. 2015, 6, 372. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Adam, H.; Díaz-Mendoza, M.; Zurczak, M.; González-Schain, N.D.; Suárez-López, P. Graft-transmissible induction of potato tuberization by the microRNA miR172. Development 2009, 136, 2873–2881. [Google Scholar] [CrossRef]
- Folkes, L.; Moxon, S.; Woolfenden, H.C.; Stocks, M.B.; Szittya, G.; Dalmay, T.; Moulton, V. PAREsnip: A tool for rapid genome-wide discovery of small RNA/target interactions evidenced through degradome sequencing. Nucleic Acids Res. 2012, 40, e103. [Google Scholar] [CrossRef]
- Lin, S.S.; Chen, Y.; Lu, M.Y.J. Degradome Sequencing in Plants. In Plant MicroRNAs. Methods in Molecular Biology; Folter, S.D., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1932, pp. 197–213. [Google Scholar]
- Mweetwa, A.M.; Hunter, D.; Poe, R.; Harich, K.C.; Ginzberg, I.; Veilleux, R.E.; Tokuhisa, J.G. Steroidal glycoalkaloids in Solanum chacoense. Phytochemistry 2012, 75, 32–40. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Brousse, C.; Liu, Q.; Beauclair, L.; Deremetz, A.; Axtell, M.J.; Bouche, N. A non-canonical plant microRNA target site. Nucleic Acids Res. 2014, 42, 5270–5279. [Google Scholar] [CrossRef]
- McWilliam, H.; Li, W.; Uludag, M.; Squizzato, S.; Park, Y.M.; Buso, N.; Cowley, A.P.; Lopez, R. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013, 41, W597–W600. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Commun. Ecol. Pack. 2007, 10, 631–637. [Google Scholar]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Varkonyi-Gasic, E. Stem-loop qRT-PCR for the detection of plant microRNAs. Plant Epigenetics Methods Protoc. 2017, 1456, 163–175. [Google Scholar]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mao, J.; Mo, Z.; Yuan, G.; Xiang, H.; Visser, R.G.; Bai, Y.; Liu, H.; Wang, Q.; van der Linden, C.G. The CBL-CIPK network is involved in the physiological crosstalk between plant growth and stress adaptation. Plant Cell Environ. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Budak, H.; Kantar, M.; Bulut, R.; Akpinar, B.A. Stress responsive miRNAs and isomiRs in cereals. Plant Sci. 2015, 235, 1–13. [Google Scholar] [CrossRef]
- Bhogireddy, S.; Mangrauthia, S.K.; Kumar, R.; Pandey, A.K.; Singh, S.; Jain, A.; Budak, H.; Varshney, R.K.; Kudapa, H. Regulatory non-coding RNAs: A new frontier in regulation of plant biology. Funct. Integr. Genom. 2021, 21, 313–330. [Google Scholar] [CrossRef]
- Zhou, B.; Fan, P.; Li, Y.; Yan, H.; Xu, Q. Exploring miRNAs involved in blue/UV-A light response in Brassica rapa reveals special regulatory mode during seedling development. BMC Plant Biol. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, G.; Zhang, W. UV-B responsive microRNA genes in Arabidopsis thaliana. Mol. Syst. Biol. 2007, 3, 103. [Google Scholar] [CrossRef]
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-tolerance to biotic and abiotic stresses in plants: A focus on resistance to aphid infestation. J. Exp. Bot. 2016, 67, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Li, Z.-G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: Key regulators and possible mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Fasani, E.; DalCorso, G.; Costa, A.; Zenoni, S.; Furini, A. The Arabidopsis thaliana transcription factor MYB59 regulates calcium signalling during plant growth and stress response. Plant Mol. Biol. 2019, 99, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Sabehat, A.; Weiss, D.; Lurie, S. Heat-shock proteins and cross-tolerance in plants. Physiol. Plant. 1998, 103, 437–441. [Google Scholar] [CrossRef]
- Yang, F.; Qiao, Y.; Wang, Y.-Q.; Li, Q.; Li, D.; Wang, Y.-S.; Chai, W.-W. Genome-Wide Identification of HSP90 Genes and Expression Analysis under High Temperature and Drought Stresses in Potato. Acta Agric. Boreali. Occident. Sin. 2022, 30, 690–702. [Google Scholar]
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet. 2014, 10, e1004664. [Google Scholar] [CrossRef]
- Pelletier, M.K.; Burbulis, I.E.; Winkel-Shirley, B. Disruption of specific flavonoid genes enhances the accumulation of flavonoid enzymes and end-products in Arabidopsis seedlings. Plant Mol. Biol. 1999, 40, 45–54. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W.J.; Vu, T.T.; Jeong, C.Y.; Hong, S.-W.; Lee, H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017, 36, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Robe, K.; Bettembourg, M.; Navarro, N.; Rofidal, V.; Santoni, V.; Gaymard, F.; Vignols, F.; Roschzttardtz, H.; Izquierdo, E. The transcription factor bHLH121 interacts with bHLH105 (ILR3) and its closest homologs to regulate iron homeostasis in Arabidopsis. Plant Cell 2020, 32, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Li, Y.; Cai, Y.; Li, C.; Pu, M.; Lu, C.; Yang, Y.; Liang, G. bHLH121 functions as a direct link that facilitates the activation of FIT by bHLH IVc transcription factors for maintaining Fe homeostasis in Arabidopsis. Mol. Plant 2020, 13, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-X.; Wang, L.-J.; Zhao, B.; Shan, C.-M.; Zhang, Y.-H.; Chen, D.-F.; Chen, X.-Y. Progressive regulation of sesquiterpene biosynthesis in Arabidopsis and Patchouli (Pogostemon cablin) by the miR156-targeted SPL transcription factors. Mol. Plant 2015, 8, 98–110. [Google Scholar] [CrossRef]
- Krits, P.; Fogelman, E.; Ginzberg, I. Potato steroidal glycoalkaloid levels and the expression of key isoprenoid metabolic genes. Planta 2007, 227, 143–150. [Google Scholar] [CrossRef]
- Maya-Bernal, J.L.; Ávila, A.; Ruiz-Gayosso, A.; Trejo-Fregoso, R.; Pulido, N.; Sosa-Peinado, A.; Zúñiga-Sánchez, E.; Martínez-Barajas, E.; Rodríguez-Sotres, R.; Coello, P. Expression of recombinant SnRK1 in E. coli. Characterization of adenine nucleotide binding to the SnRK1. 1/AKINβγ-β3 complex. Plant Sci. 2017, 263, 116–125. [Google Scholar] [CrossRef]
- Thelander, M.; Olsson, T.; Ronne, H. Snf1-related protein kinase 1 is needed for growth in a normal day–night light cycle. EMBO J. 2004, 23, 1900–1910. [Google Scholar] [CrossRef]
- Ginzberg, I.; Thippeswamy, M.; Fogelman, E.; Demirel, U.; Mweetwa, A.M.; Tokuhisa, J.; Veilleux, R.E. Induction of potato steroidal glycoalkaloid biosynthetic pathway by overexpression of cDNA encoding primary metabolism HMG-CoA reductase and squalene synthase. Planta 2012, 235, 1341–1353. [Google Scholar] [CrossRef]
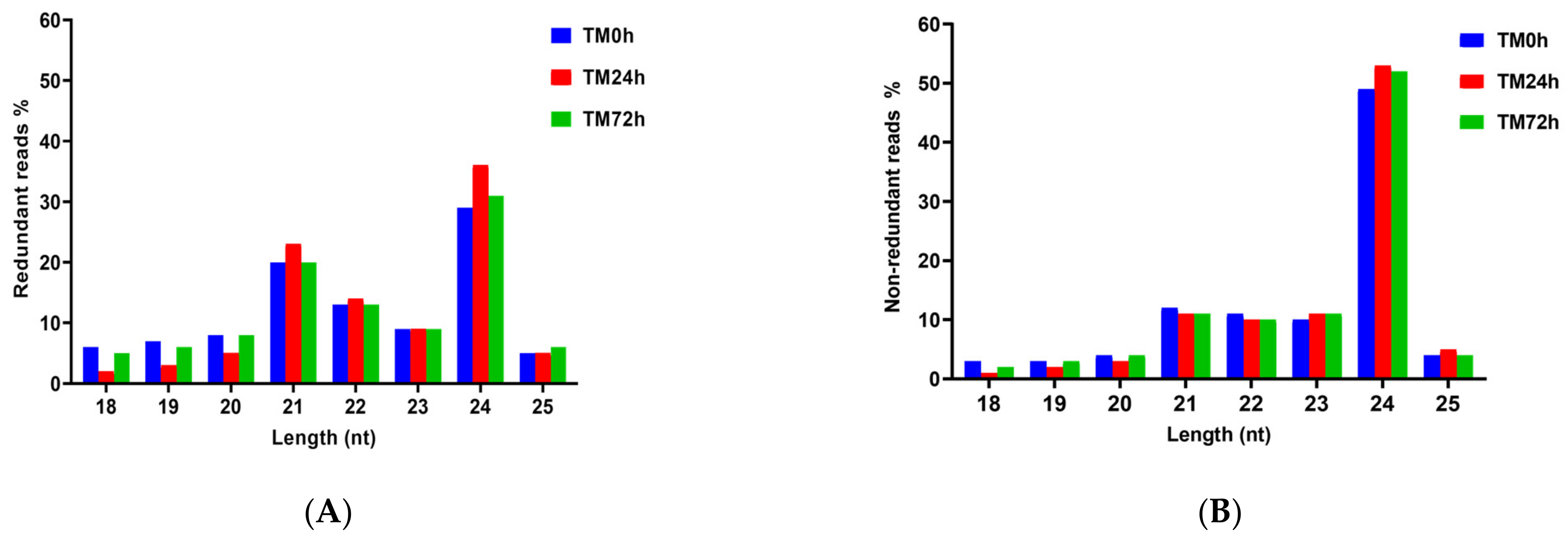








| Libraries | TM0h | TM24h | TM72h | |||
|---|---|---|---|---|---|---|
| Total | Unique | Total | Unique | Total | Unique | |
| Raw reads | 8,387,733 | 1,876,413 | 14,878,292 | 4,727,875 | 9,443,424 | 2,573,468 |
| 3ADT and length filter | 5,430,900 | 631,689 | 6,967,261 | 1,187,156 | 4,966,215 | 860,198 |
| Junk reads | 15,997 | 13,089 | 58,133 | 46,220 | 26,152 | 19,826 |
| Rfam | 275,638 | 14,587 | 661,780 | 20,447 | 579,642 | 17,007 |
| mRNA | 206,800 | 11,482 | 681,823 | 40,578 | 354,377 | 16,525 |
| Repeats | 8383 | 268 | 14,181 | 353 | 19,755 | 346 |
| rRNA | 218,120 | 11,214 | 515,113 | 13,967 | 471,560 | 12,224 |
| tRNA | 39,211 | 1861 | 102,225 | 3447 | 75,462 | 2767 |
| snoRNA | 3278 | 214 | 9412 | 495 | 4807 | 329 |
| snRNA | 943 | 175 | 4318 | 660 | 2082 | 286 |
| other Rfam RNA | 14,086 | 1123 | 30,712 | 1878 | 25,731 | 1401 |
| valid reads | 2,508,266 | 1,207,773 | 6,628,896 | 3,436,592 | 3,605,238 | 1,662,556 |
| Category * | Total Pre-miRNA | Total Unique miRNA | TM0h Pre-miRNA | TM0h Unique miRNA | TM24h Pre-miRNA | TM24h Unique miRNA | TM72h Pre-miRNA | TM72h Unique miRNA |
|---|---|---|---|---|---|---|---|---|
| Group 1a | 141 | 198 | 113 | 136 | 141 | 197 | 111 | 147 |
| Group1b | 39 | 60 | 30 | 46 | 39 | 60 | 34 | 51 |
| Group2a | 61 | 70 | 54 | 59 | 61 | 70 | 50 | 57 |
| Group2b | 213 | 177 | 174 | 134 | 213 | 176 | 186 | 149 |
| Group3 | 48 | 48 | 30 | 30 | 47 | 47 | 32 | 32 |
| Group4 | 174 | 179 | 119 | 112 | 173 | 178 | 125 | 117 |
| Sample | TD0h (Number) | TD24h (Number) | TD72h (Number) |
|---|---|---|---|
| Raw Reads | 20,749,711 | 20,114,323 | 19,663,065 |
| Unique Raw Reads | 7,961,145 | 7,939,131 | 7,482,735 |
| Reads < 15 nt after removing 3′ adaptor | 160,233 | 151,083 | 148,593 |
| Mappable Reads | 20,589,478 | 19,963,240 | 19,514,472 |
| Unique reads < 15nt after removing 3′ adaptor | 62,376 | 60,612 | 58,916 |
| Unique Mappable Reads | 7,898,769 | 7,878,519 | 7,423,819 |
| Mapped Reads | 12,438,537 | 11,769,904 | 11,982,016 |
| Unique Mapped Reads | 4,316,514 | 4,241,085 | 4,107,240 |
| Number of input Transcript | 44,851 | 44,851 | 44,851 |
| Number of Covered Transcript | 36,626 | 36,894 | 36,462 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Yang, F.; Li, Q.; Ren, P.; An, P.; Li, D.; Xiao, J. Combined Small RNA and Degradome Sequencing Reveals Important Roles of Light-Responsive microRNAs in Wild Potato (Solanum chacoense). Agronomy 2023, 13, 1763. https://doi.org/10.3390/agronomy13071763
Qiao Y, Yang F, Li Q, Ren P, An P, Li D, Xiao J. Combined Small RNA and Degradome Sequencing Reveals Important Roles of Light-Responsive microRNAs in Wild Potato (Solanum chacoense). Agronomy. 2023; 13(7):1763. https://doi.org/10.3390/agronomy13071763
Chicago/Turabian StyleQiao, Yan, Fang Yang, Qian Li, Panrong Ren, Peipei An, Dan Li, and Junfei Xiao. 2023. "Combined Small RNA and Degradome Sequencing Reveals Important Roles of Light-Responsive microRNAs in Wild Potato (Solanum chacoense)" Agronomy 13, no. 7: 1763. https://doi.org/10.3390/agronomy13071763
APA StyleQiao, Y., Yang, F., Li, Q., Ren, P., An, P., Li, D., & Xiao, J. (2023). Combined Small RNA and Degradome Sequencing Reveals Important Roles of Light-Responsive microRNAs in Wild Potato (Solanum chacoense). Agronomy, 13(7), 1763. https://doi.org/10.3390/agronomy13071763





