Ecophysiological Responses of Rice (Oryza sativa L.) to Drought and High Temperature
Abstract
:1. Introduction
2. High-Temperature Stress
3. Drought
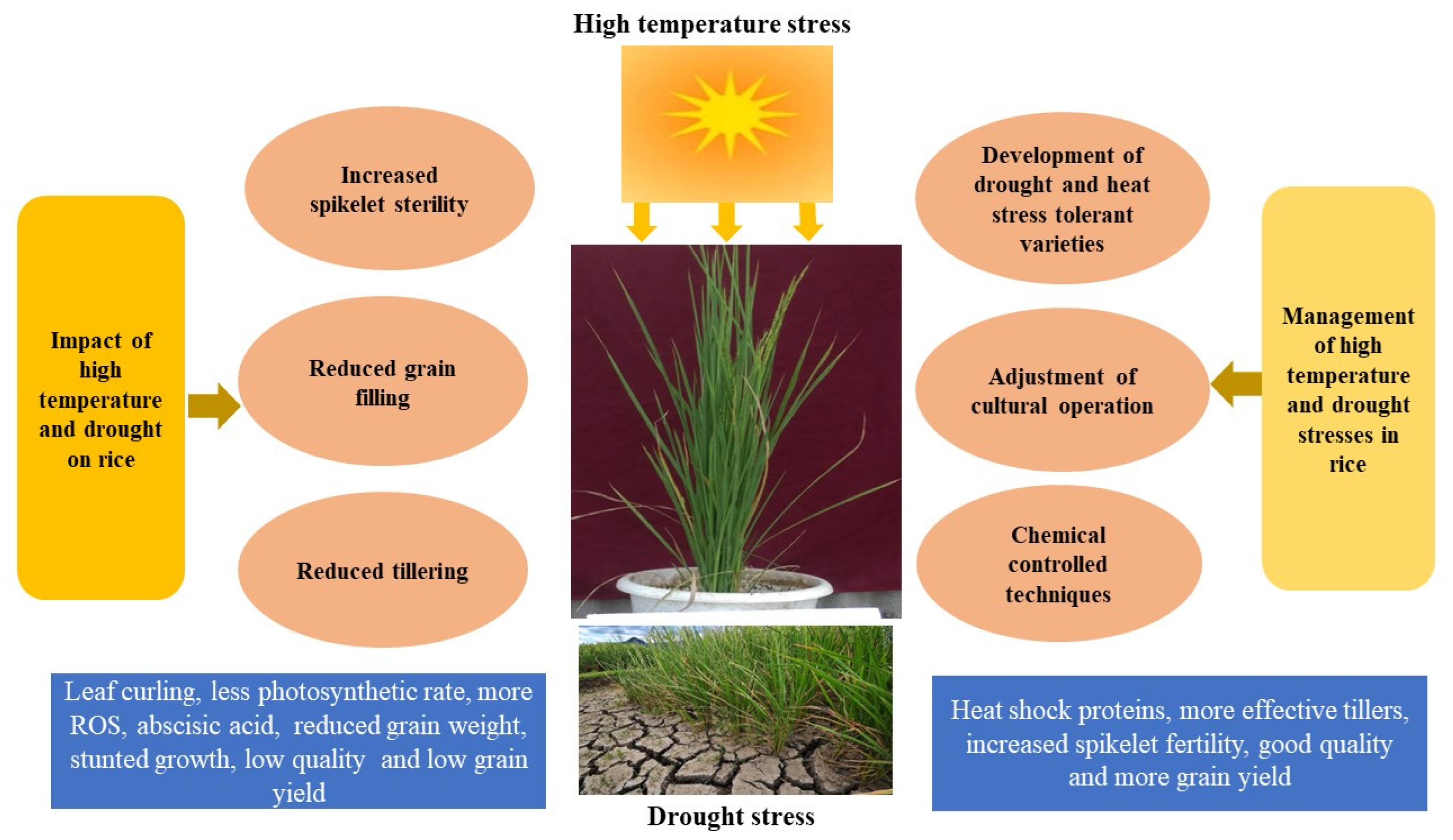
4. Impact of High-temperature and Drought Stresses on Different Growth Stages of Rice
4.1. Impact on the Reproductive Stage
4.2. Impact on Gametophyte and Pollen Development
4.3. Impact on Pollination and Fertilization
4.4. Impact on Seed Setting
5. Biochemical and Physiological Impact of High Temperature and Drought Stress
Role of Phytohormones and Antioxidants
6. Molecular Basis of High-Temperature and Drought Stresses
7. Management Strategies
7.1. Improvement in Rice Crop Management Practices
- Under severe drought and temperature conditions, rice nurseries can be sown early in the morning, or direct sowing can be carried out [81];
- Mulching with paddy straw, peat, or wood chips can be implemented to prevent soil moisture loss in rice crops;
- The use of compost or manure can minimize evapotranspiration and provide nutrients to the soil after decomposition;
- Conservation tillage practices can enhance water conservation, protect the soil surface from harsh weather conditions, and reduce evapotranspiration, thereby maintaining soil health;
- Deep tillage can improve soil permeability and porosity, allowing for maximum water absorption;
- Crop rotation can be practiced to enhance water-holding capacity and improve soil structure throughout the seasons;
- Adding green manure to the soil can improve moisture-holding capacity and enhance soil quality;
- Rainwater harvesting and strip cropping are soil and water conservation techniques that reduce runoff and optimize water usage for irrigation;
- Interplanting and mixed cropping of different crops at different times and durations can provide better water utilization and improve overall crop resilience;
- Contour plowing can be adopted to retain more water in the soil and ensure its even distribution across the cropped area.
7.2. Development of Heat- and Drought-Stress-Tolerant Varieties
7.3. Genetic Engineering
7.4. Chemical Control Technology
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischer, G.; Orduz-Rodríguez, J.O. Ecofisiología en frutales. In Manual Para el Cultivo de Frutales en el Trópico; Fischer, G., Ed.; Produmedios: Bogota, Columbia, 2012; pp. 54–72. [Google Scholar]
- Jiménez-Arias, D.; Garci, J.F.; Morales-Sierra, S.; Sua, E.; Pe, J.A.; Luis, J.C.; Garrido-Orduña, C.; Herrera, A.J.; Valde, F.; Sandalio, L.M.; et al. Menadione sodium bisulphite (MSB): Beyond seed-soaking. Root T pretreatment with MSB primes salt stress tolerance in tomato plants. Environ. Exp. Bot. 2019, 157, 161–170. [Google Scholar] [CrossRef]
- Gursoy, M.; Balkan, A.; Ulukan, H. Ecophysiological responses to stresses in plants: A general approach. Pak. J. Biol. Sci. 2012, 15, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Talaat, N.B.; Shawky, B.T. 24-Epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.). Environ. Exp. Bot. 2012, 82, 80–88. [Google Scholar] [CrossRef]
- Zhong, M.; Song, R.; Wang, Y.; Shu, S.; Sun, J.; Guo, S. TGase regulates salt stress tolerance through enhancing bound polyamines-T mediated antioxidant enzymes activity in tomato. Environ. Exp. Bot. 2020, 179, 104191. [Google Scholar] [CrossRef]
- Isik, G. Ecophysiological responses of Solanum lycopersicum L. To different levels of salt stress. Pak. J. Bot. 2022, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; Volume 4, ISBN 1815-6797. [Google Scholar]
- Menguer, P.K.; Sperotto, R.A.; Ricachenevsky, F.K. Awalkonthewildside: Oryza species as source for rice abiotic stresss tolerance. Genet. Mol. Biol. 2017, 40, 238–252. [Google Scholar] [CrossRef] [Green Version]
- Piveta, L.B.; Roma-Burgos, N.; Noldin, J.A.; Viana, V.E.; Oliveira, C.d.; Lamego, F.P.; Avila, L.A.D. Molecular and physiological responses of rice and weedy rice to heat and drought stress. Agriculture 2021, 11, 9. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nat. Cell Biol. 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Palmgren, M.; Edenbrandt, A.K.; Vedel, S.E.; Andersen, M.M.; Landes, X.; Østerberg, J.T.; Falhof, J.; Olsen, L.I.; Christensen, S.B.; Sandøe, P.; et al. Are we ready for back-to-nature crop breeding? Trends Plant Sci. 2015, 20, 155–164. [Google Scholar] [CrossRef]
- de Freitas, G.P.M.; Basu, S.; Ramegowda, V.; Thomas, J.; Benitez, L.C.; Braga, E.B.; Pereira, A. Physiological and transcriptional responses to low-temperature stress in rice genotypes at the reproductive stage. Plant Signal. Behav. 2019, 14, e1581557. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.S.; Uzair, M.; Raza, A.; Habib, M.; Xu, Y.; Yousuf, M.; Yang, S.H.; Khan, M.R. Uncovering the Research Gaps to Alleviate the Negative Impacts of Climate Change on Food Security: A Review. Front. Plant Sci. 2022, 13, 927535. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Huang, J.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Agarwal, P.; Reddy, M.K.; Sopory, S.K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep. 2006, 25, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Bagirov, V.; Treshkin, S.; Korobka, A.; Dereka, F.; Garkusha, S.; Kovalev, V.; Esaulova, L.; Kizinek, S. Scientific support of the rice growing industry of the agroindustrial complex of the Russian Federation in solving the problems of food security. E3S Web Conf. 2020, 210, 05006. [Google Scholar] [CrossRef]
- Zafar, S.A.; Arif, M.H.; Uzair, M.; Rashid, U.; Naeem, M.K.; Rehman, O.U.; Rehman, N.; Zaid, I.U.; Farooq, M.S.; Zahra, N.; et al. Agronomic and Physiological Indices for Reproductive Stage Heat Stress Tolerance in Green Super Rice. Agronomy 2022, 12, 1907. [Google Scholar] [CrossRef]
- Papademetriou, M.K.; Dent, F.J.; Herath, E.M. Bridging the Rice Yield Gap in the Asia Pacific Region; Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific: Bangkok, Thailand, 2000; Available online: http://coin.fao.org/coin-static/cms/media/9/13171760277090/2000_16_high.pdf (accessed on 11 June 2023).
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef] [Green Version]
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quiñones, C.O.; Vidal, A.C.; Ouwerkerk, P.B.F.; Van Rie, J.; Fabre, D.; Jagadish, K.S.V.; Dingkuhn, M. Effect of carbohydrates and night temperature on night respiration in rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Mahat, J.; Shrestha, J.; Madhav, K.C.; Paudel, K. Influence of high-temperature stress on rice growth and development. A review. Heliyon 2022, 8, e12651. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Ashraf, M.Y.; Khaliq, B.; Sun, M.; Hussain, S.; Gao, Z.-Q.; Noor, H.; Alam, S. Mechanisms and adaptation strategies to improve heat tolerance in rice. A review. Plants 2019, 8, 508. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, R.; Ning, H.; Luo, Q. Changes in climate extremes and their impact on wheat yield in Tianshan Mountains region, northwest China. Environ. Earth Sci. 2016, 75, 1228. [Google Scholar] [CrossRef]
- Ambavaram, M.M.R.; Basu, S.; Krishnan, A.; Ramegowda, V.; Batlang, U.; Rahman, L.; Baisakh, N.; Pereira, A. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress. Nat. Commun. 2014, 5, 5302. [Google Scholar] [CrossRef] [Green Version]
- Yong, C.; Ouyang, S.; Zhao, Y.; Tie, L.; Shao, C.; Duan, H. Plant responses to high temperature and drought: A bibliometrics analysis. Front. Plant Sci. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Berard, A.; Ben Sassi, M.; Kaisermann, A.; Renault, P. Soil microbial community responses to heat wave components: Drought and high temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar] [CrossRef]
- Simontacchi, M.; Egalatro, A.; Artuso, F.E.; Santa-María, G.E. Plant Survivalina Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef] [Green Version]
- Ehong, Y.; Ezhang, H.; Ehuang, L.; Eli, D.; Song, F. Overexpression of a Stress-Responsive NAC Transcription Factor Gene ONAC022 Improves Drought and Salt Tolerance in Rice. Front. Plant Sci. 2016, 7, 4. [Google Scholar]
- Divya, K.; Bhatnagar-Mathur, P.; Sharma, K.K.; Reddy, P.S. Heat Shock Proteins (Hsps) Mediated Signalling Pathways during Abiotic Stress Conditions; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 499–516. [Google Scholar]
- Lavania, D.; Kumar, R.; Goyal, I.; Rana, S.; Grover, A. Genetic improvement of rice crop under high temperature stress: Bridging plant physiology with molecular biology. Indian J. Plant Physiol. 2016, 21, 391–408. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Ji, Q.; Zhang, F.; Li, Y.; Fan, J.; Hou, X.; Yan, F.; Liu, X.; Gong, K. Effects of various soil water potential thresholds for drip irrigation on soil salinity, seed cotton yield and water productivity of cotton in northwest China. Agric. Water Manag. 2023, 279, 108172. [Google Scholar] [CrossRef]
- Zargar, S.M.; Mir, R.A.; Ebinezer, L.B.; Masi, A.; Hami, A.; Manzoor, M.; Salgotra, R.K.; Sofi, N.R.; Mushtaq, R.; Rohila, J.S.; et al. Physiological and multi-omics approaches for explaining drought stress tolerance and supporting sustainable production of rice. Front. Plant Sci. 2022, 12, 803603. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Ramegowda, Y.; Ramegowda, V.; Karaba, N.N.; Sreeman, S.M.; Udayakumar, M. Combined Drought and Heat Stress in Rice: Responses, Phenotyping and Strategies to Improve Tolerance. Rice Sci. 2021, 28, 233–242. [Google Scholar] [CrossRef]
- Sharma, R.; Sudan, J.; Sharma, R.; Kumari, S.; Salgotra, R.K.; Singh, R. Terminal heat stress-responsive genes analysis in heat susceptible HDR77 genotype of wheat (Triticum aestivum L.) by using semi-quantative RTPCR. Electron. J. Plant Breed. 2017, 8, 1124–1132. [Google Scholar] [CrossRef]
- Shi, W.; Yin, X.; Struik, P.C.; Xie, F.; Schmidt, R.C.; Jagadish, K.S.V. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crop Res. 2016, 190, 18–25. [Google Scholar] [CrossRef]
- Zafar, S.A.; Uzair, M.; Khan, M.R.; Patil, S.B.; Fang, J.; Zhao, J.; Singla-Pareek, S.L.; Pareek, A.; Li, X. DPS1 regulates cuticle development and leaf senescence in rice. Food Energy Secur. 2021, 10, e27. [Google Scholar]
- Jagadish, S.K.; Craufurd, P.Q.; Wheeler, T. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Wang, C.; Liu, K.; Tian, X. Detrimental effects of heat stress on grain weight and quality in rice (Oryza sativa L.) are aggravated by decreased relative humidity. Peer J. 2021, 9, e11218. [Google Scholar] [CrossRef]
- Haider, S.; Raza, A.; Iqbal, J.; Shaukat, M.; Mahmood, T. Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: A brief appraisal. Mol. Biol. Rep. 2022, 49, 5771–5785. [Google Scholar] [CrossRef]
- Easterling, W.E.; Aggarwal, P.K.; Batima, P.; Brander, K.M.; Erda, L.; Howden, S.M.; Kirilenko, A.; Morton, J.; Soussana, J.-F.; Schmidhuber, J. Food fibre and forest products. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 273–313. [Google Scholar]
- Karwa, S.; Bahuguna, R.N.; Chaturvedi, A.K.; Maurya, S.; Arya, S.S.; Chinnusamy, V.; Pal, M. Phenotyping and characterization of heat stress tolerance at reproductive stage in rice (Oryza sativa L.). Acta Physiol. Plant. 2020, 42, 29. [Google Scholar] [CrossRef]
- Zafar, S.A.; Hameed, A.; Ashraf, M.; Khan, A.S.; Li, X.; Siddique, K.H. Agronomic, physiological and molecular characterisation of rice mutants revealed the key role of reactive oxygen species and catalase in high-temperature stress tolerance. Funct. Plant Biol. 2020, 47, 440–453. [Google Scholar] [CrossRef]
- Lyman, N.B.; Jagadish, K.S.V.; Nalley, L.L.; Dixon, B.L.; Siebenmorgen, T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 2013, 8, e72157. [Google Scholar] [CrossRef]
- Liu, W.; Yin, T.; Zhao, Y.; Wang, X.; Wang, K.; Shen, Y.; Ding, Y.; Tang, S. Effects of high temperature on rice grain development and quality formation based on proteomics comparative analysis under field warming. Front. Plant Sci. 2021, 12, 746180. [Google Scholar] [CrossRef]
- Snider, J.L.; Oosterhuis, D.M.; Loka, D.A.; Kawakami, E.M. High temperature limits in vivo pollen tube growth rates by altering diurnal carbohydrate balance in field-grown Gossypium hirsutum pistils. J. Plant Physiol. 2011, 168, 1168–1175. [Google Scholar] [CrossRef]
- Aryan, S.; Gulab, G.; Habibi, N.; Kakar, K.; Sadat, M.I.; Zahid, T.; Rashid, R.A. Phenological and physiological responses of hybrid rice under different high-temperature at seedling stage. Bull. Natl. Res. Cent. 2022, 46, 45. [Google Scholar] [CrossRef]
- Nagai, T.; Makino, A. Differences between rice and wheat in temperature responses of photosynthesis and plant growth. Plant Cell Physiol. 2009, 50, 744–755. [Google Scholar] [CrossRef] [Green Version]
- Taratima, W.; Chuanchumkan, C.; Maneerattanarungroj, P.; Trunjaruen, A.; Theerakulpisut, P.; Dongsansuk, A. Effect of heat stress on some physiological and anatomical characteristics of rice (Oryza sativa L.) cv. KDML105 Callus and Seedling. Biology 2022, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, A.G.; Jiménez, M.J.; Siebenmorgen, T.J. Rice fissuring response to high drying and tempering temperatures. J. Food Eng. 2003, 59, 61–69. [Google Scholar] [CrossRef]
- Cooper, S.; Cooper, N.T.W.; Siebenmorgen, T.J.; Counce, P.A. Effects of night time temperature during kernel development on rice physicochemical properties. Cereal Chem. 2008, 85, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Ha, K.Y.; Lee, J.K.; Lee, S.Y.; Lee, J.S. Grain quality characteristics for brewing in rice. Korean J. Crop Sci. 1994, 39, 38–44. [Google Scholar]
- Asaoka, M.; Okuno, K.; Fuwa, H. Effect of environmental temperature at the milky stage on Amylose content and fine structure of amylopectin of waxy and nonwaxy endosperm starches of rice (L.). Agric. Biol. Chem. 1985, 49, 373–379. [Google Scholar]
- Bhardwaj, R.; Salgotra, R.K.; Sharma, M. Studies on correlation of amylose content and grain dimensions in Basmati rice (Oryza sativa L.). Electron. J. Plant Breed. 2019, 10, 364–369. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yuki, K.; Park, S.Y.; Ohya, T. Carbohydrate metabolism in the developing endosperm of Rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K. Drought stress responses and its management in rice. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 177–200. [Google Scholar]
- Bouman, B.A.M.; Toung, T.P. Field water management to save water and increase its productivity in irrigated lowland rice. Agric. Water Manag. 2001, 49, 11–30. [Google Scholar] [CrossRef]
- Moonmoon, S.; Md Tariqul, I. Effect of drought stress at different growth stages on yield and yield components of six rice (Oryza sativa L.) genotypes. Fundam. Appl. Agri. 2017, 2, 285–289. [Google Scholar] [CrossRef]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- O’Toole, J.C. Adaptation of rice to drought-prone environments. In Drought Resistance in Crops with Emphasis on Rice; IRRI: Manila, Philippines, 1982; pp. 195–216. [Google Scholar]
- Blum, A. Drought tolerance- Is it a complex trait? In Field Screening for Drought Tolerance in Crop Plants with Emphasis on Rice, Proceedings of the International Workshop on Field Screening for Drought Tolerance in Rice, ICRISAT, Patancheru, India, 11–14 December 2000; ICRISAT and The Rockefeller Foundation: New York, NY, USA, 2002; pp. 17–22. [Google Scholar]
- Zubaer, M.A.; Chowdhury, A.K.M.B.B.; Islam, M.Z.; Ahmed, T.; Hasan, M.A. Effects of water stress on growth and yield attributes of Aman rice genotypes. Int. J. Sust. Crop Prod. 2007, 2, 25–30. [Google Scholar]
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.N.; Francia, E.; Mare, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants. An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Alou, I.N.; Steyn, J.M.; Annandale, J.G.; Van der Laan, M. Growth, phenological, and yield response of upland rice (Oryza sativa L. cv. Nerica 4®) to water stress during different growth stages. Agric. Water Manag. 2018, 198, 39–52. [Google Scholar] [CrossRef]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef]
- Davatgar, N.; Neishabouri, M.; Sepaskhah, A.; Soltani, A. Physiological and morphological responses of rice (Oryza sativa L.) to varying water stress management strategies. Int. J. Plant Prod. 2012, 3, 19–32. [Google Scholar]
- Anjum, S.A.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and development responses of crop plants under drought stress: A review. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, J.; Qian, Q.; Shang, L. Enhancement of heat and drought stress tolerance in rice by genetic manipulation: A systematic review. Rice 2022, 15, 67. [Google Scholar] [CrossRef]
- Prathap, V.; Ali, K.; Singh, A.; Vishwakarma, C.; Krishnan, V.; Chinnusamy, V.; Tyagi, A. Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol. Biochem. 2019, 142, 440–451. [Google Scholar]
- Mukamuhirwa, A.; Persson Hovmalm, H.; Ortiz, R.; Nyamangyoku, O.; Prieto–Linde, M.L.; Ekholm, A.; Johansson, E. Effect of intermittent drought on grain yield and quality of rice (Oryza sativa L.) grown in Rwanda. J. Agron. Crop Sci. 2020, 206, 252–262. [Google Scholar] [CrossRef]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V. High-temperature effects on rice growth, yield, and grain quality. Adv. Agron. 2011, 111, 87–206. [Google Scholar]
- Gaballah, M.M.; Metwally, A.M.; Skalicky, M.; Hassan, M.M.; Brestic, M.; El Sabagh, A.; Fayed, A.M. Genetic diversity of selected rice genotypes under water stress conditions. Plants 2021, 10, 27. [Google Scholar] [CrossRef]
- You, C.; Chen, L.; He, H.; Wu, L.; Wang, S.; Ding, Y.; Ma, C. iTRAQ-based proteome profile analysis of superior and inferior spikelets at early grain filling stage in japonica rice. BMC Plant Biol. 2017, 17, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Lou, D.; Wang, H.; Liang, G.; Yu, D. OsSAPK2 confers abscisic acid sensitivity and tolerance to drought stress in rice. Front. Plant Sci. 2017, 8, 993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Wu, S.; Zhang, Y.; Xu, T.; Guo, F.; Tang, H.; Li, X.; Wang, P.; Qian, W.; Xue, Y. A high temperature-dependent mitochondrial lipase EXTRA GLUME1 promotes floral phenotypic robustness against temperature fluctuation in rice (Oryza sativa L.). PLoS Genet. 2016, 12, e1006152. [Google Scholar] [CrossRef] [Green Version]
- Sarvestani, Z.T.; Pirdashti, H.; Sanavy, S.A.; Balouchi, H. Study of water stress effects in different growth stages on yield and yield components of different rice (Oryza sativa L.) cultivars. Pak. J. Biol. Sci. 2008, 15, 1303–1309. [Google Scholar] [CrossRef] [Green Version]
- Brunel-Muguet, S.; D’Hooghe, P.; Bataillé, M.-P.; Larré, C.; Kim, T.-H.; Trouverie, J.; Avice, J.-C.; Etienne, P.; Dürr, C. Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2015, 6, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, Y.; Peter, P.; Finka, A.; Cicekli, C.; Vígh, L.; Goloubinoff, P. Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal. Behav. 2010, 5, 1530–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begcy, K.; Dresselhaus, T. Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod. 2018, 31, 343–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Henry, A.; Sreenivasulu, N. Rice yield formation under high day and night temperatures-A prerequisite to ensure future food security. Plant Cell Environ. 2020, 43, 1595–1608. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Zhou, J.; Hu, S.; Chen, H.; Xiang, J.; Zhang, Y.; Zeng, Y.; Shi, Q.; Zhu, D. Research progress on heat stress of rice at flowering stage. Rice Sci. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Zhang, G. The next generation of rice: Inter-subspecific Indica-Japonica hybrid rice. Front. Plant Sci. 2022, 13, 857896. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. High temperature at flowering inhibits swelling of pollen grains, a driving force for thecae dehiscence in rice (Oryza sativa L.). Plant Prod. Sci. 2000, 3, 430–434. [Google Scholar] [CrossRef]
- Das, S.; Krishnan, P.; Nayak, M.; Ramakrishnan, B. High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot. 2014, 101, 36–46. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Raveendran, M.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Endo, M.; Tsuchiya, T.; Hamada, K.; Kawamura, S.; Yano, K.; Ohshima, M.; Higashitani, A.; Watanabe, M.; Kawagishi-Kobayashi, M. High temperatures cause male sterility in rice plants with transcriptional alterations during pollen development. Plant Cell Physiol. 2009, 50, 1911–1922. [Google Scholar] [CrossRef]
- Liu, X.H.; Lyu, Y.S.; Yang, W.; Yang, Z.T.; Lu, S.J.; Liu, J.X. A membrane-associated NAC transcription factor OsNTL3 is involved in thermotolerance in rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Huang, R.; Wai, H.P.; Xiong, H.; Shen, X.; He, H.; Yan, S. Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice. Physiol. Mol. Biol. Plants 2017, 23, 817–825. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Schmidt, R.C.; Struik, P.C.; Yin, X.; Jagadish, S.V.K. Pollen germination and in vivo fertilization in response to high-temperature during flowering in hybrid and inbred rice. Plant Cell Environ. 2018, 41, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.; Feng, B.; Zhang, C.; Yang, Y.; Yang, X.; Chen, T.; Zhao, X.; Zhang, X.; Jin, Q.; Tao, L. Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Front. Plant Sci. 2016, 7, 1637. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Cui, K.; Hu, Q.; Wang, W.; Nie, L.; Huang, J.; Peng, S. Enclosed stigma contributes to higher spikelet fertility for rice (Oryza sativa L.) subjected to heat stress. Crop J. 2019, 7, 335–349. [Google Scholar] [CrossRef]
- Firon, N.; Shaked, R.; Peet, M.M.; Pharr, D.M.; Zamski, E.; Rosenfeld, K.; Althan, L.; Pressman, E. Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Sci. Hortic. 2006, 3, 212–217. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef]
- Kobayashi, K.; Matsui, T.; Murata, Y.; Yamamoto, M. Percentage of dehisced thecae and length of dehiscence control pollination stability of rice cultivars at high temperatures. Plant Prod. Sci. 2011, 14, 89–95. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Chen, T.; Feng, B.; Fu, W.; Yan, J.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 2018, 11, 14. [Google Scholar] [CrossRef]
- Wang, Y.L.; Zhang, Y.P.; Zhu, D.F.; Xiang, J.; Wu, H.; Chen, H.Z.; Zhang, Y.K. Effect of heat stress on spikelet degeneration and grain filling at panicle initiation period of rice. Acta Agron. Sin. 2016, 42, 1402–1410. [Google Scholar] [CrossRef]
- Wu, C.; Cui, K.; Wang, W.; Li, Q.; Fahad, S.; Hu, Q.; Huang, J.; Nie, L.; Mohapatra, P.K.; Peng, S. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front. Plant Sci. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, G.F.; Zhang, C.X.; Yang, Y.J.; Xiong, J.; Yang, X.Q.; Zhang, X.F.; Jin, Q.Y.; Tao, L.X. Male parent plays more important role in heat tolerance in three-line hybrid rice. Rice Sci. 2015, 22, 116–122. [Google Scholar]
- Cao, Y.Y.; Chen, Y.H.; Chen, M.X.; Wang, Z.Q.; Wu, C.F.; Bian, X.C.; Yang, J.C.; Zhang, J.H. Growth characteristics and endosperm structure of superior and inferior spikelets of indica rice under high-temperature stress. Biol. Plant. 2016, 60, 532–542. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, H.; Zhao, C.; Chen, G.; Zou, Y. Amino acid content in rice grains is affected by high temperature during the early grain-filling period. Sci. Rep. 2019, 9, 2700. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.-Z.; Pan, G.; Wang, F.-B.; Wei, K.-S.; Li, Z.-W.; Shi, C.-H.; Wei, G.; Cheng, F.-M. Effect of high temperature on the expressions of genes encoding starch synthesis enzymes in developing rice endosperms. J. Integr. Agric. 2015, 14, 642–659. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, S.; Li, G.; Liu, Z.; Ding, C.; Chen, L.; Wang, S.; Ding, Y. Application of nitrogen fertilizer at heading stage improves rice quality under elevated temperature during grain-filling stage. Crop Sci. 2017, 57, 2183–2192. [Google Scholar] [CrossRef] [Green Version]
- Hemati, A.; Moghiseh, E.; Amirifar, A.; Mofidi-Chelan, M.; Asgari Lajayer, B. Physiological Effects of Drought Stress in Plants. In Plant Stress Mitigators; Vaishnav, A., Arya, S., Choudhary, D.K., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; He, D.; Wang, W.; Yuan, S. Exogenous trehalose protects photosystem II by promoting cyclic electron flow under heat and drought stresses in winter wheat. Plant Biol. 2021, 23, 770–776. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Fukuoka, M.; Hasegawa, T. Integrated micrometeorology model for panicle and canopy temperature (IM2PACT) for rice heat stress studies under climate change. J. Agrl. Meteorol. 2011, 67, 233. [Google Scholar] [CrossRef] [Green Version]
- Wada, H.; Hatakeyama, Y.; Nakashima, T.; Nonami, H.; Erra-Balsells, R.; Hakata, M.; Nakata, K.; Hiraoka, K.; Onda, Y. On-site single pollen metabolomics reveals varietal differences in phosphatidylinositol synthesis under heat stress conditions in rice. Sci. Rep. 2010, 10, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Lin, H.X. Molecular regulation and genetic control of rice thermal response. Crop J. 2021, 9, 497–505. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic reprogramming in chloroplasts under heat stress in plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plantarum. 2019, 165, 644–663. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Hakata, M.; Wada, H.; Masumoto-Kubo, C.; Tanaka, R.; Sato, H.; Morita, S. Development of a new heat tolerance assay system for rice spikelet sterility. Plant Methods 2017, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanamachi, K.; Miyazaki, M.; Matsuo, K.; Suriyasak, C.; Tamada, A.; Matsuyama, K.; Iwaya-Inoue, M.; Ishibashi, Y. Differential responses to high temperature during maturation in heat-stress tolerant cultivars of Japonica rice. Plant Prod. Sci. 2016, 19, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.; Wang, X.; Mubeen, I.; Kamran, M.; Kanwal, I.; Díaz, G.A.; Abbas, A.; Parveen, A.; Atiq, M.N.; Alshaya, H.; et al. Phytohormones trigger drought tolerance in crop plants: Outlook and future perspectives. Front. Plant Sci. 2022, 12, 799318. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H.M. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Samal, P.; Babu, S.C.; Mondal, B.; Mishra, S.N. The global rice agriculture towards 2050: An inter-continental perspective. Outlook Agri. 2022, 51, 164–172. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the. Intergovernmental Panel Climate Change; Team, R.K.P., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; pp. 117–130. [Google Scholar] [CrossRef] [Green Version]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant 2018, 162, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An over view. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Velten, J.; Xin, Z.; Stout, J. Characterization of maize inbred lines for drought and heat tolerance. J. Soil Water Conserv. 2012, 67, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Gooding, M.J.; Ellis, R.H.; Shewry, P.R.; Schofield, J.D. Effects of restricted water availability and increased temperature on the grain filling, drying and quality of winter wheat. J. Cereal Sci. 2003, 37, 295–309. [Google Scholar] [CrossRef]
- Dixit, S.; Swamy, B.P.M.; Vikram, P.; Ahmed, H.U.; Cruz, M.T.S.; Amante, M.; Atri, D.; Leung, H.; Kumar, A. Fine mapping of QTLs for rice grain yield under drought reveals sub-QTLs conferring a response to variable drought severities. Theor. Appl. Genet. 2012, 125, 155–169. [Google Scholar] [CrossRef]
- Dixit, S.; Singh, A.; Cruz, M.T.S.; Maturan, P.T.; Amante, M.; Kumar, A. Multiple major QTL lead to stable yield performance of rice cultivars across varying drought intensities. BMC Genet. 2014, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.H.; Lin, C.W.; Chen, J.C.; Lin, Y.C.; Cheng, S.Y.; Liu, T.H.; Jan, F.J.; Wu, S.T.; Thseng, F.S.; Ku, H.M. Tagging rice drought- related QTL with SSR DNA markers. Crop. Environ. Bioinform. 2007, 4, 65–76. [Google Scholar]
- Venuprasad, R.; Dalid, C.O.; Del Valle, M.; Zhao, D.; Espiritu, M.; Cruz, M.T.S.; Amante, M.; Kumar, A.; Atlin, G.N. Identification and characterization of large-effect quantitative trait loci for grain yield under lowland drought stress in rice using bulk- segregant analysis. Theor. Appl. Genet. 2009, 120, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ramchander, S.; Raveendran, M.; Robin, S. Mapping QTLs for physiological traits associated with drought tolerance in rice (Oryza sativa L.). J. Investig. Genom. 2016, 3, 56–61. [Google Scholar]
- Qu, Y.Y.; Mu, P.; Zhang, H.L.; Chen, C.Y.; Gao, Y.M.; Tian, Y.X.; Wen, F.; Li, Z.C. Mapping QTLs of root morphological traits at different growth stages in rice. Genetica 2008, 133, 187–200. [Google Scholar] [CrossRef]
- Mishra, K.K.; Vikram, P.; Yadaw, R.B.; Swamy, B.P.M.; Dixit, S.; Cruz, M.T.S.; Maturan, P.; Marker, S.; Kumar, A. qDTY12.1: A locus with a consistent effect on grain yield under drought in rice. BMC Genet. 2013, 14, 12. [Google Scholar] [CrossRef] [Green Version]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.V.K.; Kusolwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, Q.; Tang, M.; Zhang, X.; Pan, Y.; Yang, X.; Gao, G.; Lv, R.; Tao, W.; Jiang, L.; et al. QTL mapping and identification of candidate genes for heat tolerance at the flowering stage in rice. Front Genet. 2021, 22, 621871. [Google Scholar] [CrossRef]
- Ye, C.; Ishimaru, T.; Lambio, L.; Li, L.; Long, Y.; He, Z.; Htun, T.; Tang, S.; Su, Z. Marker-assisted pyramiding of QTLs for heat tolerance and escape upgrades heat resilience in rice (Oryza sativa L.). Theor. Appl. Genet. 2022, 135, 1345–1354. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Gupta, B.B.; Bhat, J.A.; Sharma, S. Genetic diversity and population structure of basmati rice (Oryza sativa L.) Germplasm collected from North Western Himalayas using trait linked SSR markers. PLoS ONE 2015, 10, e0131858. [Google Scholar] [CrossRef] [Green Version]
- Zaid, I.U.; Zahra, N.; Habib, M.; Naeem, M.K.; Asghar, U.; Uzair, M.; Latif, A.; Rehman, A.; Ali, G.M.; Khan, M.R. Estimation of genetic variances and stability components of yield-related traits of Green Super Rice at multi-environmental conditions of Pakistan. Agronomy 2022, 12, 1157. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Burgos, N.R.; Oosterhuis, D.M. Temperature and drought impacts on rice production: An agronomic perspective regarding short- and long-term adaptation measures. Water Resour. Rural. Develop. 2017, 9, 12–27. [Google Scholar] [CrossRef]
- Langridge, P.; Reynolds, M.P. Genomic tools to assist breeding for drought tolerance. Curr. Opin. Biotechnol. 2015, 32, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Zargar, S.M. Potential of genetic and genomic resources for genetic improvement of food crops. In Rediscovery of Genetic and Genomic Resources for Future Food Security; Salgotra, R.K., Zargar, S.M., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Gupta, C.; Salgotra, R.K.; Mahajan, G. Future threats and opportunities facing crop wild relatives and landrace diversity. In Rediscovery of Genetic and Genomic Resources for Future Food Security; Salgotra, R.K., Zargar, S.M., Eds.; Springer: Singapore, 2020; pp. 351–364. [Google Scholar] [CrossRef]
- Lafitte, H.; Li, Z.; Vijayakumar, C.; Gao, Y.; Shi, Y.; Xu, J.; Fu, B.; Yu, S.; Ali, A.; Domingo, J. Improvement of rice drought tolerance through backcross breeding: Evaluation of donors and selection in drought nurseries. Field Crops Res. 2006, 97, 77–86. [Google Scholar] [CrossRef]
- Gupta, M.; Salgotra, R.K.; Chauhan, B.S. Next-generation sequencing technologies and their implications for efficient utilization of genetic resources. In Rediscovery of Genetic and Genomic Resources for Future Food Security; Salgotra, R.K., Zargar, S.M., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Salgotra, R.K.; Stewart, N. Functional markers for precision plant breeding. Intl. J. Mol. Sci. 2020, 21, 4792. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.S.; Behera, P.K.; Kumar, V.; Lenka, S.K.; Panda, D. Physiological characterization and allelic diversity of selected drought tolerant traditional rice (Oryza sativa L.) landraces of Koraput, India. Physiol. Mol. Biol. Plants 2018, 24, 1035–1046. [Google Scholar] [CrossRef]
- Wei, H.; Liu, J.; Wang, Y.; Huang, N.; Zhang, X.; Wang, L.; Zhang, J.; Tu, J.; Zhong, X. A dominant major locus in chromosome 9 of rice (Oryza sativa L.) confers tolerance to high temperature at seedling stage. J. Hered. 2013, 104, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Salgotra, R.K.; Stewart, C.N., Jr. Genetic Augmentation of Legume Crops Using Genomic Resources and Genotyping Platforms for Nutritional Food Security. Plants 2022, 11, 1866. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Gupta, B.B. Plant genetic resources and traditional/indigenous knowledge: Potentials and challenges. In Plant Genetic Resources and Traditional Knowledge for Food Security; Salgotra, R.K., Gupta, B.B., Eds.; Springer: Singapore, 2015. [Google Scholar] [CrossRef]
- Bakhsh, A.; Hussain, T. Engineering crop plants against abiotic stress: Current achievements and prospects. Emir. J. Food Agric. 2015, 27, 24–39. [Google Scholar] [CrossRef]
- Jovovic, Z.; Andjelkovic, V.; Przulj, N.; Mandic, D. Untapped genetic diversity of wild relatives for crop Improvement. In Rediscovery of Genetic and Genomic Resources for Future Food Security; Springer: Singapore, 2020; pp. 25–65. [Google Scholar]
- Priyanka, B.; Sekhar, K.; Sunita, T.; Reddy, V.D.; Rao, K.V. Characterization of expressed sequence tags (ESTs) of pigeonpea (Cajanus cajan L.) and functional validation of selected genes for abiotic stress tolerance in Arabidopsis thaliana. Mol. Genet. Genom. 2010, 283, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.M.; Hauptli, H.; Crossway, A.; Knauf, V.C. Gene transfer in crop improvement. Sciences 1987, 236, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, S.; Boyett, V.A.; Khodakovskaya, M. Enhancement of drought tolerance in rice by silencing of the OsSYT-5 gene. PLoS ONE 2021, 16, e0258171. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kushwaha, N.K.; Ram, H.; Soni, P. Genome editing for improving abiotic stress tolerance in rice. In Genome Engineering for Crop Improvement; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 314–332. [Google Scholar]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Singh, H.; Trivedi, P.K.; Jain, M.; Yadav, S.R. State-of-the-art in CRISPR technology and engineering drought, salinity, and thermo-tolerant crop plants. Plant Cell Rep. 2021, 41, 815–831. [Google Scholar] [CrossRef]
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma functionality and fertility are reduced by heat and drought co-stress in wheat. Front. Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Nayak, A.K.; Tripathi, R.; Katara, J.L.; Bihari, P.; Lal, B.; Gautam, P. Boron application improves yield of rice cultivars under high temperature stress during vegetative and reproductive stages. Int. J. Biometeorol. 2018, 62, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Dhaubhadel, S.; Browning, K.S.; Gallie, D.R.; Krishna, P. Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 2002, 2, 681–691. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Ihsan, Z.; Shah, A.N.; Wu, C.; Yousaf, M.; Nasim, W.; Alharby, H. Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front. Plant Sci. 2016, 7, 1250. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Akram, N.; Al-Qurainy, F.; Foolad, M.R. Drought tolerance: Roles of organic osmolytes, growth regulators, and mineral nutrients. Adv. Agron. 2011, 111, 249–296. [Google Scholar]
- Kim, J.-M.; To, T.K.; Matsui, A.; Tanoi, K.; Kobayashi, N.I.; Matsuda, F.; Habu, Y.; Ogawa, D.; Sakamoto, T.; Matsunaga, S. Acetate-mediated novel survival strategy against drought in plants. Nat. Plants 2017, 3, 17097. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef]
| Morphological | Physiological | Biochemical |
|---|---|---|
| Leaf rolling | Reduction in photosynthesis | Activate oxidative reactions |
| Root length decrease | Cell membrane damage | Increase in mannitol |
| Reduced fruit width and fruit weight | Phytohormone imbalance | Accumulation of hydrogen peroxide |
| Shortened period for days to booting, heading, and anthesis | Reduced starch biosynthesis | Electrolyte leakage |
| Decrease in effective tillers, grains per panicle, and kernel weight | Photosynthesis damages | Accumulation of secondary metabolites |
| Early maturity | Disturbance of carbohydrate metabolism | Increases phenols |
| Increase in spikelet sterility | Repression of photosynthesis genes | Increases flavonoids |
| Shortened duration of grain filling | Down regulation of photosynthesis | Increases proline |
| Increased the proportion of abnormal seeds | More carbohydrate accumulation in sink | Increase in inositol |
| Reduction in grain yield | Increase in CO2/O2 ratio | Increase in sorbitol |
| Reduced the rates of pollen | Decrease in chlorophyll (chl) content, chl a fluorescence, decreased photosystem II (PSII) | Disruption of proteins and enzymes in cell membrane |
| Reduced pollen tube | Thylakoid membrane damage | Activation of stress-responsive genes, e.g., heat shock protein genes |
| Lesser number of pollen grain | Increase in leaf temperature | Activation of antioxidant |
| Decrease in pollen grain germination on stigma | Senescence of functional leaves | Excessive production of ROS |
| Lesser pollen grain swelling | Higher water use efficiency | Reduces hemicellulose and cellulose |
| Impede pollination and fertilization | Higher canopy transpiration | Decrease in the CO2 assimilation rate |
| Tapetum degeneration | Decrease stomatal conductance and stomata opening | Decrease in growth regulator production such as auxin, IBA, cytokinin, etc. |
| Distort floral organs | Decrease relative water content (RWC) | Production of primarily superoxide and hydrogen peroxide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgotra, R.K.; Chauhan, B.S. Ecophysiological Responses of Rice (Oryza sativa L.) to Drought and High Temperature. Agronomy 2023, 13, 1877. https://doi.org/10.3390/agronomy13071877
Salgotra RK, Chauhan BS. Ecophysiological Responses of Rice (Oryza sativa L.) to Drought and High Temperature. Agronomy. 2023; 13(7):1877. https://doi.org/10.3390/agronomy13071877
Chicago/Turabian StyleSalgotra, Romesh Kumar, and Bhagirath Singh Chauhan. 2023. "Ecophysiological Responses of Rice (Oryza sativa L.) to Drought and High Temperature" Agronomy 13, no. 7: 1877. https://doi.org/10.3390/agronomy13071877
APA StyleSalgotra, R. K., & Chauhan, B. S. (2023). Ecophysiological Responses of Rice (Oryza sativa L.) to Drought and High Temperature. Agronomy, 13(7), 1877. https://doi.org/10.3390/agronomy13071877








