Metabolomic Insights into Primary and Secondary Metabolites Variation in Common and Glutinous Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Reagents
2.2. Metabolite Extraction and UPLC-MS/MS Analysis
2.3. Identification and Quantification of Metabolites
2.4. Data Analysis
3. Results
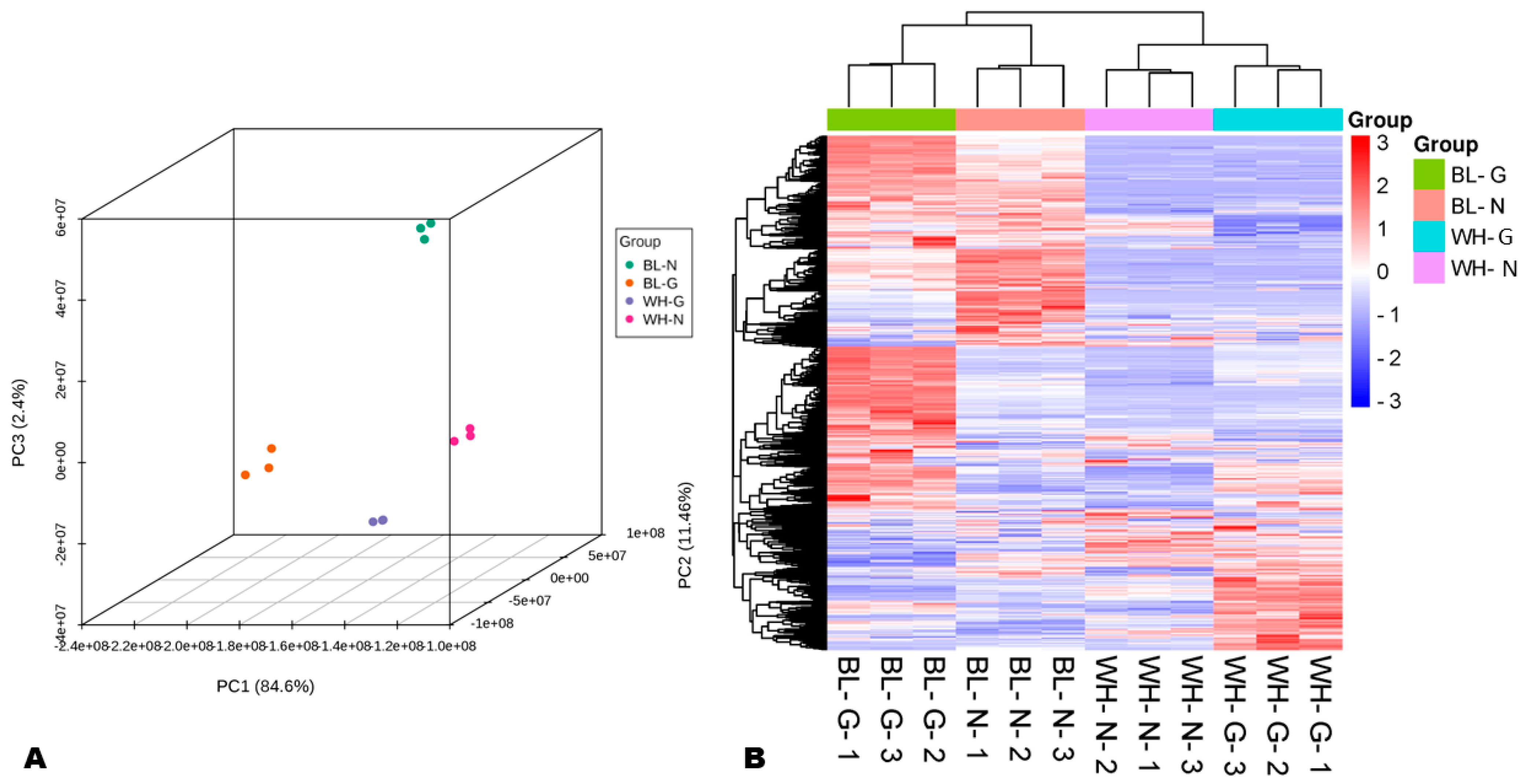
3.1. Distribution and Variation of Metabolites in Common and Glutinous Rice
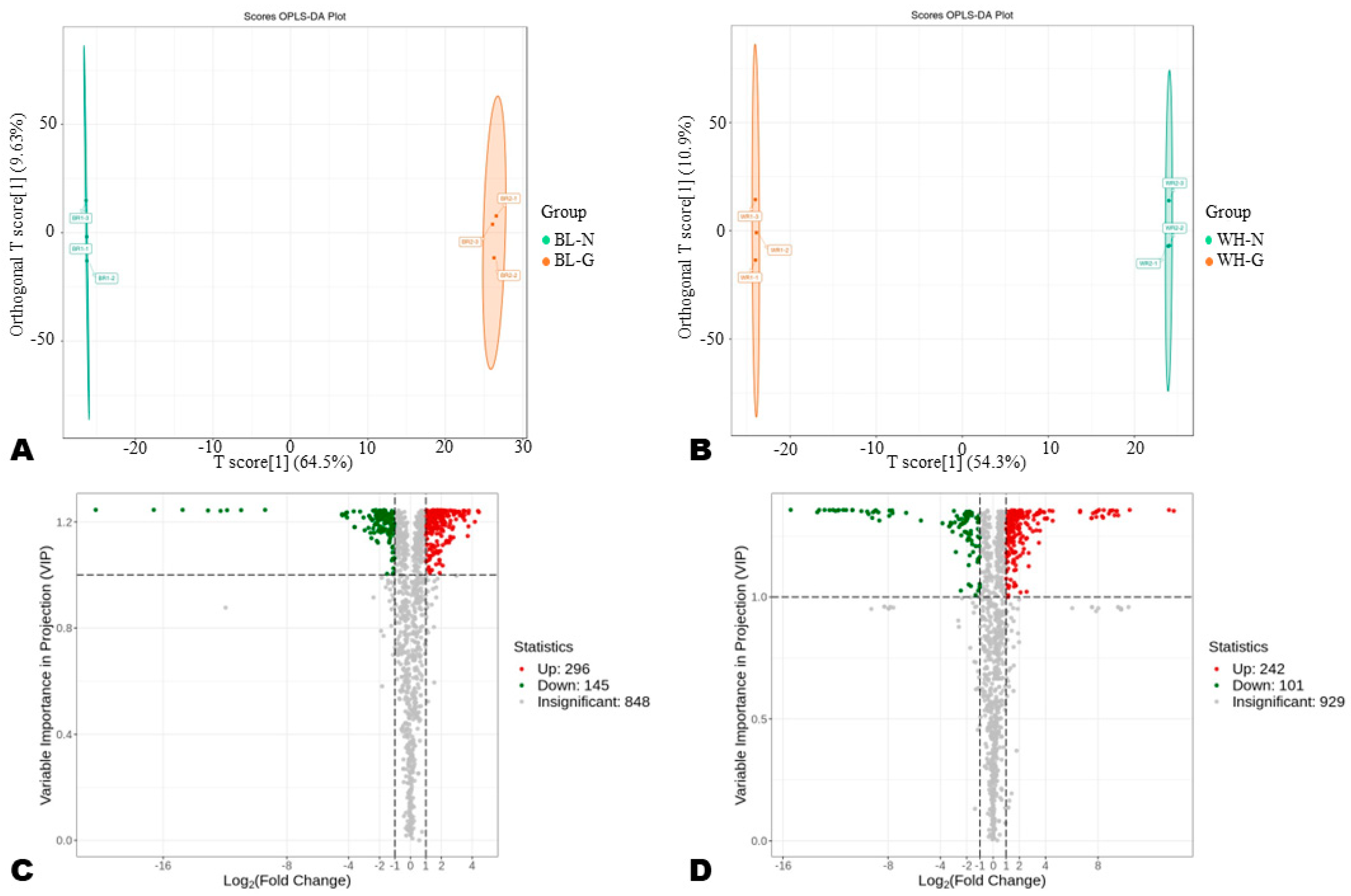
3.2. Differentially Accumulated Metabolites in Common and Glutinous Rice
3.3. Variation in Nutrients and Active Compounds
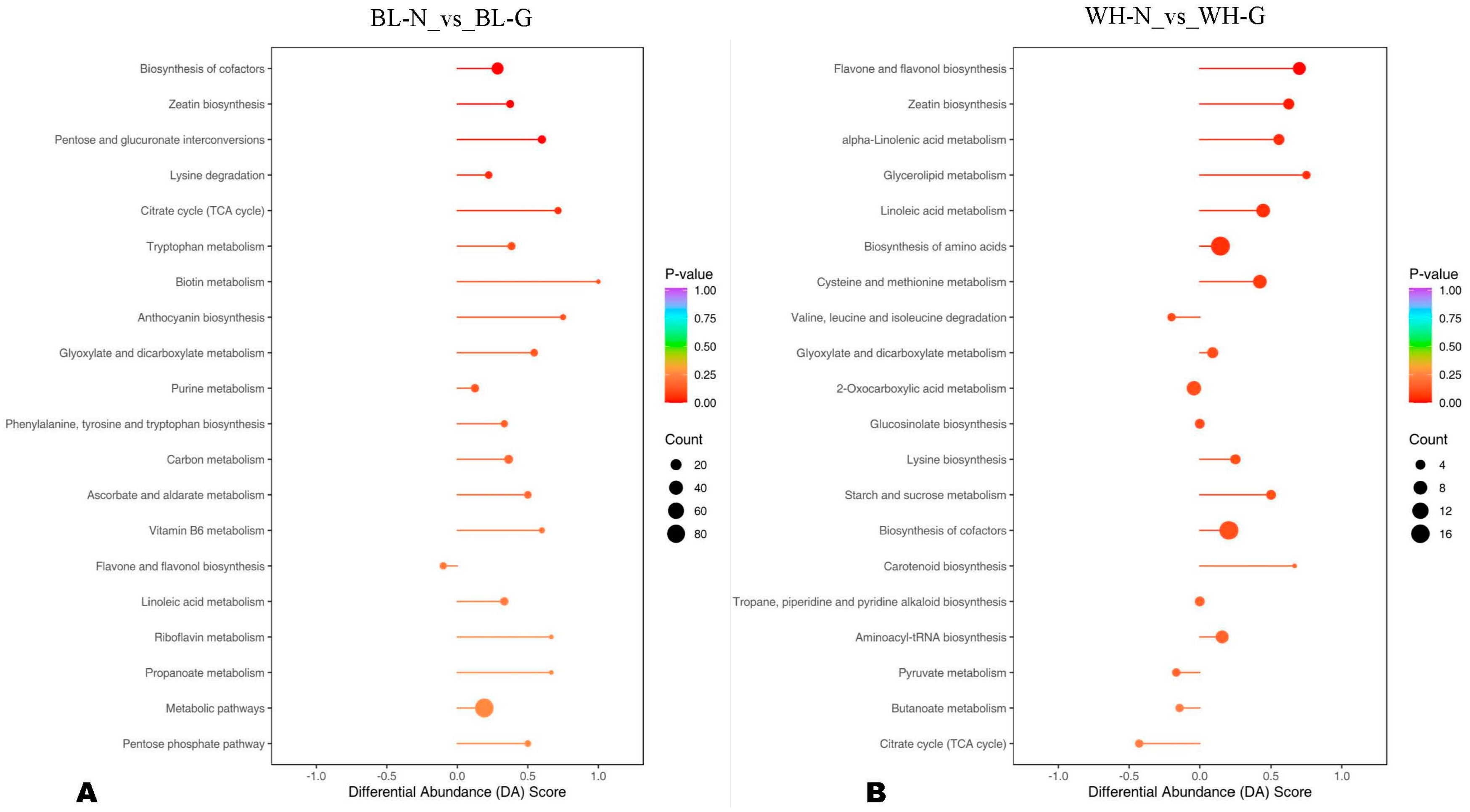
3.4. Major Differentially Regulated Metabolic Pathways
3.5. Variation Characteristics of Vitamins in Common and Glutinous Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Li, A.; Sun, C.; Zhang, J.; Hu, J.; Wang, S.; Zhou, N.; Xiong, Q. Rice Quality-Related Metabolites and the Regulatory Roles of Key Metabolites in Metabolic Pathways of High-Quality Semi-Glutinous Japonica Rice Varieties. Foods 2022, 11, 3676. [Google Scholar] [CrossRef] [PubMed]
- Sudan, J.; Urwat, U.; Farooq, A.; Pakhtoon, M.M.; Zaffar, A.; Naik, Z.A.; Batool, A.; Bashir, S.; Mansoor, M.; Sofi, P.A.; et al. Explicating Genetic Architecture Governing Nutritional Quality in Pigmented Rice. PeerJ 2023, 11, e15901. [Google Scholar] [CrossRef] [PubMed]
- Rathna Priya, T.S.; Eliazer Nelson, A.R.L.; Ravichandran, K.; Antony, U. Nutritional and Functional Properties of Coloured Rice Varieties of South India: A Review. J. Ethn. Foods 2019, 6, 11. [Google Scholar] [CrossRef]
- Melini, V.; Panfili, G.; Fratianni, A.; Acquistucci, R. Bioactive Compounds in Rice on Italian Market: Pigmented Varieties as a Source of Carotenoids, Total Phenolic Compounds and Anthocyanins, before and after Cooking. Food Chem. 2019, 277, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Gomez-Alonso, S.; Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Hermosin-Gutierrez, I.; Arlorio, M. Phenolics Characterization and Antioxidant Activity of Six Different Pigmented Oryza sativa L. Cultivars Grown in Piedmont (Italy). Food Res. Int. 2014, 65, 282–290. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-Oxidative Analysis, and Identification and Quantification of Anthocyanin Pigments in Different Coloured Rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cui, D.; Ma, X.; Han, B.; Han, L. Comparative Analysis of Rice Reveals Insights into the Mechanism of Colored Rice via Widely Targeted Metabolomics. Food Chem. 2022, 399, 133926. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Cros, G.; Yokota, T.; Crozier, A. Phytochemical Profiles of Black, Red, Brown, and White Rice from the Camargue Region of France. J. Agric. Food Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Rashid, M.A.R.; Zhang, Y.; Zhao, Y.; Pan, Y. Genome Wide Association Study on Development and Evolution of Glutinous Rice. BMC Genom. Data 2022, 23, 33. [Google Scholar] [CrossRef]
- Gautam, R.K.; Langyan, S.; Devi, S.V.; Singh, R.; Semwal, D.P.; Ali, S.; Mangat, G.S.; Sarkar, S.; Bagchi, T.B.; Roy, S.; et al. Genetic Resources of Sticky Rice in India: Status and Prospects. Genet. Resour. Crop Evol. 2023, 70, 95–106. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Hidayah, N.; Saputro, I.E.; Lovillo, M.P. Study of Glutinous and Non-Glutinous Rice (Oryza sativa) Varieties on Their Antioxidant Compounds. In Proceedings of the International Conference on Plant, Marine and Environmental Sciences (PMES-2015), Kuala Lumpur, Malaysia, 1–2 January 2015; International Institute of Chemical, Biological & Environmental Engineering. pp. 26–31. [Google Scholar]
- Burešová, I.; Červenka, L.; Šebestíková, R.; Augustová, M.; Jarošová, A. Applicability of Flours from Pigmented and Glutinous Rice in Gluten-Free Bread Baking. Foods 2023, 12, 1324. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, G.; Tian, W.; Yang, S.; Chen, J.; Wang, F.; Li, Y. Normal Rice Flours Perform Better in Gluten-Free Bread than Glutinous Rice Flours. J. Food Sci. 2022, 87, 554–566. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Santhanaraju Vairappan, C. Biochemical Properties of Rice Wine Produced from Three Different Starter Cultures. J. Trop. Biol. Conserv. 2013, 10, 31–41. [Google Scholar]
- Chanthathamrongsiri, N.; Prompanya, C.; Leelakanok, N.; Jiangseubchatveera, N.; Semangoen, T.; Nuurai, P.; Khawsuk, W.; Petchsomrit, A. Rice Extract: Antioxidant Activities and Formulations. J. Appl. Pharm. Sci. 2022, 12, 126–133. [Google Scholar] [CrossRef]
- Guo, H.; Chariyakornkul, A.; Phannasorn, W.; Mahatheeranont, S.; Wongpoomchai, R. Phytochemical Profile and Chemopreventive Properties of Cooked Glutinous Purple Rice Extracts Using Cell-Based Assays and Rat Model. Foods 2022, 11, 2333. [Google Scholar] [CrossRef]
- Guo, H.; Punvittayagul, C.; Vachiraarunwong, A.; Phannasorn, W.; Wongpoomchai, R. Cancer Chemopreventive Potential of Cooked Glutinous Purple Rice on the Early Stages of Hepatocarcinogenesis in Rats. Front. Nutr. 2022, 9, 1–14. [Google Scholar] [CrossRef]
- Liang, C.; Guan, Z.; Wei, K.; Yu, W.; Wang, L.; Chen, X.; Wang, Y. Characteristics of Antioxidant Capacity and Metabolomics Analysis of Flavonoids in the Bran Layer of Green Glutinous Rice (Oryza sativa L. Var. Glutinosa Matsum). Sci. Rep. 2023, 13, 16372. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Sun, C.; Li, A.; Zhang, J.; Shi, Q.; Zhang, Y.; Hu, J.; Zhou, N.; Wei, H.; Liu, B.; et al. Metabolomics and Biochemical Analyses Revealed Metabolites Important for the Antioxidant Properties of Purple Glutinous Rice. Food Chem. 2022, 389, 133080. [Google Scholar] [CrossRef]
- Nakayama, T.; Nagai, Y.; Uehara, Y.; Nakamura, Y.; Ishii, S.; Kato, H.; Tanaka, Y. Eating Glutinous Brown Rice Twice a Day for 8 Weeks Improves Glycemic Control in Japanese Patients with Diabetes Mellitus. Nutr. Diabetes 2017, 7, e273–e276. [Google Scholar] [CrossRef]
- Nakayama, T.; Nagai, Y.; Yasunaka, Y.; Uraguchi, T.; Wada, Y.; Sone, M.; Tanaka, Y. Effect of Eating Glutinous Brown Rice Twice a Day for 6 Weeks on Serum 1,5-Anhydroglucitol in Japanese Subjects without Diabetes. J. Nutr. Metab. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Nayak, S.N.; Aravind, B.; Malavalli, S.S.; Sukanth, B.S.; Poornima, R.; Bharati, P.; Hefferon, K.; Kole, C.; Puppala, N. Omics Technologies to Enhance Plant Based Functional Foods: An Overview. Front. Genet. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tiozon, R.J.N.; Sartagoda, K.J.D.; Serrano, L.M.N.; Fernie, A.R.; Sreenivasulu, N. Metabolomics Based Inferences to Unravel Phenolic Compound Diversity in Cereals and Its Implications for Human Gut Health. Trends Food Sci. Technol. 2022, 127, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Mu, X.; Liu, J.; Li, B.; Liu, H.; Zhang, B.; Xiao, P. Plant Metabolomics: A New Strategy and Tool for Quality Evaluation of Chinese Medicinal Materials. Chin. Med. 2022, 17, 45. [Google Scholar] [CrossRef]
- Dossou, S.S.K.; Xu, F.; Cui, X.; Sheng, C.; Zhou, R.; You, J.; Tozo, K.; Wang, L. Comparative Metabolomics Analysis of Different Sesame (Sesamum indicum L.) Tissues Reveals a Tissue-Specific Accumulation of Metabolites. BMC Plant Biol. 2021, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, H.; You, Z.; Zhang, Z.; Zhu, H.; He, M.; He, J.; Duan, X.; Jiang, Y.; Yun, Z. Multiple Metabolomics Comparatively Investigated the Pulp Breakdown of Four Dragon Fruit Cultivars during Postharvest Storage. Food Res. Int. 2023, 164, 112410. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, Q.; Qiao, C.; Song, J.; Cheng, C.L.; Xu, H. Chemical Markers for the Quality Control of Herbal Medicines: An Overview. Chin. Med. 2008, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, Y.; Lin, J.; Wang, J.; Zhang, X.; Yan, T.; Jia, Y. Chemical Constituents and Mechanisms from Hemerocallis Citrina Baroni with Anti-Neuroinflammatory Activity. J. Funct. Foods 2023, 102, 105427. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Huang, Y.; Zhou, Q. Widely Targeted Metabolomics Analysis Reveals New Biomarkers and Mechanistic Insights on Chestnut (Castanea mollissima Bl.) Calcification Process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luoa, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Dossou, S.S.K.; Xu, F.; You, J.; Zhou, R.; Li, D.; Wang, L. Widely Targeted Metabolome Profiling of Different Colored Sesame (Sesamum Indicum L.) Seeds Provides New Insight into Their Antioxidant Activities. Food Res. Int. 2022, 151, 110850. [Google Scholar] [CrossRef]
- Kefale, H.; Segla Koffi Dossou, S.; Li, F.; Jiang, N.; Zhou, R.; Wang, L.; Zhang, Y.; Li, D.; You, J.; Wang, L. Widely Targeted Metabolic Profiling Provides Insights into Variations in Bioactive Compounds and Antioxidant Activity of Sesame, Soybean, Peanut, and Perilla. Food Res. Int. 2023, 174, 113586. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, S.; Yang, Y.; Liu, J.; Zhuang, Y. Integrated Metabolome and Transcriptome Analysis Reveals a Regulatory Network of Fruit Peel Pigmentation in Eggplant (Solanum melongena L.). Int. J. Mol. Sci. 2022, 23, 3475. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, S.; Chen, D.; Wu, X.; Cai, Q. Polyphenols and Phytohormones Profiling of Pre-Harvest Sprouting Resistant and Susceptible Wheat Genotypes. SN Appl. Sci. 2023, 5, 249. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Prom-u-thai, C.; Rerkasem, B. Rice Quality Improvement. A Review. Agron. Sustain. Dev. 2020, 40, 28. [Google Scholar] [CrossRef]
- Qiu, S.; Abbaspourrad, A.; Padilla-Zakour, O.I. Changes in the Glutinous Rice Grain and Physicochemical Properties of Its Starch upon Moderate Treatment with Pulsed Electric Field. Foods 2021, 10, 395. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Guo, S.; Li, Y.; Yin, H.; Tian, L.; Cheng, G.; Li, Y. Red Rice Seed Coat Targeting SPHK2 Ameliorated Alcoholic Liver Disease via Restored Intestinal Barrier and Improved Gut Microbiota in Mice. Nutrients 2023, 15, 4176. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.J.L.; Yang, D.; Pranata, H.P.; Tai, E.S.; Liu, M.H. Postprandial Glycemic and Lipidemic Effects of Black Rice Anthocyanin Extract Fortification in Foods of Varying Macronutrient Compositions and Matrices. NPJ Sci. Food 2023, 7, 59. [Google Scholar] [CrossRef]
- Song, H.; Shen, X.; Zhou, Y.; Zheng, X. Black Rice Anthocyanins Alleviate Hyperlipidemia, Liver Steatosis and Insulin Resistance by Regulating Lipid Metabolism and Gut Microbiota in Obese Mice. Food Funct. 2021, 12, 10160–10170. [Google Scholar] [CrossRef]
- Suwannasom, N.; Thepmalee, C.; Khoothiam, K.; Thephinlap, C. Evaluation of Anti-Hyperglycemia and Complications of Red and Black Thai Jasmine Rice Cultivars in Streptozotocin-Induced Diabetic Rats. Molecules 2022, 27, 8043. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, D.; Ji, Y.; Liu, Y.; Xu, L.; Guo, Y. Dietary Supplementation of Black Rice Anthocyanin Extract Regulates Cholesterol Metabolism and Improves Gut Microbiota Dysbiosis in C57BL/6J Mice Fed a High-Fat and Cholesterol Diet. Mol. Nutr. Food Res. 2020, 64, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice Quality: How Is It Defined by Consumers, Industry, Food Scientists, and Geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Y.; Feng, S.; Wu, Z.; Zheng, D.; Feng, N. Joint Analysis of Transcriptome and Metabolome Revealed the Difference in Carbohydrate Metabolism between Super Hybrid Rice and Conventional Rice under Salt Stress. Plant Stress 2023, 10, 100251. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Shan, S.; Cao, F.; Chen, G.; Zou, Y.; Huang, M.; Abou-Elwafa, S.F. Proteomic Profiling Reveals Differentially Expressed Proteins Associated with Amylose Accumulation during Rice Grain Filling. BMC Genom. 2020, 21, 714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sampathkumar, A.; Kerber, S.M.L.; Swart, C.; Hille, C.; Seerangan, K.; Graf, A.; Sweetlove, L.; Fernie, A.R. A Moonlighting Role for Enzymes of Glycolysis in the Co-Localization of Mitochondria and Chloroplasts. Nat. Commun. 2020, 11, 4509. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Jiang, Y. Amino Acids in Rice Grains and Their Regulation by Polyamines and Phytohormones. Plants 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhang, S.; Gu, M.; Xu, G. Function, Transport, and Regulation of Amino Acids: What Is Missing in Rice? Crop J. 2021, 9, 530–542. [Google Scholar] [CrossRef]
- Yibo, C.; Zhidong, W.; Chongrong, W.; Hong, L.; Daoqiang, H.; Degui, Z.; Lei, Z.; Yangyang, P.; Rong, G.; Shaochuan, Z. Comparisons of Metabolic Profiles for Carbohydrates, Amino Acids, Lipids, Fragrance and Flavones During Grain Development in Indica Rice Cultivars. Rice Sci. 2022, 29, 155–165. [Google Scholar] [CrossRef]
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Tryptophan: A Precursor of Signaling Molecules in Higher Plants BT—Hormones and Plant Response; Gupta, D.K., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 273–289. ISBN 978-3-030-77477-6. [Google Scholar]
- Ghosh, P.; Banerjee, A.; Roychoudhury, A. Dissecting the Biochemical and Molecular-Genetic Regulation of Diverse Metabolic Pathways Governing Aroma Formation in Indigenous Aromatic Indica Rice Varieties. Mol. Biol. Rep. 2023, 50, 2479–2500. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Huang, J.; Ren, J.; Xiao, X.; Li, Y.; Zhai, L.; Yan, X.; Yun, Y.; Yang, Q.; Tang, Q.; et al. Metabolomic Insights into Primary and Secondary Metabolites Variation in Common and Glutinous Rice (Oryza sativa L.). Agronomy 2024, 14, 1383. https://doi.org/10.3390/agronomy14071383
Zhao M, Huang J, Ren J, Xiao X, Li Y, Zhai L, Yan X, Yun Y, Yang Q, Tang Q, et al. Metabolomic Insights into Primary and Secondary Metabolites Variation in Common and Glutinous Rice (Oryza sativa L.). Agronomy. 2024; 14(7):1383. https://doi.org/10.3390/agronomy14071383
Chicago/Turabian StyleZhao, Mingchao, Jingfen Huang, Junfang Ren, Xiaorong Xiao, Yapeng Li, Linan Zhai, Xiaowei Yan, Yong Yun, Qingwen Yang, Qingjie Tang, and et al. 2024. "Metabolomic Insights into Primary and Secondary Metabolites Variation in Common and Glutinous Rice (Oryza sativa L.)" Agronomy 14, no. 7: 1383. https://doi.org/10.3390/agronomy14071383




