Melatonin Increases Drought Resistance through Regulating the Fine Root and Root Hair Morphology of Wheat Revealed with RhizoPot
Abstract
:1. Introduction
2. Materials and Methods
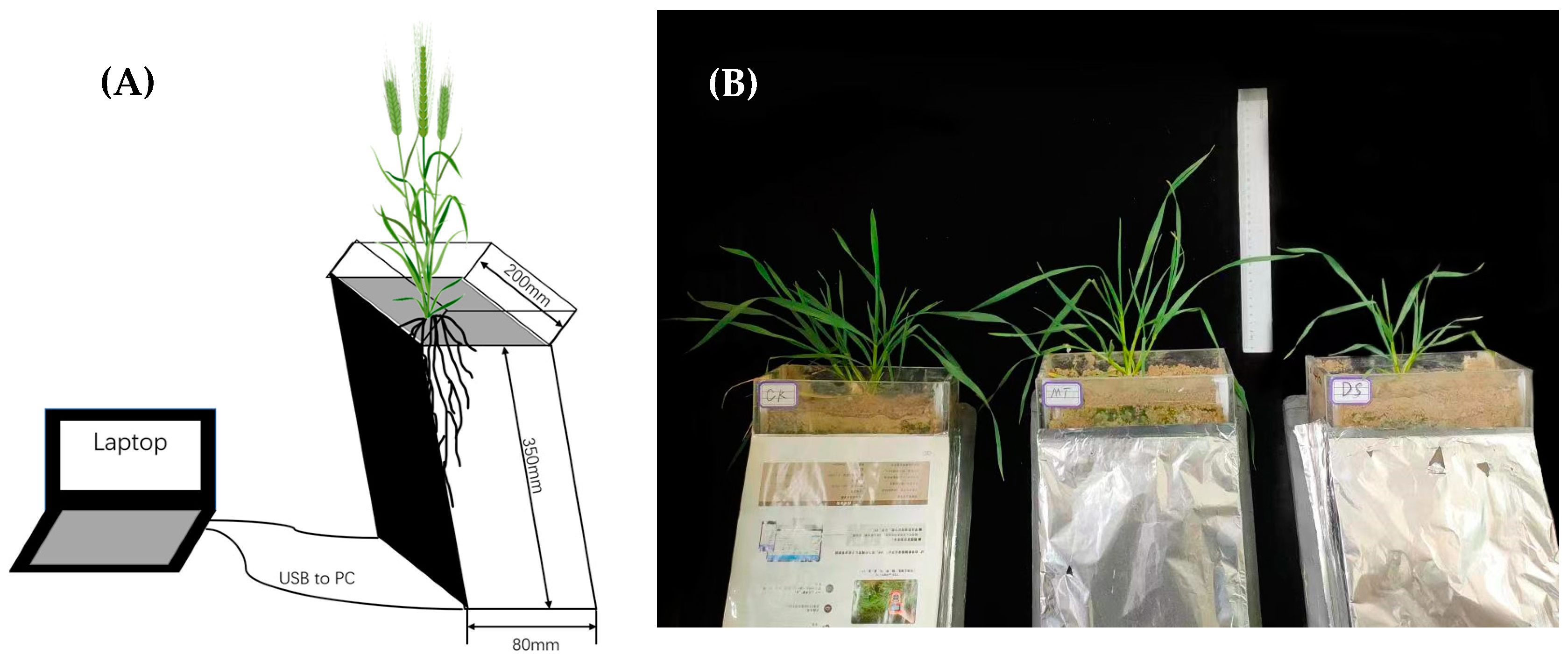
2.1. Plant Materials and Growth Conditions
2.2. Measurements of Morphological Traits of Aboveground Plant and Photosynthetic Indicators
2.3. Stomata Detection and Pore Measurement
2.4. Assessment of Antioxidant Enzymes Activities, Hydrogen Peroxide Content, Soluble Protein Content and Detection of H2O2
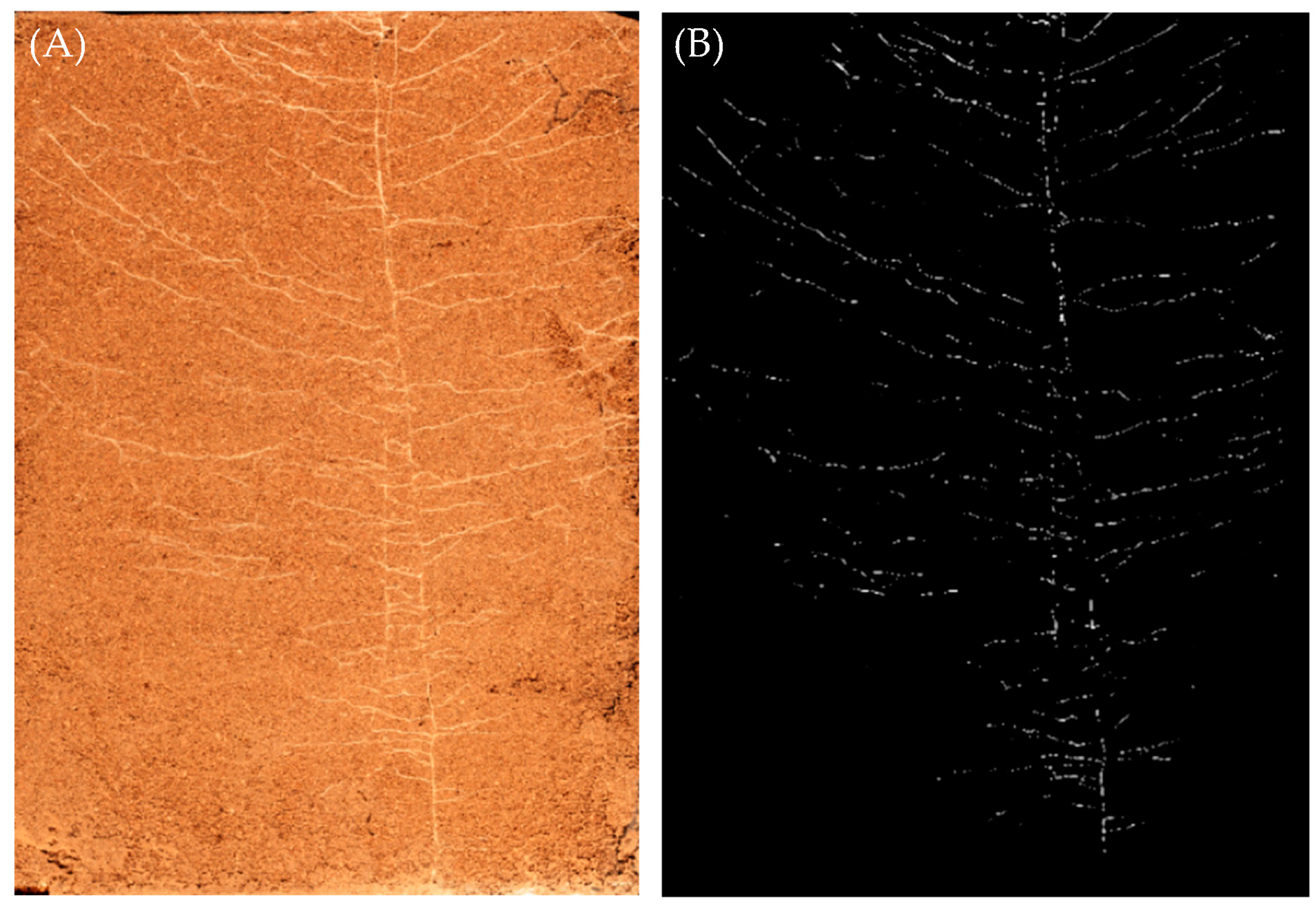
2.5. Collection and Calculation of Root Growth Data
2.6. Statistical Analysis
3. Results
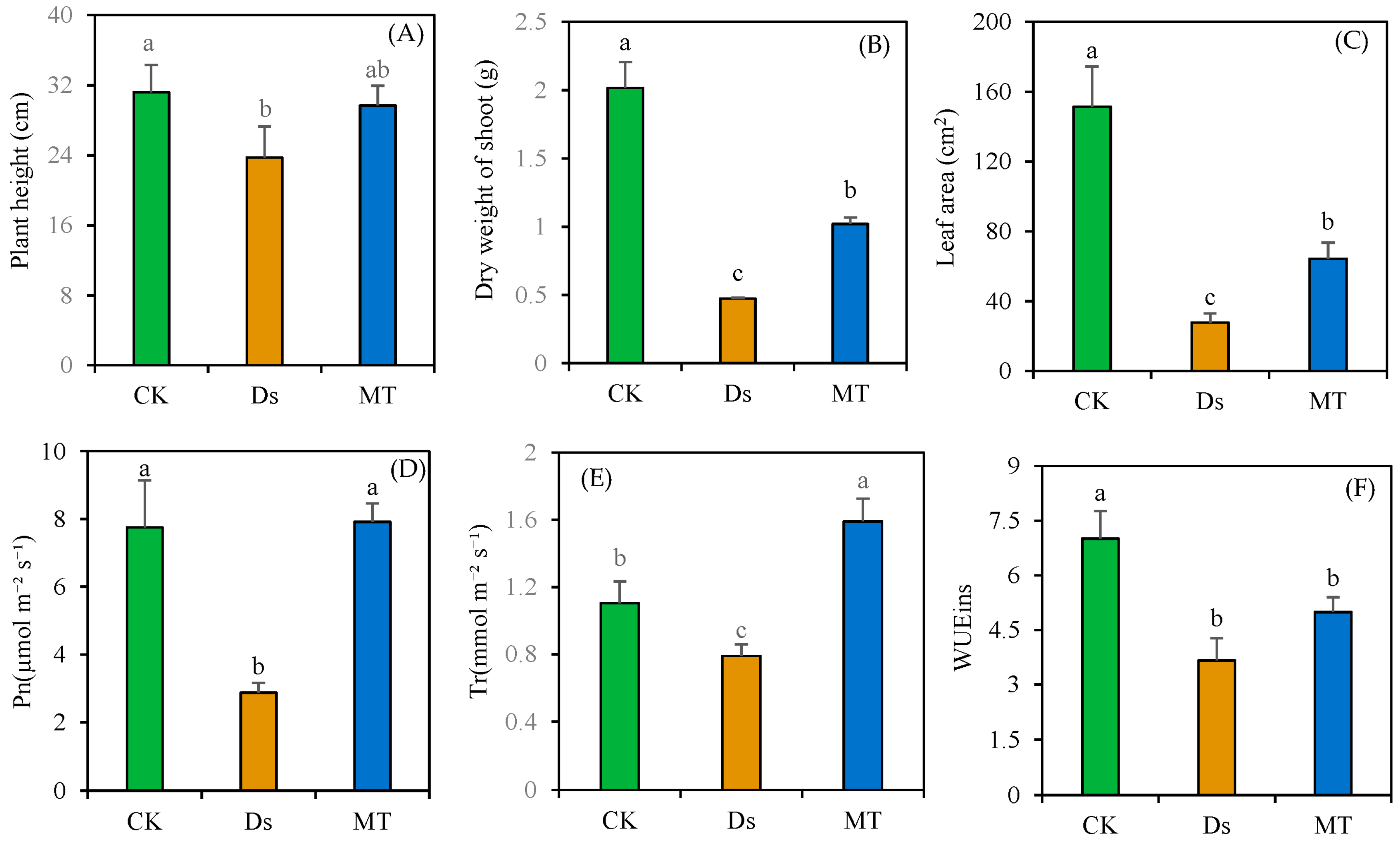
3.1. Effect of MT on Wheat Shoot Morphology under Drought Stress
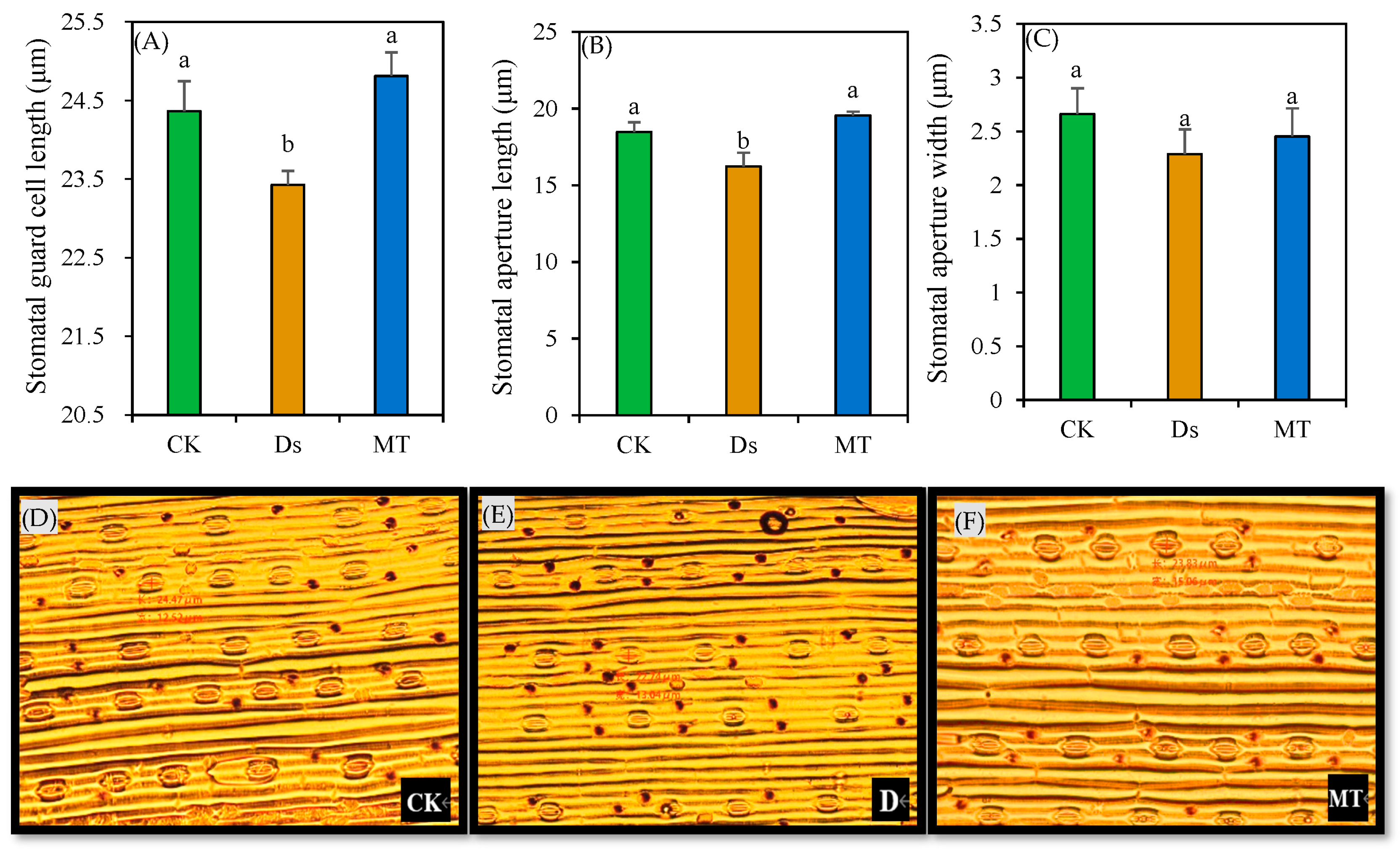
3.2. Length and Width of Stomatal Aperture
3.3. Antioxidant Enzymes Activities, Hydrogen Peroxide Content, Soluble Protein Content and Detection of H2O2
3.4. Root Morphology Indicators of Wheat Plants under Different Treatments
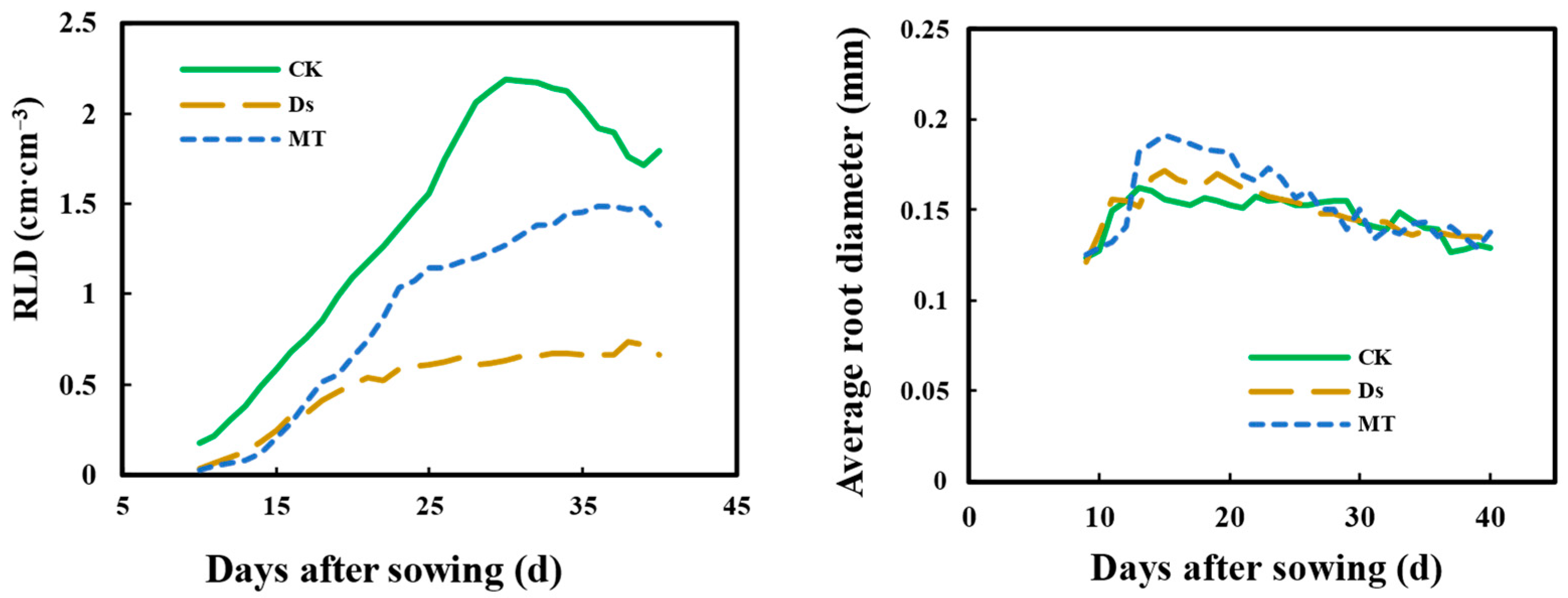
3.5. Effect of MT on Root Length Density (RLD) and Average Root Diameter of Wheat Fine Roots under Drought Condition
3.6. Effect of MT on Specific Root Length, Specific Root Surface Area, and Specific Root Volume of Wheat
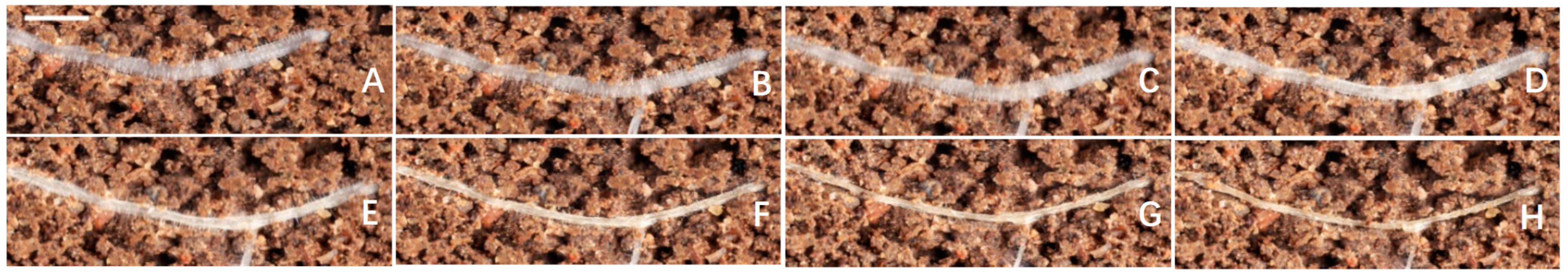
3.7. Effect of MT on Root Hair Phenotypes under Drought Condition
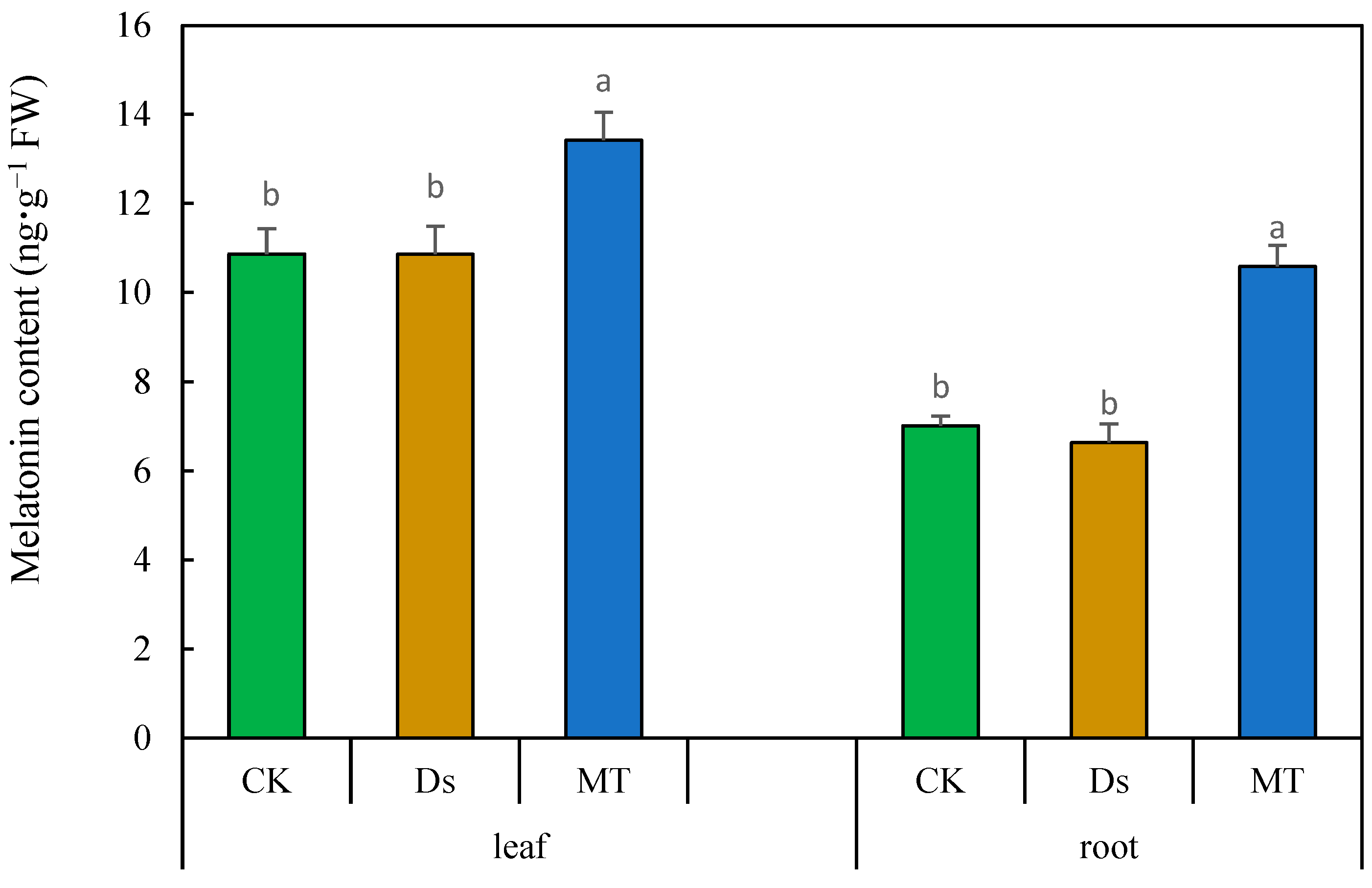
3.8. Effect of MT on Content of Melatonin in Wheat under Drought Condition
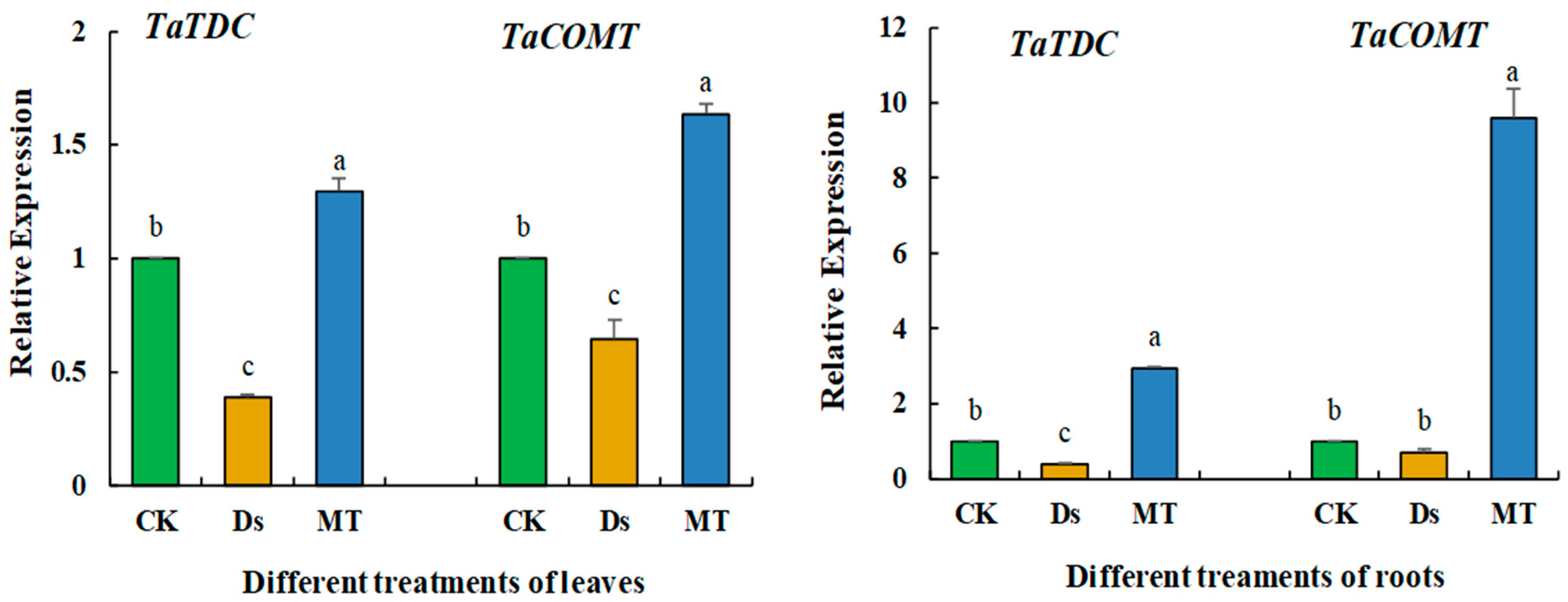
3.9. Relative Expression of Caffeic Acid-3-O-Methyltransferase Gene (TaCOMT) and Tryptophan Decarboxylase Gene (TaTDC)
4. Discussion
4.1. MT Effectively Improves Aboveground Morphology of Wheat Plants under Drought Condition
4.2. MT Mitigates Oxidative Damage on Physiology Function of Wheat Shoot and Root under Drought Condition
4.3. MT Effectively Improves Root Characteristics of Wheat under Drought Condition
4.4. Root Hair Phenotypes Are Sensitive to Drought Conditions and MT Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Bureau of Statistics of China. 2018. Available online: http://www.stats.gov.cn/tjsj/ndsj/2018/indexeh.htm (accessed on 10 April 2019).
- Zhao, J.; Han, T.; Wang, C.; Jia, H.; Worqlul, A.W.; Norelli, N.; Zeng, Z.H.; Chu, Q.Q. Optimizing irrigation strategies to synchronously improve the yield and water productivity of winter wheat under interannual precipitation variability in the North China Plain. Agr. Water Manag. 2020, 240, 106298. [Google Scholar] [CrossRef]
- Sallam, A.; Mourad, A.M.I.; Hussain, W.; Baenziger, P.S. Genetic variation in drought tolerance at seedling stage and grain yield in low rainfall environments in wheat (Triticum aestivum L.). Euphytica 2018, 214, 169. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Gu, F.; Liu, H.; Kang, G.; Feng, W.; Wang, Y.; Guo, T. Tillage and irrigation increase wheat root systems at deep soil layer and grain yields in lime concretion black soil. Sci. Rep. 2021, 11, 6394. [Google Scholar] [CrossRef] [PubMed]
- Bianco, D.M.; Kepinski, S. Building a future with root architecture. J. Exp. Bot. 2018, 69, 5319–5323. [Google Scholar] [CrossRef] [PubMed]
- Laclau, J.P.; da Silva, E.A.; Lambais, G.; Bernoux, M.; le Maire, G.; Stape, J.L.; Stape, J.L.; Jean-Pierre, B.; de Moraes Gonçalves, J.L.; Jourdan, C.; et al. Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Front. Plant Sci. 2013, 4, 243. [Google Scholar] [CrossRef] [Green Version]
- Haling, R.E.; Brown, L.K.; Bengough, A.G.; Valentine, T.A.; White, P.J.; Young, I.M.; George, T.S. Root hair length and rhizosheath mass depend on soil porosity, strength and water content in barley genotypes. Planta 2014, 239, 643–651. [Google Scholar] [CrossRef]
- Giri, J.; Bhosale, R.; Huang, G.; Pandey, B.K.; Parker, H.; Zappala, S.; Bennett, M.J. Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat. Commun. 2018, 9, 1408. [Google Scholar] [CrossRef] [Green Version]
- Karas, B.J.; Ross, L.; Novero, M.; Amyot, L.; Shrestha, A.; Inada, S.; Nakano, M.; Sakai, T.; Bonetta, D.; Sato, S.; et al. Intragenic complementation at the Lotus japonicus CELLULOSE SYNTHASE-LIKE D1 locus rescues root hair defects. Plant Physiol. 2021, 186, 2037–2050. [Google Scholar] [CrossRef]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges, and perspective. Plant J. 2022, 110, 23–42. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.; Shah, F.; Shah, F.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Bawa, G.; Feng, L.; Shi, J.; Chen, G.; Cheng, Y.; Luo, J.; Wang, X. Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway. Funct. Plant Biol. 2020, 47, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin improves drought resistance in maize seedlings by enhancing the antioxidant system and regulating abscisic acid metabolism to maintain stomatal opening under peg-induced drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Ye, J.; Deng, X.P.; Wang, S.W.; Yin, L.N.; Chen, D.Q.; Xiong, B.L.; Wang, X.Y. Effects of melatonin on growth, photosynthetic characteristics and antioxidant system in seedling of wheat under drought stress. J. Triticeae Crop 2015, 35, 1275–1283. [Google Scholar]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Li, D.; Batchelor, W.D.; Zhang, D.; Miao, H.; Li, H.; Song, S.; Li, R. Analysis of melatonin regulation of germination and antioxidant metabolism in different wheat cultivars under polyethylene glycol stress. PLoS ONE 2020, 15, e0237536. [Google Scholar] [CrossRef]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.X.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef]
- Liang, C.; Li, A.; Yu, H.; Li, W.; Liang, C.; Guo, S.; Zhang, R.; Chu, C. Melatonin regulates root architecture by modulating auxin response in rice. Front. Plant Sci. 2017, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Sarropoulou, V.N.; Therios, I.N.; Dimassi-Theriou, K.N. Melatonin promotes adventitious root regeneration in in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus × P. canescens), and MxM 60 (P. avium × P. mahaleb). J. Pineal Res. 2012, 52, 38–46. [Google Scholar] [CrossRef]
- Chen, Z.P.; Gu, Q.; Yu, X.L.; Huang, L.Q.; Xu, S.; Wang, R.; Shen, W.; Shen, W.B. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018, 121, 1127–1136. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Yang, K.; Wang, Y.; Shi, Z. Melatonin facilitates lateral root development by coordinating PAO-derived hydrogen peroxide and RBOH-derived superoxide radical. Free Radic. Biol. Med. 2019, 143, 534–544. [Google Scholar] [CrossRef]
- Huang, X.; Chen, S.; Li, W.; Tang, L.; Zhang, Y.; Yang, N.; Zou, Y.; Zhai, X.; Xiao, N.; Liu, W.; et al. ROS regulated reversible protein phase separation synchronizes plant flowering. Nat. Chem. Biol. 2021, 17, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.W.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanumly copersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.X.; Rutto, L.; Katuuramu, D. Melatonin acts synergistically with auxin to promote lateral root development through fine tuning auxin transport in Arabidopsis thaliana. PLoS ONE 2019, 14, e0221687. [Google Scholar] [CrossRef] [Green Version]
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; López-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Cary, A.J.; Liu, W.; Howell, S.H. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995, 107, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Růzicka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.P.; Wang, Y.S.; Bai, H.W.; Li, H.; Zhang, C.Z.; Chen, J.; Xu, W.M. Melatonin-ROS signal module regulates plant lateral root development. Plant Signal Behav. 2021, 16, e1901447. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Chen, Z.; Yang, B.; Komatsu, S.; Zhou, S. Proteomic analysis reveals the effects of melatonin on soybean root tips under flooding stress. J. Proteom. 2020, 232, 104064. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Li, C. Fine root and root hair morphology of cotton under drought stress revealed with Rhizopot. J. Agron. Crop Sci. 2020, 206, 679–693. [Google Scholar] [CrossRef]
- Zhu, L.X.; Liu, L.T.; Sun, H.C.; Zhang, Y.J.; Liu, X.W.; Wang, N.; Chen, J.; Zhang, K.; Bai, Z.Y.; Wang, G.Y.; et al. The responses of lateral roots and root hairs to nitrogen stress in cotton based on daily root measurements. J. Agron. Crop Sci. 2021, 208, 89–105. [Google Scholar] [CrossRef]
- Liu, Z.; Qian, J.C.; Liu, B.M.; Zhong, K.; Wu, Y.J. Effects of the magnetic resonance imaging contrast agent Gd-DTPA on plant growth and root imaging in rice. PLoS ONE 2014, 9, e100246. [Google Scholar] [CrossRef] [PubMed]
- Steda, T.; Haberle, J.; Klimeová, J.; Klimek-Kopyra, A.; Stredova, H.; Bodner, G.; Chloupek, O. Field phenotyping of plant roots by electrical capacitance—A standardized methodological protocol for application in plant breeding: A Review. Int. Agrophys. 2020, 34, 173–184. [Google Scholar] [CrossRef]
- Ljudmilla, B.; Hardy, R.; Thomas, N. Surveying the plant’s world by magnetic resonance imaging. Plant J. 2012, 70, 129–146. [Google Scholar]
- Liu, X.W.; Dong, X.J.; Xue, Q.W.; Leskovar, D.I.; Jifon, J.; Butnor, J.R.; Marek, T. Ground penetrating radar (GPR) detects fine roots of agricultural crops in the field. Plant Soil 2018, 423, 517–531. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Li, D.; Wang, N.; Sun, H.; Zhang, Y.; Zhang, K.; Li, A.; Bai, Z.; Li, C.; et al. In situ root phenotypes of cotton seedlings under phosphorus stress revealed through RhizoPot. Front. Plant Sci. 2021, 12, 716691. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Wang, H.; Li, D.; Li, R.; Li, Y. Further study on the method of leaf area calculation in winter wheat. J. Triticeae Crop 2018, 38, 455–459. [Google Scholar]
- Li, D.; Zhang, D.; Wang, H.; Li, Y.; Li, R. Physiological response of plants to polyethylene glycol (PEG-6000) by exogenous melatonin application in wheat. Zemdirbyste 2017, 104, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Liu, L.; Li, H.; Zhang, S.; Fu, X.; Zhai, X.; Yang, N.; Shen, J.; Li, R.; Li, D. Exogenous melatonin promotes the salt tolerance by removing active oxygen and maintaining ion balance in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 12, 787062. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Liu, L.; Zhu, L.; Kang, J.; Wang, N.; Shao, L. High-throughput in situ root image segmentation based on the improved deeplabv3+ method. Front. Plant Sci. 2020, 11, 576791. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Muhammad, Z.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative effect of melatonin improves drought tolerance by regulating growth, photosynthetic traits and leaf ultrastructure of maize seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G. Genetic improvement of water-use efficiency and yield of dryland wheat. In Proceedings of the 9th International Wheat Genetics Symposium, Saskatoon, SK, Canada, 2–7 August 1998; Slinkard, A.E., Ed.; Saskatchewan University: Saskatoon, SK, Canada, 1998; Volume 1, pp. 57–60. [Google Scholar]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [Green Version]
- Gowda, V.R.; Henry, A.; Yamauchi, A.; Shashidhar, H.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crop Res. 2011, 122, 1–13. [Google Scholar] [CrossRef]
- Lilley, J.M.; Kirkegaard, J.A. Seasonal variation in the value of subsoil water to wheat: Simulation studies in southern New South Wales. Aust. J. Agr. Res. 2008, 58, 1115–1128. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Gao, J.F.; Han, F.; Zhang, J.Y.; Xia, L.; Ji, H.; Li, H.B.; Liu, B.T. The covariation and plasticity of root traits drive different rice genotypes to adapt to the nitrogen environment. J. Plant Nutr. Fertil. 2022, 28, 611–621. [Google Scholar]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in micro-tom tomato: Improved drought tolerance via the regulation of the photosynthetic apparatus, membrane stability, osmoprotectants, and root system. Life 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; You, J.; Li, J.Z.; Wang, Y.P.; Chan, Z.L. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Lal, M.K.; Kumar, R.; Chourasia, K.N.; Naga, K.C.; Kumar, D.; Das, S.K.; Zinta, G. Mechanistic insights on melatonin mediated drought stress mitigation in plants. Physiol. Plantarum 2020, 172, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yan, X.; Liao, H. 3D reconstruction and dynamic modeling of root architecture in situ and its application to crop phosphorus research. Plant J. 2009, 60, 1096–1108. [Google Scholar] [CrossRef]


| Organs | Treatments | SOD Activity (U/g FW) | POD Activity (U/g FW) | CAT Activity (U/mg prot.) | Soluble Protein Content (μg/mL) | H2O2 Content (mmol/g prot.) | MDA Content (nmol/g) |
|---|---|---|---|---|---|---|---|
| Leaf | CK | 501.71 ± 33.11 b | 593.17 ± 20.57 a | 191.87 ± 21.86 b | 2514.51 ± 286.35 b | 2663.92 ± 19.12 b | 355.00 ± 21.79 c |
| Ds | 556.15 ± 50.94 ab | 621.53 ± 42.13 a | 241.19 ± 2.86 a | 3444.91 ± 369.34 a | 3694.54 ± 18.83 a | 445.00 ± 30.41 a | |
| MT | 612.40 ± 12.86 a | 582.75 ± 17.39 a | 137.13 ± 13.83 c | 2201.49 ± 201.49 b | 2355.71 ± 20.00 c | 412.67 ± 20.60 b | |
| Root | CK | 797.48 ± 29.10 a | 550.35 ± 12.03 a | 39.02 ± 6.14 b | 210.12 ± 13.73 b | 597.74 ± 8.23 b | 26.67 ± 2.89 ab |
| Ds | 636.90 ± 7.20 c | 544.56 ± 13.03 a | 73.98 ± 17.21 a | 248.17 ± 9.82 a | 661.25 ± 6.61 a | 38.3 ± 12.58 a | |
| MT | 689.21 ± 36.75 b | 576.39 ± 51.15 a | 70.73 ± 15.45 a | 233.47 ± 22.47 ab | 652.99 ± 1.66 a | 16.67 ± 2.89 b |
| Treatment | Root Length (RL, cm) | Area of Contour (AC, cm2) | Root Surface Area (RCA, cm2) | Average Root Diameter (ARD, mm) | Root Volume (RV, cm3) |
|---|---|---|---|---|---|
| CK | 16,672.25 ± 520.02 a | 530.48 ± 81.91 a | 1666.55 ± 257.31 a | 0.33 ± 0.05 a | 14.03 ± 4.48 a |
| Ds | 7807.56 ± 1420.07 c | 230.68 ± 11.42 b | 724.71 ± 35.89 b | 0.30 ± 0.04 a | 5.49 ± 0.61 b |
| MT | 14,086.05 ± 1630.21 b | 452.74 ± 63.48 a | 1422.33 ± 199.42 a | 0.32 ± 0.05 a | 13.51 ± 0.93 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Guo, L.; Sun, H.; Wu, J.; Liu, L.; Wang, J.; Wang, B.; Wang, Q.; Sun, Z.; Li, D. Melatonin Increases Drought Resistance through Regulating the Fine Root and Root Hair Morphology of Wheat Revealed with RhizoPot. Agronomy 2023, 13, 1881. https://doi.org/10.3390/agronomy13071881
Zhang Z, Guo L, Sun H, Wu J, Liu L, Wang J, Wang B, Wang Q, Sun Z, Li D. Melatonin Increases Drought Resistance through Regulating the Fine Root and Root Hair Morphology of Wheat Revealed with RhizoPot. Agronomy. 2023; 13(7):1881. https://doi.org/10.3390/agronomy13071881
Chicago/Turabian StyleZhang, Zhihui, Li Guo, Hongchun Sun, Jinhua Wu, Liantao Liu, Jianwei Wang, Biao Wang, Qianyi Wang, Zhimei Sun, and Dongxiao Li. 2023. "Melatonin Increases Drought Resistance through Regulating the Fine Root and Root Hair Morphology of Wheat Revealed with RhizoPot" Agronomy 13, no. 7: 1881. https://doi.org/10.3390/agronomy13071881




