Exploring the Role of Novel Biostimulators in Suppressing Oxidative Stress and Reinforcing the Antioxidant Defense Systems in Cucurbita pepo Plants Exposed to Cadmium and Lead Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site, Plant Material, and Water Analysis
2.2. Experimental Layout
2.3. Preparation of Garlic Extract (GE), Onion Extract (OE) and Bee-Honey Solution (BHs)
2.4. Sampling
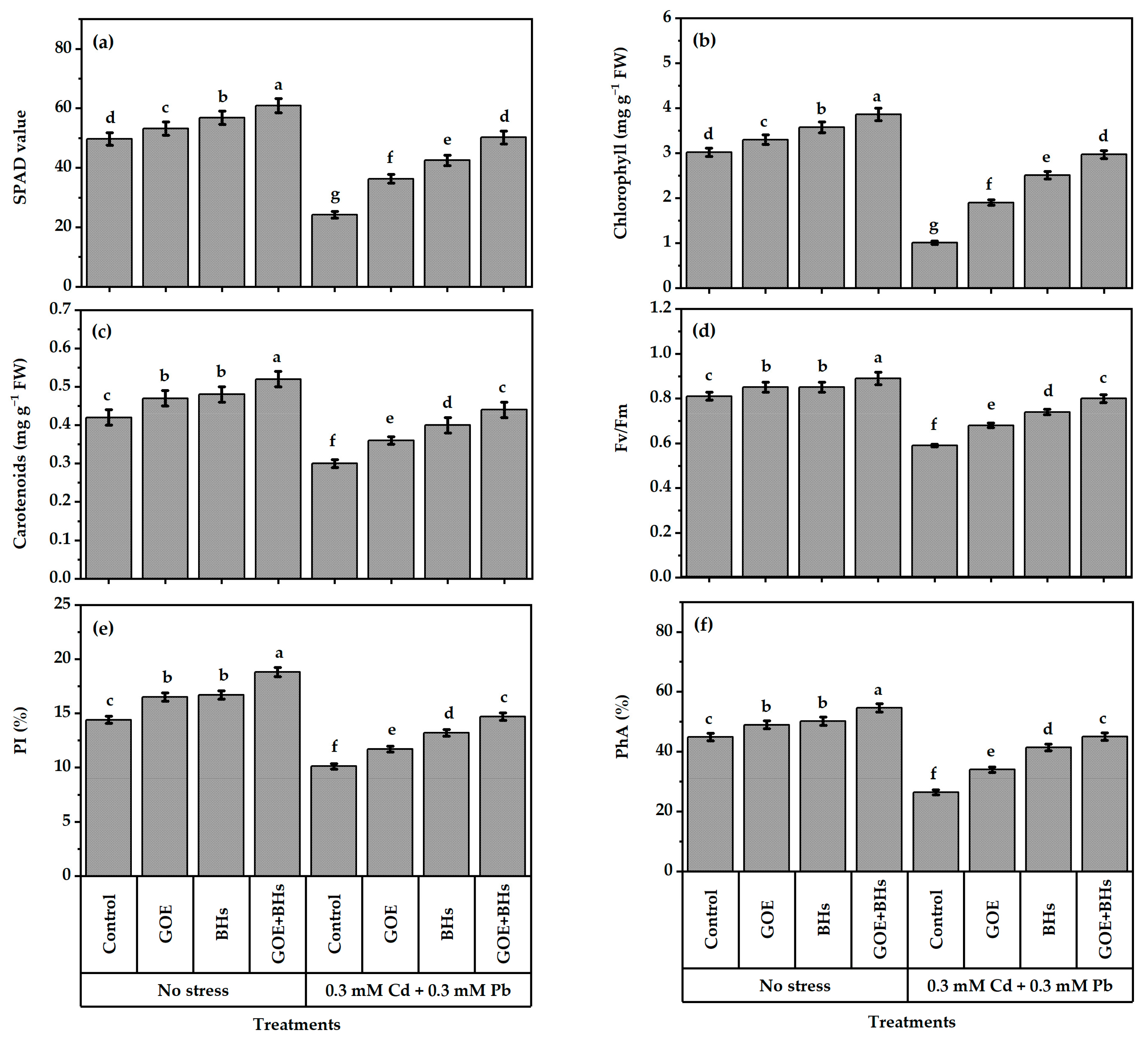
2.5. Evaluation of the Efficiency of Photosynthesis
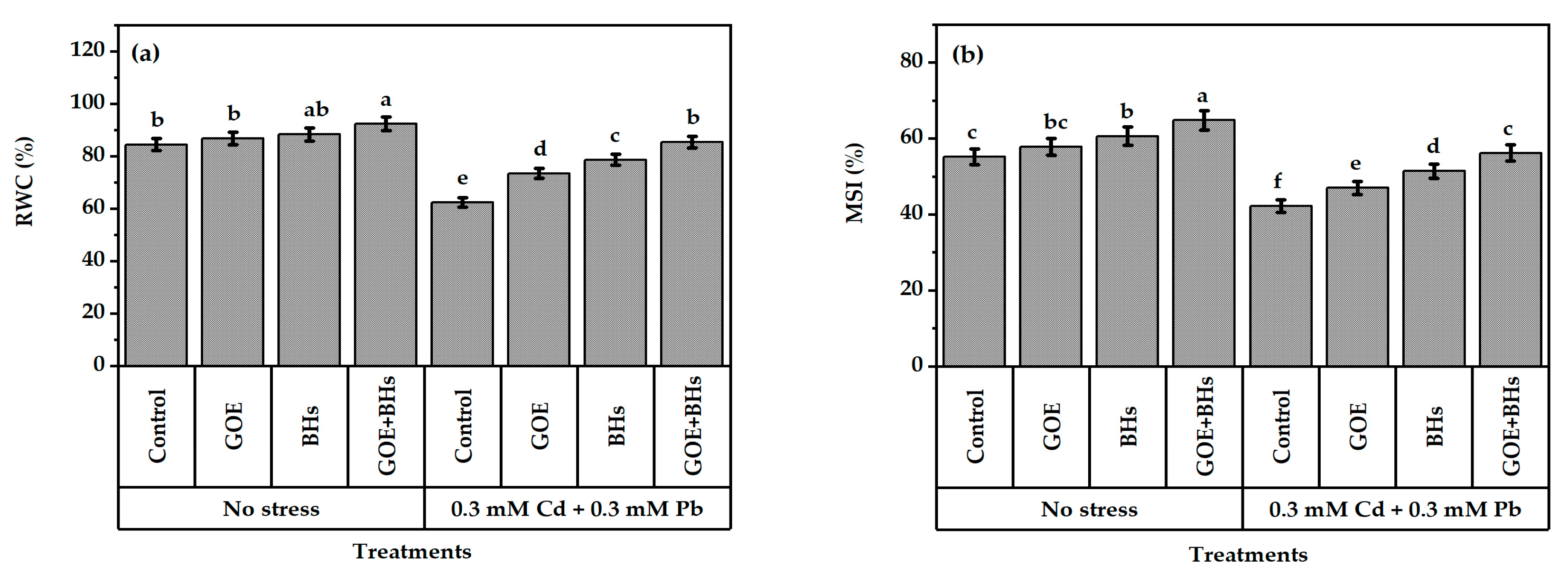
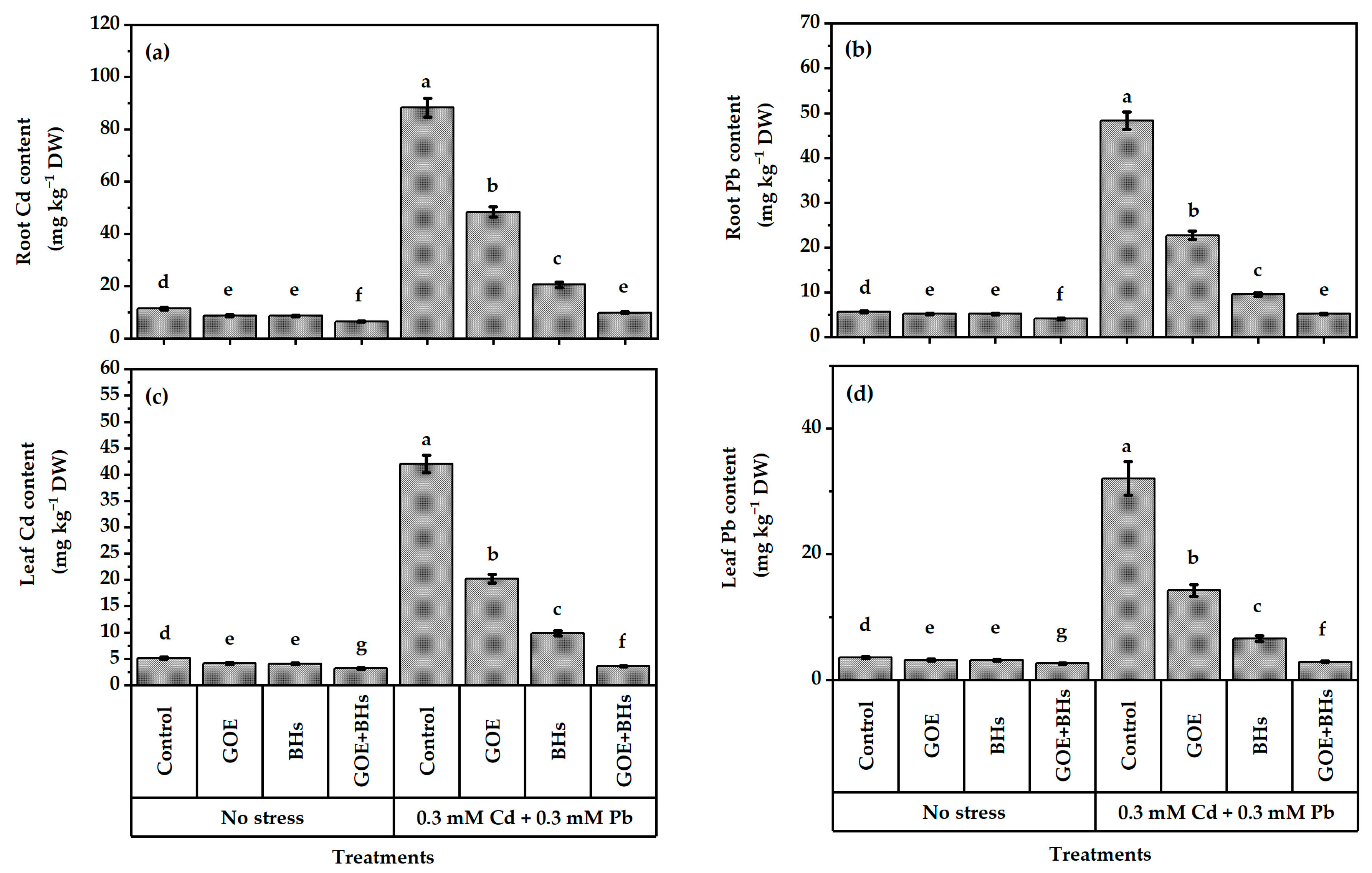
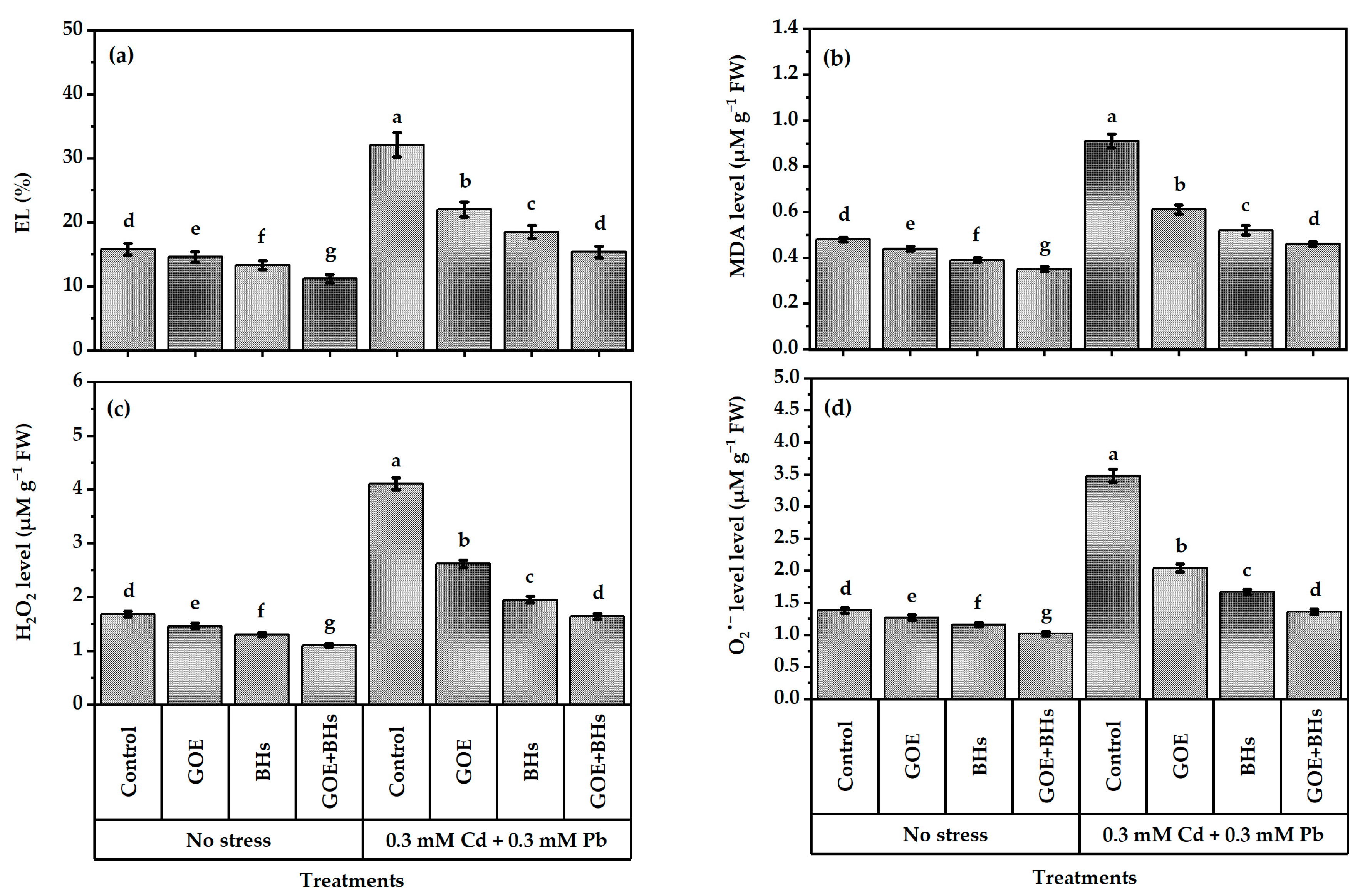
2.6. Assessment of Leaf Integrity, Levels of Cadmium (Cd), Lead (Pb), and Oxidative Stress Markers
2.7. Assessment of Osmo-Protectors and Antioxidant Contents
2.8. Assaying Enzyme Activities and Enzyme Gene Expressions
2.9. Assessment of Hormonal Content
2.10. Assessment of Growth, Yield, and Fruit Quality Characteristics
2.11. Data Analysis
3. Results
3.1. Photosynthetic Activity of Plants
3.2. Leaf Tissue Stability
3.3. Content of Cadmium (Cd) and Lead (Pb) in Leaves and Roots
3.4. Membrane Damage Linked to Oxidative Stress and Indicators of Oxidative Stress
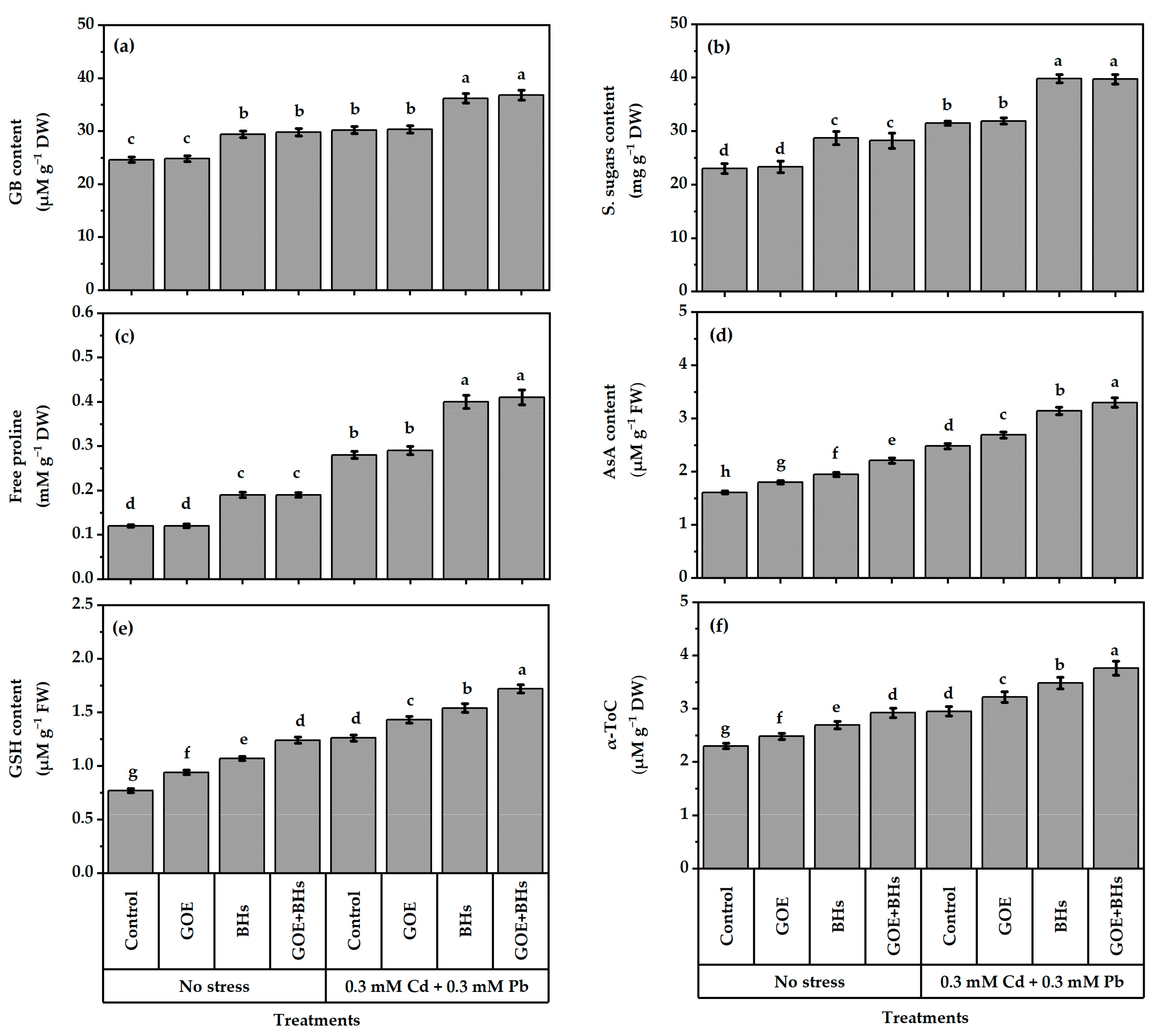
3.5. Osmo-Protectors (OPs) and Non-Enzymatic Antioxidants
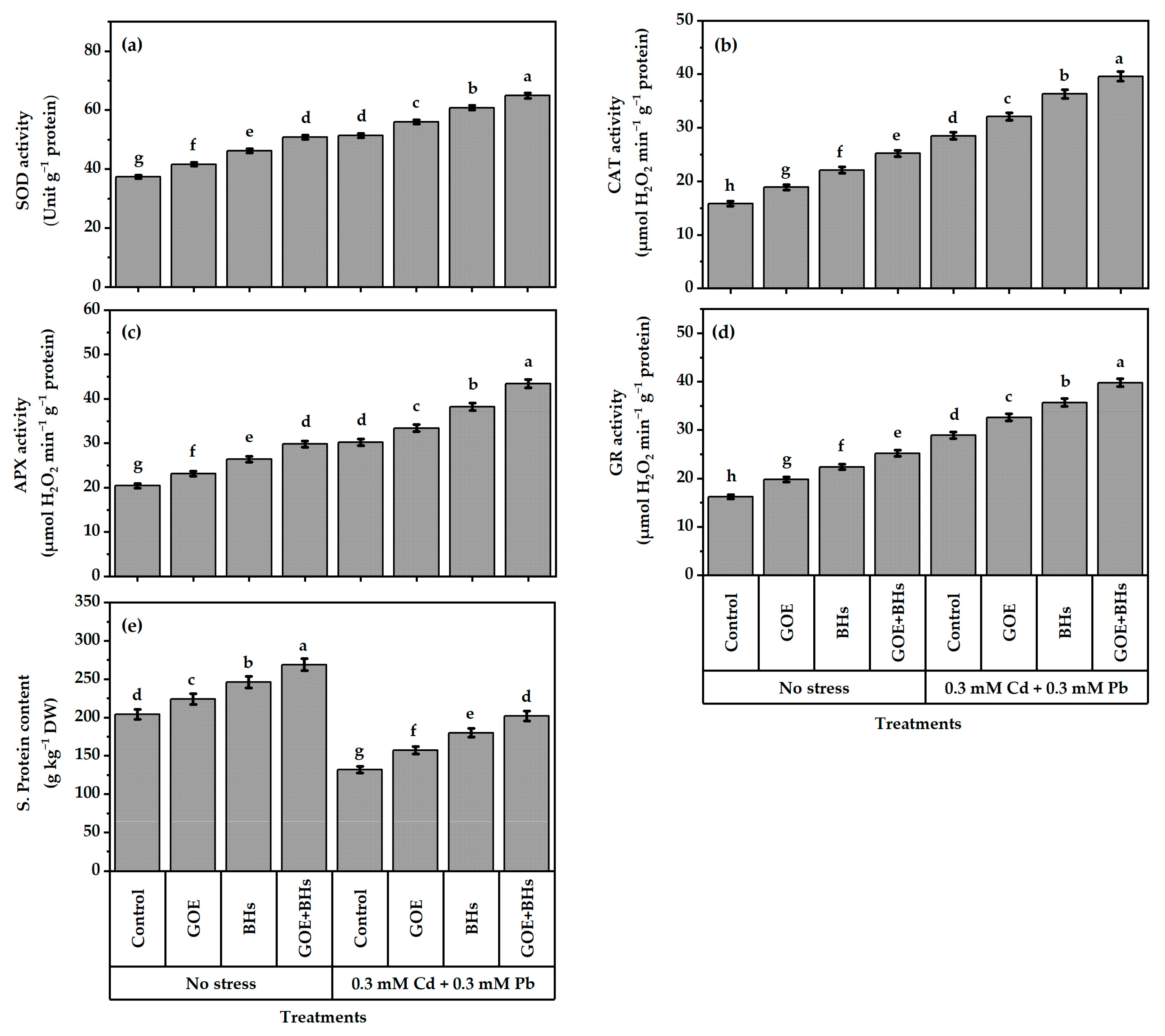
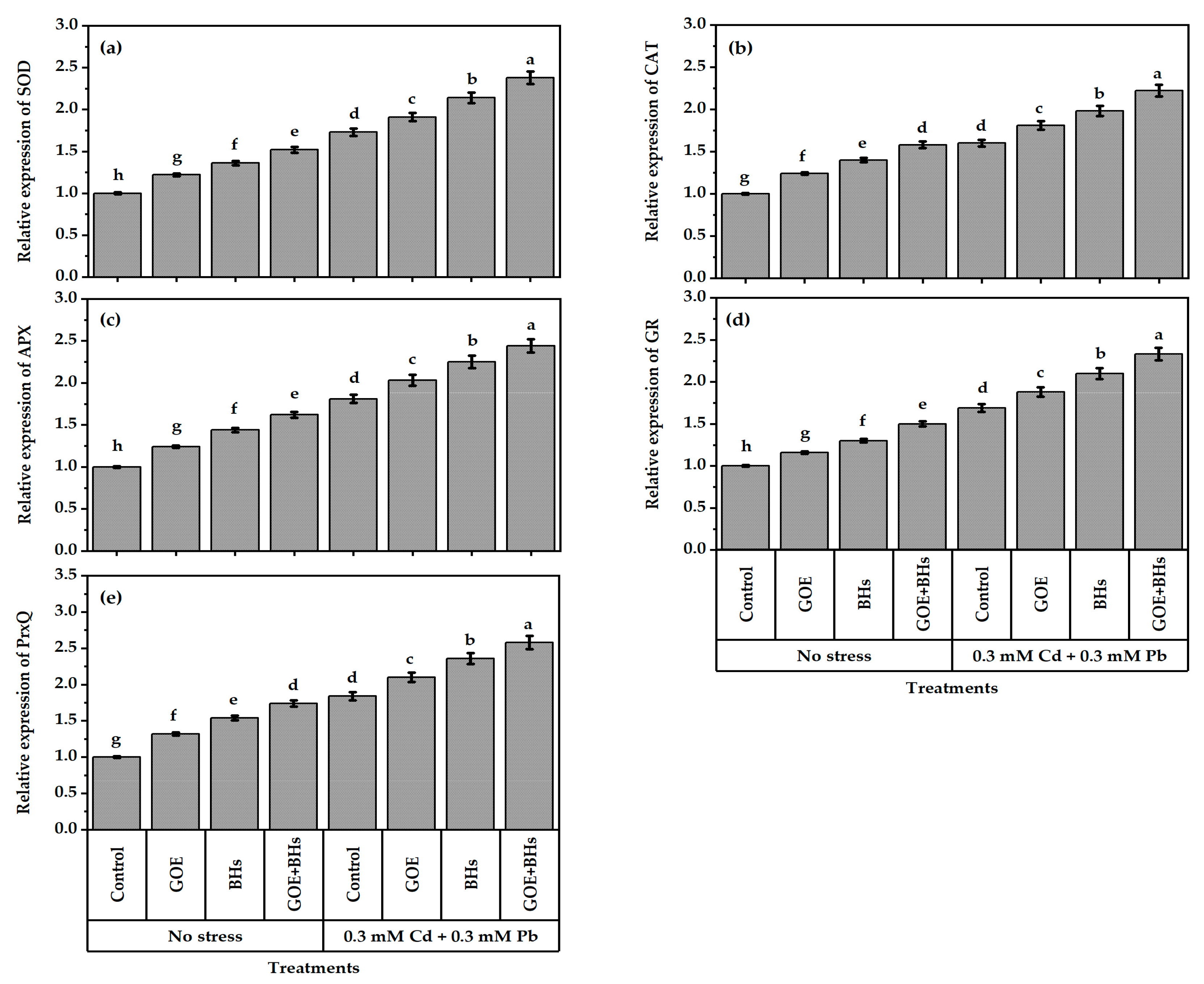
3.6. Antioxidant Defense System Components
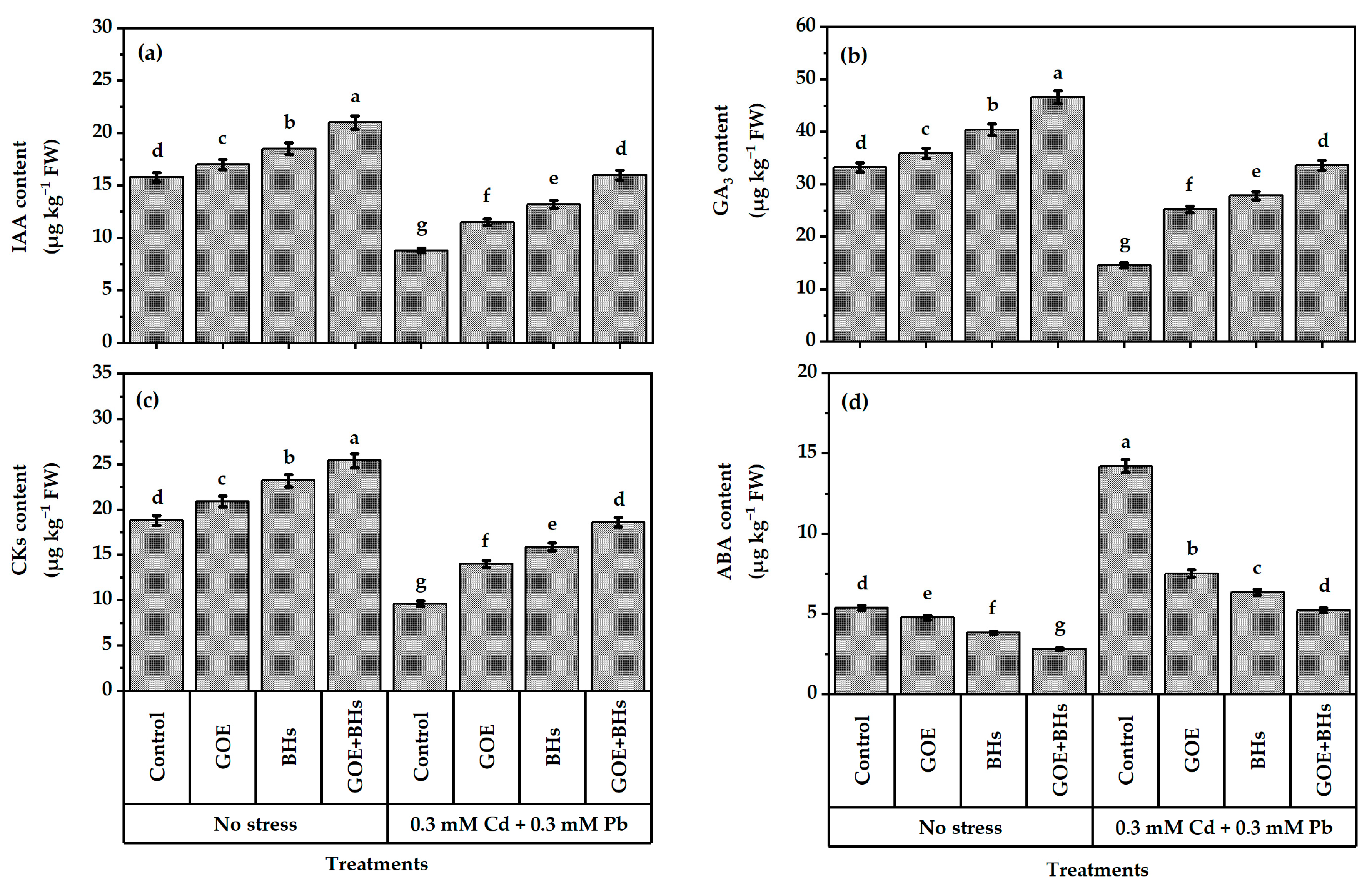
3.7. Content of Phytohormones in Plant Leaves
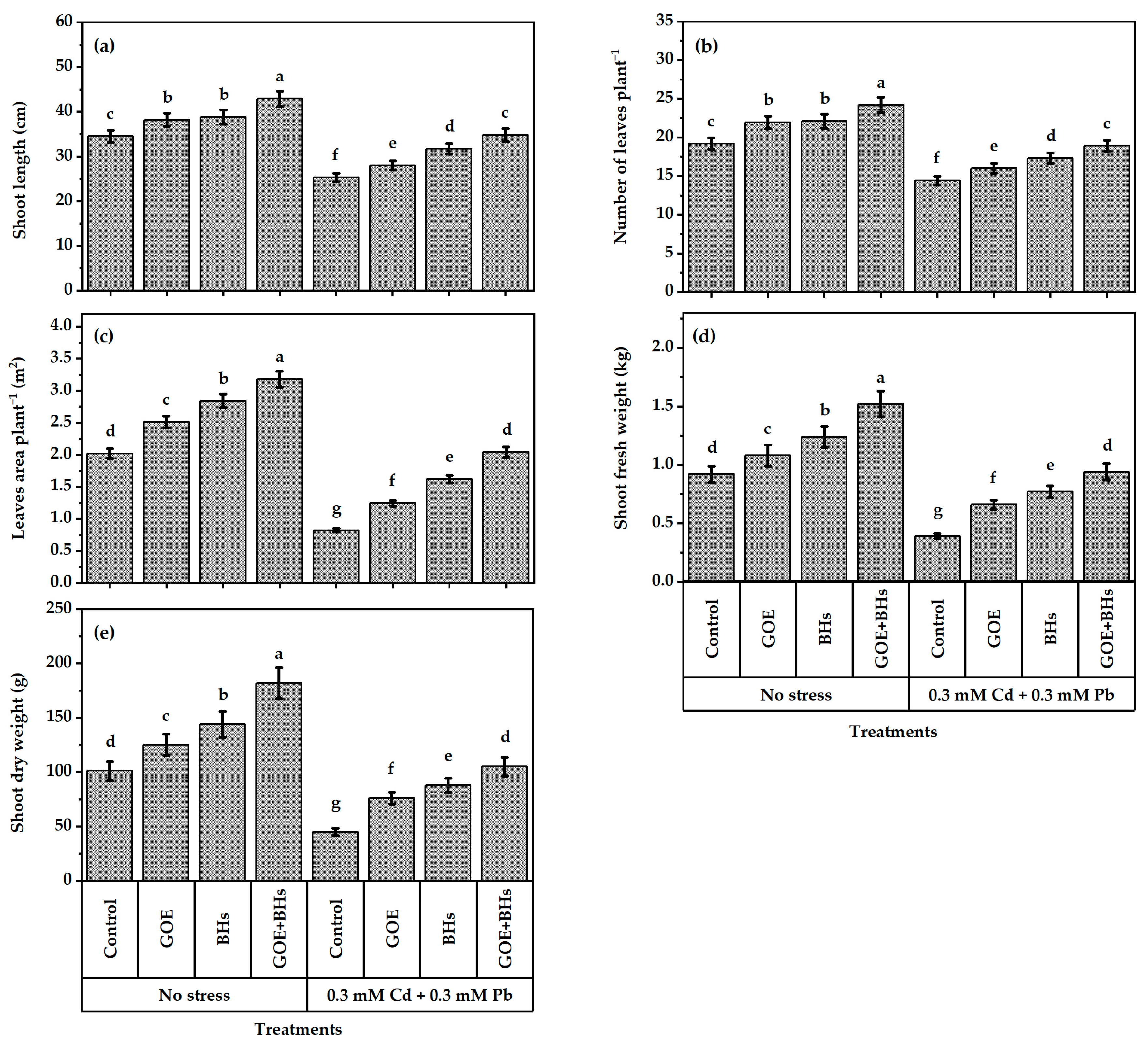
3.8. Growth and Yield Traits
3.9. Fruit Quality Traits
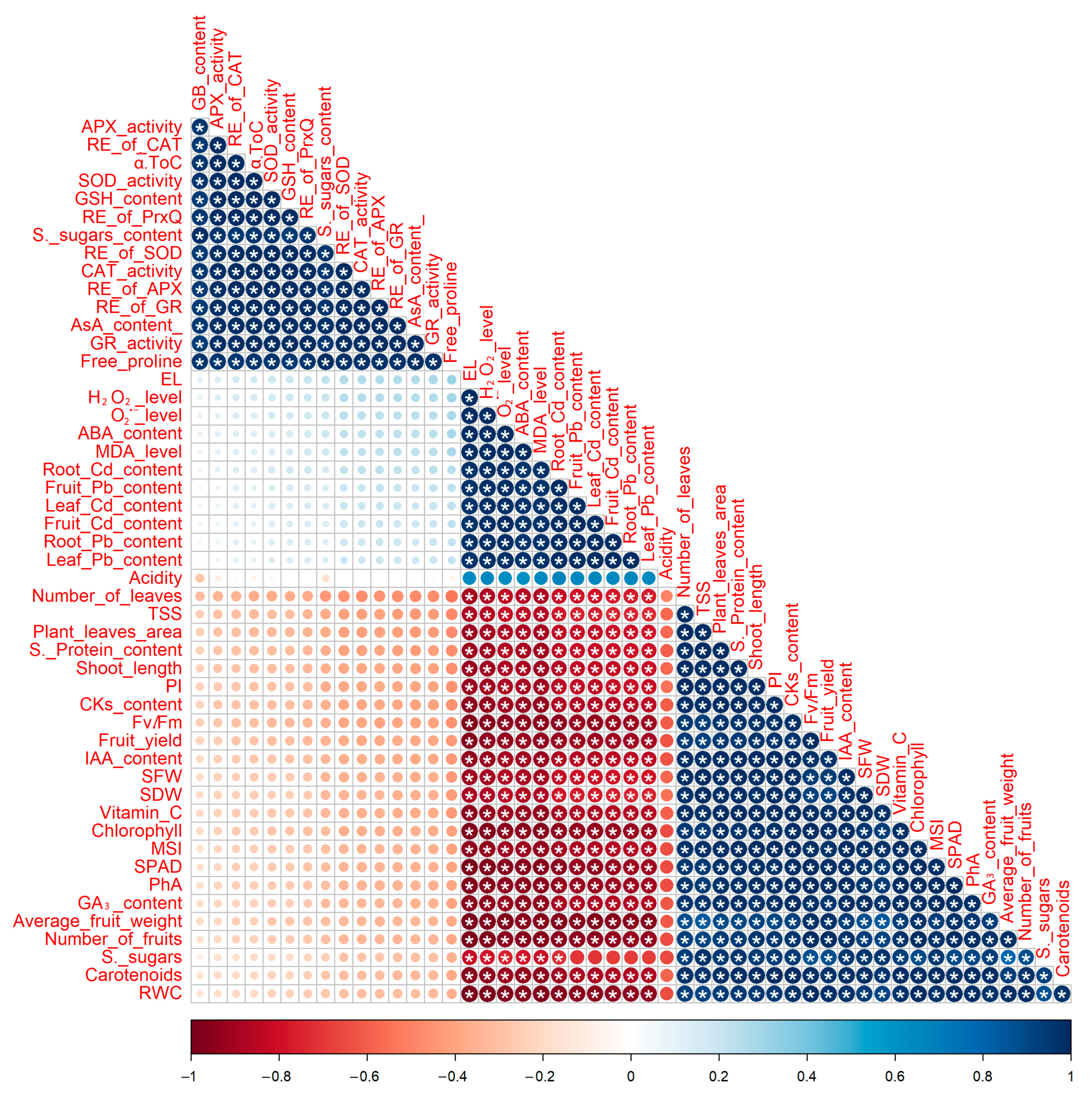
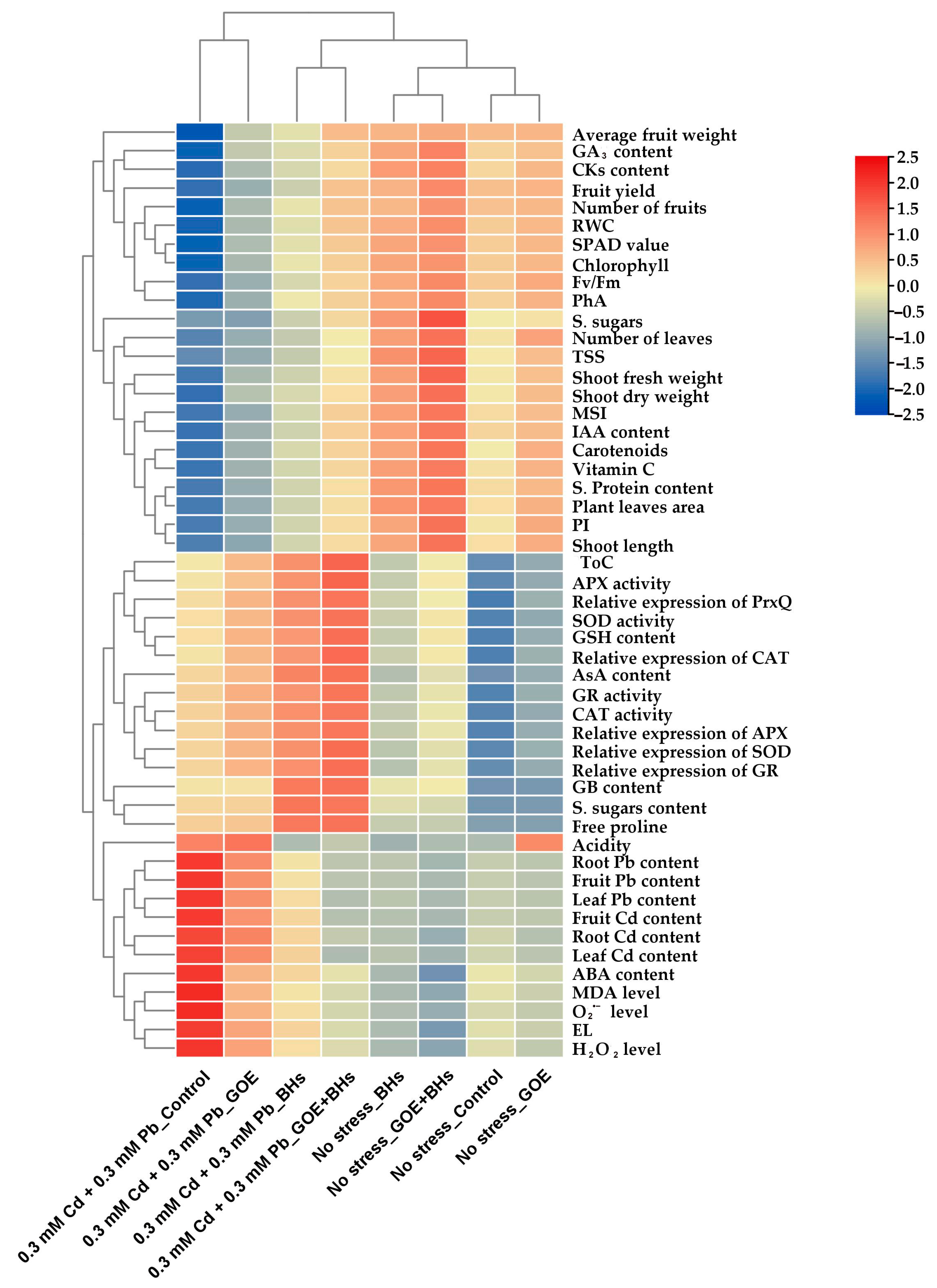
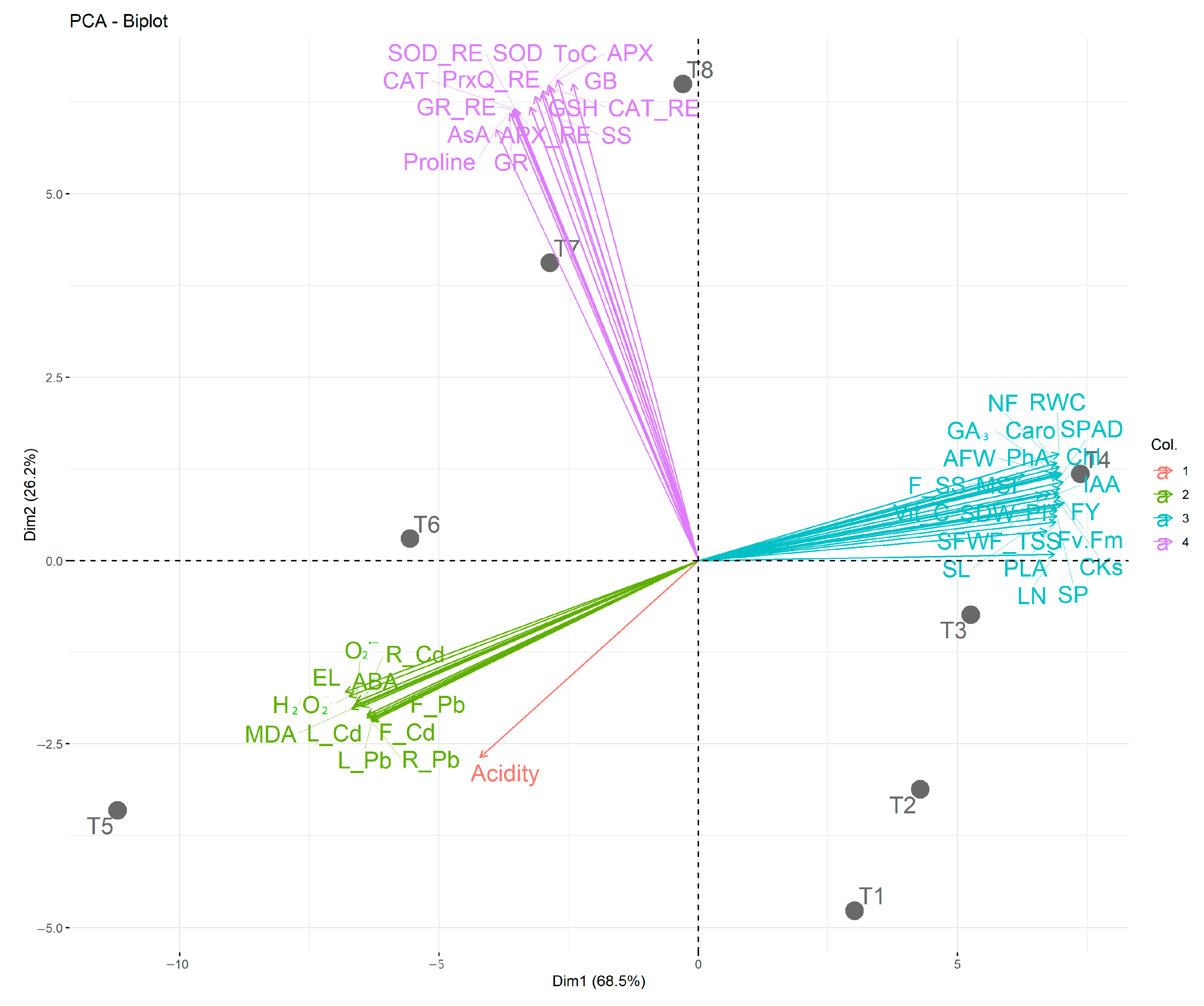
3.10. Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galal, T.M. Health hazards and heavy metals accumulation by summer squash (Cucurbita pepo L.) cultivated in contaminated soils. Environ. Monit. Assess. 2016, 188, 434. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.T.; Eyidogan, F.; Yucel, M.; Öktem, H.A. Functional roleof nitric oxide under abiotic stress conditions. In Nitric Oxide Actionin Abiotic Stress Responses in Plants; Khan, M., Mobin, M., Mohammad, F., Corpas, F., Eds.; Springer: Cham, Switzerland, 2015; p. 2141. [Google Scholar] [CrossRef]
- Sahay, S.; Gupta, M. An update on nitric oxide and its benignrole in plant responses under metal stress. Nitric Oxide 2017, 67, 3952. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bak, J.; Gzyl, J.; Rucinska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The new insights into cadmium sensing. Front. Plant Sci. 2014, 5, 245. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav. 2013, 8, e23681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarhan, M.G.R.; Abd Elhafeez, A.M.; Bashandy, S.O. Evaluation of Heavy Metals Concentration as Affected by Vehicular Emission in Alluvial Soil at Middle Egypt Conditions. Egypt. J. Soil Sci. 2021, 60, 337–354. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cadmium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Morphophysiological and phytochemical responses to cadmium and lead stress in coriander (Coriandrum sativum L.). Ind. Crops Prod. 2021, 171, 113979. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.; Zia-ur-Rehman, M.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef]
- Alharby, F.H.; Alzahrani, H.S.; Hakeem, K.; Alsamadany, H.; Desoky, E.-S.M.; Rady, M.M. Silymarin-Enriched Biostimulant Foliar Application Minimizes the Toxicity of Cadmium in Maize by Suppressing Oxidative Stress and Elevating Antioxidant Gene Expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef]
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.G.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Ali, E.F.; Aljarani, A.M.; Mohammed, F.A.; Desoky, E.-S.M.; Mohamed, I.A.A.; Rady, M.M.; El-Sharnouby, M.; Tammam, S.A.; Hassan, F.A.S.; Shaaban, A. Exploring the potential enhancing effects of trans-zeatin and silymarin on the productivity and antioxidant defense capacity of cadmium-stressed wheat. Biology 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator—Review. Antioxidants. 2020, 9, 681. [Google Scholar] [CrossRef]
- Desoky, E.M.; Elrys, A.S.; Rady, M.M. Integrative Moringa and Licorice extracts application improves performance and reduces fruit contamination content of pepper plants grown on heavy metals-contaminated saline soil. Ecotoxicol. Environ. Saf. 2019, 169, 50–60. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Metwally, M.A.S.; Rady, M.M.; Qiulan, H.; Ihtisham, M.; Zandi, P.; Abbas, M.; Yan, K.; Zhao, X.; Li, J.; et al. Interplay of Silymarin and Clove Fruit Extract Effectively Enhances Cadmium Stress Tolerance in Wheat (Triticum aestivum). Front. Plant Sci. 2023, 14, 1144319. [Google Scholar] [CrossRef]
- Rady, M.M.; Alshallash, K.S.; Desoky, E.-S.M.; Taie, H.A.A.; Mohamed, I.A.A.; El-Badri, A.M.; Howladar, S.M.; AbdelKhalik, A. Synergistic effect of trans-zeatin and silymarin on mitigation of cadmium stress in chili pepper through modulating the activity of antioxidant enzymes and gene expressions. J. Appl. Res. Med. Aromat. Plants 2023. in Press. [Google Scholar] [CrossRef]
- Kusvuran, A.; Bilgici, M.; Kusvuran, S.; Bilgici, M.; Kuvuran, V.; Nazli, R. The effect of different organic matters on plant growth regulation and nutritional components under salt stress in sweet sorghum [Sorghum bicolor (L.) Moench.]. Maydica 2021, 66, 1–9. [Google Scholar]
- Tarfayah, D.A.M.M.; Ahmed, S.M.A.; Rady, M.M.; Mohamed, I.A.A. Alleviating saline-calcareous stress in Atriplex nummularia seedlings by foliar spraying with silymarin-enriched bee-honey solution. Labyrinth: Fayoum J. Sci. Interdiscip. Stud. 2023, 1, 11–20. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Abou-Sreea, A.I.B.; Azzam, C.R.; Al-Taweel, S.K.; Abdel-Aziz, R.M.; Belal, H.E.E.; Rady, M.M.; Abdel-Kader, A.A.S.; Majrashi, A.; Khaled, K.A.M. Natural Biostimulant Attenuates Salinity Stress Effects in Chili Pepper by Remodeling Antioxidant, Ion, and Phytohormone Balances, and Augments Gene Expression. Plants. 2021, 10, 2316. [Google Scholar] [CrossRef]
- Desoky, E.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Ali, E.F.; Mersal, G.A.M.; Rady, M.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Rehman, H.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced application of glutathione as an antioxidant with organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The Potential Role of Microbial Biostimulants in the Amelioration of Climate Change-Associated Abiotic Stresses on Crops. Front. Microbiol. 2022, 12, 829099. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant extracts-importance in sustainable agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- Rady, M.M.; Seif El-Yazal, M.A. Response of Anna apple dormant buds and carbohydrate metabolism during floral bud break to onion extract. Sci. Hortic. 2013, 155, 78–84. [Google Scholar] [CrossRef]
- Rady, M.M.; Seif El-Yazal, M.A. Garlic extract as a novel strategy to hasten dormancy release in buds of ‘Anna’ apple trees. South Afr. J. Bot. 2014, 92, 105–111. [Google Scholar] [CrossRef]
- Suru, S.M. Onion and garlic extracts lessen cadmium-induced nephrotoxicity in rats. Biometals 2008, 21, 623–633. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.; Abdelkhalik, A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants. 2021, 10, 748. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.; Rady, M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- Alghamdi, S.A.; Alharby, H.F.; Bamagoos, A.A.; Zaki, S.-n.S.; Abdelhamed, A.M.; Desoky, E.-S.M.; Mohamed, I.A.A.; Rady, M.M. Rebalancing Nutrients, Reinforcing Antioxidant and Osmoregulatory Capacity, and Improving Yield Quality in Drought-Stressed Phaseolus vulgaris by Foliar Application of a Bee-Honey Solution. Plants 2023, 12, 63. [Google Scholar] [CrossRef]
- Alsamadany, H.; Alharby, H.F.; Al-Zahrani, H.S.; Kuşvuran, A.; Kuşvuran, S.; Rady, M.M. Selenium fortification stimulates antioxidant-and enzyme gene expression-related defense mechanisms in response to saline stress in Cucurbita pepo. Sci. Hortic. 2023, 312, 111886. [Google Scholar] [CrossRef]
- Ponce, V.M.; Pandey, R.P.; Ercan, S. Characterization of drought across the climate spectrum. J. Hydrol. Eng. ASCE 2000, 5, 222–224. [Google Scholar] [CrossRef]
- Rady, M.M.; Rehman, H. Supplementing organic biostimulants into growing media enhances growth and nutrient uptake of tomato transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Abdulmajeed, A.M.; Alharbi, B.M.; Alharby, H.F.; Abualresh, A.M.; Badawy, G.A.; Semida, W.M.; Rady, M.M. Simultaneous action of silymarin and dopamine enhances defense mechanisms related to antioxidants, polyamine metabolic enzymes, and tolerance to cadmium stress in Phaseolus vulgaris. Plants 2022, 11, 3069. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris L. Plant Physiol. 1949, 24, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Jagendorf, A.T. Oxidation and reduction of pyridine nucleotides by purified chloroplasts. Arch. Biochem. Biophys. 1956, 62, 141–150. [Google Scholar] [CrossRef]
- Avron, M. Photophosphorylation by Swis- chard chloroplasts chloroplasts. Biochim. Biophys. Acta 1960, 40, 257–272. [Google Scholar] [CrossRef]
- Osman, A.S.; Rady, M.M. Effect of humic acid as an additive to growing media to enhance the production of eggplant and tomato transplants. J. Hortic. Sci. Biotechnol. 2014, 89, 237–244. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef] [Green Version]
- Kubiś, J. Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J. Plant Physiol. 2008, 165, 397–406. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Madhava Rao, K.V.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, A.; Berardino, R.; De Pinto, M.C.; Sanità Di Toppi, L.; Storelli, M.M.; Tommasi, F.; De Gara, L. Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol. 2008, 49, 362–374. [Google Scholar] [CrossRef]
- Baker, H.; Frank, O.; Deangelis, B.; Feingold, S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr. Rep. Int. 1951, 21, 531–536. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.; Bakker, O.; van den Hoff, M.J.B.; Karlen, Y.; Moorman, A.F.M. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Nehela, Y.; Hijaz, F.; Elzaawely, A.A.; El-Zahaby, H.M.; Killiny, N. Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC-MS-based method. J. Plant Physiol. 2016, 199, 12–17. [Google Scholar] [CrossRef]
- Rady, M.M.; Talaat, N.B.; Abdelhamid, M.T.; Shawky, B.T.; Desoky, E.S.M. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 2019, 94, 777–789. [Google Scholar] [CrossRef]
- Ünyayar, S.; Topcuoglu, S.F.; Ünyayar, A. A modified method for extraction and identification of indole-3-acetic acid (IAA), gibberellic acid (GA3), abscisic acid (ABA) and zeatin produced by Phanerochaete chrysosporium ME446. Bulg. J. Plant Physiol. 1996, 22, 105–110. [Google Scholar]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacea oleracea) chloroplast: The effect of hydrogen peroxide and paraquat. Biochem. J. 1992, 210, 899–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; 2000p. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons, Inc.: London, UK, 1984; pp. 13–175. [Google Scholar]
- Waller, R.A.; Duncan, D.B. A Bayes rule for the symmetric multiple comparison problem. J. Amer. Stat. Assoc. 1969, 64, 1484–1504. [Google Scholar]
- Kaya, C.; Akram, N.A.; Sürücü, A.; Ashraf, M. Alleviating effect of nitric oxide on oxidative stress and antioxidant defence system in pepper (Capsicum annuum L.) plants exposed to cadmium and lead toxicity applied separately or in combination. Sci. Hortic. 2019, 255, 52–60. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Rafiq, M.T.; Aziz, R.; Yang, X.; Xiao, W.; Stoffella, P.J.; Saghir, A.; Azam, M.; Li, T. Phytoavailability of cadmium (Cd) to Pak choi (Brassica chinensis L.) grown in Chinese soils: A model to evaluate the impact of soil Cd pollution on potential dietary toxicity. PLoS ONE 2014, 9, e111461. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2018, 25, 25668–25680. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Akladious, S.A. Influence of garlic extract on enzymatic and non-enzymatic antioxidants in soybean plants (Glycine max) grown under drought stress. Life Sci. J. 2014, 11, 46–58. [Google Scholar]
- Abd-Elhamied, A. The influence of the foliar application of urea, licorice and garlic extract on nutrients uptake and yield of wheat plants grown on sandy soil. A comparative study. J. Soil Sci. Agric. Eng. 2017, 8, 157–162. [Google Scholar] [CrossRef]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere. 2020, 242, 125112. [Google Scholar] [CrossRef]
- Yıldırım, E.; Ekinci, M.; Turan, M.; Güleray, A.G.A.R.; Selda, Ö.R.S.; Dursun, A.; Kul, R.; Balci, T. Impact of cadmium and lead heavy metal stress on plant growth and physiology of rocket (Eruca sativa L.). Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi. 2019, 22, 843–850. [Google Scholar]
- Rasool, M.; Anwar-ul-Haq, M.; Jan, M.; Akhtar, J.; Ibrahim, M.; Iqbal, J. Phytoremedial potential of maize (Zea mays L.) hybrids against cadmium (Cd) and lead (Pb) toxicity. Pure Appl. Biol. 2020, 9, 1932–1945. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- Taie, H.A.A.; Seif El-Yazal, M.A.; Ahmed, S.M.A.; Rady, M.M. Polyamines modulate growth, antioxidant activity and genomic DNA in heavy metals-stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Naeem, F.; Shahid, M.; Abbas, G.; Shah, N.S.; Amjad, M.; Bakhat, H.F.; Imran, M.; Niazi, N.K.; Murtaza, G. A multivariate analysis of physiological and antioxidant responses and health hazards of wheat under cadmium and lead stress. Environ. Sci. Pollut. Res. 2019, 26, 362–370. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of Biostimulants as a New Approach for the Improvement of Phytoremediation Performance—A Review. Plants. 2022, 11, 1946. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, A.S.; Selem, E.; Mohsen, A.A.A.; Arnaout, S.M.A.I.; El-Sappah, A.H.; El-Tarabily, K.A.; Desoky, E.-S.M. Spirulina platensis extract improves the production and defenses of the common bean grown in a heavy metals-contaminated saline soil. J. Environ. Sci. 2023, 129, 240–257. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Effect of biosorptive removal of cadmium ions from hydroponic solution containing indigenous garlic peel and mercerized garlic peel on lettuce productivity. Sci. Hortic. 2022, 293, 110727. [Google Scholar] [CrossRef]
- Yaseen, W.; Iqbal, M.; Hussain, I.; Khaliq, S.; Ashraf, M.A. Exogenous menadione sodium bisulphite increases pigments, osmoprotectants and alters metabolism to attenuate cadmium toxicity on growth and yield in summer squash (Cucurbita pepo). Int. J. Agric. Biol. 2021, 25, 1321–1330. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Saini, S.; Kaur, N.; Pati, P.K. Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol. Environ. Saf. 2021, 223, 112578. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 38, 739–752. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Jinadasa, N.; Collins, D.; Holford, P.; Milham, P.J.; Conroy, J.P. Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): Is cadmium tolerance necessarily desirable in food crops? Environ. Sci. Pollut. Res. 2016, 23, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmad, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef] [PubMed]
- Arsenov, D.; Zupunski, M.; Borisev, M.; Nikolic, N.; Orlovic, S.; Pilipovic, A.; Pajevic, S. Exogenously applied citric acid enhances antioxidant defense and phytoextraction of cadmium by willows (Salix spp.). Water Air Soil Pollut. 2017, 228, 221. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Qin, G.; Wang, H.; Zhu, Y.; Zhang, K.; Yang, H. Growth, accumulation, and antioxidative responses of two Salix genotypes exposed to cadmium and lead in hydroponic culture. Environ. Sci. Pollut. Res. 2019, 26, 19770–19784. [Google Scholar] [CrossRef]
- Ma, J.; Saleem, M.H.; Alsafran, M.; Al Jabri, H.; Rizwan, M.; Nawaz, M.; Ali, S.; Usman, K. Response of cauliflower (Brassica oleracea L.) to nitric oxide application under cadmium stress. Ecotoxicol. Environ. Saf. 2022, 243, 113969. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; Behera, C.; et al. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef]


| Treatments | Description | |
|---|---|---|
| Cd + Pb | Bio-Stimulator | |
| 0 mM | Control | No stress + Three foliar sprays of distilled water 15, 22, and 29 days after transplanting (DaT). |
| GOE | No stress + Three foliar sprays of 3.0% GOE (3-FS.GOE) 15, 22, and 29 DaT. | |
| BHs | No stress + Three foliar sprays of 1.5% BHs (3-FS.BHs) 15, 22, and 29 DaT. | |
| GOE + BHs | No stress + Two foliar sprays of 2.0% GOE (2-FS.GOE) 12 and 24 DaT + Two foliar sprays of 1.0% BHs (2-FS.BHs) 18 and 30 DaT. | |
| 0.3 mM + 0.3 mM | Control | Irrigating squash plants with water containing 0.3 mM Cd + 0.3 mM Pb throughout the experiments. |
| GOE | Irrigating squash plants with water containing 0.3 mM Cd + 0.3 mM Pb throughout the experiments + 3-FS.GOE 15, 22, and 29 DaT. | |
| BHs | Irrigating squash plants with water containing 0.3 mM Cd + 0.3 mM Pb throughout the experiments + 3-FS.BHs 15, 22, and 29 DaT. | |
| GOE + BHs | Irrigating squash plants with water containing 0.3 mM Cd + 0.3 mM Pb throughout the experiments + 2-FS.GOE 12 and 24 DaT + 2-FS.BHs 18 and 30 DaT. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rady, M.M.; Salama, M.M.M.; Kuşvuran, S.; Kuşvuran, A.; Ahmed, A.F.; Ali, E.F.; Farouk, H.A.; Osman, A.S.; Selim, K.A.; Mahmoud, A.E.M. Exploring the Role of Novel Biostimulators in Suppressing Oxidative Stress and Reinforcing the Antioxidant Defense Systems in Cucurbita pepo Plants Exposed to Cadmium and Lead Toxicity. Agronomy 2023, 13, 1916. https://doi.org/10.3390/agronomy13071916
Rady MM, Salama MMM, Kuşvuran S, Kuşvuran A, Ahmed AF, Ali EF, Farouk HA, Osman AS, Selim KA, Mahmoud AEM. Exploring the Role of Novel Biostimulators in Suppressing Oxidative Stress and Reinforcing the Antioxidant Defense Systems in Cucurbita pepo Plants Exposed to Cadmium and Lead Toxicity. Agronomy. 2023; 13(7):1916. https://doi.org/10.3390/agronomy13071916
Chicago/Turabian StyleRady, Mostafa M., Mohamed M. M. Salama, Sebnem Kuşvuran, Alpaslan Kuşvuran, Atef F. Ahmed, Esmat F. Ali, Hamada A. Farouk, Ashraf Sh. Osman, Khaled A. Selim, and Amr E. M. Mahmoud. 2023. "Exploring the Role of Novel Biostimulators in Suppressing Oxidative Stress and Reinforcing the Antioxidant Defense Systems in Cucurbita pepo Plants Exposed to Cadmium and Lead Toxicity" Agronomy 13, no. 7: 1916. https://doi.org/10.3390/agronomy13071916
APA StyleRady, M. M., Salama, M. M. M., Kuşvuran, S., Kuşvuran, A., Ahmed, A. F., Ali, E. F., Farouk, H. A., Osman, A. S., Selim, K. A., & Mahmoud, A. E. M. (2023). Exploring the Role of Novel Biostimulators in Suppressing Oxidative Stress and Reinforcing the Antioxidant Defense Systems in Cucurbita pepo Plants Exposed to Cadmium and Lead Toxicity. Agronomy, 13(7), 1916. https://doi.org/10.3390/agronomy13071916






