Humate-Coated Urea as a Tool to Decrease Nitrogen Losses in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment
2.3. Chemical Analyses
2.4. Analysis of the Intensity of the Nitrogen and Carbon Cycle Processes
Computation of Additional Parameters of the State of Soil Microbial Communities
2.5. Statistics
3. Results
3.1. Content of N-NH4, N-NO3, and Soil pH
3.2. Gaseous N Losses
3.3. Activity of Soil Microbial Community
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matson, P.A.; Naylor, R.; Ortiz-Monasterio, I. Integration of environmental, agronomic, and economic aspects of fertilizer management. Science 1998, 280, 112–115. [Google Scholar] [CrossRef] [Green Version]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kesse, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Chalk, P.M.; Smith, C.J. The role of agroecosystems in chemical pathways of N2O production. Agric. Ecosyst. Environ. 2020, 290, 106783. [Google Scholar] [CrossRef]
- Lasis, A.A.; Akinremi, O.O.; Zhang, Q.; Kumaragamage, D. Efficiency of fall versus spring applied urea-based fertilizers treated with urease and nitrification inhibitors I. Ammonia volatilization and mitigation by NBPT. Soil Sci. Soc. Am. J. 2020, 84, 949–962. [Google Scholar] [CrossRef]
- Gould, W.D.; Hagedorn, C.; McCready, R.G.L. Urea transformations and fertilizer efficiency in soil. Adv. Agron. 1986, 40, 209–238. [Google Scholar]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 1–10. [Google Scholar] [CrossRef]
- McCarty, G. Modes of action of nitrification inhibitors. Biol. Fertil. Soils 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Matczuk, D.; Siczek, A. Effectiveness of the use of urease inhibitors in agriculture: A review. Int. Agrophys 2021, 35, 197–208. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Subbarao, G.; Sahrawat, K.L.; Nakahara, K.; Ishikawa, T.; Kishii, M.; Rao, I.; Nardi, P.; Bonnett, D.; Berry, W.; Suenaga, K.; et al. Biological nitrification inhibition—A novel strategy to regulate nitrification in agricultural systems. Adv. Agron. 2012, 114, 249–302. [Google Scholar]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Change Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Cruchaga, S.; Lasa, B.; Jauregui, I.; González-Murua, C.; Aparicio-Tejo, P.M.; Ariz, I. Inhibition of endogenous urease activity by NBPT application reveals differential N metabolism responses to ammonium or nitrate nutrition in pea plants: A physiological study. Plant Soil 2013, 373, 813–827. [Google Scholar] [CrossRef] [Green Version]
- Artola, E.; Cruchaga, S.; Ariz, I.; Moran, J.F.; Garnica, M.; Houdusse, F.; Aparicio-Tejo, P.M. Effect of N-(n-butyl) thiophosphoric triamide on urea metabolism and the assimilation of ammonium by Triticum aestivum L. Plant Growth Regul. 2011, 63, 73–79. [Google Scholar] [CrossRef]
- Lam, S.K.; Suter, H.; Mosier, A.R.; Chen, D. Using nitrification inhibitors to mitigate agricultural N2O emission: A double-edged sword? Global. Change Biol. 2017, 23, 485–489. [Google Scholar] [CrossRef]
- MacCarthy, P. The principles of humic substances. Soil Sci. 2001, 166, 738–751. [Google Scholar] [CrossRef]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the potential role of humic products in modifying biological properties of the soil-a review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Tan, W.; Koopal, L.K.; Wang, M.; Liu, F.; Norde, W. Influence of soil humic and fulvic acid on the activity and stability of lysozyme and urease. Environ. Sci. Technol. 2013, 47, 5050–5056. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Li, Z.; Zhang, C.; Wan, C.; Zhang, Y.; Lee, D.-J. Inhibition of urease activity by humic acid extracted from sludge fermentation liquid. Bioresour. Technol. 2019, 290, 121767. [Google Scholar] [CrossRef]
- Ameera, A.R.; Osumanu, H.A.; Nik, M.; Mohamadu, B.J. Reducing ammonia loss from urea by mixing with humic and fulvic acids isolated from coal. Am. J. Env. Sci. 2009, 5, 420–426. [Google Scholar]
- Devi, N.S.; Saha, D. Influence of organic matter vis-a-vis humic acid on the transformation of inorganic and organic forms of nitrogen in a typic haplustept soil. Commun. Soil Sci. Plant Anal. 2017, 48, 1042–1051. [Google Scholar] [CrossRef]
- Dong, L.; Córdova-Kreylos, A.L.; Yang, J.; Yuan, H.; Scow, K.M. Humic acids buffer the effects of urea on soil ammonia oxidizers and potential nitrification. Soil Biol. Biochem. 2009, 41, 1612–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.E.; Daigle, J.Y.; Leblanc, P.; Paulin, R.; Ghanem, I. Nitrogen availability and nitrate leaching from organo-mineral fertilizers. Can. J. Soil Sci. 1993, 73, 197–208. [Google Scholar] [CrossRef]
- Shen, Y.; Lin, H.; Gao, W.; Li, M. The effects of humic acid urea and polyaspartic acid urea on reducing nitrogen loss compared with urea. J. Sci. Food Agric. 2020, 100, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Rose, M.T.; Wong, V.N.L.; Cavagnaro, T.R.; Patti, A.F. Nitrogen dynamics in soil fertilized with slow release brown coal-urea fertilizers. Sci. Rep. 2018, 8, 14577. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ding, F.; Zhang, J.; Qi, X.; Gu, R.; Wu, Q.; Li, C. Effects of activated humic acid-urea on nitrogen use efficiency and its driving factors under wheat-maize rotation system. Chin. J. Eco-Agric. 2016, 24, 1310–1319. [Google Scholar]
- Jing, J.; Zhang, S.; Yuan, L.; Li, Y.; Zhang, Y.; Wen, Y.; Zhao, B. Humic acid complex formation with urea alters its structure and enhances biomass production in hydroponic maize. J. Sci. Food Agric. 2022, 102, 3636–3643. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Saha, B.K.; Rose, M.T.; Wong, V.N.; Cavagnaro, T.R.; Patti, A.F. A slow release brown coal-urea fertilizer reduced gaseous N loss from soil and increased silver beet yield and N uptake. Sci. Total Environ. 2019, 649, 793–800. [Google Scholar] [CrossRef]
- Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Caballero-Molada, M.; Atares, S.; García, C.; Vicente, O. New eco-friendly polymeric-coated urea fertilizers enhanced crop yield in wheat. Agronomy 2020, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Kou, M.; Tang, Z.; Zhang, A.; Li, H. The use of humic acid urea fertilizer for increasing yield and utilization of nitrogen in sweet potato. Plant Soil Environ. 2017, 63, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Kong, B.; Wu, Q.; Li, Y.; Zhu, T.; Ming, Y.; Li, C.; Li, C.; Wang, F.; Jiao, S.; Shi, L.; et al. The application of humic acid urea improves nitrogen use efficiency and crop yield by reducing the nitrogen loss compared with urea. Agriculture 2022, 12, 1996. [Google Scholar] [CrossRef]
- Yakimenko, O.S.; Terekhova, V.A. Humic preparations and the assessment of their biological activity for certification purposes. Eurasian. Soil Sci. 2011, 44, 1222–1230. [Google Scholar] [CrossRef]
- Yakimenko, O.; Khundzhua, D.; Izosimov, A.; Yuzhakov, V.; Patsaeva, S. Source indicator of commercial humic products: UV-Vis and fluorescence proxies. J. Soils Sediments 2018, 18, 1279–1291. [Google Scholar] [CrossRef]
- Glickman, T.S. (Ed.) Glossary of Meteorology; American Meteorological Soc: Boston, MA, USA, 2000; p. 855. [Google Scholar]
- Berg, B.; Wessén, B.; Ekbohm, G. Nitrogen level and decomposition in Scots pine needle litter. Oikos 1982, 38, 291–296. [Google Scholar] [CrossRef]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Arshad, M.; Frankenberger, W.T. Production and stability of ethylene in soil. Biol. Fertil. Soils 1990, 10, 29–34. [Google Scholar] [CrossRef]
- Smith, A.M. Ethylene production by bacteria in reduced microsites in soil and some implications to agriculture. Soil Biol. Biochem. 1976, 8, 293–298. [Google Scholar] [CrossRef]
- Zavarzina, A.G.; Vanifatova, N.G.; Stepanov, A.A. Fractionation of humic acids according to their hydrophobicity, size, and charge-dependent mobility by the salting-out method. Euras. Soil Sci. 2008, 41, 1294–1301. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, B.; Lin, Z.; Wen, Y.; Li, Y. Effects of value-added urea on wheat yield and N use efficiency and the distribution of residual N in soil profiles. J. Plant Nutr. Fertil. 2014, 20, 620–628. [Google Scholar]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Effects of urea enhanced with different weathered coal-derived humic acid components on maize yield and fate of fertilizer nitrogen. J. Integr. Agric. 2019, 18, 656–666. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, X. Effects of humic acid nitrogen fertilization on corn yield, nitrogen utilization and nitrogen loss. J. Plant Nut. Fertil. 2016, 22, 1232–1239. [Google Scholar]
- Gao, S.; Zhang, S.; Yuan, L.; Li, Y.; Wen, Y.; Xu, J.; Hu, S.; Zhao, B. Humic acids incorporated into urea at different proportions increased winter wheat yield and optimized fertilizer-nitrogen fate. Agronomy 2022, 12, 1526. [Google Scholar] [CrossRef]
- Rose, M.T.; Perkins, E.L.; Saha, B.K.; Tang, E.C.W.; Cavagnaro, T.R.; Jackson, W.R.; Hapgood, K.P.; Hoadley, A.F.A.; Patti, A.F. A slow release nitrogen fertilizer produced by simultaneous granulation and superheated steam drying of urea with brown coal. Chem. Biol. Technol. Agric. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.K.; Rose, M.T.; Wong, V.; Cavagnaro, T.R.; Patti, A.F. Hybrid brown coal-urea fertilizer reduces nitrogen loss compared to urea alone. Sci. Total Environ. 2017, 601, 1496–1504. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Lee, S.; Byrne, R. Effects of humic acids from vermicomposts on plant growth. Eur. J. Soil Biol. 2006, 42, S65–S69. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Lee, S.; Edwards, C.A.; Arancon, N.Q.; Metzger, J.D. The influence of humic acids derived from earthwormprocessed organic wastes on plant growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef]
- Brookes, P.C. The use of microbial parameters in monitoring soil pollution by heavy metals. Biol. Fertil. Soils 1995, 19, 269–279. [Google Scholar] [CrossRef]
- Leita, L.; De Nobili, M.; Mondini, C.; Muhlbachova, G.; Marchiol, L.; Bragato, G.; Contin, M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fertil. Soils 1999, 28, 371–376. [Google Scholar] [CrossRef]

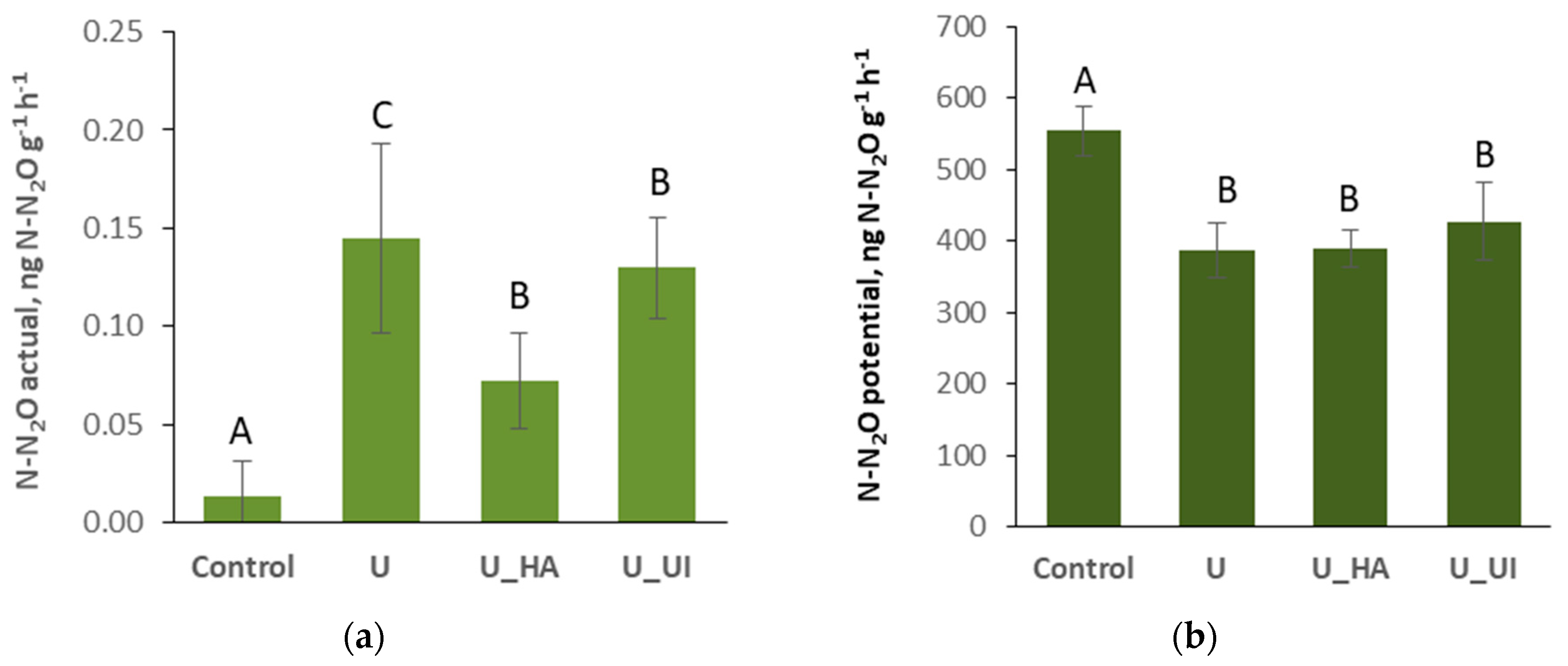
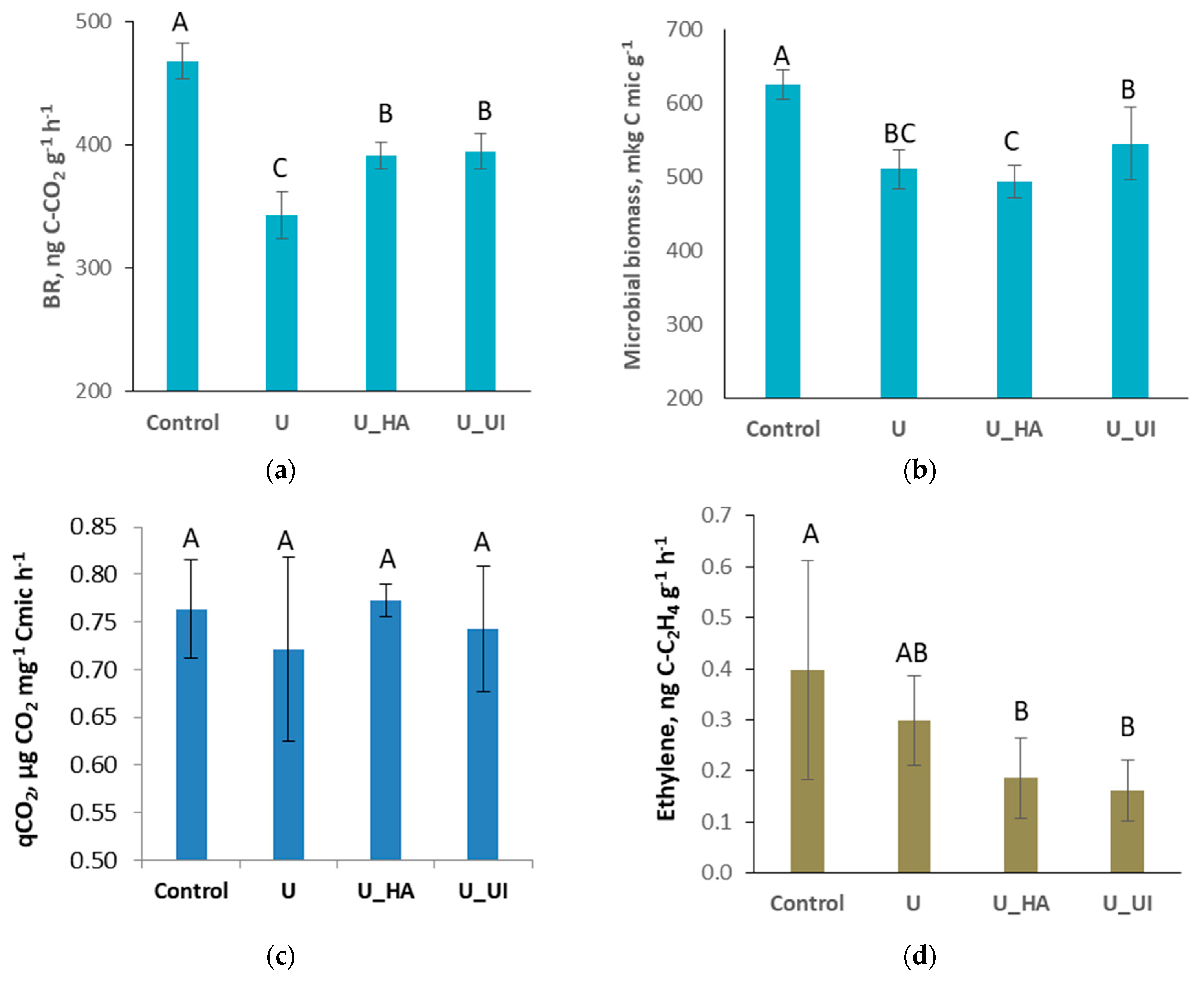
| Treatment | N-NH4 | N-NO3 | N-N2O, % |
|---|---|---|---|
| Control | NA 1 | NA 1 | 9.6 |
| U | 1.25 | 97.2 | 100.0 |
| U_HA | 1.93 | 88.2 | 50.1 |
| U_UI | 2.34 | 85.5 | 89.7 |
| Treatment | BR | SIR | Cmic | Ethylene |
|---|---|---|---|---|
| Control | 100.0 | 100.0 | 100.0 | 100.0 |
| U | 76.8 | 81.7 | 81.8 | 75.3 |
| U_HA | 85.1 | 79.0 | 87.3 | 46.9 |
| U_UI | 81.0 | 87.2 | 79.0 | 40.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsakov, K.; Stepanov, A.; Pozdnyakov, L.; Yakimenko, O. Humate-Coated Urea as a Tool to Decrease Nitrogen Losses in Soil. Agronomy 2023, 13, 1958. https://doi.org/10.3390/agronomy13081958
Korsakov K, Stepanov A, Pozdnyakov L, Yakimenko O. Humate-Coated Urea as a Tool to Decrease Nitrogen Losses in Soil. Agronomy. 2023; 13(8):1958. https://doi.org/10.3390/agronomy13081958
Chicago/Turabian StyleKorsakov, Konstantin, Alexey Stepanov, Lev Pozdnyakov, and Olga Yakimenko. 2023. "Humate-Coated Urea as a Tool to Decrease Nitrogen Losses in Soil" Agronomy 13, no. 8: 1958. https://doi.org/10.3390/agronomy13081958
APA StyleKorsakov, K., Stepanov, A., Pozdnyakov, L., & Yakimenko, O. (2023). Humate-Coated Urea as a Tool to Decrease Nitrogen Losses in Soil. Agronomy, 13(8), 1958. https://doi.org/10.3390/agronomy13081958





