Effect of Different Ratios of Red and Blue Light on Maximum Stomatal Conductance and Response Rate of Cucumber Seedling Leaves
Abstract
:1. Preface
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Design
2.3. Item Determination
2.3.1. Morphological Indicators
2.3.2. Determination of Blade Structure Parameters
2.3.3. Measurement of Photosynthetic Properties
2.3.4. Stomatal Characteristics
2.3.5. Stomatal Movement Measurements
2.3.6. RNA Extraction and RT-PCR for Gene Expression Analysis
2.4. Stomatal Movement Rate Analysis
2.5. Statistical Analysis
3. Results
3.1. Effect on Morphological Indicators of Cucumber Seedlings
3.2. Effect of Different Red and Blue Light Ratios on Leaf Structure
3.3. Effect on Photosynthetic Properties
3.4. Effect on Stomatal Characteristics
3.5. Effect on Stomatal Dynamics
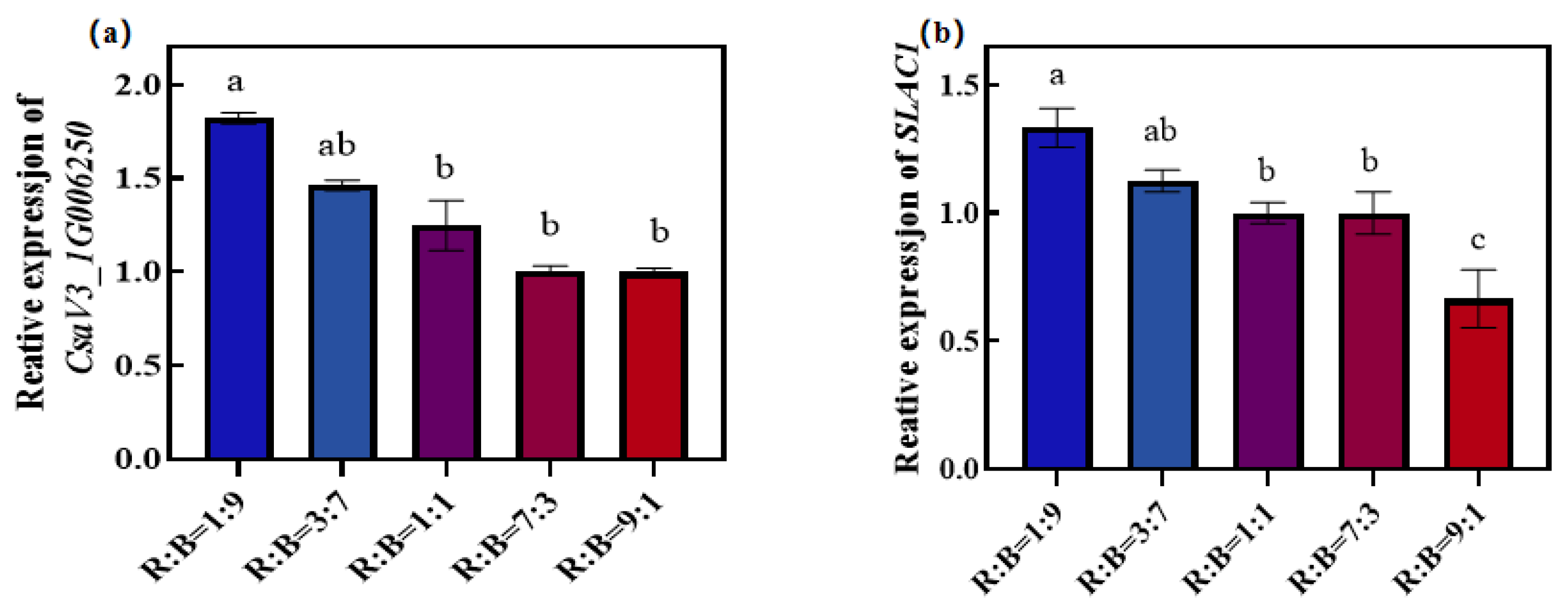
3.6. Effect on Stomatal Development and Motility Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zeiger, E.; Farquhar, G.D.; Cowan, I.R. Stomatal Function; Stanford University Press: Redwood City, CA, 1987. [Google Scholar]
- Kollist, H.; Nuhkat, M.; Roelfsema, M.R. Closing gaps: Linking elements that control stomatal movement. New Phytol. 2014, 203, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Naumburg, E.; Ellsworth, D.S. Photosynthetic sunfleck utilization potential of understory saplings growing under elevated CO2 in face. Oecologia 2000, 122, 163–174. [Google Scholar] [CrossRef]
- Lawson, T.; von Caemmerer, S.; Baroli, I. Photosynthesis and stomatal behaviour. Prog. Bot. 2010, 72, 265–304. [Google Scholar] [CrossRef]
- Matthews, J.S.A.; Vialet-Chabrand, S.R.M.; Lawson, T. Diurnal variation in gas exchange: The balance between Carbon Fixation and water loss. Plant Physiol. 2017, 174, 614–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Barradas, V.L.; Jones, H.G.; Clark, J.A. Stomatal responses to changing irradiance in Phaseolus vulgaris L. J. Exp. Bot. 1994, 45, 931–936. [Google Scholar] [CrossRef]
- Fischer, R.A.; Rees, D.; Sayre, K.D.; Lu, Z.-M.; Condon, A.G.; Saavedra, A.L. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 1998, 38, 1467–1475. [Google Scholar] [CrossRef]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A.; Centritto, M. Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Front. Plant Sci. 2016, 7, 1568. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, G.; Haworth, M.; Wahbi, S.; Mahmood, T.; Zuomin, S.; Centritto, M. Abscisic acid induces rapid reductions in mesophyll conductance to carbon dioxide. PLoS ONE 2016, 11, e0148554. [Google Scholar] [CrossRef] [Green Version]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of stomatal development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAusland, L.; Vialet-Chabrand, S.; Davey, P.; Baker, N.R.; Brendel, O.; Lawson, T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016, 211, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Vialet-Chabrand, S.; Lawson, T. Dynamic Leaf Energy Balance: Deriving stomatal conductance from thermal imaging in a dynamic environment. J. Exp. Bot. 2019, 70, 2839–2855. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A. Speedy small stomata? J. Exp. Bot. 2014, 65, 1415–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2006, 143, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2018, 221, 371–384. [Google Scholar] [CrossRef]
- Doheny-Adams, T.; Hunt, L.; Franks, P.J.; Beerling, D.J.; Gray, J.E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 547–555. [Google Scholar] [CrossRef]
- Dutton, C.; Hõrak, H.; Hepworth, C.; Mitchell, A.; Ton, J.; Hunt, L.; Gray, J.E. Bacterial infection systemically suppresses stomatal density. Plant Cell Environ. 2019, 42, 2411–2421. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Jiao, Y.; Lau, O.S.; Deng, X.W. Light-regulated transcriptional networks in higher plants. Nat. Rev. Genet. 2007, 8, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Kong, D.; Yang, J.; Wang, H. Light regulation of stomatal development and patterning: Shifting the paradigm from Arabidopsis to grasses. Plant Commun. 2020, 1, 100030. [Google Scholar] [CrossRef]
- Assmann, S.M.; Shimazaki, K. The multisensory guard cell. stomatal responses to blue light and Abscisic Acid1. Plant Physiol. 1999, 119, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelfsema, M.R.; Hedrich, R. In the light of stomatal opening: New insights into ‘The watergate’. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Shimazaki, K.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light regulation of stomatal movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiger, E. The biology of stomatal guard cells. Annu. Rev. Plant Physiol. 1983, 34, 441–474. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Raschke, K. Separation and measurement of direct and indirect effects of light on Stomata. Plant Physiol. 1981, 68, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Hetherington, A. Faculty opinions recommendation of Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat. Cell Biol. 2006, 8, 391–397. [Google Scholar] [CrossRef]
- Mott, K.A.; Sibbernsen, E.D.; Shope, J.C. The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ. 2008, 31, 1299–1306. [Google Scholar] [CrossRef]
- Sibbernsen, E.; Mott, K.A. Stomatal responses to flooding of the intercellular air spaces suggest a vapor-phase signal between the mesophyll and the guard cells. Plant Physiol. 2010, 153, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Mott, K.A.; Peak, D. Testing a vapour-phase model of stomatal responses to humidity. Plant Cell Environ. 2012, 36, 936–944. [Google Scholar] [CrossRef]
- Lu, P.; Outlaw, W.H., Jr.; Smith, B.G.; Freed, G.A. Plant, Cell, and Molecular Mechanisms of Abscisic-Acid Regulation of Stomatal Apertures. A New Mechanism for the Regulation of Stomatal-Aperture Size in Intact Leaves: Accumulation of Mesophyll-Derived Sucrose in the Guard-Cell Wall of Vicia Faba L; USDOE Office of Energy Research: Washington, DC, USA, 1996. [Google Scholar] [CrossRef] [Green Version]
- Outlaw, W.H., Jr. Integration of cellular and physiological functions of guard cells. Crit. Rev. Plant Sci. 2003, 22, 503–529. [Google Scholar] [CrossRef]
- Kang, Y.; Outlaw, W.H.; Andersen, P.C.; Fiore, G.B. Guard-cell apoplastic sucrose concentration ? A link between leaf photosynthesis and stomatal aperture size in the apoplastic phloem loader vicia faba L. Plant Cell Environ. 2007, 30, 551–558. [Google Scholar] [CrossRef]
- Lawson, T. The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J. Exp. Bot. 2003, 54, 1743–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Lawson, T.; Simkin, A.J.; Kelly, G.; Granot, D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014, 203, 1064–1081. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Terashima, I.; Fujita, T.; Wang, Y. Coordination between photosynthesis and stomatal behavior. In The Leaf: A Platform for Performing Photosynthesis; Springer: Cham, Switzerland, 2018; pp. 141–161. [Google Scholar]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2008, 181, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Ando, E.; Kinoshita, T. Red light-induced phosphorylation of plasma membrane H+-atpase in stomatal guard cells. Plant Physiol. 2018, 178, 838–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tominaga, M.; Kinoshita, T.; Shimazaki, K. Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant Cell Physiol. 2001, 42, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, P.E. Blue Light Regulation of stomata in wheat seedlings. i. influence of red background illumination and initial conductance level. Physiol. Plant. 1986, 66, 202–206. [Google Scholar] [CrossRef]
- Chen, Y.H.; Hu, L.; Punta, M.; Bruni, R.; Hillerich, B. Homologue structure of the SLAC1 anion channel for closing stomata in leaves. Nature 2010, 467, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Hikosaka, S.; Goto, E.; Takasuna, H.; Kudou, T. Effects of light quality and light period on flowering of everbearing strawberry in a closed plant production system. Acta Hortic. 2012, 956, 107–112. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, K.-H.; Oh, M.-M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Di, Q.; Li, J.; Du, Y.; Wei, M.; Shi, Q.; Li, Y.; Yang, F. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. J. Plant Growth Regul. 2020, 40, 1477–1492. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth and acclimation of impatiens, salvia, petunia, and tomato seedlings to blue and red light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Van Labeke, M.-C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [Green Version]
- Boardman, N.K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Shengxin, C.; Chunxia, L.; Xuyang, Y.; Song, C.; Xuelei, J.; Xiaoying, L.; Zhigang, X.; Rongzhan, G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the Rosette Stage. Front. Plant Sci. 2016, 7, 1144. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, X.; Gao, L.; Chen, Q.; Qu, M. Blue Light is more essential than red light for maintaining the activities of Photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Monte, E.; Tepperman, J.M.; Al-Sady, B.; Kaczorowski, K.A.; Alonso, J.M.; Ecker, J.R.; Li, X.; Zhang, Y.; Quail, P.H. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc. Natl. Acad. Sci. USA 2004, 101, 16091–16098. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar] [CrossRef]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef]
- Gitziii, D.; Liugitz, L.; Britz, S.; Sullivan, J. Ultraviolet-B effects on stomatal density, water-use efficiency, and stable carbon isotope discrimination in four glasshouse-grown soybean cultivars. Environ. Exp. Bot. 2005, 53, 343–355. [Google Scholar] [CrossRef]
- Barillot, R.; Frak, E.; Combes, D.; Durand, J.-L.; Escobar-Gutiérrez, A.J. What determines the complex kinetics of stomatal conductance under blueless par in Festuca arundinacea? subsequent effects on leaf transpiration. J. Exp. Bot. 2010, 61, 2795–2806. [Google Scholar] [CrossRef] [Green Version]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
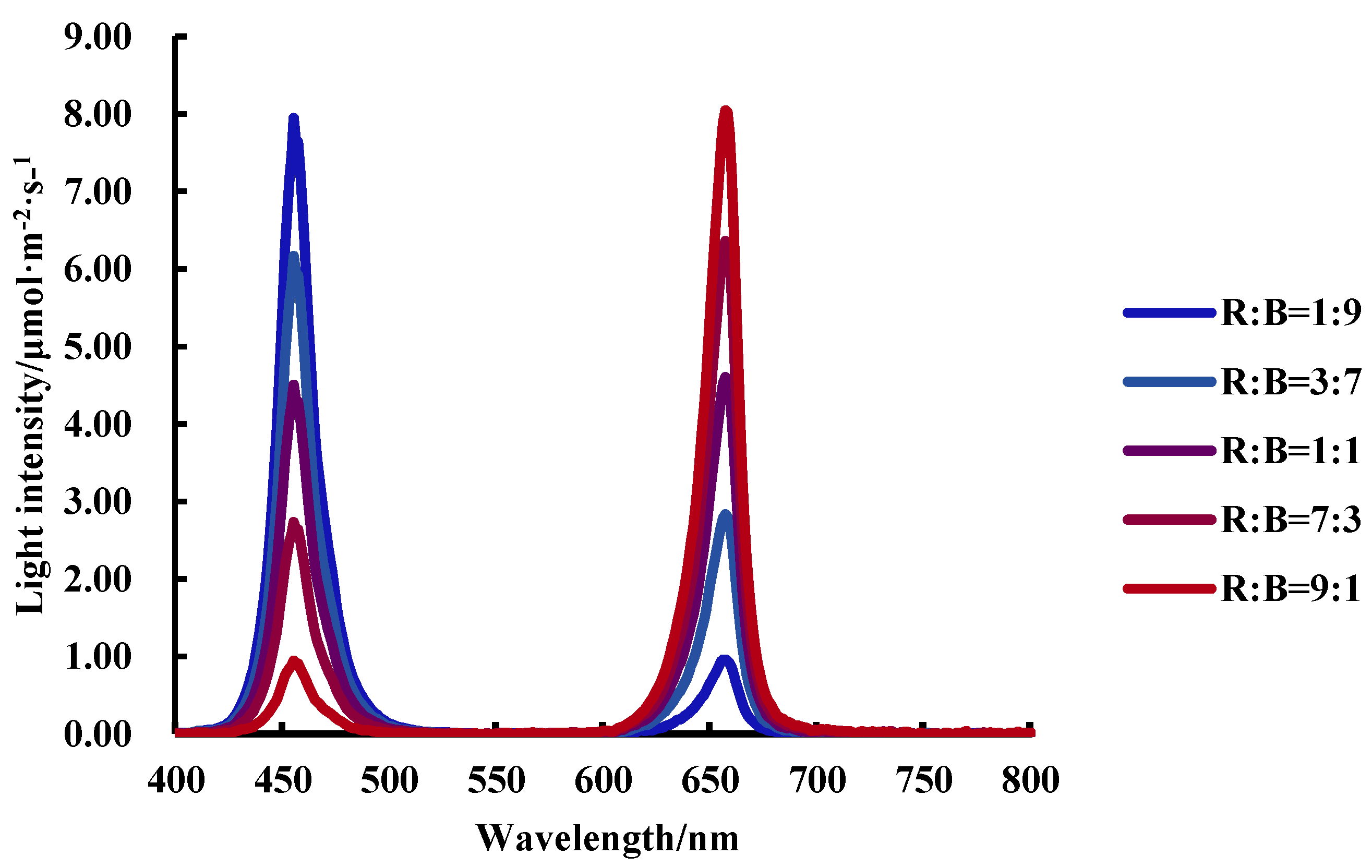
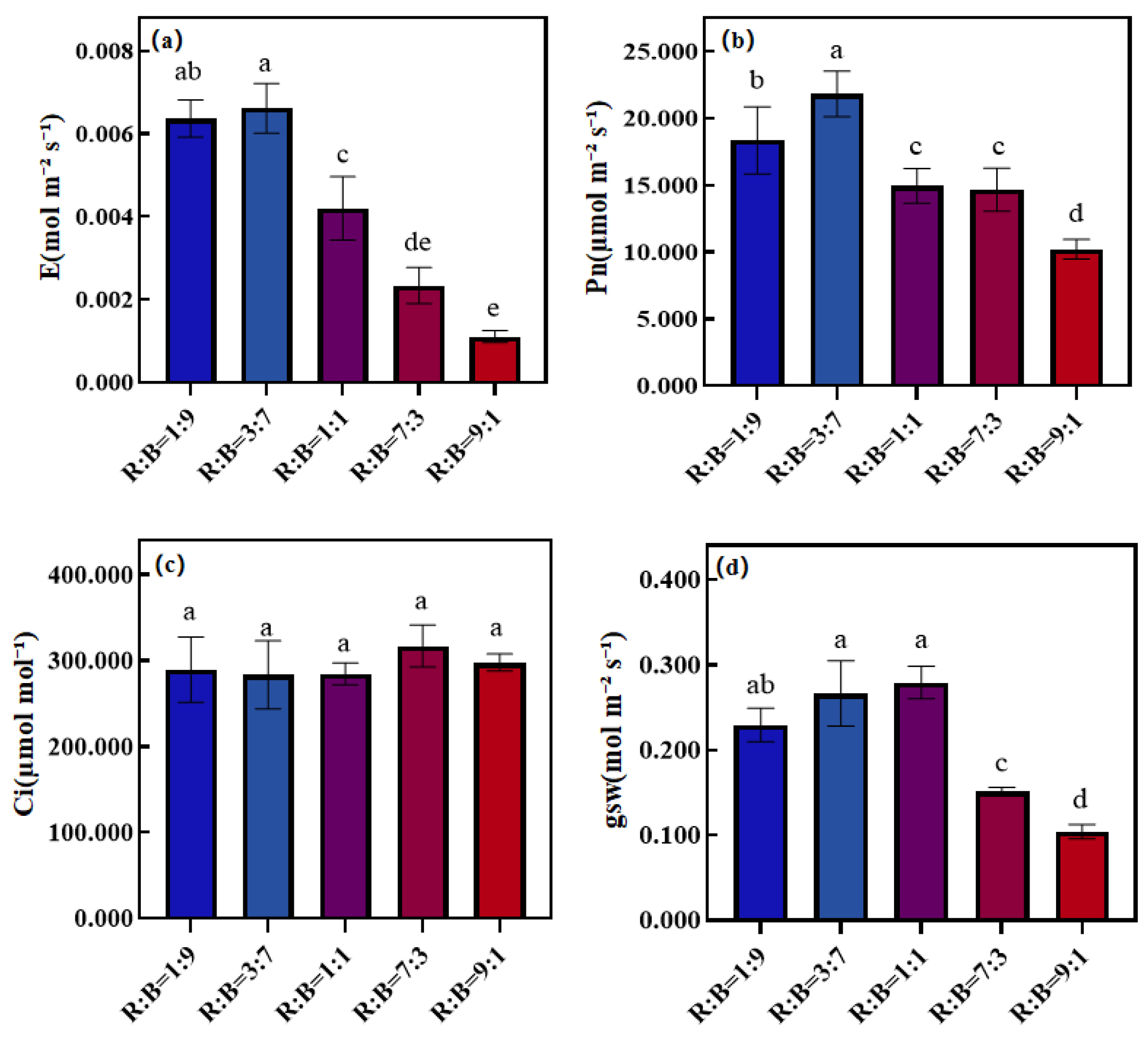
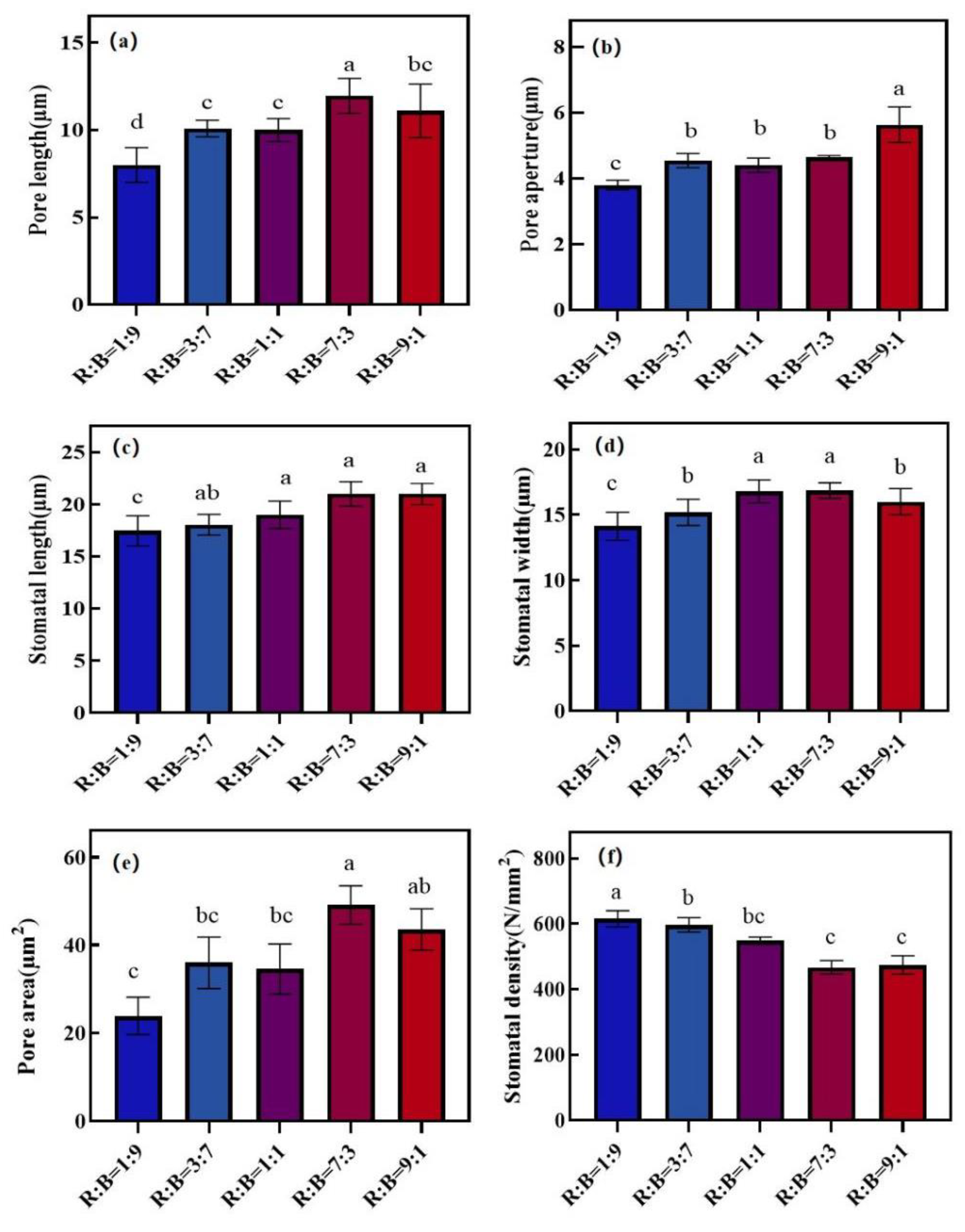
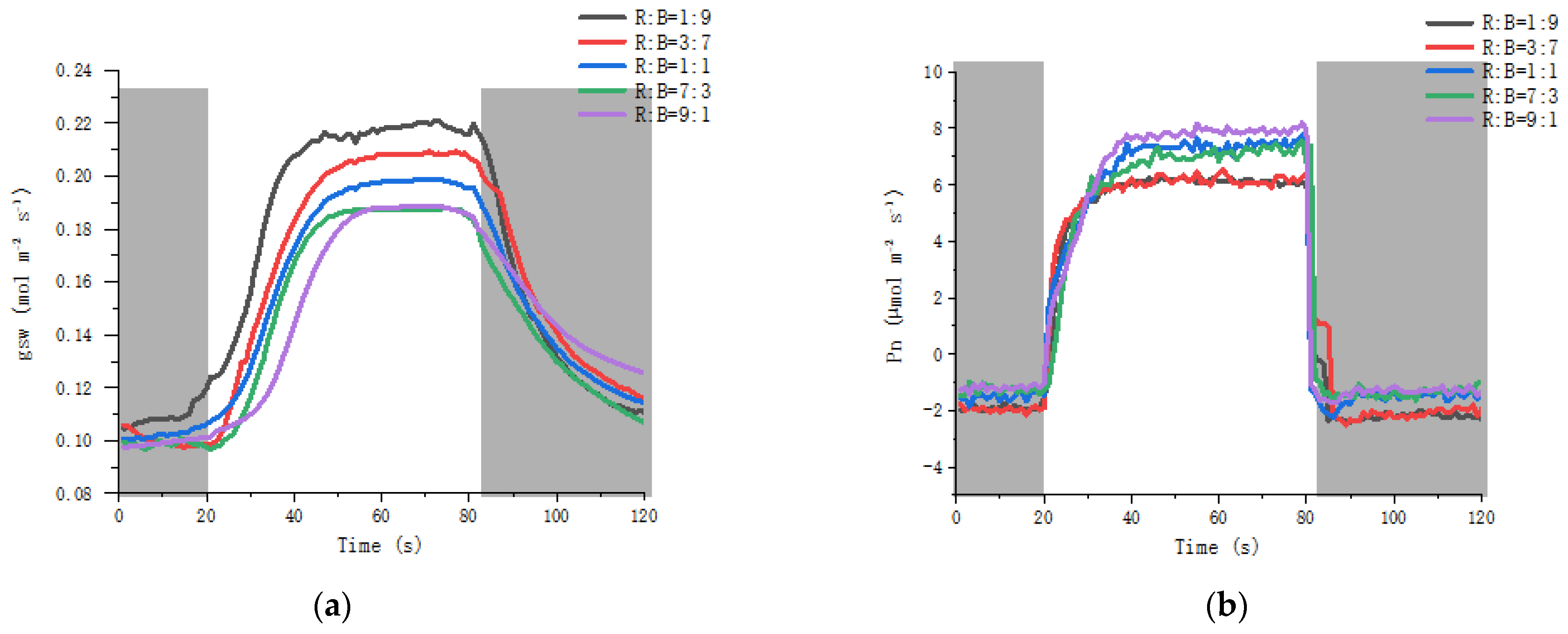

| Treatments | Red Light (μmol·m−2·s−1) | Blue Light (μmol·m−2·s−1) | R:B | PAR (μmol·m−2·s−1) |
|---|---|---|---|---|
| R:B = 1:9 | 20 | 180 | 1:9 | 200 |
| R:B = 3:7 | 60 | 140 | 3:7 | 200 |
| R:B = 1:1 | 100 | 100 | 1:1 | 200 |
| R:B = 7:3 | 140 | 60 | 7:3 | 200 |
| R:B = 9:1 | 180 | 20 | 9:1 | 200 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Action | 5′-CACCAAGCCCAAGAAGATC-3′ | 5′-TAAACCTAATCACCACCAGC-3′ |
| CsaV3_1G006250 | 5′-CAGTGCCTACTGAATCAAGCGACTC-3′ | 5′-GTAGGTACTGCCACGATTGGATGG-3′ |
| SLAC1 | 5′-CTCGGCCAACAATGACATCAGC-3′ | 5′-CAAACCCGTTTCCAGCGACATC-3′ |
| Treatments | Plant Height (cm) | Stem Diameter (mm) | Total Leaf Area (cm2) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|
| R:B = 1:9 | 16.020 ± 1.260 d | 6.320 ± 1.005 a | 492.935 ± 34.325 c | 18.624 ± 2.804 c | 1.644 ± 0.162 a |
| R:B = 3:7 | 17.270 ± 0.610 d | 5.960 ± 1.109 a | 557.733 ± 54.466 b | 20.910 ± 0.595 bc | 1.818 ± 0.170 a |
| R:B = 1:1 | 20.320 ± 1.850 bc | 5.457 ± 1.22 a | 606.001 ± 12.166 ab | 22.095 ± 1.780 bc | 1.954 ± 0.136 a |
| R:B = 7:3 | 32.900 ± 3.310 a | 6.230 ± 1.017 a | 638.663 ± 15.177 a | 27.610 ± 0.690 a | 2.005 ± 0.035 a |
| R:B = 9:1 | 32.300 ± 1.630 a | 6.143 ± 1.289 a | 629.950 ± 30.9701 a | 23.731 ± 1.982 b | 1.820 ± 0.099 a |
| Treatments | Upper Epidermis (μm) | Palisade Tissue (μm) | Spongy Tissue (μm) | Inferior Epidermis (μm) | Blade Thickness (μm) |
|---|---|---|---|---|---|
| R:B = 1:9 | 15.72 ± 0.905 a | 69.001 ± 1.491 a | 88.87 ± 5.443 ab | 8.941 ± 0.547 a | 188.964 ± 5.731 a |
| R:B = 3:7 | 13.148 ± 1.026 b | 52.214 ± 1.488 bc | 92.196 ± 3.804 a | 8.27 ± 0.738 a | 182.772 ± 13.120 a |
| R:B = 1:1 | 8.854 ± 0.570 c | 48.845 ± 1.542 c | 78.079 ± 2.331 c | 7.773 ± 0.412 b | 150.424 ± 2.823 bc |
| R:B = 7:3 | 9.895 ± 0.771 c | 50.707 ± 2.333 bc | 83.172 ± 2.992 ab | 8.154 ± 0.515 a | 155.673 ± 4.864 b |
| R:B = 9:1 | 9.357 ± 0.462 c | 38.347 ± 1.946 d | 75.621 ± 3.500 c | 5.716 ± 0.341 c | 136.028 ± 4.725 c |
| Treatments | ki | Slmax | Gsmax | r0 |
|---|---|---|---|---|
| R:B = 1:9 | 22 | 5.781 | 0.221 | 0.124 |
| R:B = 3:7 | 20 | 6.004 | 0.209 | 0.099 |
| R:B = 1:1 | 26 | 6.461 | 0.199 | 0.108 |
| R:B = 7:3 | 25 | 6.185 | 0.188 | 0.097 |
| R:B = 9:1 | 30 | 7.111 | 0.189 | 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, S.; Lin, A.; Yang, Y.; Zhang, G.; Xu, P.; Wu, Y.; Yang, Z. Effect of Different Ratios of Red and Blue Light on Maximum Stomatal Conductance and Response Rate of Cucumber Seedling Leaves. Agronomy 2023, 13, 1941. https://doi.org/10.3390/agronomy13071941
Li X, Zhao S, Lin A, Yang Y, Zhang G, Xu P, Wu Y, Yang Z. Effect of Different Ratios of Red and Blue Light on Maximum Stomatal Conductance and Response Rate of Cucumber Seedling Leaves. Agronomy. 2023; 13(7):1941. https://doi.org/10.3390/agronomy13071941
Chicago/Turabian StyleLi, Xue, Shiwen Zhao, Aiyu Lin, Yuanyuan Yang, Guanzhi Zhang, Peng Xu, Yongjun Wu, and Zhenchao Yang. 2023. "Effect of Different Ratios of Red and Blue Light on Maximum Stomatal Conductance and Response Rate of Cucumber Seedling Leaves" Agronomy 13, no. 7: 1941. https://doi.org/10.3390/agronomy13071941
APA StyleLi, X., Zhao, S., Lin, A., Yang, Y., Zhang, G., Xu, P., Wu, Y., & Yang, Z. (2023). Effect of Different Ratios of Red and Blue Light on Maximum Stomatal Conductance and Response Rate of Cucumber Seedling Leaves. Agronomy, 13(7), 1941. https://doi.org/10.3390/agronomy13071941





