Evaluation of the Effect of the Vigor of Soybean Seeds Treated with Micronutrients Using X-ray Fluorescence Spectroscopy and Hyperspectral Imaging
Abstract
:1. Introduction
2. Materials and Methods
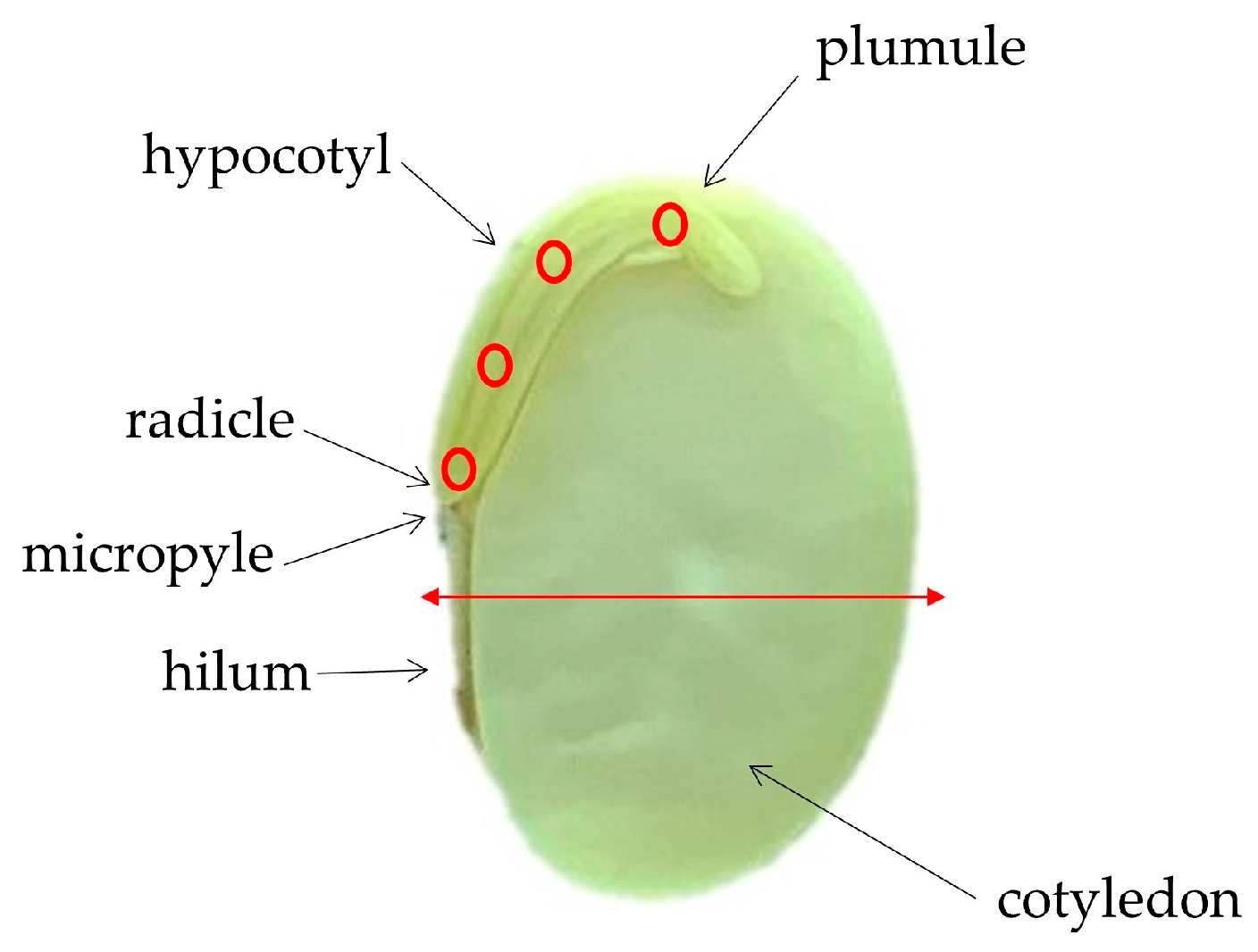
2.1. Seed Material and Classification of Vigor
2.2. Evaluation of the Uptake of Micronutrients by Seeds
2.2.1. Imbibition Curve
2.2.2. Seed Treatment
2.2.3. Analysis of Micronutrient Uptake via X-ray Fluorescence Spectroscopy (XRF)
2.2.4. Analysis of Micronutrient Uptake via Microprobe X-ray Fluorescence Spectroscopy (μ-XRF)
2.2.5. Statistical Analysis
2.3. Hyperspectral Imaging System
2.3.1. Sample Preparation
2.3.2. Hyperspectral Image Acquisition
2.3.3. Spectral Preprocessing
2.3.4. Optimal Wavelength Selection
2.3.5. Evaluation of the classification model
3. Results
3.1. Classification of Vigor
3.2. Evaluation of the Uptake of Micronutrients by Seeds
3.3. Spectral Data Analysis
3.4. Optimal Wavelengths Selected via PCA
3.5. Models Based on Selected Bands
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, Y.; Itakura, K.; Kuramoto, M.; Kaho, T.; Ohtake, N.; Hasegawa, H.; Suzuki, T.; Kondo, N. Prediction of protein and oil contents in soybeans using fluorescence excitation emission matrix. Food Chem. 2021, 365, 130403. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, A.J.; Dall’Agnol, A. The rapid soybean growth in Brazil. Oilseeds Fats Crop. Lipids 2018, 25, 58. [Google Scholar] [CrossRef] [Green Version]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean seed vigor: Uniformity and growth as key factors to improve yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef] [Green Version]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [Green Version]
- Hansel, F.D.; Oliveira, M.L. Importance of Micronutrients in Soybean Culture in Brazil; International Plant Nutrition Institute: Piracicaba, Brazil, 2016. [Google Scholar]
- Bhat, B.A.; Islam, S.T.; Ali, A.; Sheikh, B.A.; Tariq, L.; Islam, S.U.; Hassan Dar, T.U. Role of micronutrients in secondary metabolism of plants. Plant Micronutr. 2020, 13, 311–329. [Google Scholar] [CrossRef]
- Romeu, S.L.Z.; Marques, J.P.R.; Montanha, G.S.; de Carvalho, H.W.P.; Pereira, F.M.V. Chemometrics unraveling nutrient dynamics during soybean seed germination. Microchem. J. 2021, 164, 106045. [Google Scholar] [CrossRef]
- Montanha, G.S.; Dias, M.A.N.; Corrêa, C.G.; de Carvalho, H.W.P. Unfolding the fate and effects of micronutrients supplied to soybean (Glycine max (L.) Merrill) and maize (Zea mays L.) through seed treatment. J. Soil Sci. Plant Nutr. 2021, 21, 3194–3202. [Google Scholar] [CrossRef]
- Rohr, L.A.; França-Silva, F.; Corrêa, C.G.; de Carvalho, H.W.P.; Gomes-Junior, F.G. Soybean seeds treated with zinc evaluated by X-ray micro-fluorescence spectroscopy. Sci. Agric. 2023, 80, e20210131. [Google Scholar] [CrossRef]
- Amigo, J.M.; Martí, I.; Gowen, A. Hyperspectral Imaging and Chemometrics. A perfect combination for the analysis of food structure, composition and quality. Data Handl. Sci. Technol. 2013, 28, 343–370. [Google Scholar] [CrossRef]
- Elmasry, G.; Mandour, N.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Recent applications of multispectral imaging in seed phenotyping and quality monitoring-an overview. Sensors 2019, 19, 1090. [Google Scholar] [CrossRef] [Green Version]
- Amigo, J.M.; Babamoradi, H.; Elcoroaristizabal, S. Hyperspectral Image analysis. A Tutorial. Anal. Chim. Acta 2015, 896, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Kemsley, E.K.; Defernez, M.; Marini, F. Multivariate statistics: Considerations and confidences in food authenticity problems. Food Control 2019, 105, 102–112. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wu, X.; Chen, Q.; Lu, B.; Dai, C. Detection of viability of soybean seed based on fluorescence hyperspectra and CARS-SVM-AdaBoost model. J. Food Process. Preserv. 2019, 43, e14238. [Google Scholar] [CrossRef]
- Zhu, S.; Chao, M.; Zhang, J.; Xu, X.; Song, P.; Zhang, J.; Huang, Z. Identification of soybean seed varieties based on hyperspectral imaging technology. Sensors 2019, 19, 5225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.; Zhou, J.; Scaboo, A.M.; Niu, X. Nondestructive phenotyping fatty acid trait of single soybean seeds using reflective hyperspectral imagery. J. Food Process Eng. 2021, 44, e13759. [Google Scholar] [CrossRef]
- Aulia, R.; Kim, Y.; Zuhrotul Amanah, H.; Muhammad Akbar Andi, A.; Kim, H.; Kim, H.; Lee, W.H.; Kim, K.H.; Baek, J.H.; Cho, B.K. Non-Destructive prediction of protein contents of soybean seeds using near-Infrared Hyperspectral Imaging. Infrared Phys. Technol. 2022, 127, 104365. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, J.; Li, H.; Tan, K.; Zhang, X. Identification of high-oil content soybean using hyperspectral reflectance and one-dimensional convolutional neural network. Spectrosc. Lett. 2023, 56, 28–41. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Ma, C.; Yin, X.; Guo, Y.; Sun, X.; Jin, C. Application of visible-near-infrared hyperspectral imaging technology coupled with wavelength selection algorithm for rapid determination of moisture content of soybean seeds. J. Food Compos. Anal. 2023, 116, 105048. [Google Scholar] [CrossRef]
- Ministério da Agricultura, Pecuária e Abastecimento. Regras Para Análise de Sementes; Ministério da Agricultura, Pecuária e Abastecimento: Brasília, Brasil, 2009. [Google Scholar]
- Krzyzanowski, F.C.; Vieira, R.D.; França-Neto, J.B.; Marcos-Filho, J. Vigor de Sementes: Conceitos e Testes, 2nd ed.; ABRATES: Londrina, Brazil, 2020. [Google Scholar]
- Almeida, E.; Montanha, G.S.; Carvalho, H.W.P.; Marguí, E. Evaluation of energy dispersive X-ray fluorescence and total reflection X-ray fluorescence spectrometry for vegetal mass-limited sample analysis: Application to soybean root and shoots. Spectrochim. Acta 2020, 170, 105915. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 May 2023).
- Xu, P.; Sun, W.; Xu, K.; Zhang, Y.; Tan, Q.; Qing, Y.; Yang, R. Identification of defective maize seeds using hyperspectral imaging combined with deep learning. Foods 2023, 12, 144. [Google Scholar] [CrossRef]
- Bian, X.; Wang, K.; Tan, E.; Diwu, P.; Zhang, F.; Guo, Y. A selective ensemble preprocessing strategy for near-infrared spectral quantitative analysis of complex samples. Chemom. Intell. Lab. Syst. 2020, 197, 103916. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhossieni, M. Quantification of soybean seed germination response to seed deterioration under peg-induced water stress using hydrotime concept. Acta Physiol. Plant. 2018, 40, 126. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, W.M.H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer Science & Business Media: New York, NY, USA, 2012; ISBN 9781461446927. [Google Scholar]
- Rossi, R.F.; Cavariani, C.; França-Neto, J.d.B. Seed vigor, plant population and agronomic performance of soybean. Rev. Ciênc. Agrar.-Amaz. J. Agric. Environ. Sci. 2017, 60, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Filho, J. Seed Physiology of Cultivated Plants, 2nd ed.; ABRATES: Londrina, Brazil, 2015. [Google Scholar]
- Montanha, G.S.; Romeu, S.L.Z.; Marques, J.P.R.; Rohr, L.A.; De Almeida, E.; Dos Reis, A.R.; Linhares, F.S.; Sabatini, S.; De Carvalho, H.W.P. Microprobe-XRF assessment of nutrient distribution in soybean, cowpea, and kidney bean seeds: A fabaceae family case study. ACS Agric. Sci. Technol. 2022, 2, 1318–1324. [Google Scholar] [CrossRef]
- Magalhães, W.d.A.; Megaioli, T.G.; Freddi, O.d.S.; Santos, M.A. Nutrient quantification in soybeans. Rev. Ciênc. Agroambient. 2015, 13, 95–100. [Google Scholar]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc Oxide Nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Montanha, G.S.; Rodrigues, E.S.; Marques, J.P.R.; de Almeida, E.; Colzato, M.; Pereira de Carvalho, H.W. Zinc Nanocoated Seeds: An Alternative to Boost Soybean Seed Germination and Seedling Development. SN Appl. Sci. 2020, 2, 857. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.B.d.; Rodrigues Marques, J.P.; Rodak, B.W.; Galindo, F.S.; Carr, N.F.; Almeida, E.; Araki, K.; Gonçalves, J.M.; Rodrigues dos Reis, A.; van der Ent, A.; et al. Fate of nickel in soybean seeds dressed with different forms of nickel. Rhizosphere 2022, 21, 100464. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, L.; Yang, N.; Fisk, I.D.; Wei, W.; Wang, L.; Li, J.; Sun, Q.; Zeng, R. Integration of hyperspectral imaging, non-targeted metabolomics and machine learning for vigour prediction of naturally and accelerated aged sweetcorn seeds. Food Control 2023, 153, 109930. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, G.; Wang, Z.; Hu, R.; She, B.; Pan, Z.; Zhou, X.-G.; Zhang, G.; Zhang, D. Hyperspectral and imagery integrated analysis for vegetable seed vigor detection. Infrared Phys. Technol. 2023, 131, 104605. [Google Scholar] [CrossRef]












| Region of Interest | Method of Preprocessing | Wavelength (nm) |
|---|---|---|
| Cotyledons | Raw | 504.37; 506.42; 508.48; 859.17; 861.37; 863.57; 865.76; 867.96; 870.16; 872.36; 874.57; 876.77; 878.97; 881.18; 883.38; 885.59; 887.79; 890.00; 892.21; 894.42; 896.63; 898.84; 901.05; 903.26; 905.48; 907.69; 909.91 and 912.12. |
| MSC | 654.41; 656.53; 658.64; 660.76; 662.88; 665.00; 667.12; 863.57; 865.76; 867.96; 870.16; 872.36; 874.57; 876.77; 878.97; 881.18; 883.38; 885.59; 887.79; 890.00; 936.56; 938.78; 941.01; 943.24; 945.47; 947.7; 949.93 and 952.16. | |
| SNV | 599.72; 601.81; 603.91; 606.00; 608.1; 610.19; 612.29; 614.39; 616.49; 943.24; 945.47; 947.7; 949.93; 952.16; 961.1; 965.57; 967.81; 970.04; 972.28; 974.52; 976.76; 979.01; 981.25; 983.49; 985.74; 987.98; 990.23 and 992.48. | |
| Embryonic axis | Raw | 539.4; 541.47; 543.53; 545.6; 547.67; 549.75; 551.82; 558.04; 560.12; 830.7; 835.07; 837.25; 839.44; 841.63; 843.82; 846.01; 848.2; 850.4; 852.59; 854.78; 856.98; 859.17; 861.37; 863.57; 865.76; 867.96; 870.16 and 872.36. |
| MSC | 633.31; 635.41; 637.52; 639.63; 641.74; 643.85; 645.96; 648.07; 650.18; 652.3; 654.41; 656.53; 658.64; 660.76; 662.88; 665.00; 667.12; 669.24; 673.48; 923.22; 925.44; 927.66; 929.88; 932.11; 934.33; 936.56; 938.78 and 941.01. | |
| SNV | 606.00; 608.1; 610.19; 612.29; 614.39; 616.49; 618.59; 620.69; 622.79; 624.89; 626.99; 629.1; 631.2; 639.63; 795.85; 800.19; 802.36; 943.24; 974.52; 976.76; 979.01; 981.25; 983.49; 985.74; 987.98; 990.23; 992.48 and 996.97. |
| Region of Interest | Method of Preprocessing | Wavelength (nm) |
|---|---|---|
| Cotyledons | Raw | 859.17; 861.37; 863.57; 865.76; 867.96; 870.16; 872.36; 874.57; 876.77; 878.97; 881.18; 883.38; 885.59; 887.79; 890.00; 892.21; 894.42; 896.63; 898.84; 901.05; 903.23; 905.48; 907.69; 909.91; 912.12; 914.34; 916.56 and 918.78. |
| MSC | 601.81; 608.1; 610.19; 612.29; 614.39; 616.49; 618.59; 620.69; 622.79; 624.89; 626.99; 629.1; 631.2; 633.31; 635.41; 637.52; 639.63; 641.74; 643.85; 645.96; 648.07; 650.18; 652.3; 654.41; 656.53; 658.64; 660.76 and 662.88. | |
| SNV | 601.81; 610.19; 612.29; 614.39; 616.49; 618.59; 620.69; 622.79; 635.41; 637.52; 639.63; 641.74; 643.85; 645.96; 648.07; 650.18; 652.3; 654.41; 656.53; 934.33; 936.56; 938.78; 941.01; 943.24; 945.47; 947.7; 949.93 and 952.16. | |
| Embryonic axis | Raw | 756.9; 759.06; 761.22; 763.38; 765.53; 767.69; 769.85; 772.02; 774.18; 776.34; 778.51; 780.67; 782.84; 785.00; 787.17; 789.34; 791.51; 793.68; 795.85; 798.02; 800.19; 802.36; 804.54; 806.71; 808.89; 811.07; 813.24 and 817.6. |
| MSC | 597.63; 599.72; 601.81; 603.91; 606.00; 608.1; 610.19; 612.29; 614.39; 616.49; 618.59; 620.69; 622.79; 624.89; 626.99; 629.1; 631.2; 633.31; 635.41; 637.52; 639.63; 641.74; 643.85; 645.96; 648.07; 932.11; 934.33 and 936.56. | |
| SNV | 612.29; 614.39; 616.49; 618.59; 620.69; 622.79; 624.89; 626.99; 629.1; 631.2; 633.31; 635.41; 637.52; 639.63; 641.74; 970.04; 972.28; 974.52; 976.76; 979.01; 981.25; 983.49; 985.74; 987.98; 990.23; 992.48; 996.97 and 1000. |
| Region of Interest | Method of Preprocessing | Calibration Set (%) | Prediction Set (%) | ||||
|---|---|---|---|---|---|---|---|
| Overall Accuracy (%) | |||||||
| ANN | DT | PLS-DA | ANN | DT | PLS-DA | ||
| Cotyledons | Raw | 97 | 70 | 100 | 53 | 65 | 68 |
| MSC | 98 | 94 | 100 | 88 | 86 | 100 | |
| SNV | 100 | 100 | 100 | 83 | 81 | 89 | |
| Embryonic axis | Raw | 100 | 84 | 93 | 76 | 85 | 90 |
| MSC | 100 | 99 | 100 | 95 | 92 | 100 | |
| SNV | 98 | 97 | 80 | 54 | 66 | 73 | |
| Region of Interest | Method of Preprocessing | Calibration Set | Prediction Set | ||||
|---|---|---|---|---|---|---|---|
| Overall Accuracy (%) | |||||||
| ANN | DT | PLS-DA | ANN | DT | PLS-DA | ||
| Cotyledons | Raw | 94 | 92 | 100 | 86 | 73 | 97 |
| MSC | 100 | 96 | 93 | 90 | 88 | 92 | |
| SNV | 100 | 96 | 98 | 33 | 46 | 38 | |
| Embryonic axis | Raw | 98 | 100 | 98 | 43 | 50 | 41 |
| MSC | 100 | 100 | 100 | 92 | 85 | 100 | |
| SNV | 98 | 100 | 96 | 30 | 38 | 41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, R.M.; Gomes-Junior, F.G.; Carmo-Filho, A.d.S.; Ribeiro, G.d.F.R.; Rego, C.H.Q.; Iost-Filho, F.H.; Yamamoto, P.T. Evaluation of the Effect of the Vigor of Soybean Seeds Treated with Micronutrients Using X-ray Fluorescence Spectroscopy and Hyperspectral Imaging. Agronomy 2023, 13, 1945. https://doi.org/10.3390/agronomy13071945
Alves RM, Gomes-Junior FG, Carmo-Filho AdS, Ribeiro GdFR, Rego CHQ, Iost-Filho FH, Yamamoto PT. Evaluation of the Effect of the Vigor of Soybean Seeds Treated with Micronutrients Using X-ray Fluorescence Spectroscopy and Hyperspectral Imaging. Agronomy. 2023; 13(7):1945. https://doi.org/10.3390/agronomy13071945
Chicago/Turabian StyleAlves, Rafael Mateus, Francisco Guilhien Gomes-Junior, Abimael dos Santos Carmo-Filho, Glória de Freitas Rocha Ribeiro, Carlos Henrique Queiroz Rego, Fernando Henrique Iost-Filho, and Pedro Takao Yamamoto. 2023. "Evaluation of the Effect of the Vigor of Soybean Seeds Treated with Micronutrients Using X-ray Fluorescence Spectroscopy and Hyperspectral Imaging" Agronomy 13, no. 7: 1945. https://doi.org/10.3390/agronomy13071945
APA StyleAlves, R. M., Gomes-Junior, F. G., Carmo-Filho, A. d. S., Ribeiro, G. d. F. R., Rego, C. H. Q., Iost-Filho, F. H., & Yamamoto, P. T. (2023). Evaluation of the Effect of the Vigor of Soybean Seeds Treated with Micronutrients Using X-ray Fluorescence Spectroscopy and Hyperspectral Imaging. Agronomy, 13(7), 1945. https://doi.org/10.3390/agronomy13071945








