Effects of UV-B Radiation on the Chemical Composition of Azolla and Its Decomposition after Returning to the Field and Nitrogen Transformation in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Overview and Test Materials
2.2. Experimental Design
2.3. Determination of Chemical Components of Plants
2.4. Determination of Soil Nitrogen Content
2.5. Determination of Soil Enzyme Activity
2.6. Determination of the Soil Nitrogen Transformation Bacterial Community
2.7. Statistical Analysis of Data
3. Results
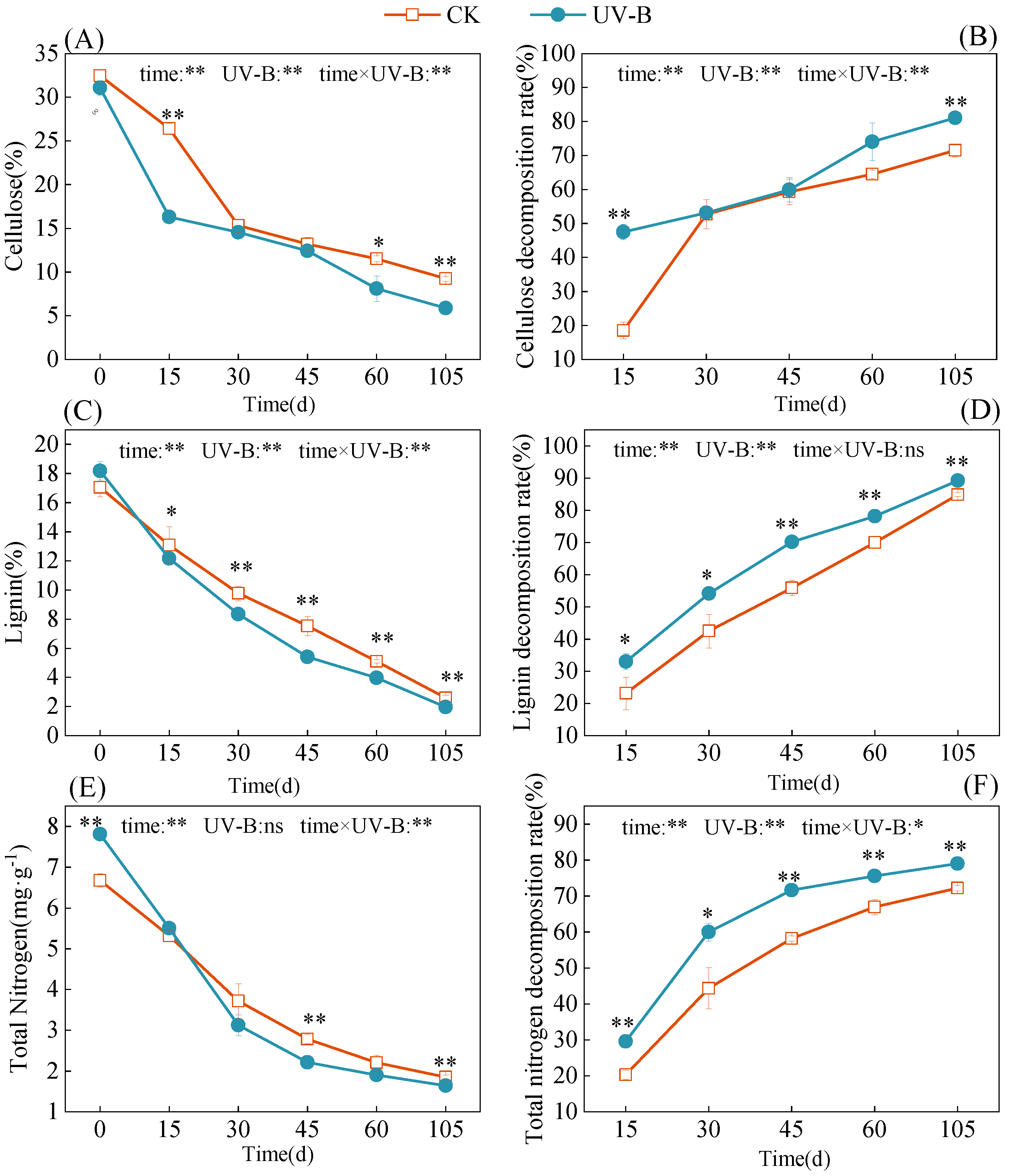
3.1. Effect of UV-B Radiation on the Chemical Composition of Azolla
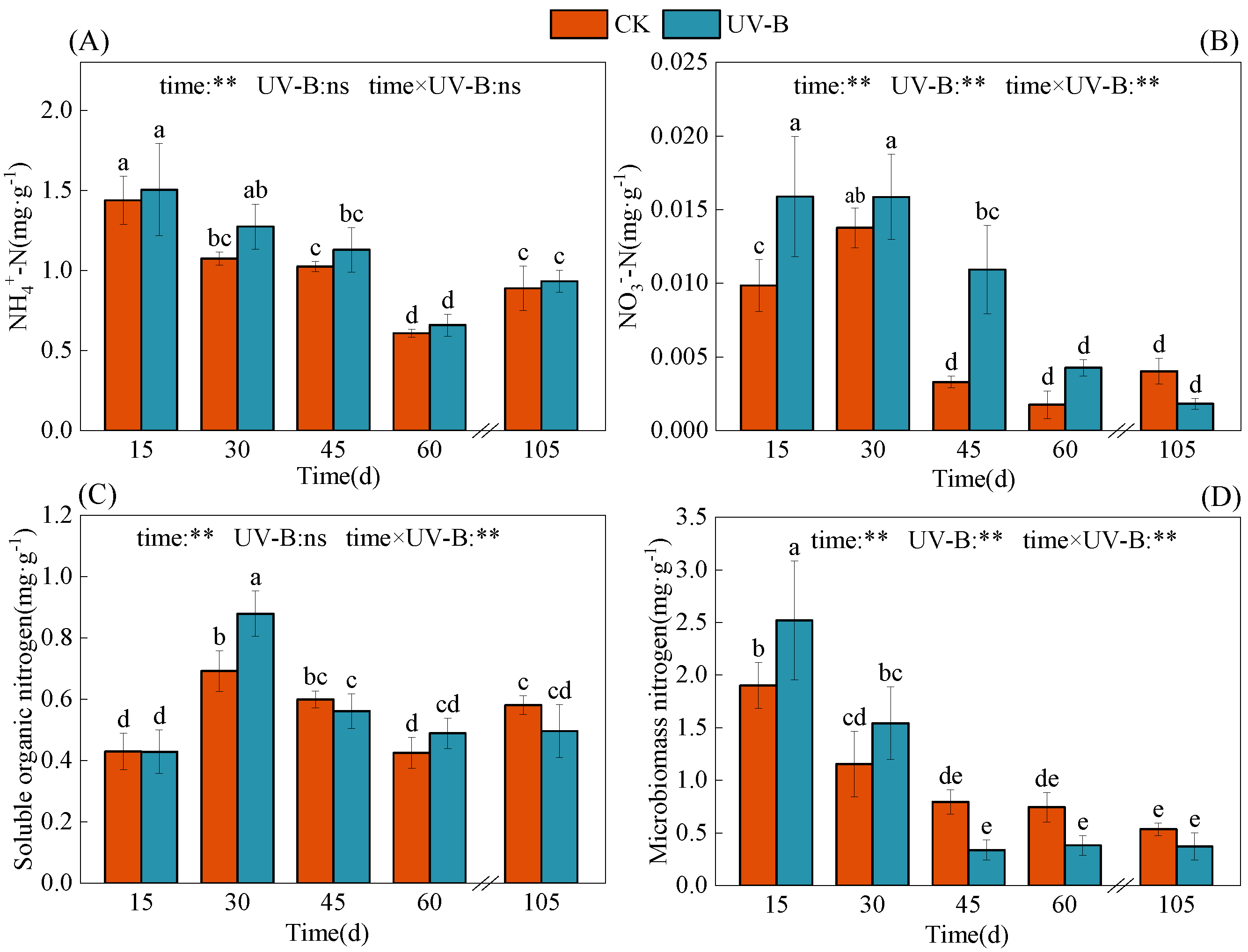
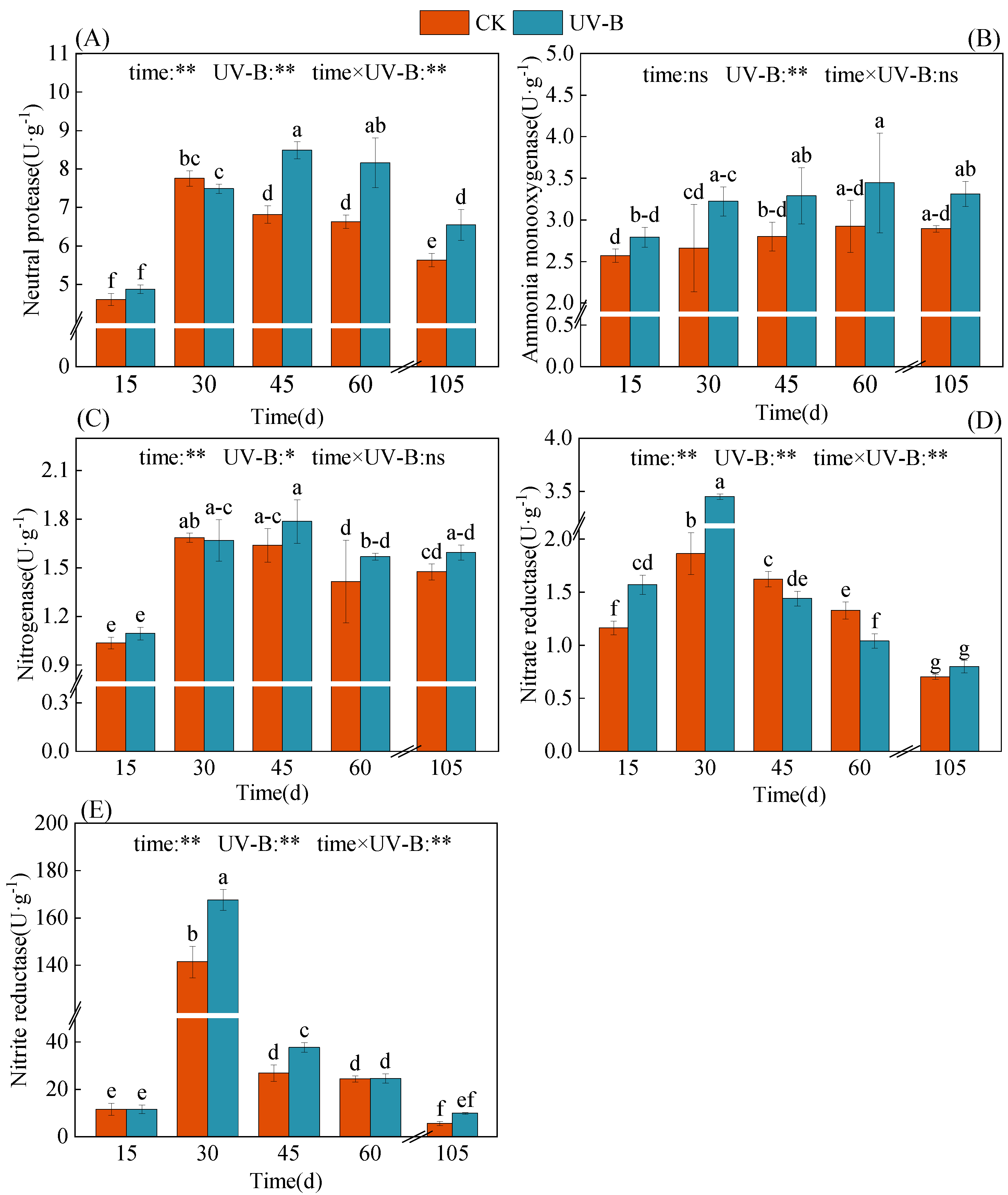
3.2. Decomposition of Azolla and Its Effect on Soil Nitrogen Transformation
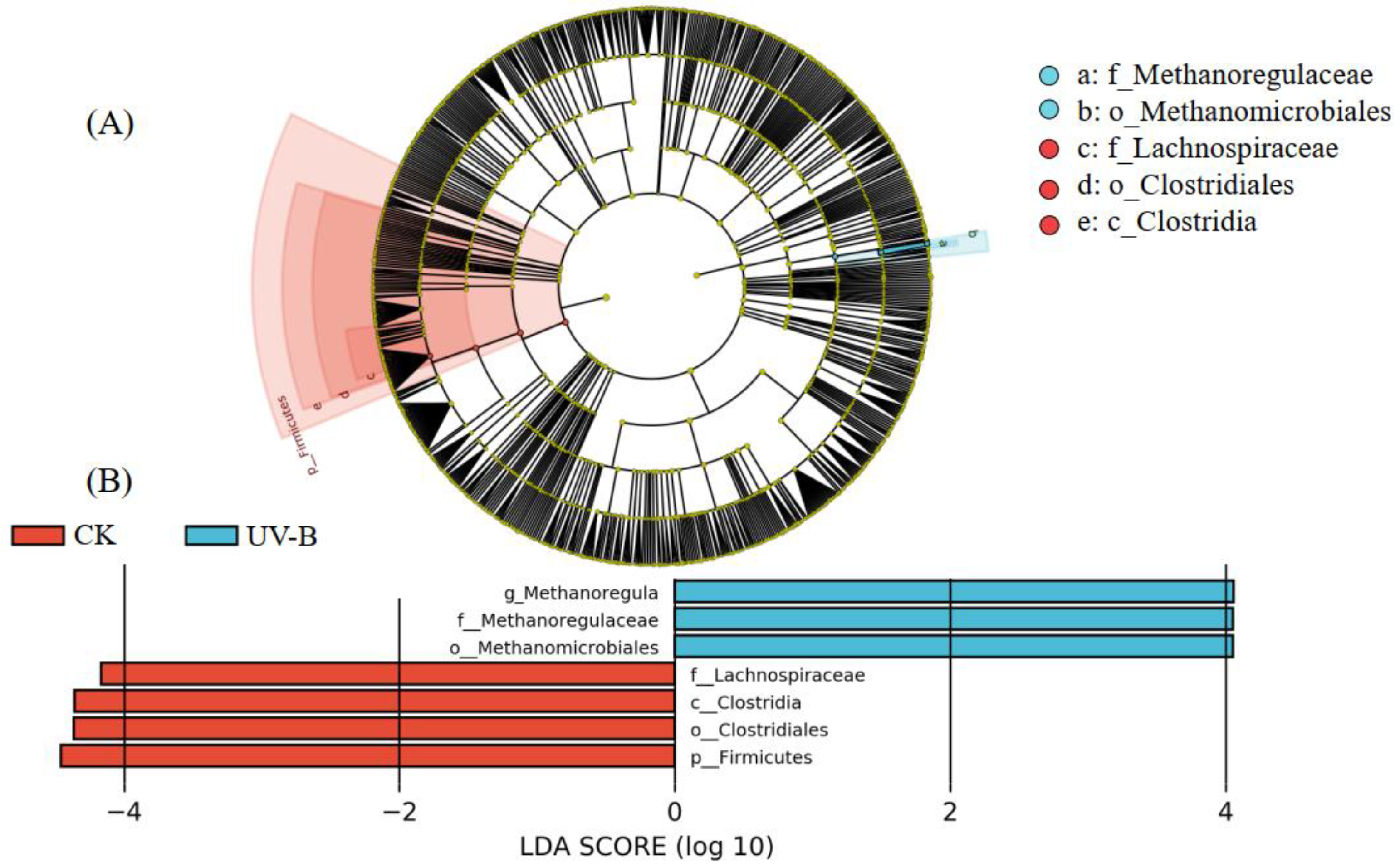
3.3. Effect of Azolla Decomposition on Soil Biological Properties
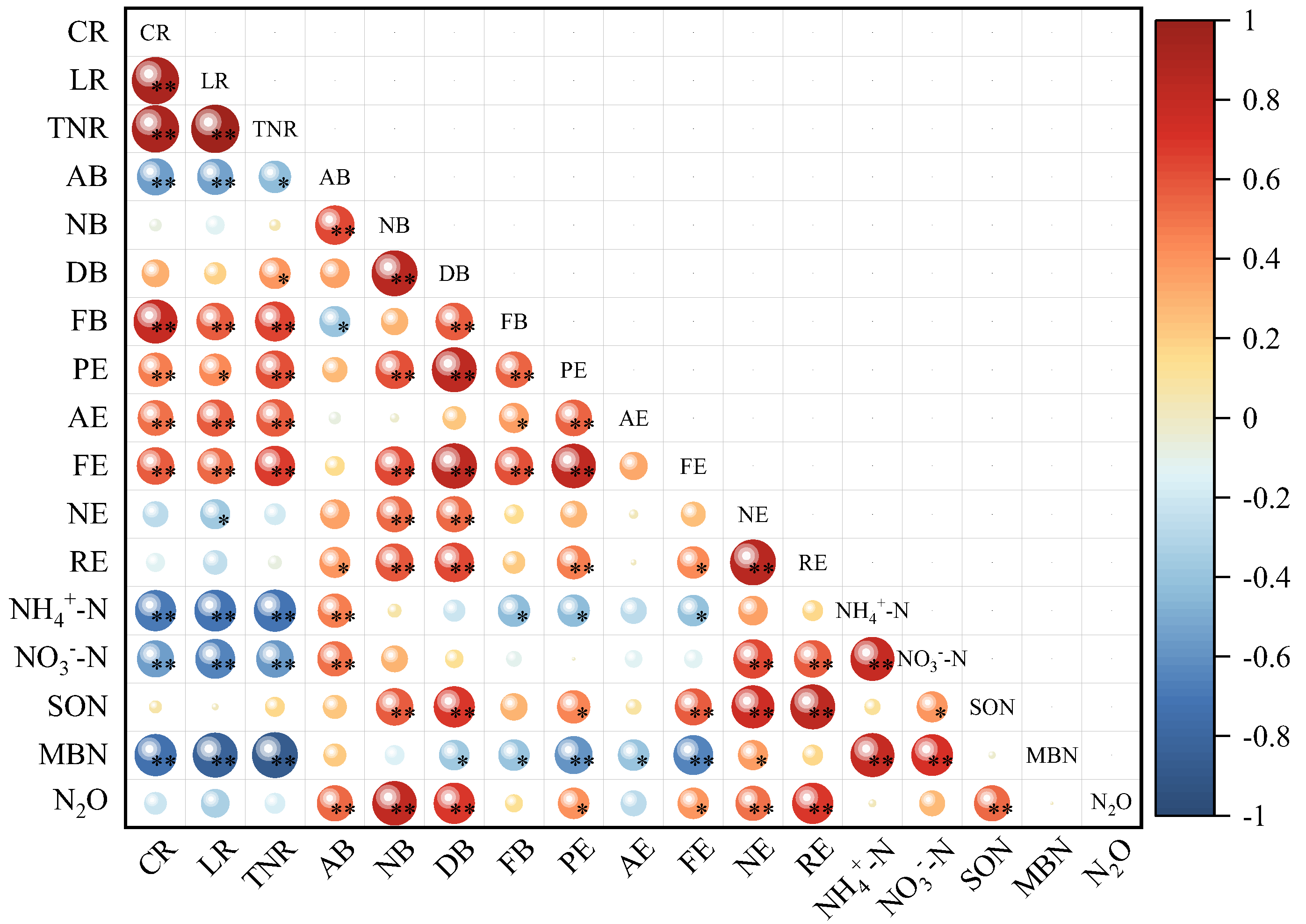
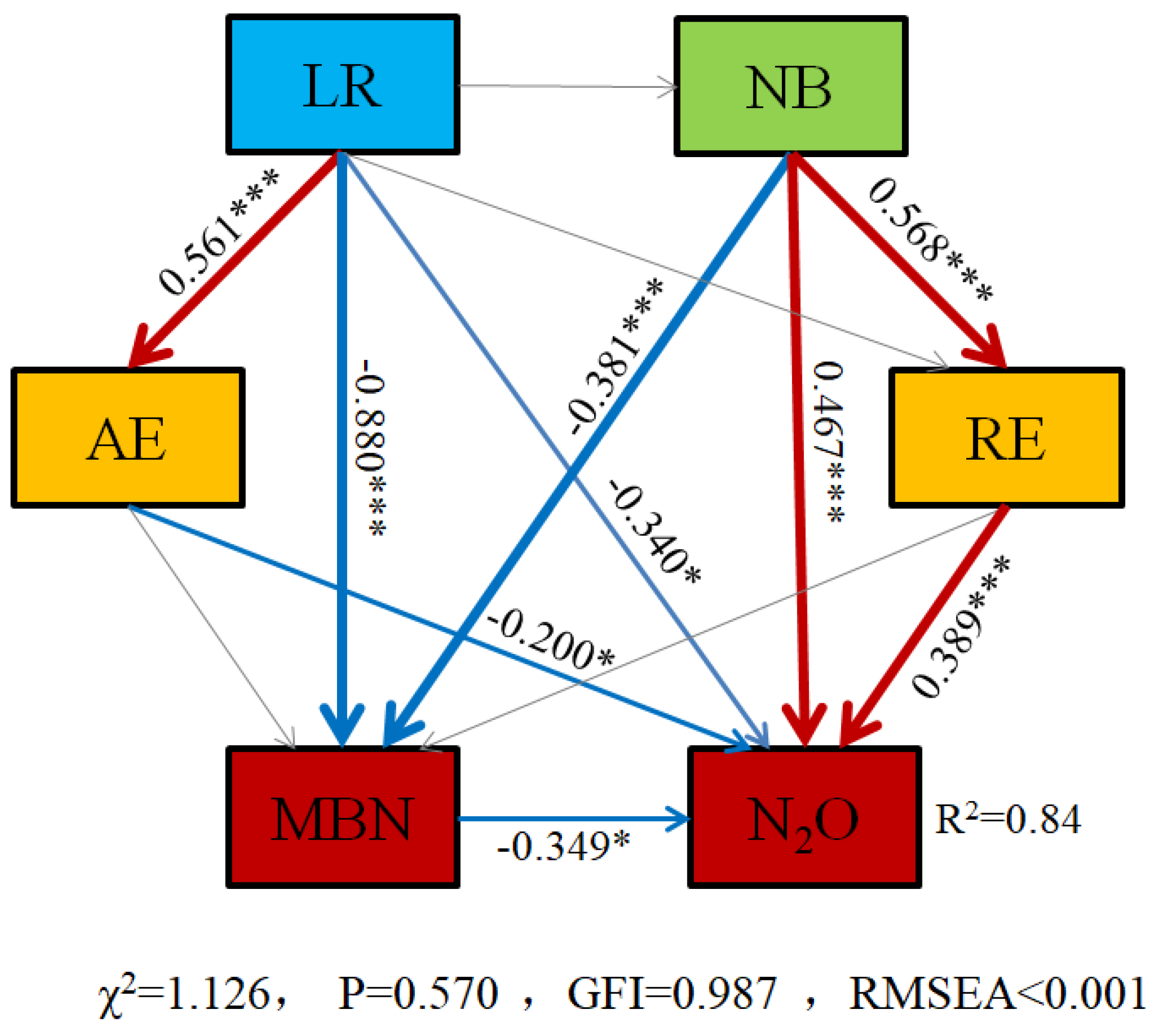
3.4. Correlation Analysis
4. Discussion
4.1. Response of Chemical Composition Change and Residue Decomposition of Azolla to Enhanced UV-B Radiation
4.2. Response of Nitrogen Transformation in Paddy Soil to Azolla Returning to Field after UV-B Radiation
4.3. Response of N2O Emission from Paddy Soil to the Application of Azolla after UV-B Radiation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, S.D.; Ivy, J.; Kinnison, D.; Mills, M.J.; Neely, R.R.; Schmidt, A. Emergence of healing in the Antarctic ozone layer. Science 2016, 353, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Pyle, J.A.; Chipperfield, M.P.; Daniel, J.S.; Park, S.; Prinn, R.G. Challenges for the recovery of the ozone layer. Nat. Geosci. 2019, 12, 592–596. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.H. Possible impacts of changes in UV-B radiation on North American trees and forests. Environ. Pollut. 2005, 137, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Spahic, I.; Gaberscik, A. Morphological, biochemical and physiological responses of Indian cress (Tropaeolum majus) to elevated UV-B radiation. Period. Biol. 2015, 117, 357–364. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Li, Z.L.; Tian, D.S.; Wang, B.X.; Wang, J.S.; Wang, S.; Han, Y.H.; Xu, X.F.; Wang, C.H.; He, N.P.; Niu, S.L. Microbes drive global soil nitrogen mineralization and availability. Glob. Chang. Biol. 2019, 25, 1078–1088. [Google Scholar] [CrossRef]
- Polvani, L.M.; Previdi, M.; England, M.R.; Chiodo, G.; Smith, K.L. Substantial twentieth-century Arctic warming caused by ozone-depleting substances. Nat. Clim. Chang. 2020, 10, 130–133. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Jiang, Y.L.; Zhou, G.S. Nitrogen cycles in terrestrial ecosystems: Climate change impacts and mitigation. Environ. Rev. 2016, 24, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Ryo, M.; Lehmann, A.; Carlos, A.; Trigueros, A.; Buchert, S.; Wulf, A.; Iwasaki, A.; Roy, J.; Yang, G.W. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 2019, 366, 886–890. [Google Scholar] [CrossRef]
- Oyange, W.A.; Chemining’wa, G.N.; Kanya, J.I.; Njiruh, P.N. Azolla fern in Mwea Irrigation Scheme and its potential nitrogen contribution in paddy rice production. J. Agric. Sci. 2019, 11, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.M.; Kumar, S.; Parihar, P.; Rachana, S. Interactive effects of herbicide and enhanced UV-B on growth, oxidative damage and the ascorbate-glutathione cycle in two Azolla species. Ecotoxicol. Environ. Saf. 2016, 133, 341–349. [Google Scholar] [CrossRef]
- Adzman, N.; Goh, S.J.; Johari, A.; Zainal Alam, M.N.H.; Kamaruddin, M.J. Preliminary study on Azolla cultivation and characterization for sustainable biomass source. J. Phys. Conf. Ser. 2022, 2259, 012018. [Google Scholar] [CrossRef]
- Yadav, R.K.; Abraham, G.; Singh, Y.V.; Singh, P.K. Advancements in the utilization of Azolla-anabaena system in relationto sustainable agricultural practices. Proc. Indian Natl. Sci. Acad. 2014, 80, 301–316. [Google Scholar] [CrossRef]
- Oinam, G.; Indira, W.; Singh, O.A.; Indrama, T.; Singh, K.O.; Koijam, L.; Silvia, C.; Sharma, A.S.; Khangembam, R.; Shamjetshabam, M.; et al. Identification of diazotrophic nostocalean yanobacteria of north eastern region of India and evaluation for nitrogenase activity and extracellular ammonium excretion. J. Appl. Biol. Biotechnol. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Kollah, B.; Patra, A.K.; Mohanty, S.R. Aquatic microphylla Azolla: A perspective paradigm for sustainable agriculture, environment and global climate change. Environ. Sci. Pollut. Res. 2016, 23, 4358–4369. [Google Scholar] [CrossRef]
- Brouwer, P.; van der Werf, A.; Schluepmann, H.; Reichart, G.J.; Nierop, K.G. Lipid yield and composition of Azolla filiculoides and the implications for biodiesel production. BioEnergy Res. 2016, 9, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.M.; Lu, P.Z.; Yang, K. Restoration using Azolla imbricata increases nitrogen functional bacterial groups and genes in soil. Appl. Microbiol. Biotechnol. 2017, 101, 3849–3859. [Google Scholar] [CrossRef]
- Fullen, M.A.; Zhu, Y.Y.; Wu, B.Z.; Li, C.Y.; Li, Y.M.; An, T.X.; Colinet, G. Agroenvironmental sustainability of the Yuanyang rice terraces in Yunnan Province, China. In Ecosystem Services of Headwater Catchments; Springer: Cham, Switzerland, 2017; pp. 117–126. [Google Scholar] [CrossRef] [Green Version]
- Li, M.L.; Zhang, Y.X.; Xu, M.; He, L.L.; Liu, L.T.; Tang, Q.S. China Eco-Wisdom: A Review of Sustainability of Agricultural Heritage Systems on Aquatic-Ecological Conservation. Multidiscip. Digit. Publ. Inst. 2020, 12, 60. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhan, G.Q.; Li, M.R.; Zhan, F.D.; Li, Y.; Zu, Y.Q.; He, Y.M. Effects of UV-B radiation and nitrogen application on the growth, mineral nutrition, and antioxidant physiology of Azolla. J. Agric. Resour. Environ. 2022, 39, 567–574. [Google Scholar] [CrossRef]
- Bao, S.D. Analysis of Soil Agronomy, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Chen, Q.Y.; Xiao, S.L.; Shi, S.Q.; Cai, L.P. Isolation of Cellulose from Poplar Wood by Nitric Acid-Ethanol Treatment and Its Effect on the Quality of Films Cast from Ionic Liquid. BioResources 2018, 13, 8943–8955. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, M.; Hou, M.; Chen, Y.M.; Sui, Y.Y.; Jiao, X.G. Regulation of nitrogen balance and yield on greenhouse eggplant under biochar addition in Mollisol. Plant Soil Environ. 2022, 68, 36–48. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Zhang, S.L.; Keay, P. Soluble organic nitrogen pools in forest soils of subtropical Australia. Plant Soil 2005, 277, 285–297. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Yang, P.; He, Q.H.; Huang, J.F.; Tong, C. Fluxes of greenhouse gases at two different aquaculture ponds in the coastal zone of southeastern China. Atmos. Environ. 2015, 115, 269–277. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.H.; Truong, H.A.; Lee, Y.J.; Lee, H. Enhanced nitrate reductase activity offers Arabidopsis ecotype Landsberg erecta better salt stress resistance than Col-0. Plant Biol. 2022, 24, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y. Enzyme Engineering, 2nd ed.; Science Press: Beijing, China, 2004. [Google Scholar]
- Li, Z.G.; Luo, Y.M.; Teng, Y. Research Method of Soil and Environment Microorganism; Science Press: Beijing, China, 2008. [Google Scholar]
- Gong, X.; Wang, S.; Wang, Z.W.; Jiang, Y.J.; Hu, Z.K.; Zheng, Y.; Chen, X.Y.; Li, H.X.; Hu, F.; Liu, M.Q.; et al. Earthworms modify soil bacterial and fungal communities through enhancing aggregation and buffering pH. Geoderma 2019, 347, 59–69. [Google Scholar] [CrossRef]
- Masood, A.; Zeeshan, M.; Abraham, G. Response of growth and antioxidant enzymes in Azollaplants (Azolla pinnata and Azolla filiculoides) exposed to UV-B. Acta Biol. Hung. 2008, 59, 247–258. [Google Scholar] [CrossRef]
- Mostafa, E.M.; Hassanm, A.M.A. The ameliorative effect of selenium on Azolla caroliniana grown under UV-B stress. Phytoprotection 2015, 95, 20. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.M.; Kumar, S.; Parihar, P.; Singh, A.; Singh, R. Evaluating the combined effects of pretilachlor and UV-B on two Azolla species. Pestic. Biochem. Physiol. 2016, 128, 45–56. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mostafa, E.M. UV-B Effect on Constituents of Azolla caroliniana. Z. Für Naturforschung C 2015, 62, 3–4. [Google Scholar] [CrossRef]
- Pancotto, V.A.; Sala, O.E.; Robson, T.M.; Caldwell, M.M.; Scopel, A.L. Direct and indirect effects of solar ultraviolet radiation on long-term decomposition. Glob. Chang. Biol. 2005, 11, 1982–1989. [Google Scholar] [CrossRef]
- Solheim, B.; Zielke, M.; Bjerke, J.W.; Rozema, J. Effects of enhanced UV-B radiation on nitrogen fixation in arctic ecosystems. Plants Clim. Chang. 2006, 182, 109–120. [Google Scholar] [CrossRef]
- Chieri, K.; Tomomi, E.; Mark, K. UV-B radiation dose requirement for suppressing intumescence injury on tomato plants. Sci. Hortic. 2017, 226, 366–371. [Google Scholar] [CrossRef]
- Singh, M.; Khan, M.; Naeem, M. Effect of nitrogen on growth, nutrient assimilation, essential oil content, yield and quality attributes in Zingiber officinale Rosc. J. Saudi Soc. Agric. Sci. 2016, 15, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Tchiaze, A.I.; Taffouo, V.D.; Fankem, H.; Kenne, M.; Baziramakenga, R.; Ekodeck, G.E.; Antoun, H. Influence of Nitrogen Sources and Plant Growth-Promoting Rhizobacteria Inoculation on Growth, Crude Fiber and Nutrient Uptake in Squash (Cucurbita moschata Duchesne ex Poir.) Plants. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 53–59. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, T.; Cai, Z.; Müller, C. Nitrogen cycling in forest soils across climate gradients in Eastern China. Plant Soil 2011, 342, 419–432. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, X.; Ding, W.; Yan, Y.; Lu, X. Effects of freeze-thaw on soil nitrogen transformation and N2O emission: A review. Acta Pedol. Sin. 2013, 50, 1032–1042. [Google Scholar] [CrossRef]
- Bhuvaneshwari, K.; Singh, P.K. Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. 3 Biotech. 2015, 5, 523–529. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Musiani, F.; Broll, V.; Evangelisti, E.; Ciurli, S. The model structure of the copper-dependent ammonia monooxygenase. J. Biol. Inorg. Chem. 2020, 25, 995–1007. [Google Scholar] [CrossRef]
- Cai, Y.J.; Ding, W.X.; Xiang, J. Mechanisms of nitrous oxide and nitric oxide production in soils: A review. Soil 2012, 44, 712–718. [Google Scholar] [CrossRef]
- Fernandes, S.O.; Javanaud, C.; Michotey, V.D.; Guasco, S.; Anschutz, P.; Bonin, P. Coupling of bacterial nitrification with denitrification and anammox supports N removal in intertidal sediments (Arcachon Bay, France). Estuar. Coast. Shelf Sci. 2016, 20, 39–50. [Google Scholar] [CrossRef]
- Xiao, H.C.; Wu, J.; Zhang, Y.X.; Li, Z.R.; Zhan, F.D.; Li, Y.; He, Y.M. Effects of enhanced UV-B radiation on nitrogen transformation in paddy soil. J. Agro-Environ. Sci. 2022, 9, 1645–1659. [Google Scholar] [CrossRef]
- Feng, B.G.; Guo, P.; Li, L.H.; Sun, Y.; Chen, W.W. Effect of nano-TiO2 onmass ratio of nitrate nitrogen and activity of nitrate reductase in soil. J. Jilin Univ. (Sci. Ed.) 2018, 56, 1570–1576. [Google Scholar] [CrossRef]
- Farrell, M.; Hill, P.W.; Farrar, J.; Bardgett, R.D.; Jones, D.L. Seasonal variation in soluble soil carbon and nitrogen across a grassland productivity gradient. Soil Biol. Biochem. 2011, 43, 835–844. [Google Scholar] [CrossRef]
- Hirsch, P.R.; Mauchline, T.H.; Clark, I.M. Culture-independent molecular techniques for soil microbial ecology. Soil Biol. Biochem. 2010, 42, 878–887. [Google Scholar] [CrossRef]
- Kou, T.J.; Cheng, X.H.; Zhu, J.G.; Xie, Z.B. The influence of ozone pollution on CO2, CH4, and N2O emissions from a Chinese subtropical rice-wheat rotation system under free-air O3 exposure. Agric. Ecosyst. Environ. 2015, 204, 72–81. [Google Scholar] [CrossRef]
- Lou, Y.S.; Ren, L.X.; Zhao, S.D.; Ren, L.X.; Zhang, Y.W.; Zhu, H.W. Effects of silicate application on CH4 and N2O emissions and global warming potentials in paddy soil under enhanced UV-B radiation. Energy Sci. Eng. 2019, 5, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
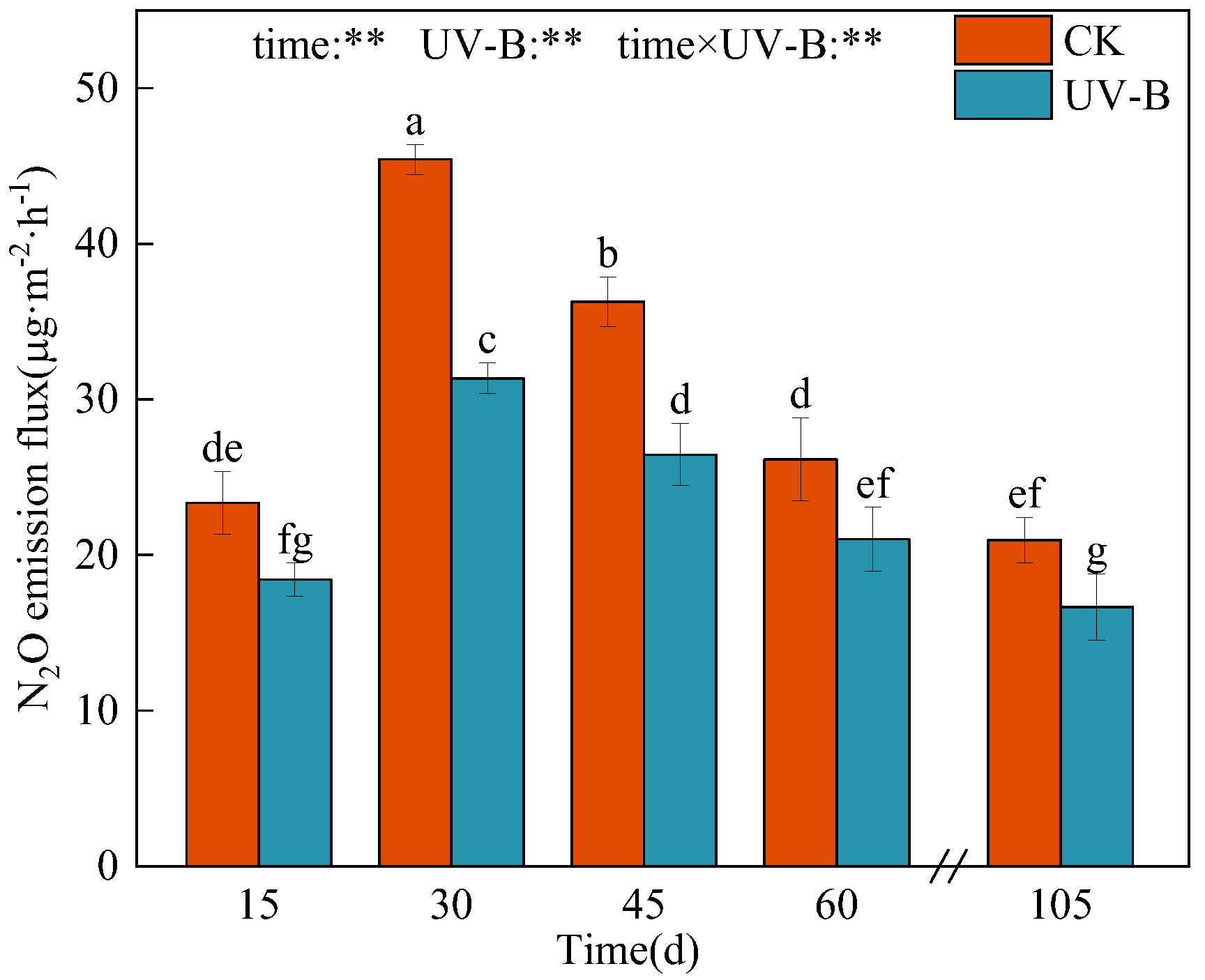


| Treatment | Cellulose (%) | Lignin (%) | Total Nitrogen (mg·g−1) |
|---|---|---|---|
| CK | 32.5 ± 0.92 | 17.1 ± 0.66 | 6.68 ± 0.16 |
| UV-B | 31.1 ± 1.1 | 18.2 ± 0.61 | 7.81 ± 0.16 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.; Li, H.; Liu, C.; Liang, X.; Xie, C.; Li, Z.; Li, Y.; Zhan, F.; He, Y. Effects of UV-B Radiation on the Chemical Composition of Azolla and Its Decomposition after Returning to the Field and Nitrogen Transformation in Soil. Agronomy 2023, 13, 1968. https://doi.org/10.3390/agronomy13081968
Chang L, Li H, Liu C, Liang X, Xie C, Li Z, Li Y, Zhan F, He Y. Effects of UV-B Radiation on the Chemical Composition of Azolla and Its Decomposition after Returning to the Field and Nitrogen Transformation in Soil. Agronomy. 2023; 13(8):1968. https://doi.org/10.3390/agronomy13081968
Chicago/Turabian StyleChang, Linxi, Haitao Li, Chengqian Liu, Xinran Liang, Chunmei Xie, Zuran Li, Yuan Li, Fangdong Zhan, and Yongmei He. 2023. "Effects of UV-B Radiation on the Chemical Composition of Azolla and Its Decomposition after Returning to the Field and Nitrogen Transformation in Soil" Agronomy 13, no. 8: 1968. https://doi.org/10.3390/agronomy13081968





