Modeling Climate Change Indicates Potential Shifts in the Global Distribution of Orchardgrass
Abstract
:1. Introduction
2. Materials and Methods
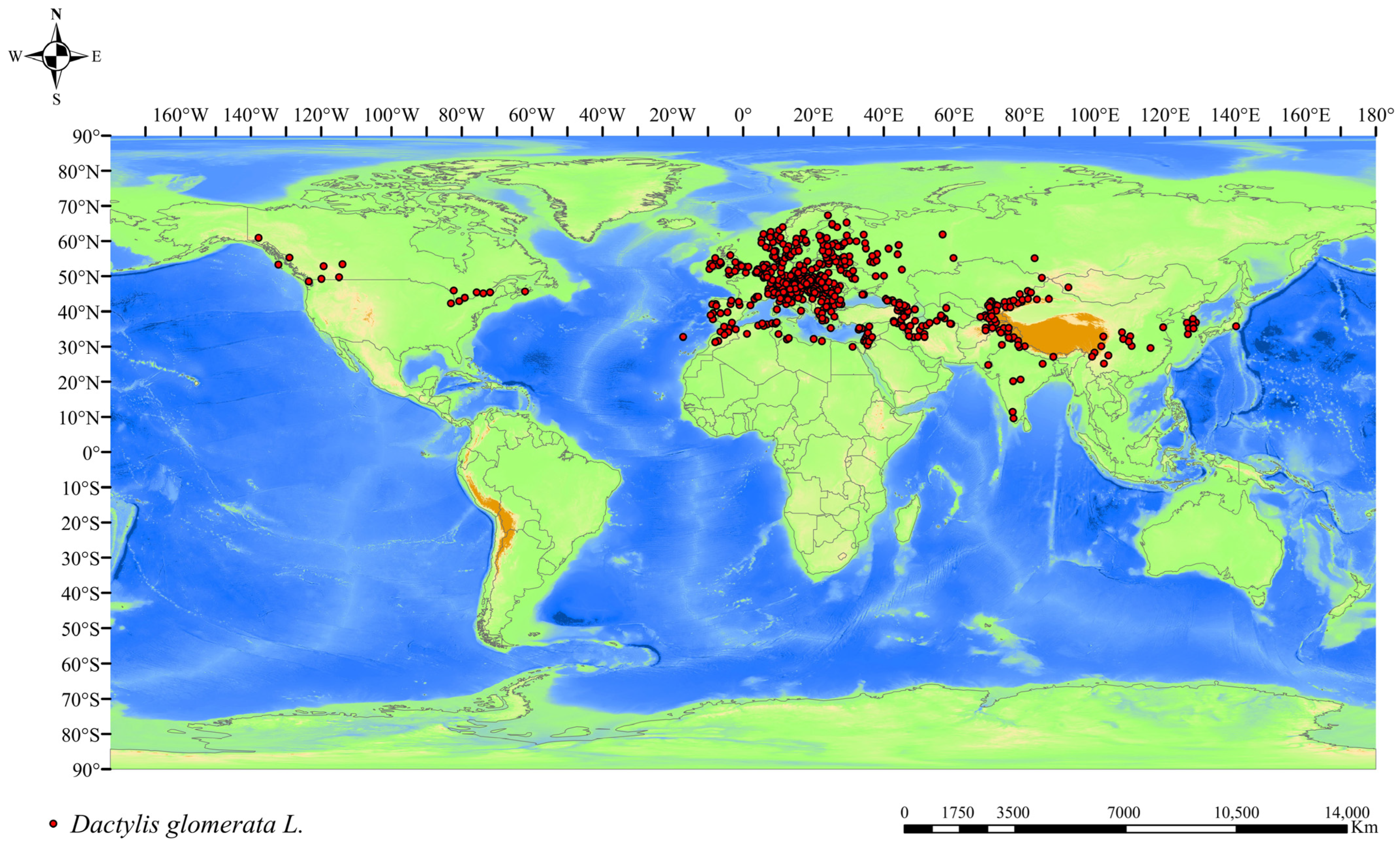
2.1. Data on Species Occurrence
2.2. Environmental Variables
2.3. Optimization of Model Parameters
2.4. Species Distribution Model
2.5. Estimation of Orchardgrass Distribution Area
3. Results
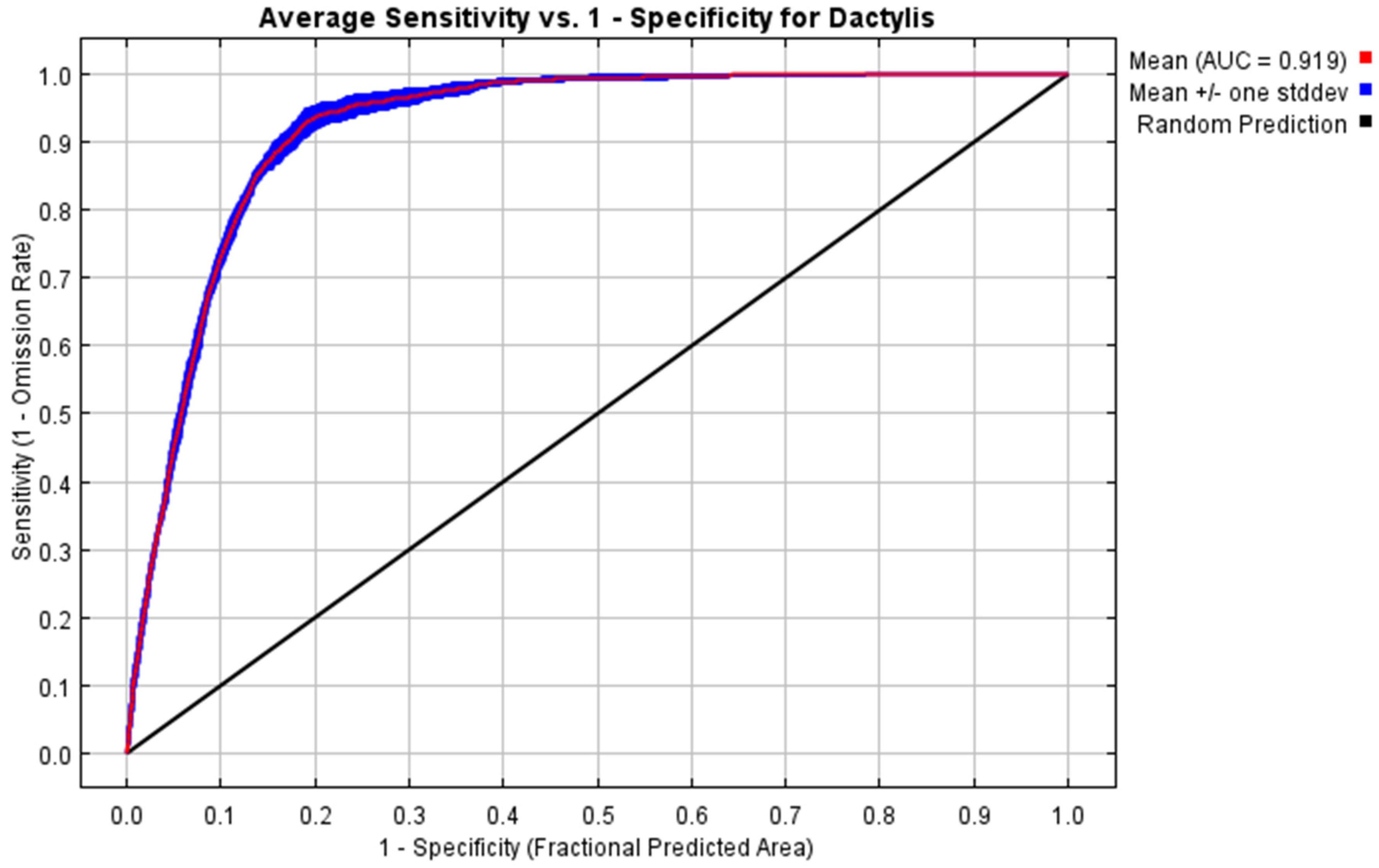
3.1. Modeling of Species Distribution
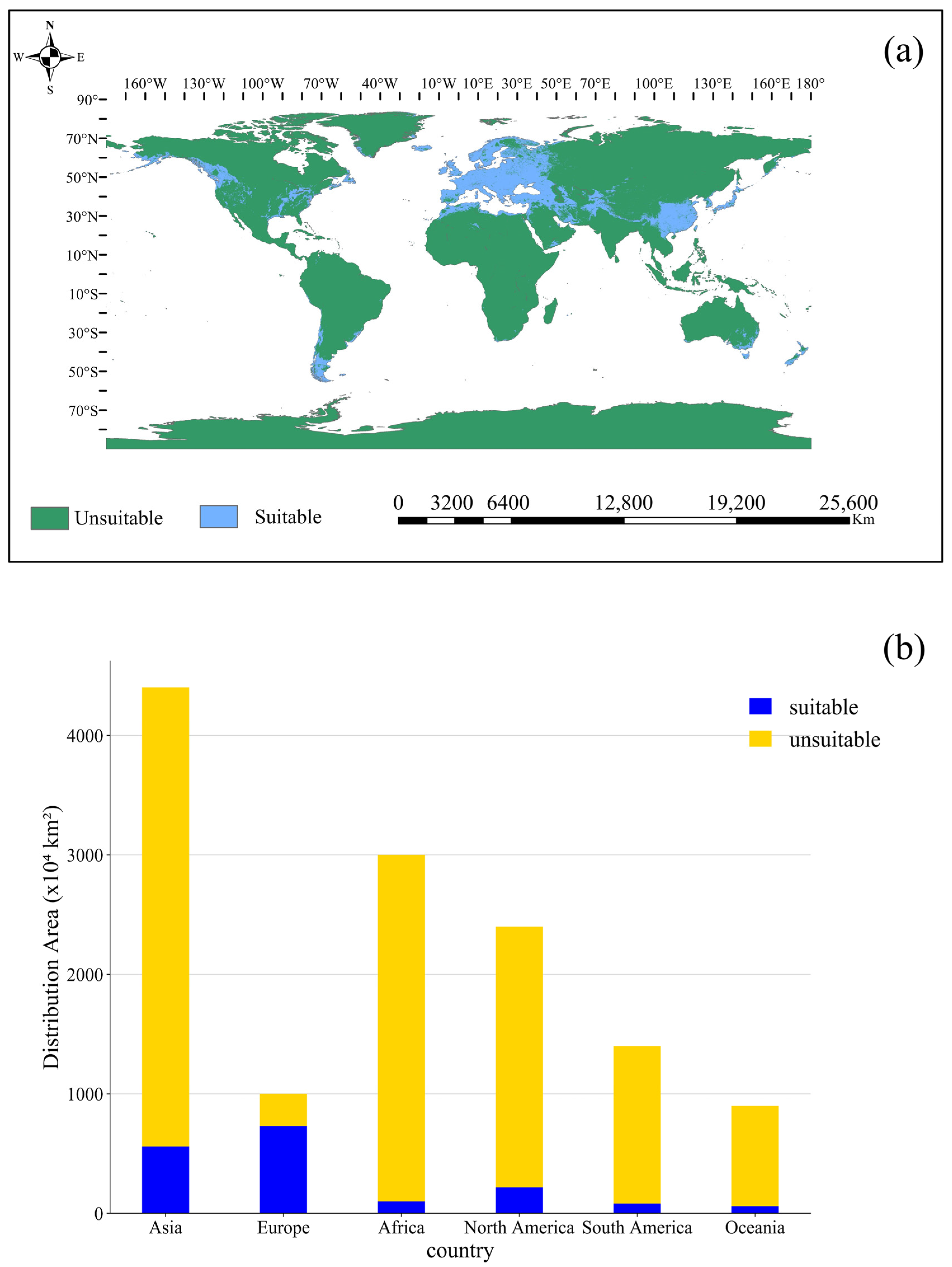
3.2. Current Suitable Distribution for Orchardgrass
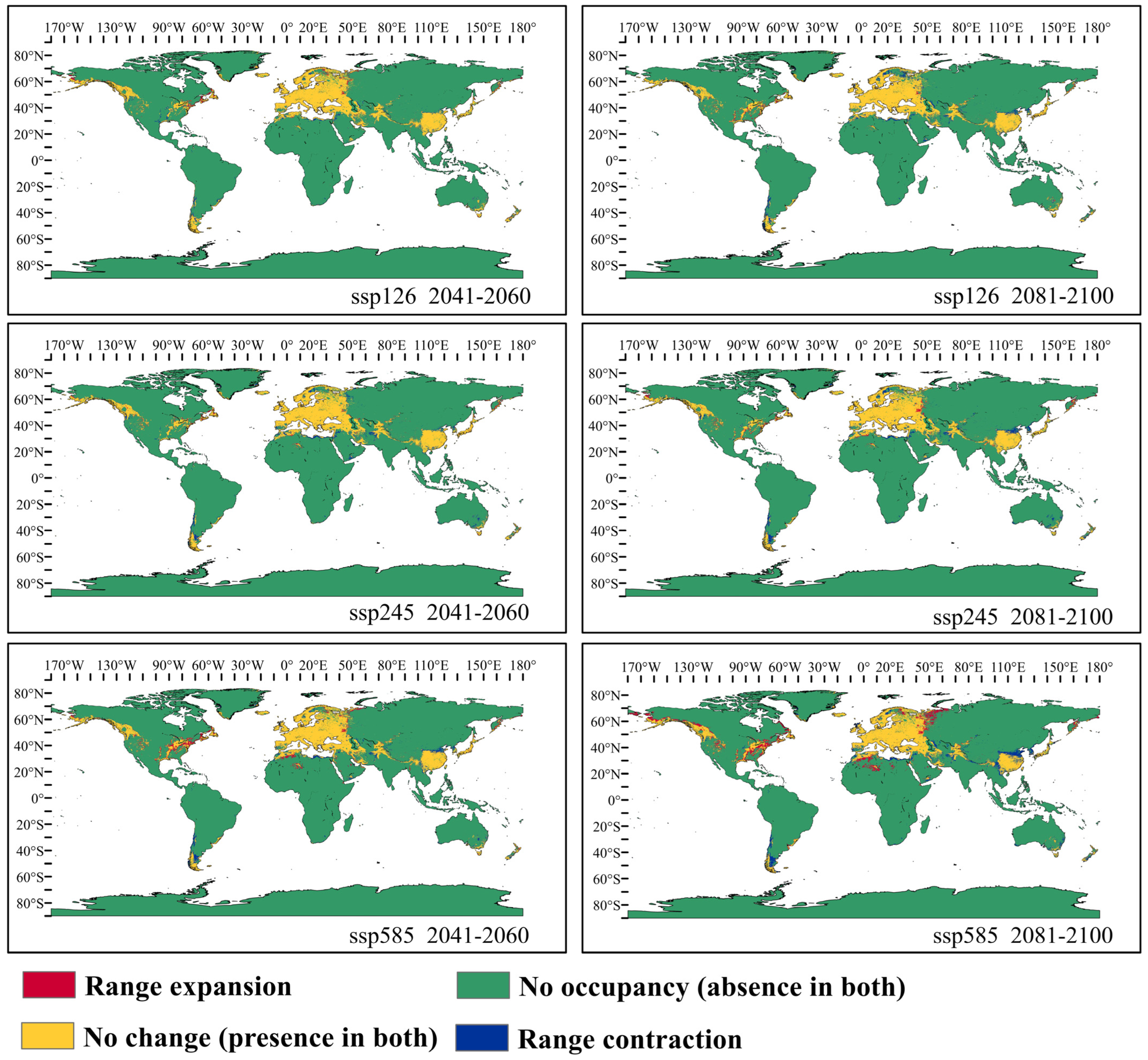
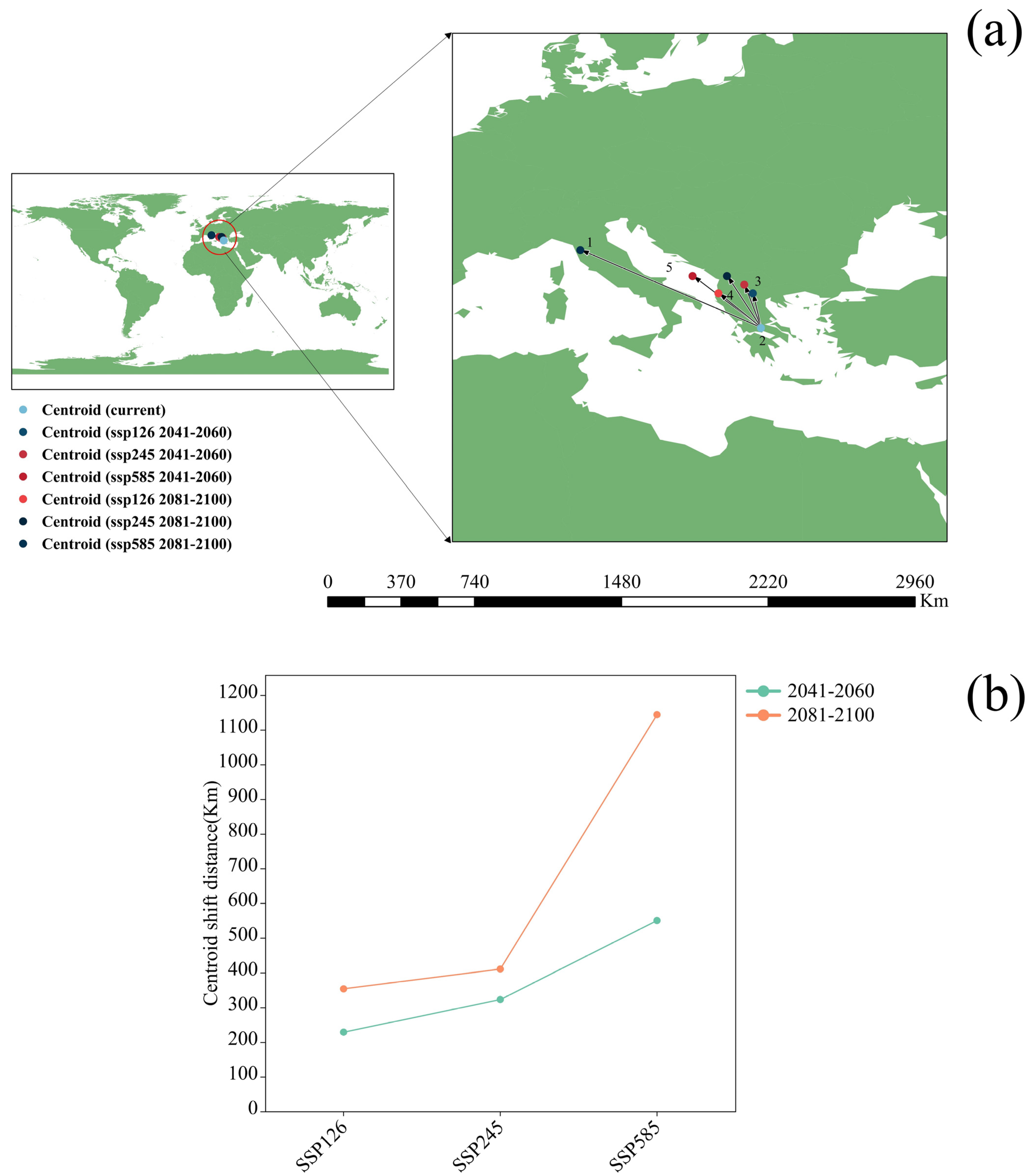
3.3. Potential Distribution of Orchardgrass under Future Climate Conditions
4. Discussion
4.1. MaxEnt Modeling
4.2. Suitable Habitat Distribution Patterns of Orchardgrass under the Current Environment
4.3. Response of Suitable Habitats for Orchardgrass to Future Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirata, M.; Yuyama, N.; Cai, H. Isolation and characterization of simple sequence repeat markers for the tetraploid forage grass Dactylis glomerata: Simple sequence repeat markers for Dactylis. Plant Breed. 2011, 130, 503–506. [Google Scholar] [CrossRef]
- Xie, W.; Bushman, B.S.; Ma, Y.; West, M.S.; Robins, J.G.; Michaels, L.; Jensen, K.B.; Zhang, X.; Casler, M.D.; Stratton, S.D. Genetic diversity and variation in North American orchardgrass (Dactylis glomerata L.) cultivars and breeding lines. Grassl. Sci. 2014, 60, 185–193. [Google Scholar] [CrossRef]
- Kole, C. (Ed.) Wild Crop Relatives: Genomic and Breeding Resources: Millets and Grasses; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Tronsmo, A.M. Resistance to Winter Stress Factors in Half-Sib Families of Dactylis glomerata, Tested in a Controlled Environment. Acta Agric. Scand. Sect. B—Soil Plant Sci. 1993, 43, 89–96. [Google Scholar] [CrossRef]
- Turner, L.R.; Donaghy, D.J.; Lane, P.A.; Rawnsley, R.P. Distribution of Water-Soluble Carbohydrate Reserves in the Stubble of Prairie Grass and Orchardgrass Plants. Agron. J. 2007, 99, 591–594. [Google Scholar] [CrossRef]
- Volaire, F. Seedling survival under drought differs between an annual (Hordeum vulgare) and a perennial grass (Dactylis glomerata). New Phytol. 2003, 160, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Volaire, F.; Conéjero, G.; Lelièvre, F. Drought survival and dehydration tolerance in Dactylis glomerata and Poa bulbosa. Funct. Plant Biol. 2001, 28, 743. [Google Scholar] [CrossRef]
- Stout, W.L.; Jung, G.A. Influences of Soil Environment on Biomass and Nitrogen Accumulation Rates of Orchardgrass. Agron. J. 1992, 84, 1011–1019. [Google Scholar] [CrossRef]
- Jensen, K.B.; Asay, K.H.; Waldron, B.L. Dry Matter Production of Orchardgrass and Perennial Ryegrass at Five Irrigation Levels. Crop Sci. 2001, 41, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Shaimi, N.; Kallida, R.; Volaire, F.; Saidi, N.; Faiz, C.A. Summer Dormancy and Drought Survival of Moroccan Ecotypes of Orchardgrass. Crop Sci. 2009, 49, 1416–1424. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Sanmartín, I. Biogeography: An Ecological and Evolutionary Approach, 7th edition. Syst. Biol. 2006, 55, 361–363. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change (Ed.) Evaluation of Climate Models. In Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014; pp. 741–866. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Lawler, J.J. Climate Change Adaptation Strategies for Resource Management and Conservation Planning. Ann. N. Y. Acad. Sci. 2009, 1162, 79–98. [Google Scholar] [CrossRef]
- Corlett, R.T.; Westcott, D.A. Will plant movements keep up with climate change? Trends Ecol. Evol. 2013, 28, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Gengping, Z.; Guoqing, L.; Wenjun, B.; Yubao, G. Ecological niche modeling and its applications in biodiversity conservation: Ecological niche modeling and its applications in biodiversity conservation. Biodivers. Sci. 2013, 21, 90–98. [Google Scholar] [CrossRef]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, L.J.; Hughes, L.; Poulsen, M. Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol. Model. 2005, 186, 251–270. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest 2. R News 2002, 2, 18–22. [Google Scholar]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists: Statistical explanation of MaxEnt. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Li, G.; Du, S.; Wen, Z. Mapping the climatic suitable habitat of oriental arborvitae (Platycladus orientalis) for introduction and cultivation at a global scale. Sci. Rep. 2016, 6, 30009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merow, C.; Smith, M.J.; Edwards, T.C.; Guisan, A.; McMahon, S.M.; Normand, S.; Thuiller, W.; Wüest, R.O.; Zimmermann, N.E.; Elith, J. What do we gain from simplicity versus complexity in species distribution models? Ecography 2014, 37, 1267–1281. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Z.; Zhu, F.; Gao, B. The impact of global warming on the potential suitable planting area of Pistacia chinensis is limited. Sci. Total Environ. 2023, 864, 161007. [Google Scholar] [CrossRef]
- Mohammadi, S.; Ebrahimi, E.; Shahriari Moghadam, M.; Bosso, L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol. Inform. 2019, 52, 7–13. [Google Scholar] [CrossRef]
- Rong, Z.; Zhao, C.; Liu, J.; Gao, Y.; Zang, F.; Guo, Z.; Mao, Y.; Wang, L. Modeling the Effect of Climate Change on the Potential Distribution of Qinghai Spruce (Picea crassifolia Kom.) in Qilian Mountains. Forests 2019, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Liu, S.; An, Y.; Sun, Y.; Zhao, S.; Liu, Y.; Li, M. Climatic factors and human disturbance influence ungulate species distribution on the Qinghai-Tibet Plateau. Sci. Total Environ. 2023, 869, 161681. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From Nucleotides to Satellite Imagery: Approaches to Identify and Manage the Invasive Pathogen Xylella fastidiosa and Its Insect Vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanço, M.C. Risk of spread of tomato yellow leaf curl virus (TYLCV) in tomato crops under various climate change scenarios. Agric. Syst. 2019, 173, 524–535. [Google Scholar] [CrossRef]
- Sultana, S.; Baumgartner, J.B.; Dominiak, B.C.; Royer, J.E.; Beaumont, L.J. Impacts of climate change on high priority fruit fly species in Australia. PLoS ONE 2020, 15, e0213820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Sterne, T.K.; Retchless, D.; Allee, R.; Highfield, W. Predictive modelling of mesophotic habitats in the north-western Gulf of Mexico. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 846–859. [Google Scholar] [CrossRef]
- Convertino, M.; Annis, A.; Nardi, F. Information-theoretic portfolio decision model for optimal flood management. Environ. Model. Softw. 2019, 119, 258–274. [Google Scholar] [CrossRef] [Green Version]
- Ardestani, E.G.; Mokhtari, A. Modeling the lumpy skin disease risk probability in central Zagros Mountains of Iran. Prev. Vet. Med. 2020, 176, 104887. [Google Scholar] [CrossRef] [PubMed]
- Hanafi-Bojd, A.A.; Vatandoost, H.; Yaghoobi-Ershadi, M.R. Climate Change and the Risk of Malaria Transmission in Iran. J. Med. Entomol. 2020, 57, 50–64. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Wang, J.-G.; Lei, Y.-H. Predicting Distribution of the Asian Longhorned Beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Natural Enemies in China. Insects 2022, 13, 687. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Yu, X.; Tao, X.; Liao, J.; Liu, S.; Xu, L.; Yuan, S.; Zhang, Z.; Wang, F.; Deng, N.; Huang, J.; et al. Predicting potential cultivation region and paddy area for ratoon rice production in China using Maxent model. Field Crops Res. 2022, 275, 108372. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Sun, C.; Xi, B.; Li, D.; Chen, Z.; He, Q.; Weng, X.; Jia, L. Global distribution of soapberries (Sapindus L.) habitats under current and future climate scenarios. Sci. Rep. 2021, 11, 19740. [Google Scholar] [CrossRef]
- Santana, P.A.; Kumar, L.; Da Silva, R.S.; Pereira, J.L.; Picanço, M.C. Assessing the impact of climate change on the worldwide distribution of Dalbulus maidis (DeLong) using MaxEnt. Pest Manag. Sci. 2019, 75, 2706–2715. [Google Scholar] [CrossRef] [Green Version]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.-P.; Cheng, F.-Y.; Hu, X.-G.; Mao, J.-F.; Xu, X.-X.; Zhong, Y.; Li, S.-Y.; Xian, H.-L. Modelling environmentally suitable areas for the potential introduction and cultivation of the emerging oil crop Paeonia ostii in China. Sci. Rep. 2019, 9, 3213. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhang, Y.; Tao, J. Predicting the Potential Distribution of Paeonia veitchii (Paeoniaceae) in China by Incorporating Climate Change into a Maxent Model. Forests 2019, 10, 190. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Li, Z.; Liu, Z.; Yang, Y.; Khoso, A.G.; Wang, L.; Liu, D. Climate change simulations revealed potentially drastic shifts in insect community structure and crop yields in China’s farmland. J. Pest Sci. 2023, 96, 55–69. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping Species Distributions with MAXENT Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias (Valentine, J.F., editor). PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aidoo, O.F.; Souza, P.G.; da Silva, R.S.; Santana, P.A., Jr.; Picanço, M.C.; Kyerematen, R.; Sètamou, M.; Ekesi, S.; Borgemeister, C. Climate-induced range shifts of invasive species (Diaphorina citri Kuwayama). Pest Manag. Sci. 2022, 78, 2534–2549. [Google Scholar] [CrossRef] [PubMed]
- Keenan, R.W.; Kruczek, M.E. The esterification of dolichol by rat liver microsomes. Biochemistry 1976, 15, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Esezobo, S.; Pilpel, N. Moisture and gelatin effects on the interparticle attractive forces and the compression behaviour of oxytetracycline formulations. J. Pharm. Pharmacol. 1977, 29, 75–81. [Google Scholar] [CrossRef]
- Srivastava, V.; Roe, A.D.; Keena, M.A.; Hamelin, R.C.; Griess, V.C. Oh the places they’ll go: Improving species distribution modelling for invasive forest pests in an uncertain world. Biol. Invasions 2021, 23, 297–349. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling: Insights into the AUC. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. Correction: The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S.V. Species-Distribution Modeling: Advantages and Limitations of Its Application. 2. MaxEnt. Biol. Bull. Rev. 2021, 11, 265–275. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.V.; Ellison, N.W. Dactylis. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 73–87. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Prevéy, J.S.; Parker, L.E.; Harrington, C.A.; Lamb, C.T.; Proctor, M.F. Climate change shifts in habitat suitability and phenology of huckleberry (Vaccinium membranaceum). Agric. For. Meteorol. 2020, 280, 107803. [Google Scholar] [CrossRef]
- Davidson, J.L.; Milthorpe, F.L. The Effect of Temperature on the Growth of Cocksfoot (Dactylis glomerata L.). Ann. Bot. 1965, 29, 407–417. [Google Scholar] [CrossRef]
- Ahmed, L.Q.; Escobar-Gutiérrez, A.J. Analysis of intra-specific variability of cocksfoot (Dactylis glomerata L.) in response to temperature during germination. Acta Physiol. Plant 2022, 44, 117. [Google Scholar] [CrossRef]
- Sanada, Y.; Gras, M.-C.; van Santen, E. Cocksfoot. In Fodder Crops and Amenity Grasses; Boller, B., Posselt, U.K., Veronesi, F., Eds.; Springer: New York, NY, USA, 2010; pp. 317–328. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hansen, W.D.; Braziunas, K.H.; Rammer, W.; Seidl, R.; Turner, M.G. It takes a few to tango: Changing climate and fire regimes can cause regeneration failure of two subalpine conifers. Ecology 2018, 99, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.B.; Finnan, J.; Hodkinson, T.R. Morphological and physiological traits for higher biomass production in perennial rhizomatous grasses grown on marginal land. GCB Bioenergy 2015, 7, 375–385. [Google Scholar] [CrossRef]
- Duan, J.; Ma, Z.; Wu, P.; Xoplaki, E.; Hegerl, G.; Li, L.; Schurer, A.; Guan, D.; Chen, L.; Duan, Y.; et al. Detection of human influences on temperature seasonality from the nineteenth century. Nat. Sustain. 2019, 2, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Archer, K.A.; Decker, A.M. Autumn-Accumulated Tall Fescue and Orchardgrass. I. Growth and Quality as Influenced by Nitrogen and Soil Temperature. Agron. J. 1977, 69, 601–605. [Google Scholar] [CrossRef]
- Lumaret, R.; Borrill, M. Cytology, genetics, and evolution in the genus dactylis. Crit. Rev. Plant Sci. 1988, 7, 55–91. [Google Scholar] [CrossRef]
- Fujimoto, F. Genetic Resources of Orchardgrass (Dactylis glomerata L.) and Related Subspecies from Warmer Regions. Jpn. Agric. Res. Q. 1993, 27, 106. [Google Scholar]
- Geiger, D.R.; Servaites, J.C. Diurnal Regulation of Photosynthetic Carbon Metabolism in C3 Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1994, 45, 235–256. [Google Scholar] [CrossRef]
- Parton, W.J.; Logan, J.A. A model for diurnal variation in soil and air temperature. Agric. Meteorol. 1981, 23, 205–216. [Google Scholar] [CrossRef]
- Qiu, J.; Bai, Y.; Coulman, B.; Romo, J.T. Using thermal time models to predict seedling emergence of orchardgrass (Dactylis glomerata L.) under alternating temperature regimes. Seed Sci. Res. 2006, 16, 261–271. [Google Scholar] [CrossRef]
- Taylor, T.H.; Cooper, J.P.; Treharne, K.J. Growth Response of Orchardgrass (Dactylis glomerata L.) to Different Light and Temperature Environments. I. Leaf Development and Senescence. Crop Sci. 1968, 8, 437–440. [Google Scholar] [CrossRef]
- Knievel, D.P.; Smith, D. Influence of Cool and Warm Temperatures and Temperature Reversal at Inflorescence Emergence on Growth of Timothy, Orchardgrass, and Tall Fescue. Agron. J. 1973, 65, 378–383. [Google Scholar] [CrossRef]

| Index | Variable | Source | Description | Unit |
|---|---|---|---|---|
| Bioclimatic variables | BIO1 | Worldclim | Annual Mean Temperature | °C |
| BIO2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C | ||
| BIO3 | Isothermality (BIO2/BIO7) (×100) | % | ||
| BIO4 | Temperature seasonality (standard deviation × 100) | °C | ||
| BIO5 | Max temperature of the warmest month | °C | ||
| BIO6 | Min temperature of the coldest month | °C | ||
| BIO7 | Temperature annual range (BIO5–BIO6) | °C | ||
| BIO8 | Mean temperature of wettest quarter | °C | ||
| BIO9 | Mean temperature of driest quarter | °C | ||
| BIO10 | Mean temperature of warmest quarter | °C | ||
| BIO11 | Mean temperature of coldest quarter | °C | ||
| BIO12 | Annual precipitation | mm | ||
| BIO13 | Precipitation of wettest month | mm | ||
| BIO14 | Precipitation of the driest month | mm | ||
| BIO15 | Precipitation seasonality (coefficient of variation) | mm | ||
| BIO16 | Precipitation of the wettest quarter | mm | ||
| BIO17 | Precipitation of the driest quarter | mm | ||
| BIO18 | Precipitation of the warmest quarter | mm | ||
| BIO19 | Precipitation of the coldest quarter | mm | ||
| Terrain variables | Elevation | Worldclim | Elevation | m |
| Soil variables | ESP | Harmonised World Soil Database | Exchangeable sodium percentage | — |
| Gravel | Volume percentage of gravel | — | ||
| OC | Percentage of organic carbon | — | ||
| PH | Soil reaction | mol·L−1 | ||
| AWC | Available water capacity | g/kg | ||
| Bulk | Cation exchange capacity | cmol (+)/kg | ||
| Clay | Percentage of clay | — | ||
| Drainage | Soil drainage class | — | ||
| CECS | Cation exchange capacity of the clay fraction | — | ||
| Sand | Percentage of sand | — |
| Code | Environmental Factor | Percent Contribution (%) | Suitable Threshold | Units |
|---|---|---|---|---|
| Bio4 | temperature seasonality | 34.9 | 411.50–1034.37 | °C |
| Bio2 | mean diurnal range | 22.9 | −0.88–10.69 | °C |
| Bio5 | max temperature of the warmest month | 7.8 | 17.08–40.84 | °C |
| Bio19 | precipitation of the coldest quarter | 6.6 | 116.56–825.40 | mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Yan, L.; Zhao, J.; Peng, J.; Xiong, Y.; Xiong, Y.; Ma, X. Modeling Climate Change Indicates Potential Shifts in the Global Distribution of Orchardgrass. Agronomy 2023, 13, 1985. https://doi.org/10.3390/agronomy13081985
Wu J, Yan L, Zhao J, Peng J, Xiong Y, Xiong Y, Ma X. Modeling Climate Change Indicates Potential Shifts in the Global Distribution of Orchardgrass. Agronomy. 2023; 13(8):1985. https://doi.org/10.3390/agronomy13081985
Chicago/Turabian StyleWu, Jiqiang, Lijun Yan, Junming Zhao, Jinghan Peng, Yi Xiong, Yanli Xiong, and Xiao Ma. 2023. "Modeling Climate Change Indicates Potential Shifts in the Global Distribution of Orchardgrass" Agronomy 13, no. 8: 1985. https://doi.org/10.3390/agronomy13081985
APA StyleWu, J., Yan, L., Zhao, J., Peng, J., Xiong, Y., Xiong, Y., & Ma, X. (2023). Modeling Climate Change Indicates Potential Shifts in the Global Distribution of Orchardgrass. Agronomy, 13(8), 1985. https://doi.org/10.3390/agronomy13081985






