Pseudomonas fluorescens RB5 as a Biocontrol Strain for Controlling Wheat Sheath Blight Caused by Rhizoctonia cerealis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
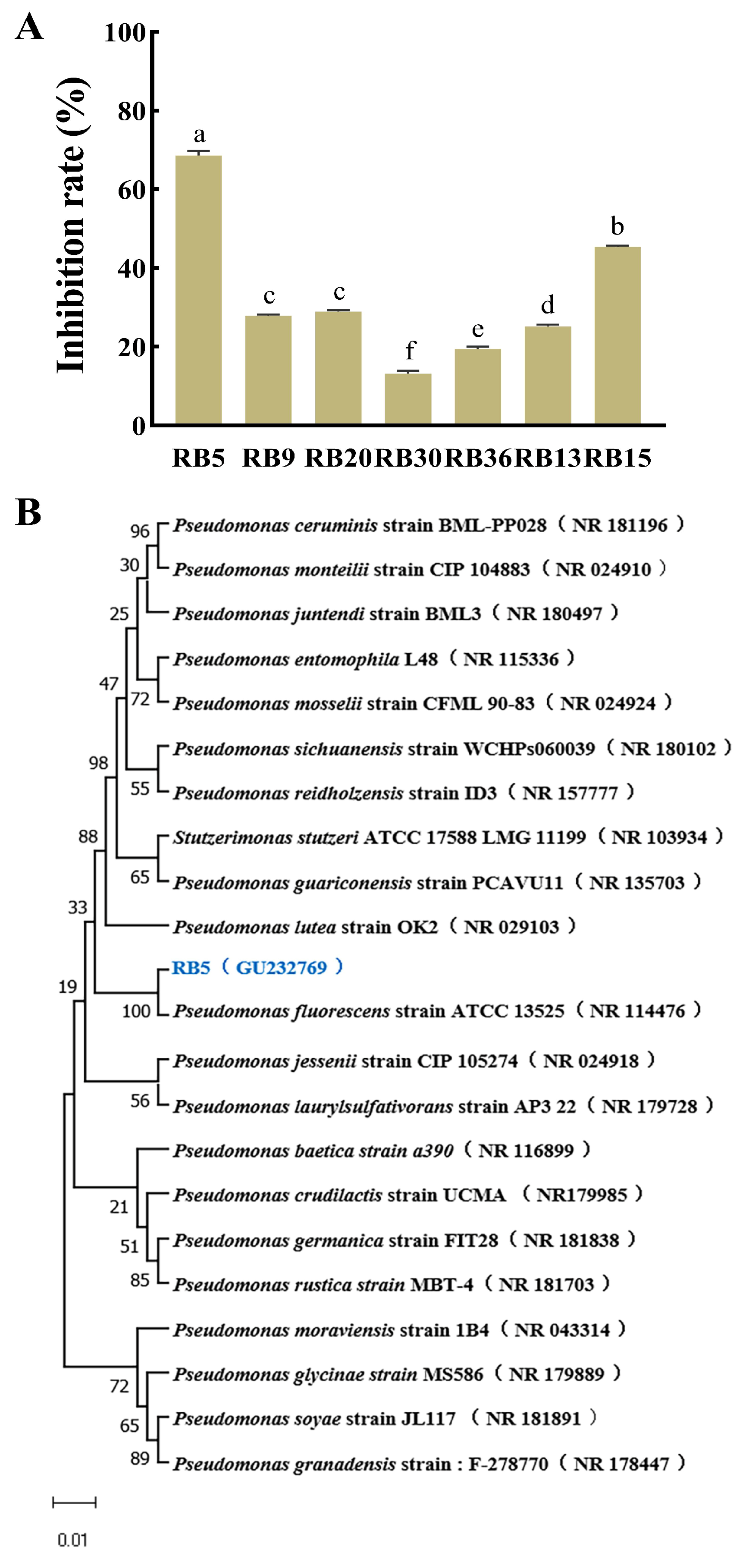
2.2. Isolation and Screening of Strains against R. cerealis
2.3. Identification of Antagonistic Strain RB5
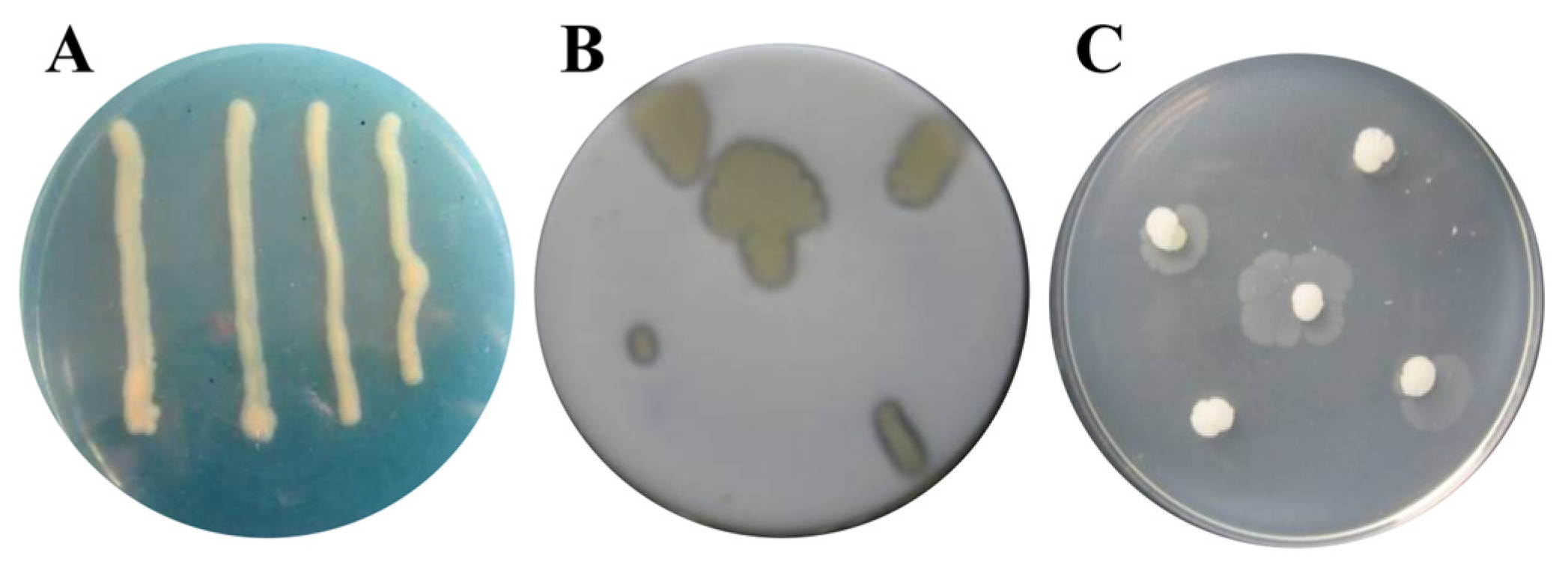
2.4. Detection of Hydrolase and Siderophore
2.5. Optimizing the Culture Conditions for RB5 with Response Surface Methodology (RSM)
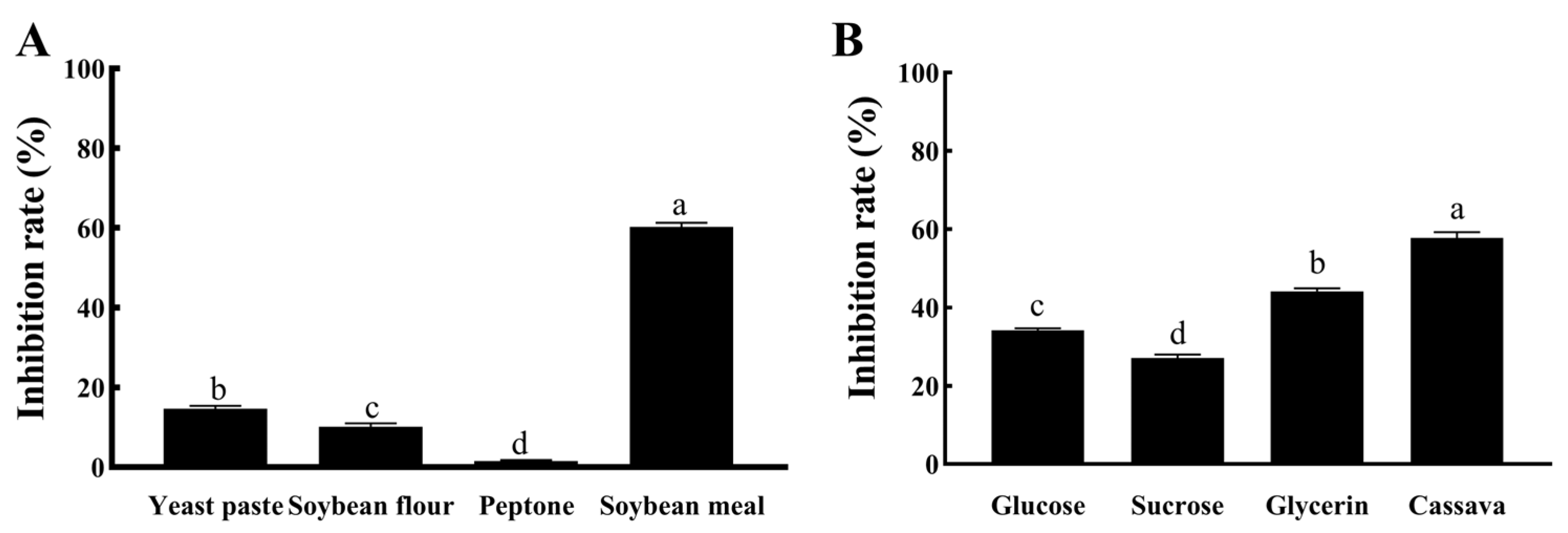
2.5.1. Single-Factor Method for Determining the Optimal Components
2.5.2. Screening of Significant Variables with Placket–Burman Design
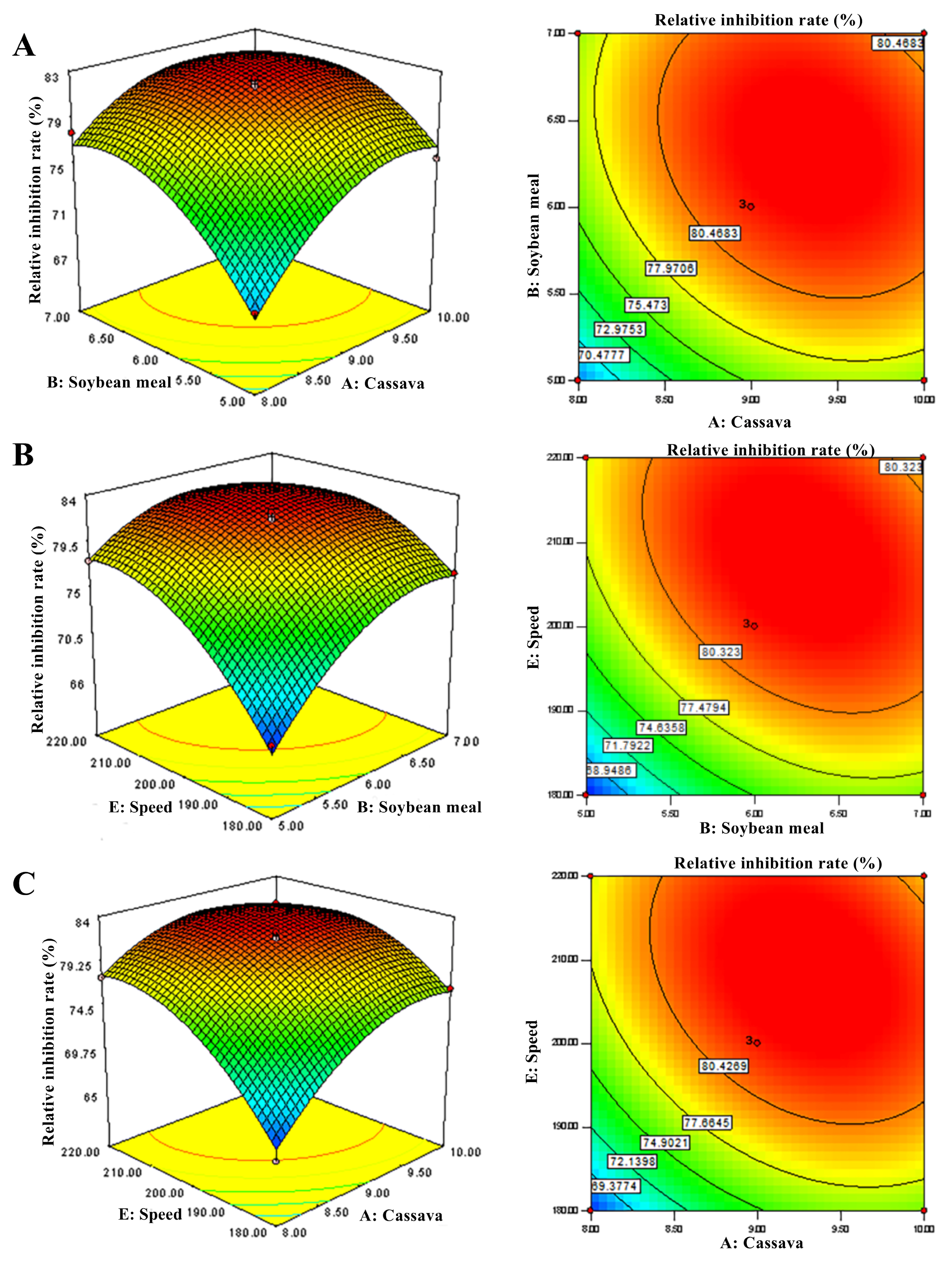
2.5.3. Optimization of the Culture Conditions with Box–Behnken Design
2.6. Detection of Antifungal Activity of RB5 Culture Filtrate
2.7. Effect on Mycelial Morphology of R. cerealis Treated with Culture Filtrate
2.8. Assay of Cell-Wall-Degrading Enzyme Activities of R. cerealis Treated with Culture Filtrate
2.8.1. Enzyme Extraction and Assay
2.8.2. Determination of Polygalacturonase (PG) Activity
2.8.3. Determination of Polymethyl Glacturonase (PMG) Activity
2.8.4. Determination of Pectin Methyl Trans Eliminase (PMTE) Activity
2.9. Pot Control Tests
2.10. Evaluate the Safety of RB5 Culture Filtrate on Animals
2.11. Statistical Analysis
3. Results
3.1. Isolation and Identification of Antagonistic Strain
3.2. Hydrolase and Siderophore Produced from Strain RB5
3.3. The Optimal Culture Conditions for RB5 Screened with Response Surface Methodology (RSM)
3.3.1. Single-Factor Optimization
3.3.2. Selection of the Optimal Variables
3.3.3. Determination of Cultivation Conditions with Box–Behnken Design
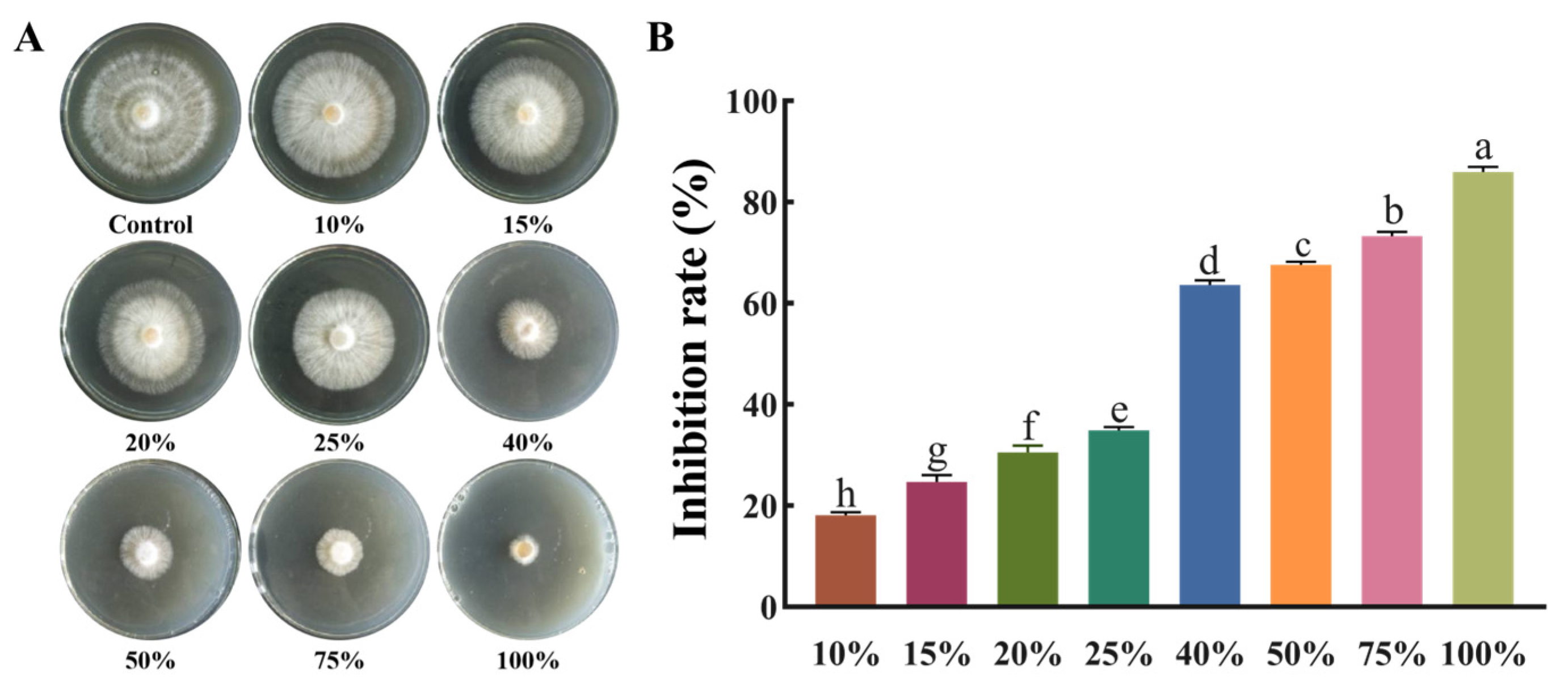
3.4. Antifungal Activity of RB5 Culture Filtrate
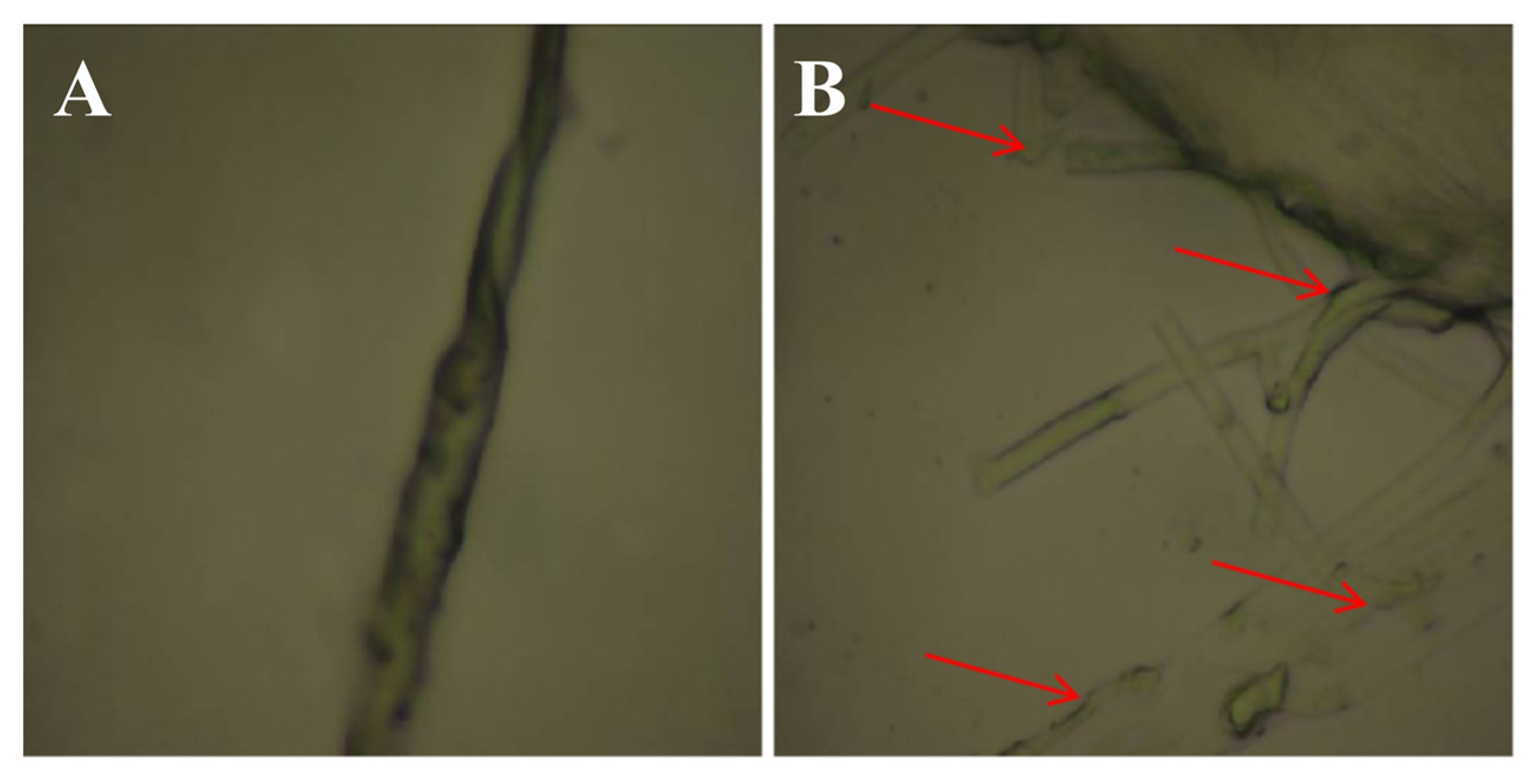
3.5. Effect of RB5 Culture Filtrate on Mycelial Morphology of R. cerealis
3.6. Effects of RB5 Culture Filtrate on Cell-Wall-Degrading Enzymes of R. cerealis
3.7. Biocontrol Effects of RB5 Culture Filtrate on Wheat Sheath Blight
3.8. Safety Evaluation of RB5 Culture Filtrate on Mice
3.8.1. Effects of RB5 Culture Filtrate on Weight and Growth of Mice
3.8.2. Effects of RB5 Culture Filtrate on Serological Physiological Indexes of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, A.; Geng, S.; Zhang, L.; Liu, D.; Mao, L. Making the bread: Insights from newly synthesized allohexaploid wheat. Mol. Plant. 2015, 8, 847–859. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Hamada, M.S.; Yin, Y.; Chen, H.; Ma, Z. The escalating threat of Rhizoctonia cerealis, the causal agent of sheath blight in wheat. Pest Manag. Sci. 2011, 67, 1411–1419. [Google Scholar] [CrossRef]
- Geng, X.; Gao, Z.; Zhao, L.; Zhang, S.; Wu, J.; Yang, Q.; Liu, S.; Chen, X. Comparative transcriptome analysis of resistant and susceptible wheat in response to Rhizoctonia cerealis. BMC Plant Biol. 2022, 22, 235. [Google Scholar] [CrossRef]
- Al-Shwaiman, H.A.; Shahid, M.; Elgorban, A.M.; Siddique, K.H.M.; Syed, A. Beijerinckia fluminensis BFC-33, a novel multi-stress-tolerant soil bacterium: Deciphering the stress amelioration, phytopathogenic inhibition and growth promotion in Triticum aestivum (L.). Chemosphere 2022, 295, 133843. [Google Scholar] [CrossRef]
- Innocenti, G.; Roberti, R.; Montanari, M.; Zakrisson, E. Efficacy of microorganisms antagonistic to Rhizoctonia cerealis and their cell-wall-degrading enzymatic activities. Mycol. Res. 2003, 107, 421–427. [Google Scholar] [CrossRef]
- McBeath, J.H.; McBeath, J. Issues in Implementing Food Security in China. In Environmental Change and Food Security in China; Huang McBeath, J., McBeath, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 243–270. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Gdanetz, K.; Trail, F. The Wheat Microbiome Under Four Management Strategies, and Potential for Endophytes in Disease Protection. Phytobiomes J. 2017, 1, 158–168. [Google Scholar] [CrossRef]
- Zheltobriukhov, V.F.; Sevriukova, G.A.; Wilk, M.; Suska-Malawska, M.; Nefedjeva, E.E.; Baibakova, E.V. Modern fungicides: Mechanisms of action, fungal resistance and phytotoxic effects. Annu. Res. Rev. Biol. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Gawande, S.J.; Soumia, P.S.; Krishna, R.; Vaishnav, A.; Ade, A.B. Biocontrol strategies: An eco-smart tool for integrated pest and diseases management. BMC Microbiol. 2022, 22, 324. [Google Scholar] [CrossRef]
- Qin, Y.; Fu, Y.; Kang, W.; Li, H.; Gao, H.; Vitalievitch, K.S.; Liu, H. Isolation and identification of a cold-adapted bacterium and its characterization for biocontrol and plant growth-promoting activity. Ecol Eng. 2017, 105, 362–369. [Google Scholar] [CrossRef]
- An, C.; Ma, S.; Liu, C.; Ding, H.; Xue, W. Burkholderia ambifaria XN08: A plant growth-promoting endophytic bacterium with biocontrol potential against sheath blight in wheat. Front. Microbiol. 2022, 13, 906724. [Google Scholar] [CrossRef]
- Peng, D.; Li, S.; Chen, C.; Zhou, M. Combined application of Bacillus subtilis NJ-18 with fungicides for control of sheath blight of wheat. Biol. Control 2014, 70, 28–34. [Google Scholar] [CrossRef]
- Sriwati, R.; Maulidia, V.; Intan, N.; Oktarina, H.; Syamsuddin; Khairan, K.; Skala, L.; Mahmud, T. Endophytic bacteria as biological agents to control Fusarium wilt disease and promote tomato plant growth. Physiol. Mol. Plant Pathol. 2023, 125, 101994. [Google Scholar] [CrossRef]
- Zhao, X.; Song, P.; Hou, D.; Li, Z.; Hu, Z. Antifungal activity, identification and biosynthetic potential analysis of fungi against Rhizoctonia cerealis. Ann. Microbiol. 2021, 71, 1. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Cong, C.; Gong, G.; Xu, Y.; Che, J.; Hou, F.; Chen, H.; Wang, L. Use of resistant Rhizoctonia cerealis strains to control wheat sheath blight using organically developed pig manure fertilizer. Sci. Total Environ. 2020, 726, 138568. [Google Scholar] [CrossRef]
- Yi, Y.; Luan, P.; Wang, K.; Li, G.; Yin, Y.; Yang, Y.; Zhang, Q.; Liu, Y. Antifungal activity and plant growth-promoting properties of Bacillus mojovensis B1302 against Rhizoctonia cerealis. Microorganisms 2022, 10, 1682. [Google Scholar] [CrossRef]
- Yi, Y.; Luan, P.; Liu, S.; Shan, Y.; Hou, Z.; Zhao, S.; Jia, S.; Li, R. Efficacy of Bacillus subtilis XZ18-3 as a biocontrol agent against Rhizoctonia cerealis on wheat. Agriculture 2022, 12, 258. [Google Scholar] [CrossRef]
- Deng, D.; Wang, H.; Zhang, Q.; Yan, Y.; Zhao, C.; Liu, Z. Evaluation of Bacillus amyloliquefaciens PEBA20 as a biocontrol agent for Rhizoctonia cerealis and Bipolaris maydis. Biocontrol. Sci. Techn. 2014, 24, 1209–1215. [Google Scholar] [CrossRef]
- Keerthana, U.; Prabhukarthikeyan, S.R.; Baite, M.S.; Yadav, M.K.; Naveen Kumar, R.; Muthu Kumar, A.; Raghu, S.; Aravindan, S.; Rath, P.C. CHAPTER 6–Fluorescent Pseudomonads: A multifaceted biocontrol agent for sustainable agriculture Fluorescent Pseudomonads: A multifaceted biocontrol agent for sustainable agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–92. [Google Scholar] [CrossRef]
- Rostami, M.; Tarighi, S.; Rahimian, H.; Taheri, P. Characterisation of rice-associated antagonistic Pseudomonads and their application in combination with plant resistance inducer molecules for the control of sheath blight disease of rice. Biocontrol. Sci. Technol. 2021, 32, 236–261. [Google Scholar] [CrossRef]
- Duffy, B. Combination of pencycuron and Pseudomonas fluorescens strain 2-79 for integrated control of rhizoctonia root rot and take-all of spring wheat. Crop Prot. 2000, 19, 21–25. [Google Scholar] [CrossRef]
- Yi, Y.; Shan, Y.; Liu, S.; Yang, Y.; Liu, Y.; Yin, Y.; Hou, Z.; Luan, P.; Li, R. Antagonistic strain Bacillus amyloliquefaciens XZ34-1 for controlling Bipolaris sorokiniana and promoting growth in wheat. Pathogens 2021, 10, 1526. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Chen, L.; Jin, X.; Ni, H. The salt-tolerant phenazine-1-carboxamide-producing bacterium Pseudomonas aeruginosa NF011 isolated from wheat rhizosphere soil in dry farmland with antagonism against Fusarium graminearum. Microbiol. Res. 2021, 245, 126673. [Google Scholar] [CrossRef]
- Bergey, D.H.; Holt, J.G.; Noel, R.K. Bergey’s Manual of Systematic Bacteriology, 9th ed.; Williams & Wilkins: Baltimore, MD, USA, 1994; Volume 1, pp. 1935–2045. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Makhdoumi Kakhki, A.; Amoozegar, M.A.; Mahmodi Khaledi, E. Diversity of hydrolytic enzymes in haloarchaeal strains isolated from salt lake. Int. J. Environ. Sci. Technol. 2011, 8, 705–714. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Production of a novel fucoidanase for the green synthesis of gold nanoparticles by Streptomyces sp. and its cytotoxic effect on HeLa Cells. Mar. Drugs. 2015, 13, 6818–6837. [Google Scholar] [CrossRef]
- Wan, C.; Li, P.; Chen, C.; Peng, X.; Li, M.; Chen, M.; Wang, J.; Chen, J. Antifungal activity of Ramulus cinnamomi explored by 1H-NMR based metabolomics approach. Molecules 2017, 22, 2237. [Google Scholar] [CrossRef]
- Marcus, L.; Barash, I.; Sneh, B.; Koltin, Y.; Finkler, A. Purification and characterization of pectolytic enzymes produced by virulent and hypovirulent isolates of Rhizoctonia solani Kuhn. Physiol. Mol. Plant Pathol. 1986, 29, 325–336. [Google Scholar] [CrossRef]
- Lowry, O.H.; Roserbrough, N.J.; Farr, A.L.; Randall, R.J. Protein mea-surement with folin phenol reagent. J. Biol. Chem. 1951, 193, 262–267. [Google Scholar] [CrossRef]
- Yu, S.; Olsen, C.E.; Marcussen, J. Methods for the assay of 1,5-anhydro-D-fructose and α-1,4-glucan lyase. Carbohyd. Res. 1997, 305, 73–82. [Google Scholar] [CrossRef]
- Figueroa, C.R.; Rosli, H.G.; Civello, P.M.; Martínez, G.A.; Herrera, R.; Moya-León, M.A. Changes in cell wall polysaccharides and cell-wall-degrading enzymes during ripening of Fragaria chiloensis and Fragaria ananassa fruits. Sci. Hortic-Amsterdam. 2010, 124, 454–462. [Google Scholar] [CrossRef]
- Jayani, R.S.; Shukla, S.K.; Gupta, R. Screening of bacterial Strains for polygalacturonase activity: Its production by Bacillus sphaericus (MTCC 7542). Enzyme Res. 2010, 306785. [Google Scholar] [CrossRef]
- Ramana Rao, T.V.; Baraiya, N.S.; Vyas, P.B.; Patel, D.M. Composite coating of alginate-olive oil enriched with antioxidants enhances postharvest quality and shelf life of Ber fruit (Ziziphus mauritiana Lamk. Var. Gola). J. Food Sci. Technol. 2016, 53, 748–756. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Jiang, X.; Zhang, H.; Mehmood, K.; Zhang, L.; Jiang, J.; Waqas, M.; Iqbal, M.; Li, J. Probiotic potential of leuconostoc pseudomesenteroides and lactobacillus strains isolated from Yaks. Front. Microbiol. 2018, 9, 2987. [Google Scholar] [CrossRef]
- Ben Ayed, H.; Nasri, R.; Jemil, N.; Ben Amor, I.; Gargouri, J.; Hmidet, N.; Nasri, M. Acute and sub-chronic oral toxicity profiles of lipopeptides from Bacillus mojavensis A21 and evaluation of their in vitro anticoagulant activity. Chem. Biol. Interact. 2015, 236, 1–6. [Google Scholar] [CrossRef]
- Metlakunta, A.S.; Soman, R.J. Safety evaluation of Bacillus coagulans SNZ 1969 in wistar rats. Regul. Toxicol. Pharm. 2020, 110, 104538. [Google Scholar] [CrossRef]
- Lemańczyk, G.; Kwaśna, H. Effects of sheath blight (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur. J. Plant Pathol. 2012, 135, 187–200. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, Z.H.; Zu, X.; Yu, X.Y.; Zhu, H.J.; Li, X.J.; Zhong, J.; Liu, E.M. Efficacy of plant growth-promoting bacteria Bacillus cereus YN917 for biocontrol of rice blast. Front. Microbiol. 2021, 12, 684888. [Google Scholar] [CrossRef]
- Myo, E.M.; Liu, B.; Ma, J.; Shi, L.; Jiang, M.; Zhang, K.; Ge, B. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol. Control 2019, 134, 23–31. [Google Scholar] [CrossRef]
- Al-Karablieh, N.; Al-Shomali, I.; Al-Elaumi, L.; Hasan, K. Pseudomonas fluorescens NK4 siderophore promotes plant growth and biocontrol in cucumber. J. Appl. Microbiol. 2022, 133, 1414–1421. [Google Scholar] [CrossRef]
- Ran, J.; Wu, Y.; Zhang, B.; Su, Y.; Lu, N.; Li, Y.; Liang, X.; Zhou, H.; Shi, J. Paenibacillus polymyxa antagonism towards Fusarium: Identification and optimisation of antibiotic production. Toxins 2023, 15, 138. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Ben Khedher, S.; Boukedi, H.; Laarif, A.; Tounsi, S. Biosurfactant produced by Bacillus subtilis V26: A potential biological control approach for sustainable agriculture development. Int. J. Recycl. Org. 2020, 10, 117–124. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall–degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Kikot, G.E.; Hours, R.A.; Alconada, T.M. Contribution of cell-wall-degrading enzymes to pathogenesis of Fusarium graminearum: A review. J. Basic. Microb. 2009, 49, 231–241. [Google Scholar] [CrossRef]
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal activity of thymol against the main fungi causing pomegranate fruit rot by suppressing the activity of cell-wall-degrading enzymes. Lwt-Food Sci. Technol. 2022, 161, 113303. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, H.; Zhou, X.; Yang, C.; Zheng, S.; Duo, J.; Mo, M.-H. Biological control tobacco bacterial wilt and black shank and root colonization by bio-organic fertilizer containing bacterium Pseudomonas aeruginosa NXHG29. Appl. Soil. Ecol. 2018, 129, 136–144. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Keerthana, U.; Raguchander, T. Antibiotic-producing Pseudomonas fluorescens mediates rhizome rot disease resistance and promotes plant growth in turmeric plants. Microbiol. Res. 2018, 210, 65–73. [Google Scholar] [CrossRef]
- Lakshmi, S.G.; Jayanthi, N.; Saravanan, M.; Ratna, M.S. Safety assesment of Bacillus clausii UBBC07, a spore forming probiotic. Toxicol. Rep. 2017, 4, 62–71. [Google Scholar] [CrossRef]
| Factors | Numbers | −1 | 1 |
|---|---|---|---|
| Cassava/(g/L) | A | 6.0 | 12.0 |
| Soybean meal/(g/L) | B | 4.0 | 9.0 |
| Temperature/°C | C | 28.0 | 35.0 |
| pH | D | 6.8 | 8.5 |
| Speed/(r/min) | E | 160.0 | 240.0 |
| Amount of inoculation/% | F | 2.0 | 2.5 |
| Culture time/h | G | 48.0 | 60.0 |
| Factors | Numbers | −1 | 0 | 1 |
|---|---|---|---|---|
| Cassava/(g/L) | A | 8.0 | 9.0 | 10.0 |
| Soybean meal/(g/L) | B | 5.0 | 6.0 | 7.0 |
| Speed/(r/min) | E | 180 | 200 | 220 |
| Numbers | A | B | C | D | E | F | G | Y (Inhibition Rate)/% |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 56.65 |
| 2 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 61.34 |
| 3 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 69.75 |
| 4 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 71.29 |
| 5 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 58.47 |
| 6 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 70.13 |
| 7 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 63.51 |
| 8 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 68.78 |
| 9 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 73.43 |
| 10 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 68.16 |
| 11 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | 65.67 |
| 12 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 71.35 |
| Numbers | A | B | E | Y (Inhibition Rate)/% |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 82.37 |
| 2 | −1 | −1 | 0 | 68.45 |
| 3 | 1 | −1 | 0 | 75.51 |
| 4 | −1 | 1 | 0 | 77.76 |
| 5 | 1 | 1 | 0 | 78.93 |
| 6 | 1 | 0 | 1 | 80.83 |
| 7 | −1 | 0 | −1 | 65.38 |
| 8 | 0 | 1 | −1 | 76.5 |
| 9 | 0 | 1 | 1 | 78.49 |
| 10 | 0 | −1 | −1 | 66.87 |
| 11 | 0 | 0 | 0 | 81.65 |
| 12 | 0 | −1 | 1 | 77.67 |
| 13 | 1 | 0 | −1 | 76.34 |
| 14 | 0 | 0 | 0 | 81.93 |
| 15 | −1 | 0 | 1 | 77.52 |
| Type | Sum of Squares | Degree of Freedom | Average of Squares | F Value | p Value | Significance |
|---|---|---|---|---|---|---|
| Model | 397.85 | 9 | 44.21 | 30.21 | 0.0008 | ** |
| A | 63.28 | 1 | 63.28 | 43.24 | 0.0012 | ** |
| B | 67.16 | 1 | 67.16 | 45.90 | 0.0011 | ** |
| C | 108.19 | 1 | 108.19 | 73.94 | 0.0004 | ** |
| AB | 8.67 | 1 | 8.67 | 5.93 | 0.0591 | |
| AC | 14.63 | 1 | 14.63 | 10.00 | 0.0250 | * |
| BC | 19.40 | 1 | 19.40 | 13.26 | 0.0149 | * |
| A2 | 41.26 | 1 | 41.26 | 28.20 | 0.0032 | ** |
| B2 | 44.66 | 1 | 44.66 | 30.52 | 0.0027 | ** |
| C2 | 48.46 | 1 | 48.46 | 33.12 | 0.0022 | ** |
| Residual | 7.32 | 5 | 1.46 | |||
| Lack-of-fit | 7.05 | 3 | 2.35 | 17.85 | 0.0535 | |
| Pure error | 0.26 | 2 | 0.13 | |||
| Total error | 405.16 | 14 |
| Culture Filtrate (%) | PG (U/mg) | PMG (U/mg) | PMTE (U/mg × 10−3) |
|---|---|---|---|
| 0 | 12.00 ± 0.58 a | 43.00 ± 2.08 a | 14.29 ± 2.31 a |
| 40 | 5.53 ± 0.14 b | 15.44 ± 0.17 b | 5.93 ± 0.63 b |
| 50 | 3.40 ± 0.10 c | 4.60 ± 0.32 c | 2.07 ± 0.30 c |
| 75 | 2.21 ± 0.14 d | 1.71 ± 0.03 cd | 0.87 ± 0.12 c |
| 100 | 1.46 ± 0.70 d | 0.87 ± 0.21 d | 0.76 ± 0.66 c |
| Treatments | Disease Incidence Rate (%) | Disease Index | Control Efficacy (%) |
|---|---|---|---|
| Sterile water | 97.40 ± 1.67 a | 54.15 ± 1.23 a | -- |
| Carbendazim | 34.12 ± 1.86 d | 16.26 ± 1.51 d | 70.69 ± 2.17 a |
| RB5 (1 × 105 cfu/mL) | 48.23 ± 2.23 b | 33.75 ± 1.65 b | 37.67 ± 3.51 c |
| RB5 (1 × 107 cfu/mL) | 40.78 ± 1.58 c | 20.91 ± 1.78 c | 61.38 ± 3.32 b |
| RB5 (1 × 109 cfu/mL) | 32.23 ± 3.24 d | 15.58 ± 1.34 d | 71.22 ± 1.78 a |
| Items | Body Weight | Behavior | Mortality Rate (%) | |
|---|---|---|---|---|
| Initial Weight (g) | Final Weight (g) | |||
Control  | 20.89 ± 1.72 a | 46.32 ± 1.25 a | Normal | 0 |
Control  | 20.36 ± 1.68 a | 44.64 ± 1.62 a | Normal | 0 |
Treatment  | 20.68 ± 1.74 a | 45.16 ± 1.46 a | Normal | 0 |
Treatment  | 20.12 ± 1.64 a | 44.57 ± 1.78 a | Normal | 0 |
| Items | Serological Physiological Index | ||||
|---|---|---|---|---|---|
| ALT (U·L−1) | AST (U·L−1) | ALT/AST (U·L−1) | BUN (mmoL·L−1) | CREA (μmol·L−1) | |
Control  | 57.67 ± 4.50 a | 120.33 ± 5.51 a | 2.60 ± 0.12 a | 12.45 ± 0.79 a | 24.89 ± 3.96 a |
Treatment  | 59.66 ± 5.12 a | 121.24 ± 5.69 a | 2.71 ± 0.17 a | 12.40 ± 0.88 a | 24.17 ± 2.17 a |
Control  | 46.33 ± 1.52 b | 108.67 ± 7.64 a | 1.89 ± 0.13 b | 12.39 ± 0.82 a | 19.20 ± 2.41 b |
Treatment  | 44.67 ± 1.53 b | 109.56 ± 6.51 a | 1.85 ± 0.27 b | 11.02 ± 0.37 a | 19.10 ± 0.67 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Hou, Z.; Shi, Y.; Zhang, C.; Zhu, L.; Sun, X.; Zhang, R.; Wang, Z. Pseudomonas fluorescens RB5 as a Biocontrol Strain for Controlling Wheat Sheath Blight Caused by Rhizoctonia cerealis. Agronomy 2023, 13, 1986. https://doi.org/10.3390/agronomy13081986
Yi Y, Hou Z, Shi Y, Zhang C, Zhu L, Sun X, Zhang R, Wang Z. Pseudomonas fluorescens RB5 as a Biocontrol Strain for Controlling Wheat Sheath Blight Caused by Rhizoctonia cerealis. Agronomy. 2023; 13(8):1986. https://doi.org/10.3390/agronomy13081986
Chicago/Turabian StyleYi, Yanjie, Zhipeng Hou, Yu Shi, Changfu Zhang, Lijuan Zhu, Xinge Sun, Rumeng Zhang, and Zichao Wang. 2023. "Pseudomonas fluorescens RB5 as a Biocontrol Strain for Controlling Wheat Sheath Blight Caused by Rhizoctonia cerealis" Agronomy 13, no. 8: 1986. https://doi.org/10.3390/agronomy13081986
APA StyleYi, Y., Hou, Z., Shi, Y., Zhang, C., Zhu, L., Sun, X., Zhang, R., & Wang, Z. (2023). Pseudomonas fluorescens RB5 as a Biocontrol Strain for Controlling Wheat Sheath Blight Caused by Rhizoctonia cerealis. Agronomy, 13(8), 1986. https://doi.org/10.3390/agronomy13081986








