A Visual Method of Hydroponic Lettuces Height and Leaves Expansion Size Measurement for Intelligent Harvesting
Abstract
:1. Introduction
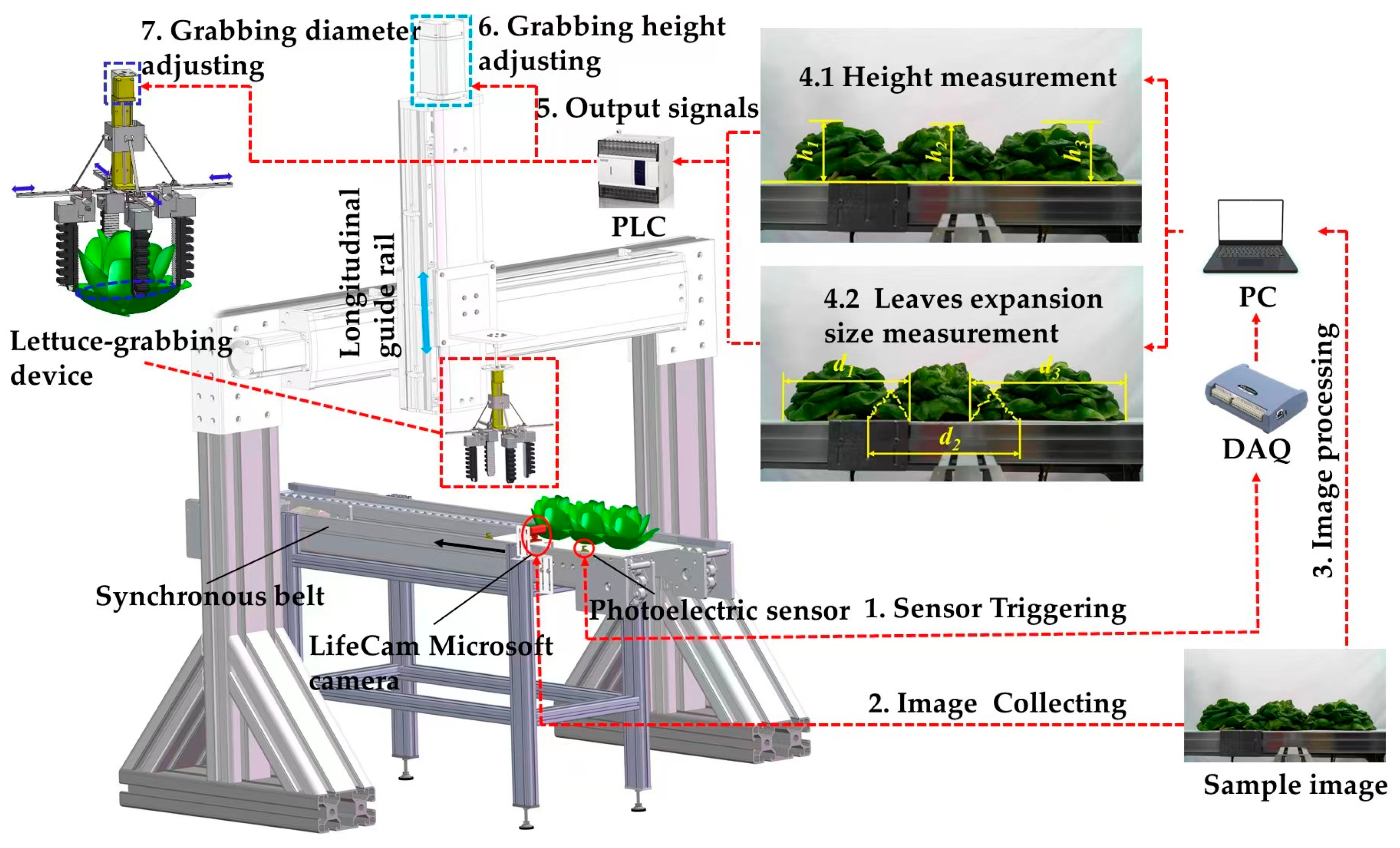
2. Materials and Device for the Experiments
2.1. Experimental Materials
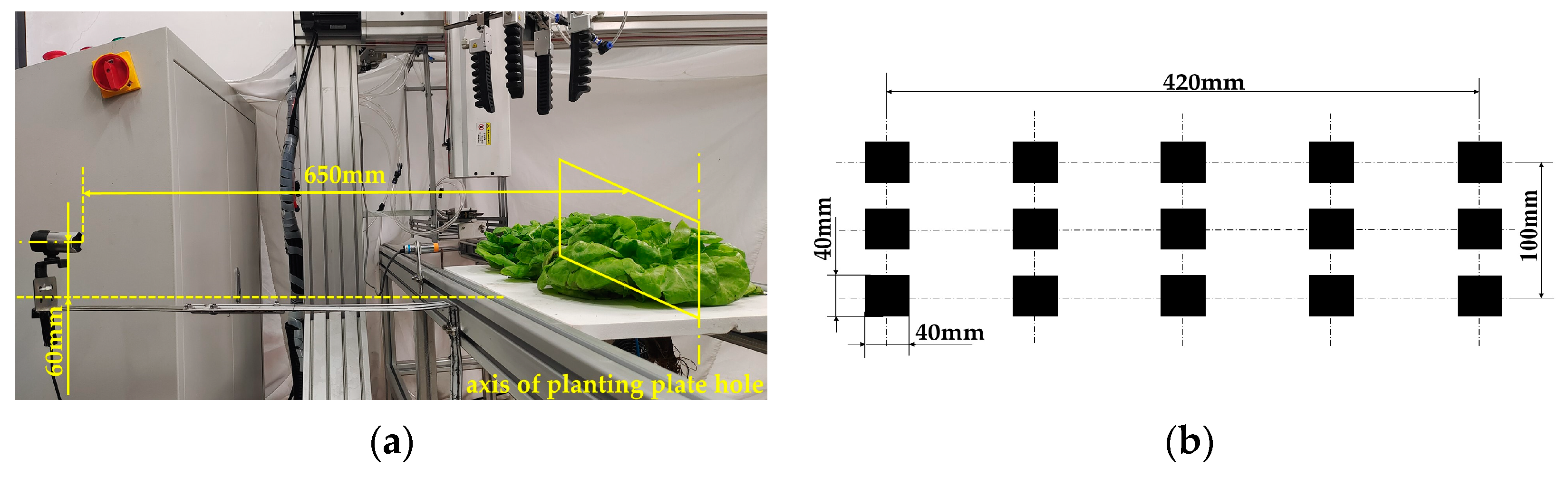
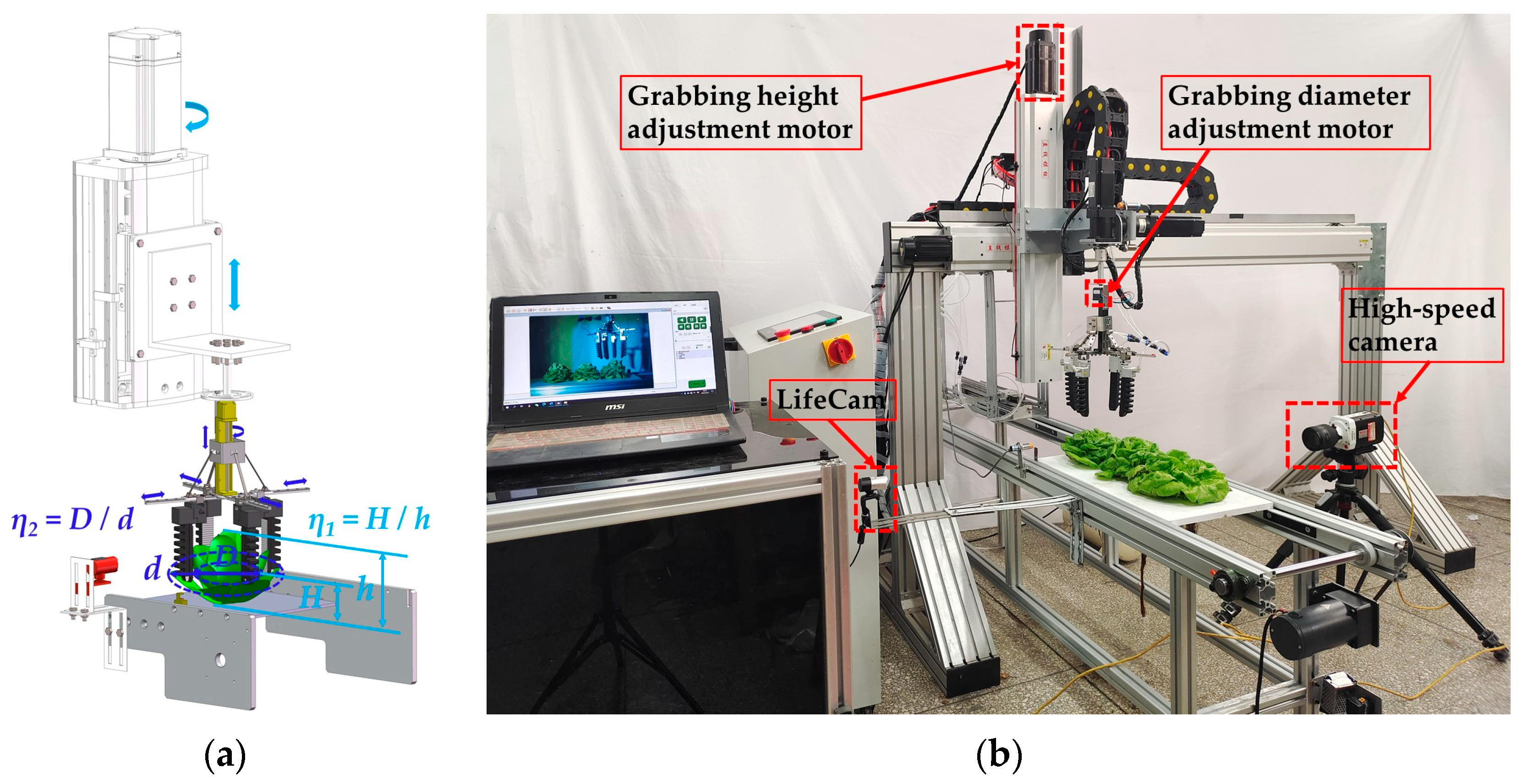
2.2. Experimental Device
3. Experimental Method
3.1. Image Acquisition and Calibration
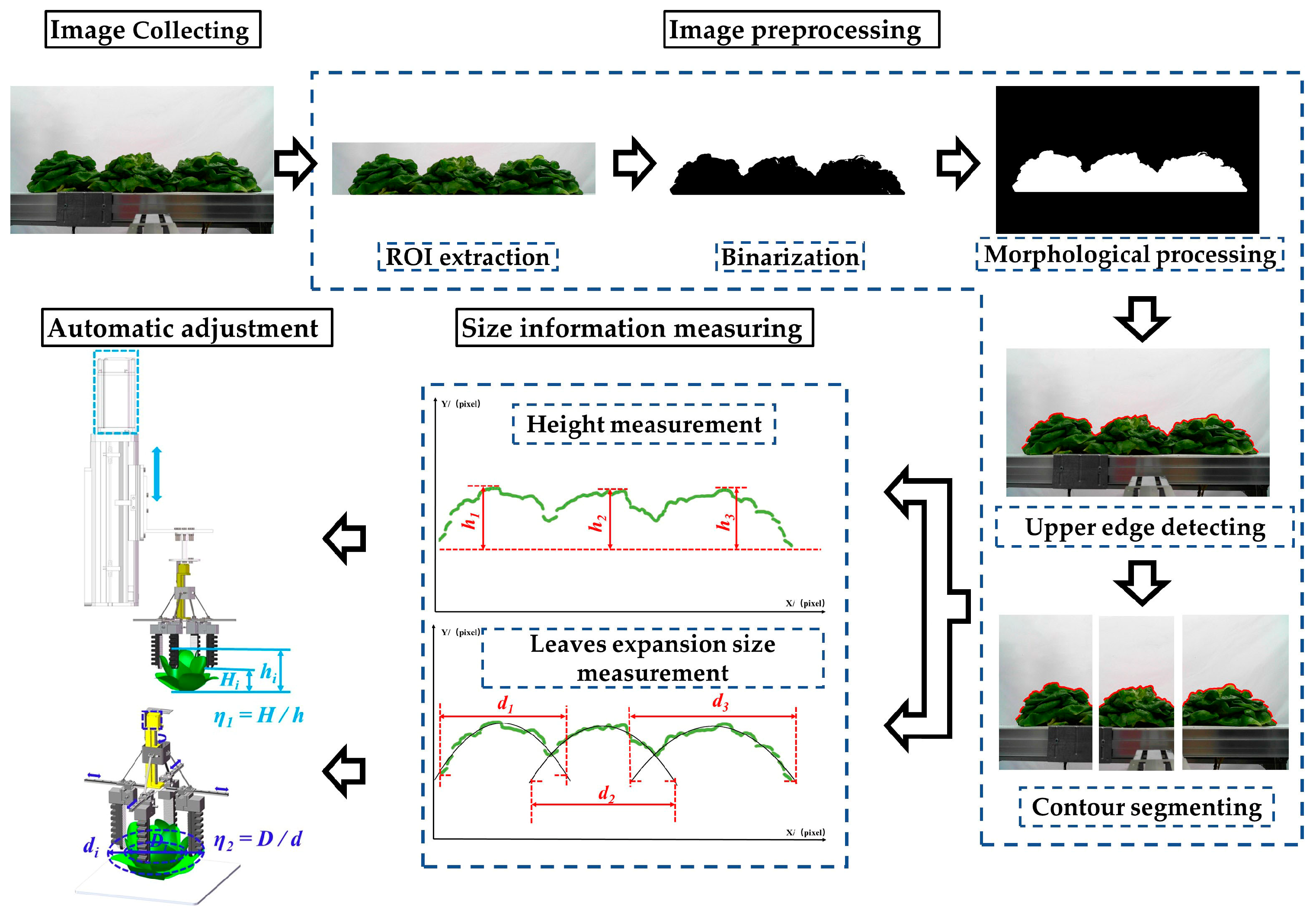
3.2. Image Preprocessing
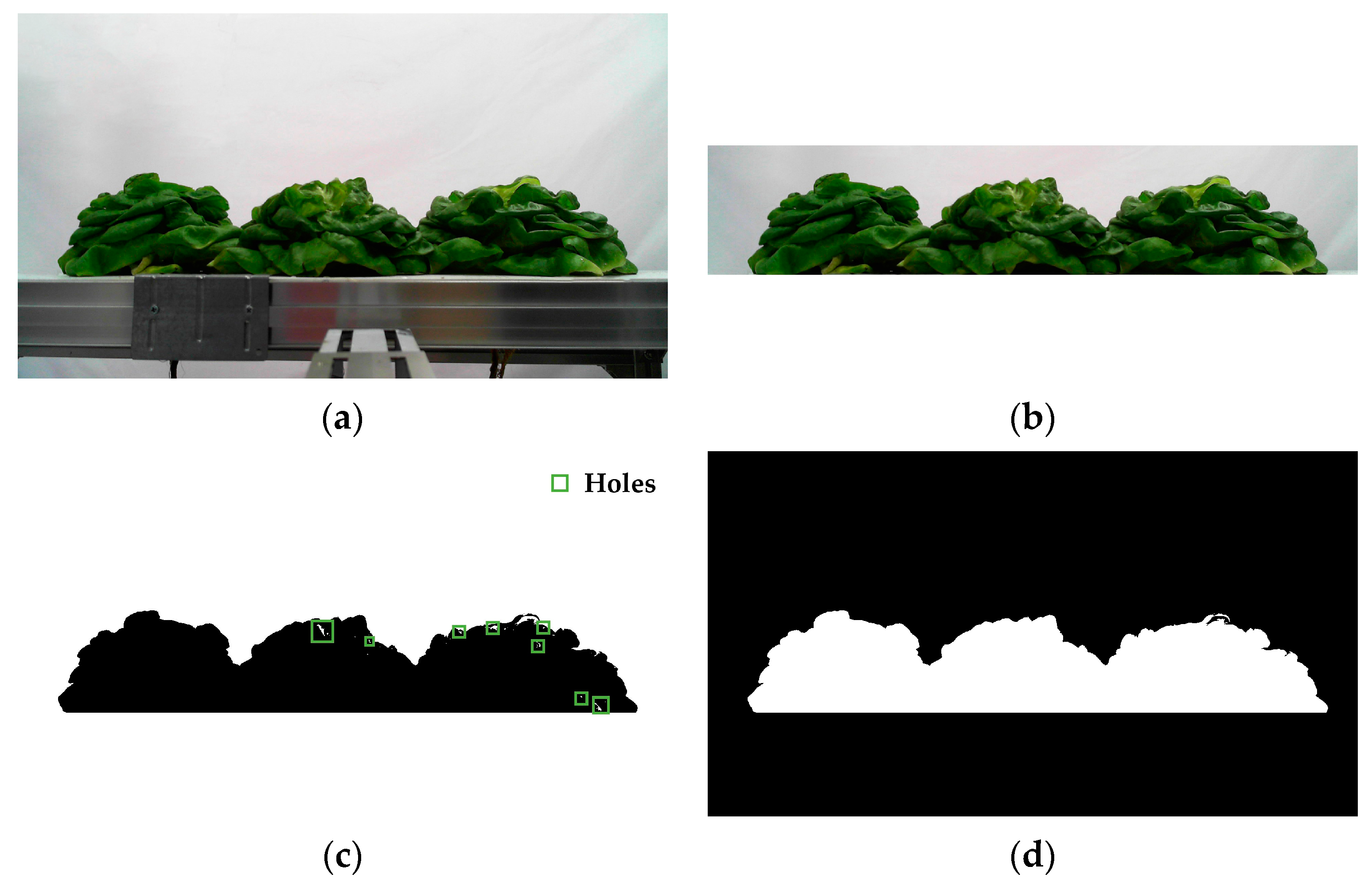
3.2.1. Foreground Extraction
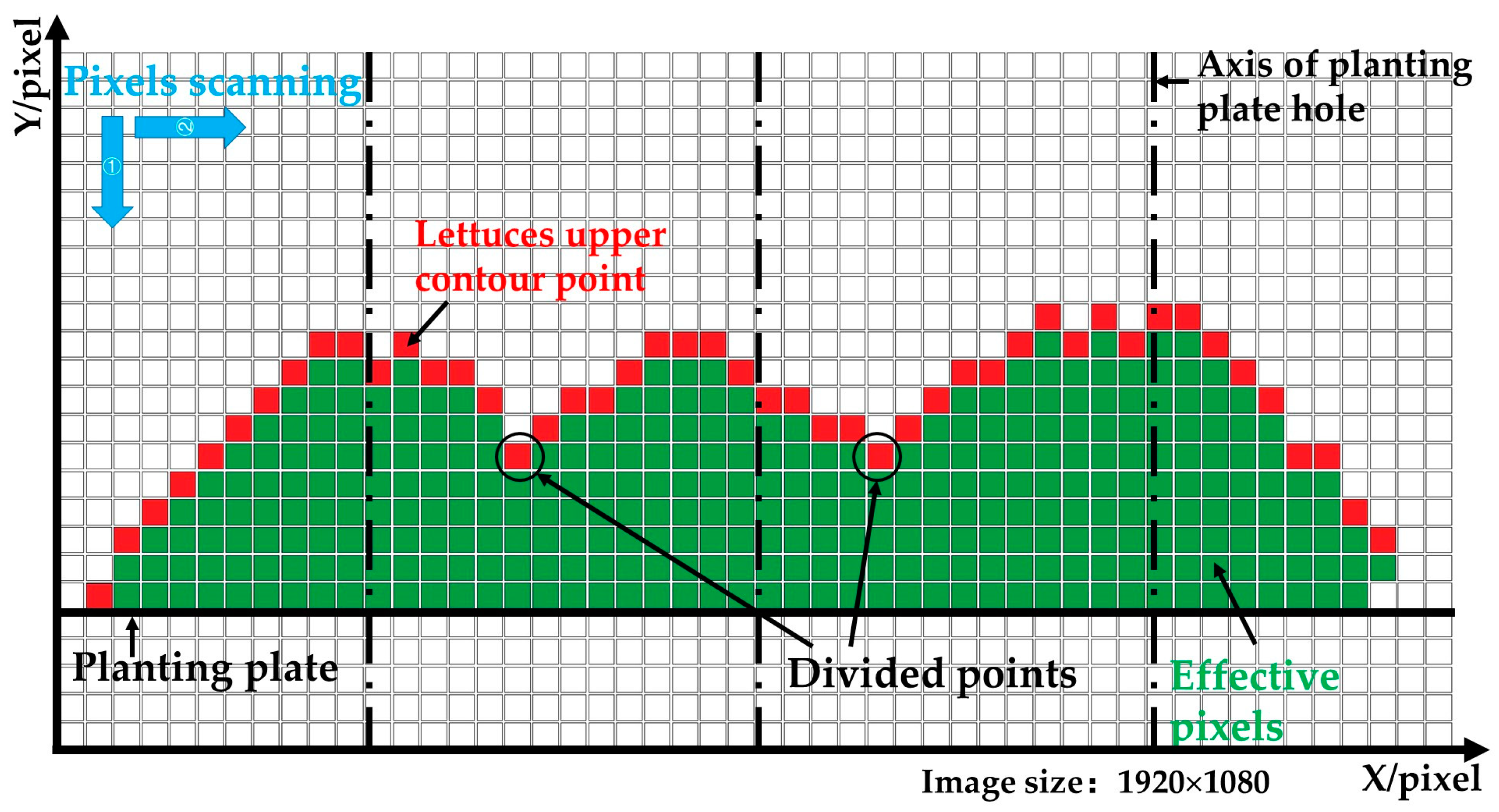
3.2.2. Detection and Segmentation of Lettuces’ Upper Contours
- (1)
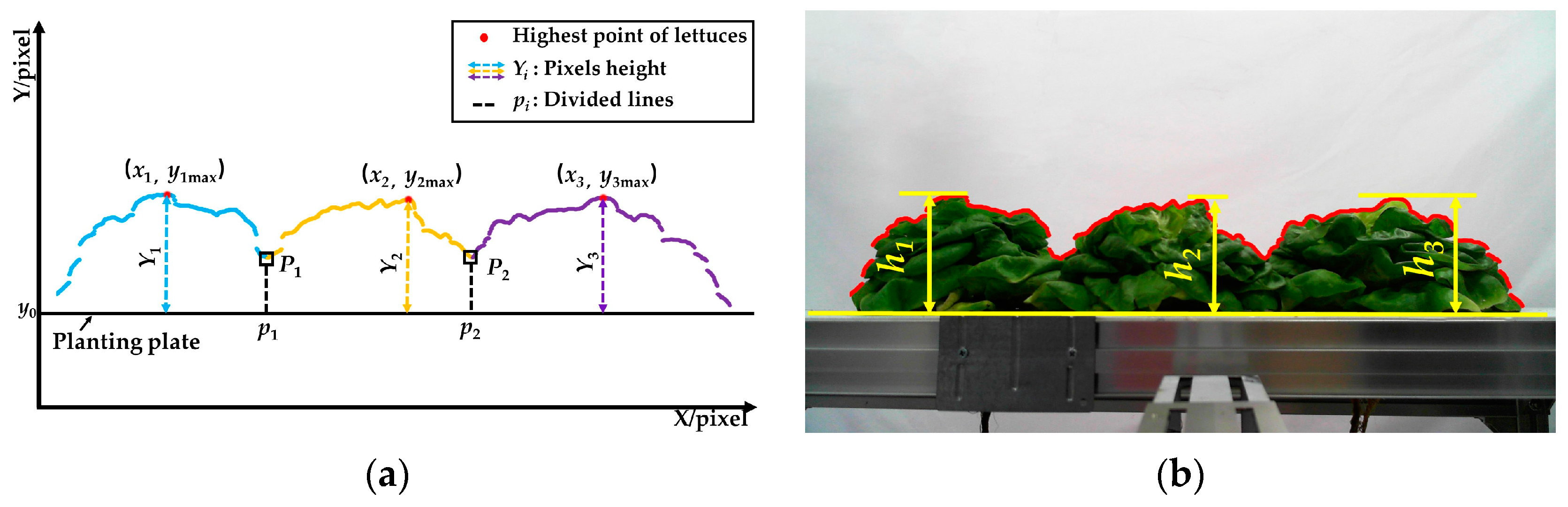
- The binarized image (Figure 5d) was converted into a matrix of 1080 × 1920. The coordinate system was established according to the matrix. In the matrix, the 1080th row was set as the X-axis, and the first column was set as the Y-axis.
- (2)
- The first column of the matrix was scanned along the Y- direction, and the coordinate value of the pixel was recorded when a gray value of 255 was first detected.
- (3)
- To detect all the coordinate values of the lettuces’ upper contours, step 2 was repeated along the X+ direction.
3.3. Measurement of Lettuce Height
3.4. Measurement of Leaves Expansion Size
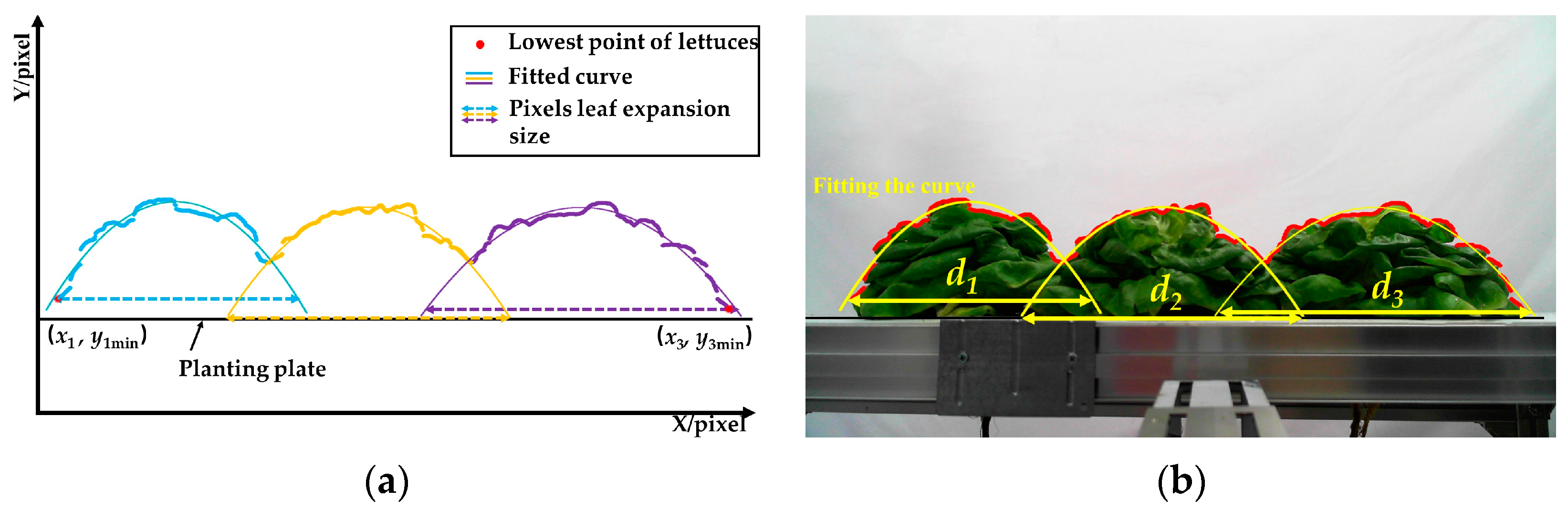
3.4.1. Position and Measurement Method for Leaves Expansion Size
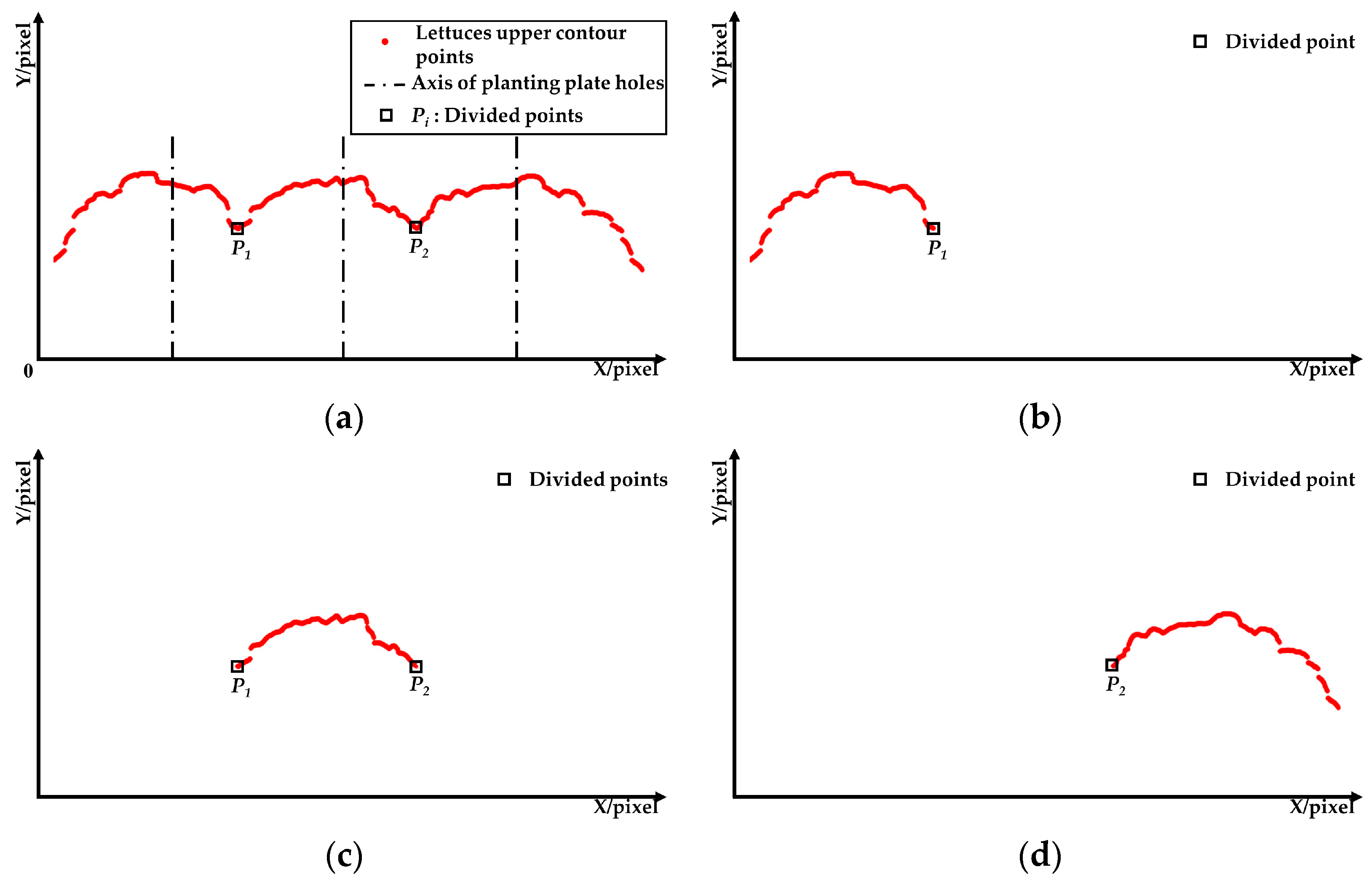
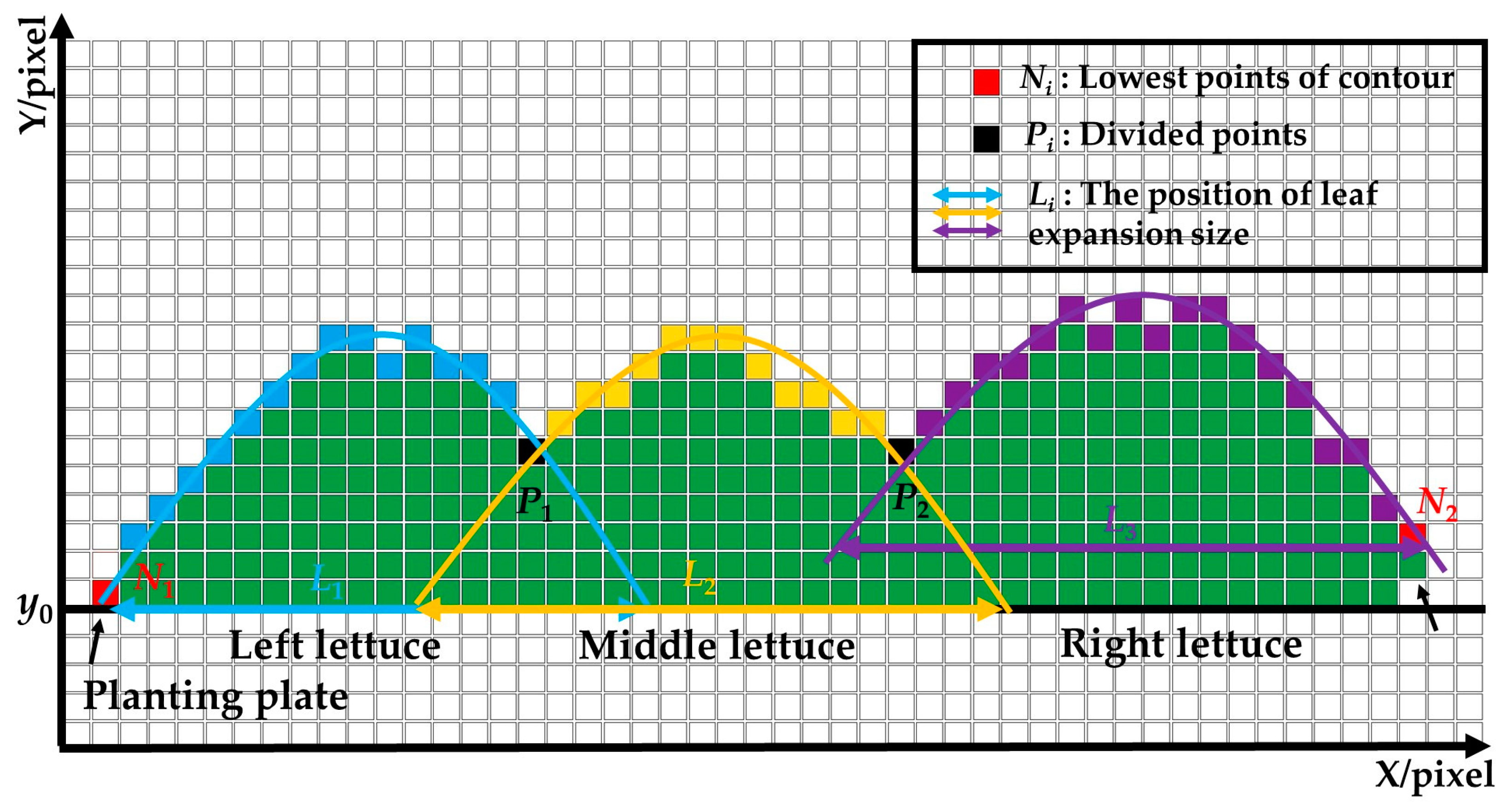
- (1)
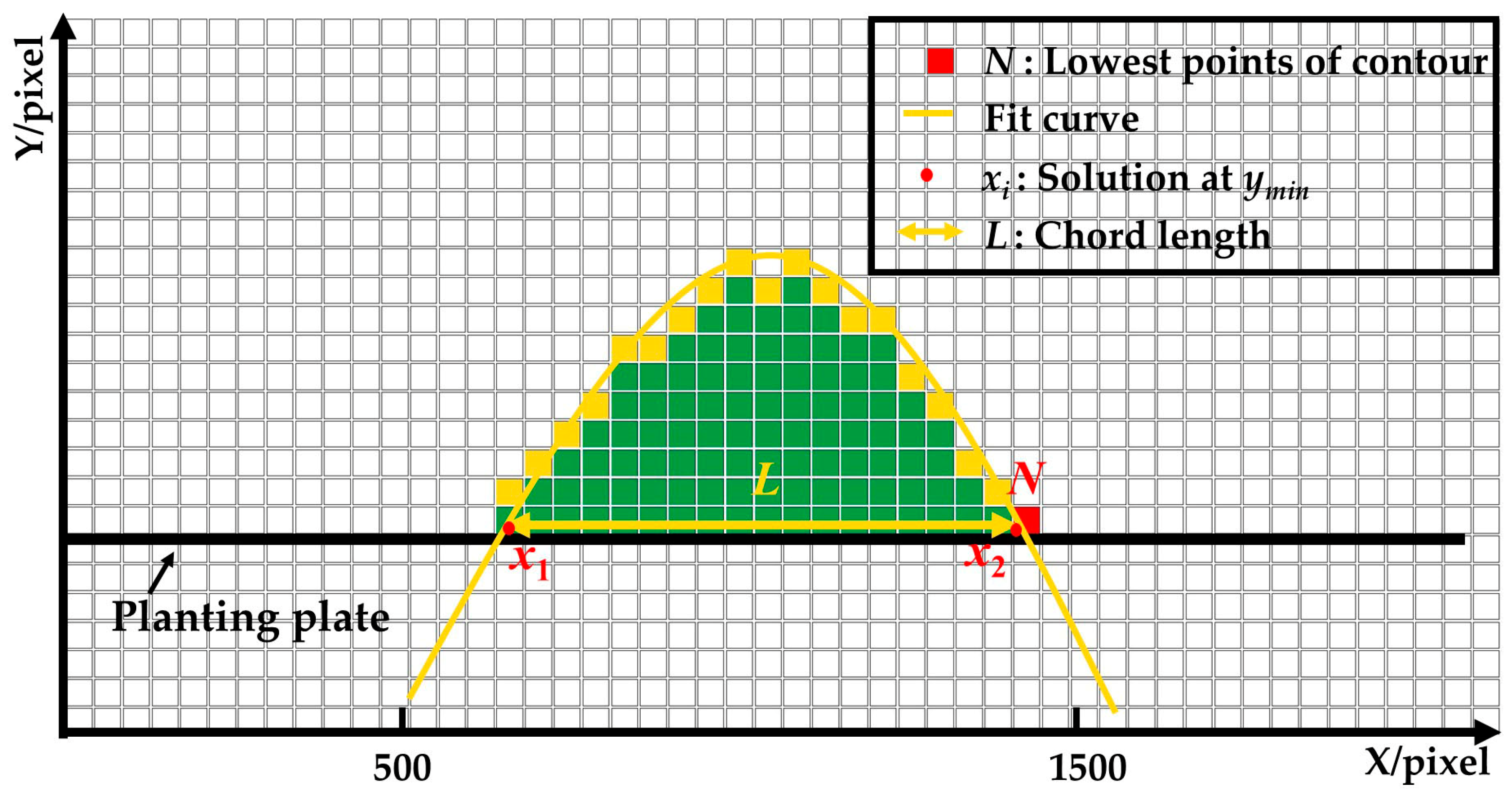
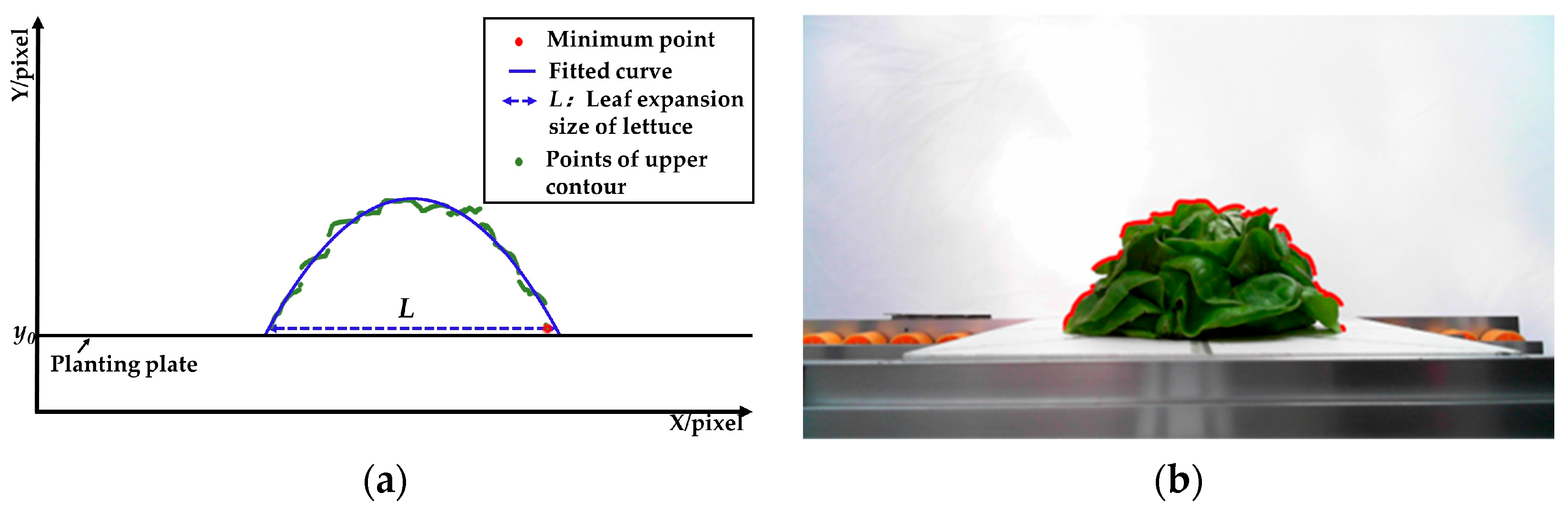
- The leaves expansion sizes of the left and right lettuces were measured according to the fitting function and the lowest point of the upper contour. Firstly, the lowest points (Ni) of the upper contours were detected and set as the measuring points of leaves expansion size. Secondly, the coordinate sets of lettuces’ upper contour were fitted to function curves. Lastly, the pixel leaves expansion sizes (L1 and L3) were calculated according to the ordinate yi of the lowest points and the fitting function.
- (2)
- The leaves of the middle lettuce overlapped with the leaves of the left and right lettuces. According to the leaves’ distribution characteristics in the pre-experiment, the planting plate’s upper surface y0 was set as the measuring point to measure the middle lettuce’s leaves expansion size. Firstly, the fitting function of the middle lettuce was established using the upper contour between P1 and P2. Then, the pixel leaves expansion size L2 was calculated according to the measuring point and the fitting function.
3.4.2. Measuring Method of Leaves Expansion Size Using Different Fitting Functions
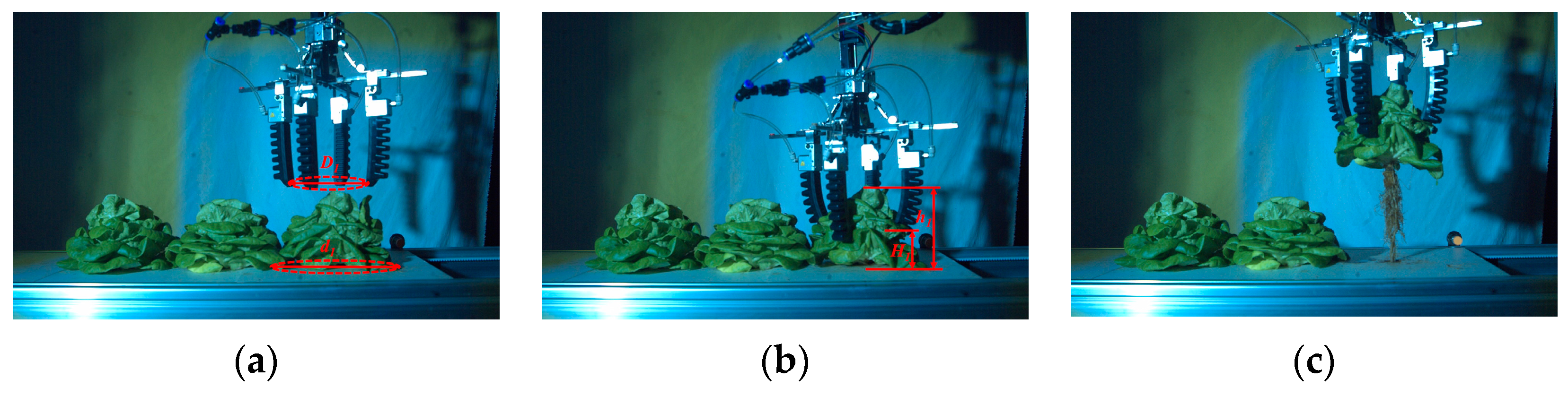
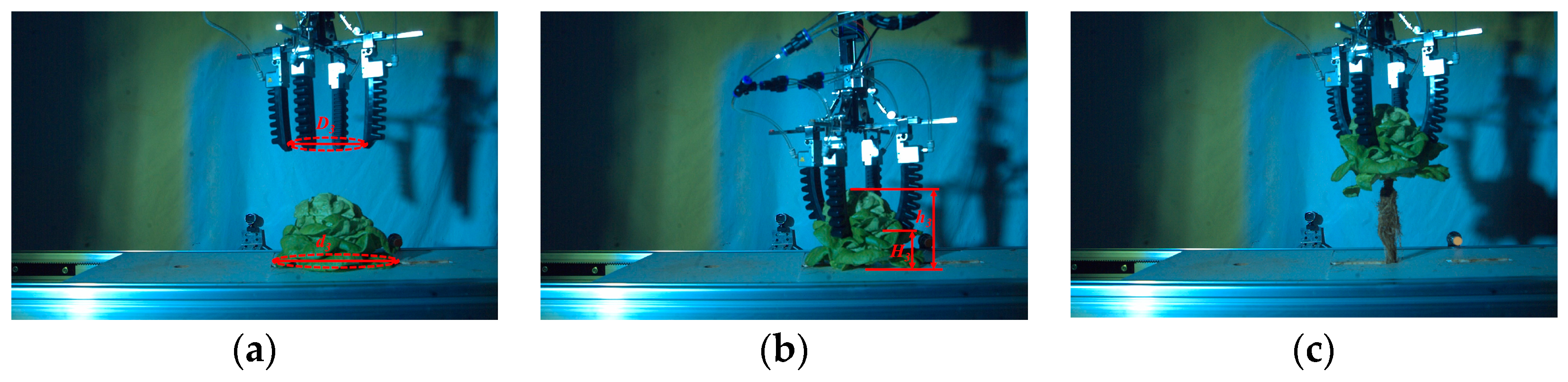
3.5. Harvesting Verification According to the Measurement Results of Lettuce Height and Leaves Expansion Size
4. Results and Discussion
4.1. Measurement Results of Lettuce Height and Leaves Expansion Size
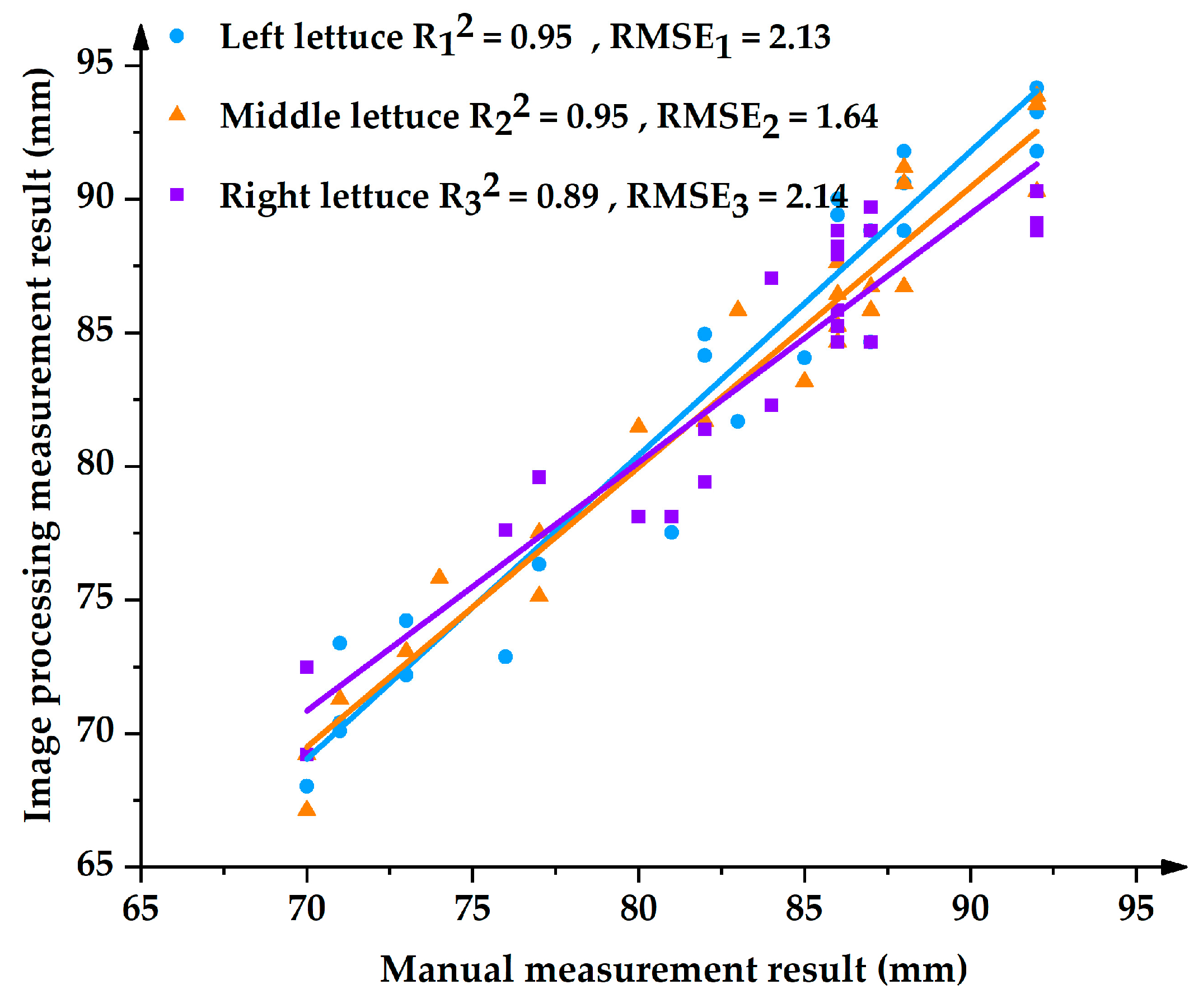
4.1.1. Measurement Results of Lettuce Height
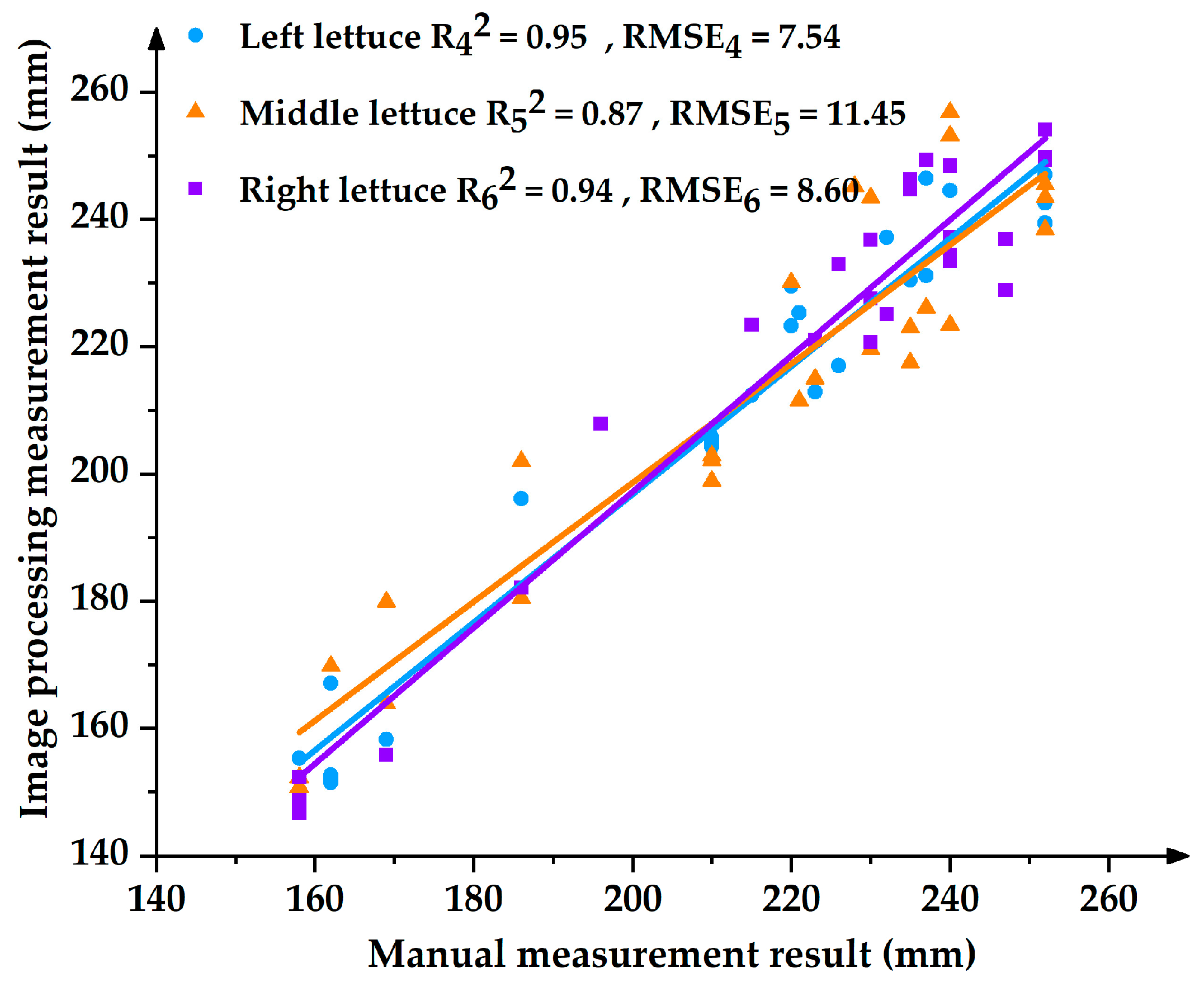
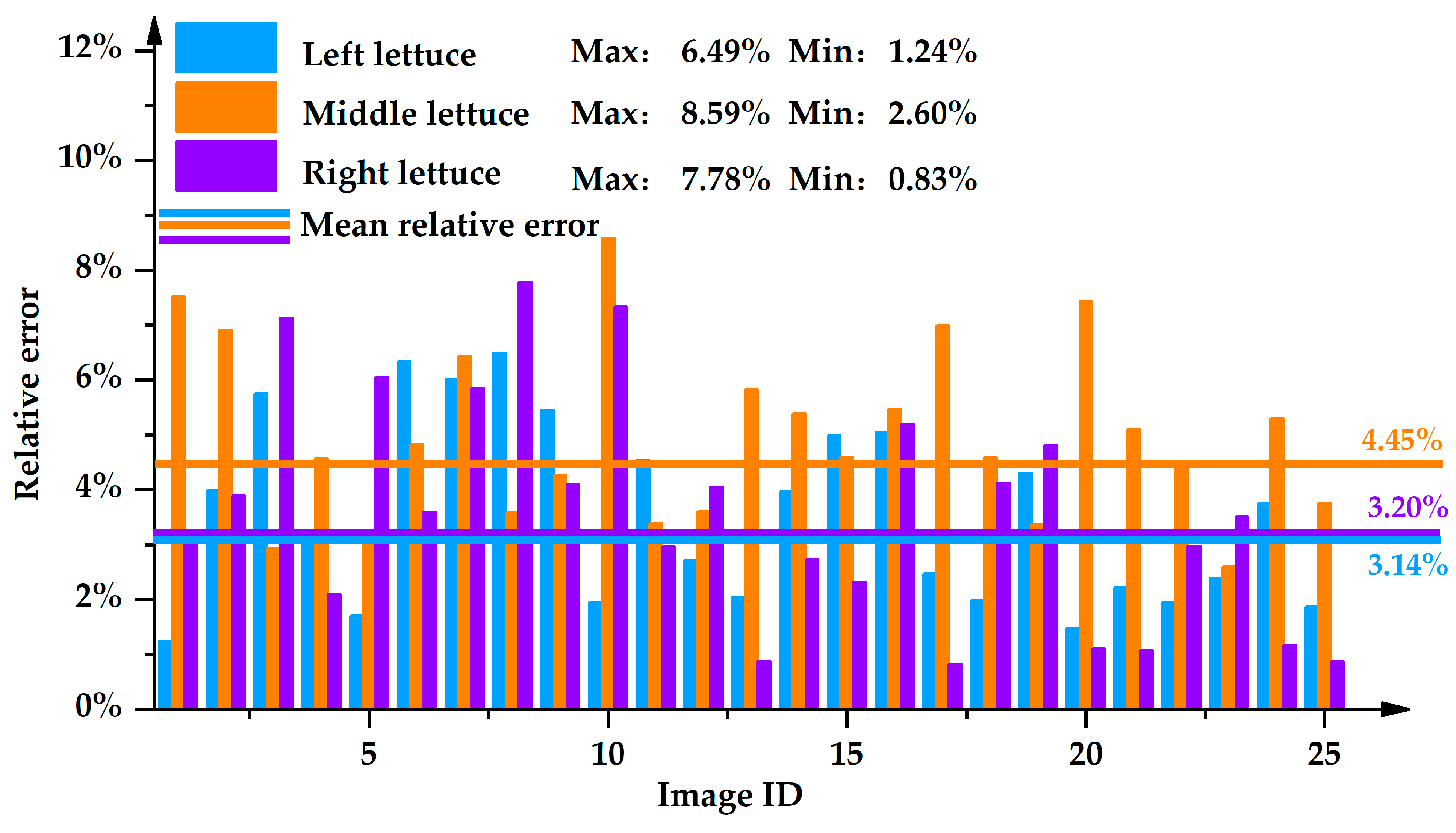
4.1.2. Analysis of Measurement Results for Leaves Expansion Size
- (1)
- The Accuracy of Different Fitting Functions for Lettuce Leaves Expansion Size
- (2)
- The Measurement Effect of the Quadratic Function for Lettuce Leaves Expansion Size in Different Positions
4.2. Verification Test of Intelligent and Continuous Harvesting
5. Conclusions
- 1.
- A measuring method of lettuce height was proposed. The maximal relative error of the lettuce height measurement was 5.58%, and the average relative error was 2.14%. The relative errors of the lettuce height for the left, middle, and right lettuces were 2.20%, 1.46%, and 2.06%, respectively.
- 2.
- The upper contour of the lettuces was fitted to different functions, and the fitting effect of the quadratic function was the best among the quadratic, cubic, and sine functions. The quadratic function was used to measure the leaves expansion size of the lettuces. The maximal relative error of leaves expansion size was 8.59%, and the average relative error was 4.03%. The relative errors of leaves expansion size for the left, middle, and right lettuces were 3.14%, 4.45%, and 3.20%, respectively.
- 3.
- The lettuce harvesting effects were verified according to the visual measurement results. The harvesting success rates of the left, middle, and right lettuces were 94%, 91%, and 96%, respectively. The injured leaves areas of the left, middle, and right lettuces were 192.6 mm2, 228.1 mm2, 205.6 mm2, respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Razmjooei, Z.; Etemadi, M.; Eshghi, S.; Ramezanian, A.; Mirazimi Abarghuei, F.; Alizargar, J. Potential Role of Foliar Application of Azotobacter on Growth, Nutritional Value and Quality of Lettuce under Different Nitrogen Levels. Plants 2022, 11, 406. [Google Scholar] [CrossRef]
- Zhang, L.; Su, W.; Tao, R.; Zhang, W.; Chen, J.; Wu, P. RNA sequencing provides insights into the evolution of lettuce and the regulation of flavonoid biosynthesis. Nat. Commun. 2017, 8, 2264. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Wang, M.; Ma, Y.; Fu, L. Effects of cutter parameters on shearing stress for lettuce harvesting using a specially developed fixture. Int. J. Agric. Biol. Eng. 2021, 14, 152–158. [Google Scholar] [CrossRef]
- Suo, R.; Wang, W.; Ma, Y.; Fu, L.; Cui, Y. Effect of different root lengths for retaining freshness of hydroponic lettuce. J. Agric. Food Res. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, C.; Cui, Y.; Fu, L.; Liu, H.; Yang, C. Design and Test of Harvester for Whole Hydroponic Lettuce with Low Damage. Trans. CSAM 2019, 50, 162–169. [Google Scholar]
- Fu, L.; Feng, Y.; Wu, J.; Liu, Z.; Gao, F.; Majeed, Y.; Al-Mallahi, A.; Zhang, Q.; Li, R.; Cui, Y. Fast and accurate detection of kiwifruit in orchard using improved YOLOv3-tiny model. Precis. Agric. 2020, 22, 754–776. [Google Scholar] [CrossRef]
- Xie, C.; Yang, C. A review on plant high-throughput phenotyping traits using UAV-based sensors. Comput. Electron. Agric. 2020, 178, 105731. [Google Scholar] [CrossRef]
- Subramanian, R.; Spalding, E.P.; Ferrier, N.J. A high throughput robot system for machine vision based plant phenotype studies. Mach. Vis. Appl. 2012, 24, 619–636. [Google Scholar] [CrossRef]
- Kolhar, S.; Jagtap, J. Plant trait estimation and classification studies in plant phenotyping using machine vision—A review. Inf. Process. Agric. 2023, 10, 114–135. [Google Scholar] [CrossRef]
- Tian, Z.; Ma, W.; Yang, Q.; Duan, F. Application status and challenges of machine vision in plant factory—A review. Inf. Process. Agric. 2022, 9, 195–211. [Google Scholar] [CrossRef]
- Li, Z.; Guo, R.; Li, M.; Chen, Y.; Li, G. A review of computer vision technologies for plant phenotyping. Comput. Electron. Agric. 2020, 176, 105672. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, H.; Zhao, X.; Fan, C.; Cheng, M. Fast reconstruction method of three-dimension model based on dual RGB-D cameras for peanut plant. Plant Methods 2023, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Stilla, U.; Xu, Y. Change detection of urban objects using 3D point clouds: A review. ISPRS J. Photogramm. Remote Sens. 2023, 197, 228–255. [Google Scholar] [CrossRef]
- Xiang, L.; Wang, D. A review of three-dimensional vision techniques in food and agriculture applications. Smart Agric. Technol. 2023, 5, 100259. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, L.; Xiang, L.; Wu, Q.; Jiang, H. Automatic Non-Destructive Growth Measurement of Leafy Vegetables Based on Kinect. Sensors 2018, 18, 806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Li, G.; Li, J.; Zheng, L.; Zhang, M.; Wang, M. Multi-phenotypic parameters extraction and biomass estimation for lettuce based on point clouds. Measurement 2022, 204, 112094. [Google Scholar] [CrossRef]
- Petropoulou, A.S.; van Marrewijk, B.; de Zwart, F.; Elings, A.; Bijlaard, M.; van Daalen, T.; Hemming, S. Lettuce Production in Intelligent Greenhouses-3D Imaging and Computer Vision for Plant Spacing Decisions. Sensors 2023, 23, 2929. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Yang, P.; Wu, Y.; Li, Y.; Guo, R. Review of Research on Fish Body Length Measurement Based on Machine Vision. Trans. CSAM 2021, 52, 207–218. [Google Scholar]
- Zhong, Y.; Gou, R.; Hu, H. Calculation of the relative distortion coefficient about camera. Opt. Tech. 2002, 28, 381–383. [Google Scholar]
- Yang, W.; Xu, X.; Duan, L.; Luo, Q.; Chen, S.; Zeng, S.; Liu, Q. High-throughput measurement of rice tillers using a conveyor equipped with x-ray computed tomography. Rev. Sci. Instrum. 2011, 82, 025102. [Google Scholar] [CrossRef]
- Iyer-Pascuzzi, A.S.; Symonova, O.; Mileyko, Y.; Hao, Y.; Belcher, H. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiol. 2010, 152, 1148–1157. [Google Scholar] [CrossRef]
- Ishikawa, T.; Hayashi, A.; Nagamatsu, S.; Kyutoku, Y.; Dan, I. Classification of Strawberry Fruit Shape by Machine Learning. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2018, XLII-2, 463–470. [Google Scholar] [CrossRef]
- Green, J.M.; Appel, H.; Rehrig, E.M.; Harnsomburana, J.; Chang, J.-F.; Balint-Kurti, P.; Shyu, C.-R. PhenoPhyte: A flexible affordable method to quantify 2D phenotypes from imagery. Plant Methods 2012, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, W.; Li, Z.; Cao, X.; Tan, S.; Qi, L. Smartphone application-based measurements of stem-base width and plant height in rice seedling. Comput. Electron. Agric. 2022, 198, 107002. [Google Scholar] [CrossRef]
- Gupta, C.; Tewari, V.K.; Machavaram, R.; Shrivastava, P. An image processing approach for measurement of chili plant height and width under field conditions. J. Saudi Soc. Agric. Sci. 2022, 21, 171–179. [Google Scholar] [CrossRef]
- Liao, G.; Wang, X.; Jin, J.; Li, J. Potato Size and Shape Detection Using Machine Vision. MATEC Web Conf. 2015, 31, 15003. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, P.; Jin, X.; Li, X.; Zhang, C.; Zhang, Y. Design and Experiment of Low Damage Flexible Harvesting Device for Hydroponic Lettuce. Trans. CSAM 2022, 53, 175–183, 210. [Google Scholar]
- Zhang, F.; Chen, Z.; Wang, Y.; Bao, R.; Chen, X.; Fu, S. Research on Flexible End-Effectors with Humanoid Grasp Function for Small Spherical Fruit Picking. Agriculture 2023, 13, 123. [Google Scholar] [CrossRef]
- Vrochidou, E.; Tsakalidou, V.N.; Kalathas, I.; Gkrimpizis, T.; Pachidis, T.; Kaburlasos, V.G. An Overview of End Effectors in Agricultural Robotic Harvesting Systems. Agriculture 2022, 12, 1240. [Google Scholar] [CrossRef]
- Goulart, R.; Jarvis, D.; Walsh, K.B. Evaluation of End Effectors for Robotic Harvesting of Mango Fruit. Sustainability 2023, 15, 6769. [Google Scholar] [CrossRef]







| Function | Quadratic | Cubic | Sine |
|---|---|---|---|
| R2 | 0.87–0.98 | 0.87–0.98 | 0.85–0.98 |
| RMSE/mm | 3.72–8.50 | 3.71–8.36 | 3.91–8.83 |
| Average Value | Standard Deviation | Coefficient of Variation (%) | ||
|---|---|---|---|---|
| Injured leaves area (mm2) | Left lettuce | 192.6 | 7.2 | 3.8 |
| Middle lettuce | 228.1 | 12.4 | 5.4 | |
| Right lettuce | 205.6 | 9.1 | 4.4 | |
| Harvesting success rate (%) | Left lettuce | 94 | 6.6 | 7.1 |
| Middle lettuce | 91 | 9.4 | 10.4 | |
| Right lettuce | 96 | 4.9 | 5.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhang, Y.; Jin, X.; Li, X.; Wang, H.; Qi, C. A Visual Method of Hydroponic Lettuces Height and Leaves Expansion Size Measurement for Intelligent Harvesting. Agronomy 2023, 13, 1996. https://doi.org/10.3390/agronomy13081996
Ma Y, Zhang Y, Jin X, Li X, Wang H, Qi C. A Visual Method of Hydroponic Lettuces Height and Leaves Expansion Size Measurement for Intelligent Harvesting. Agronomy. 2023; 13(8):1996. https://doi.org/10.3390/agronomy13081996
Chicago/Turabian StyleMa, Yidong, Yin Zhang, Xin Jin, Xinping Li, Huankun Wang, and Chong Qi. 2023. "A Visual Method of Hydroponic Lettuces Height and Leaves Expansion Size Measurement for Intelligent Harvesting" Agronomy 13, no. 8: 1996. https://doi.org/10.3390/agronomy13081996
APA StyleMa, Y., Zhang, Y., Jin, X., Li, X., Wang, H., & Qi, C. (2023). A Visual Method of Hydroponic Lettuces Height and Leaves Expansion Size Measurement for Intelligent Harvesting. Agronomy, 13(8), 1996. https://doi.org/10.3390/agronomy13081996




