Effect of Different Culture Conditions on Anthocyanins and Related Genes in Red Pear Callus
Abstract
1. Introduction
2. Materials and Methods
2.1. Different Treatments of Induced Callus Anthocyanins
2.2. Determination of the Anthocyanin Content
2.3. Determination of Total Phenol and Total Flavonoids
2.4. Determination of Antioxidant Activity
2.5. RNA Extraction
2.6. Design and Synthesis of the Primers
2.7. cDNA Compose
2.8. Related Gene Expression Determination
2.9. Statistics and Analysis
3. Results
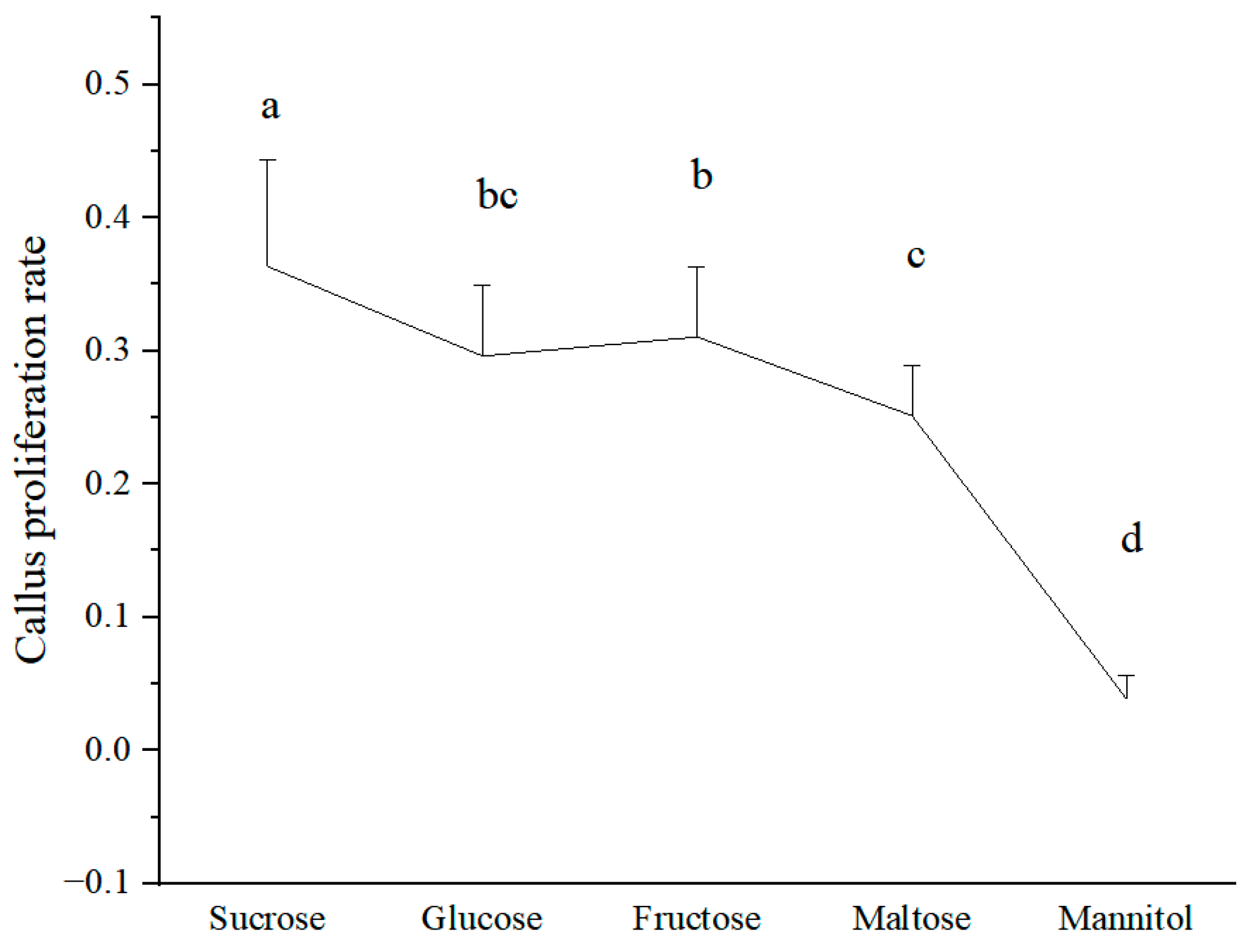
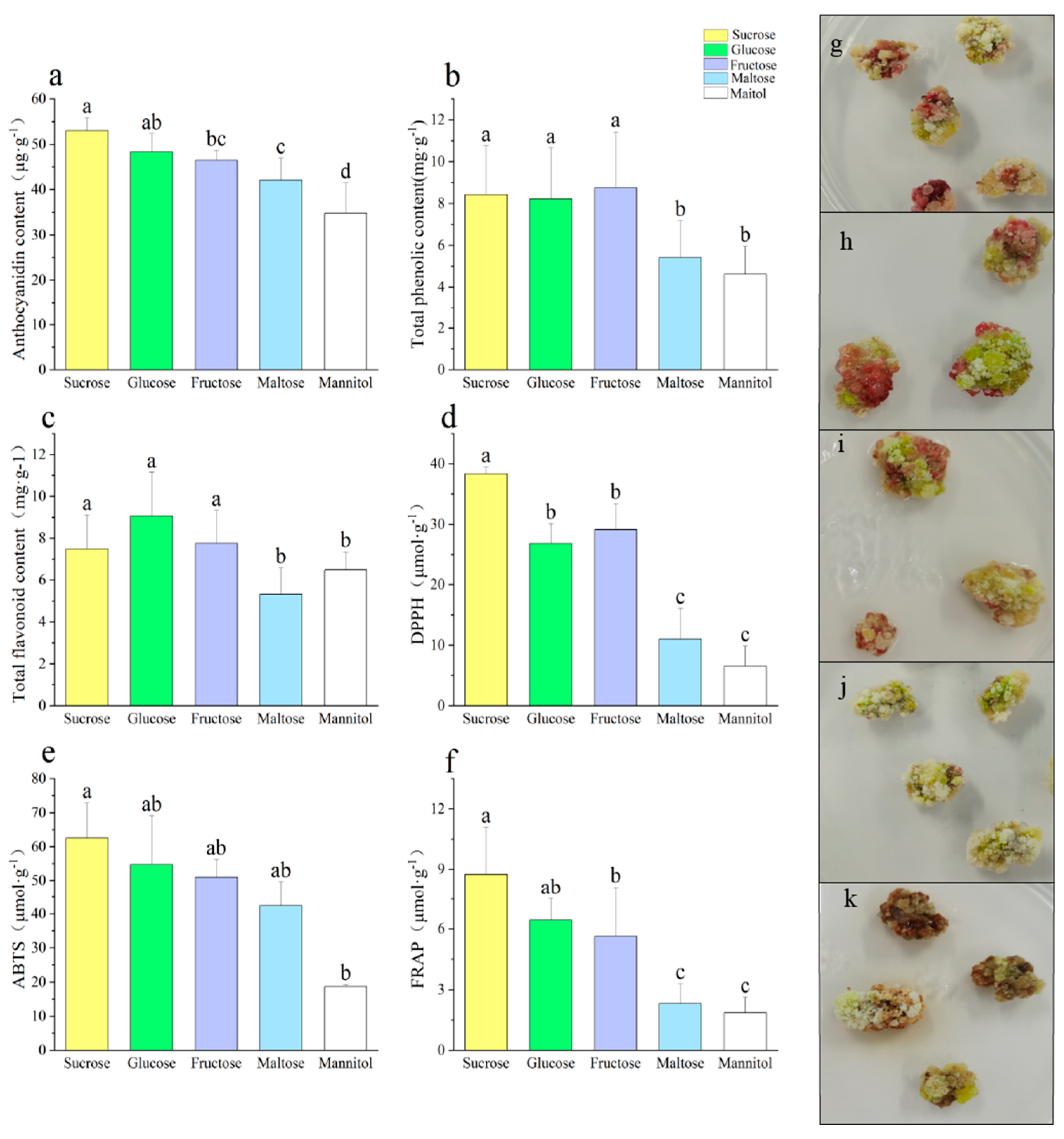
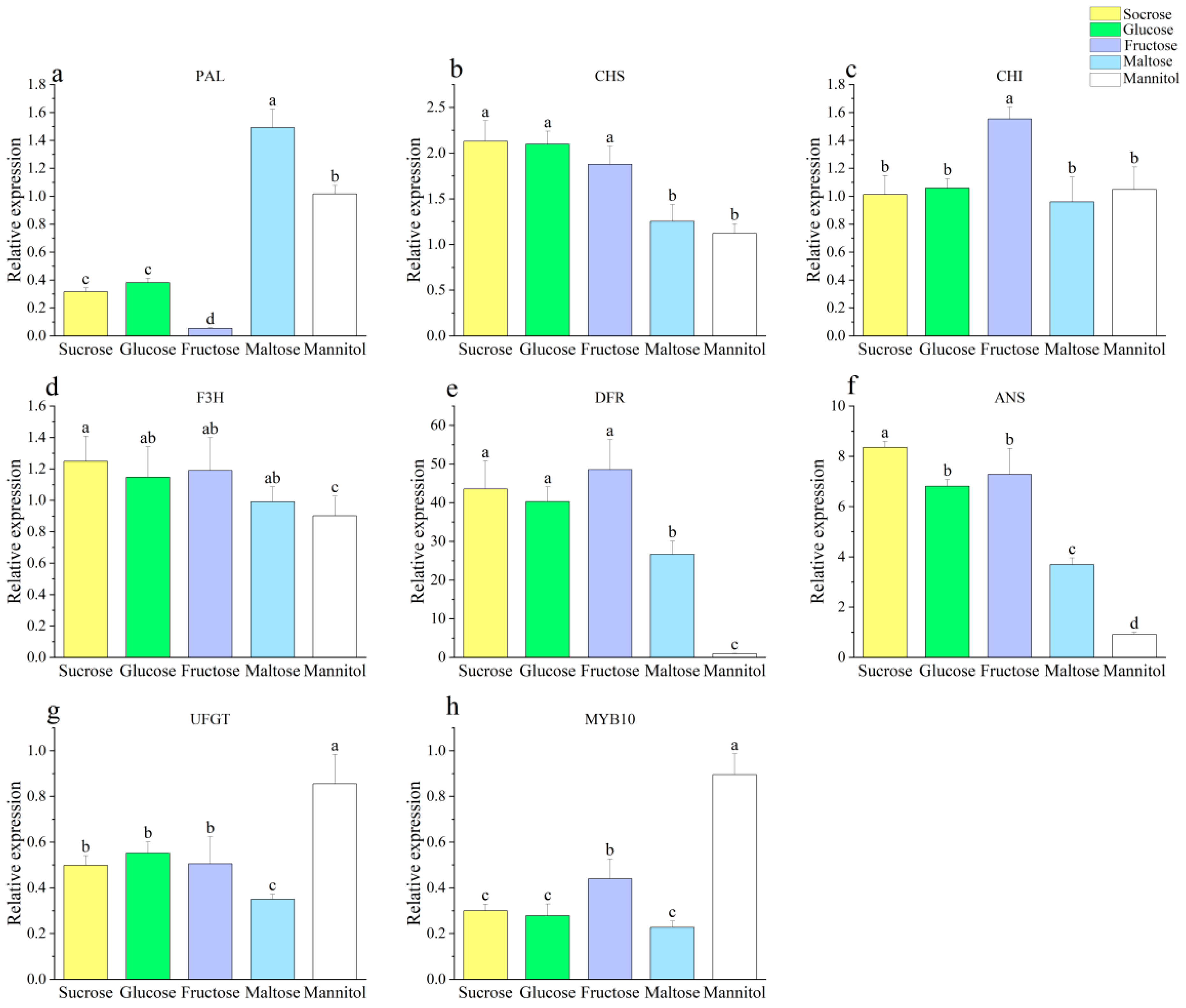
3.1. Effect of Different Sugar Sources on the Callus
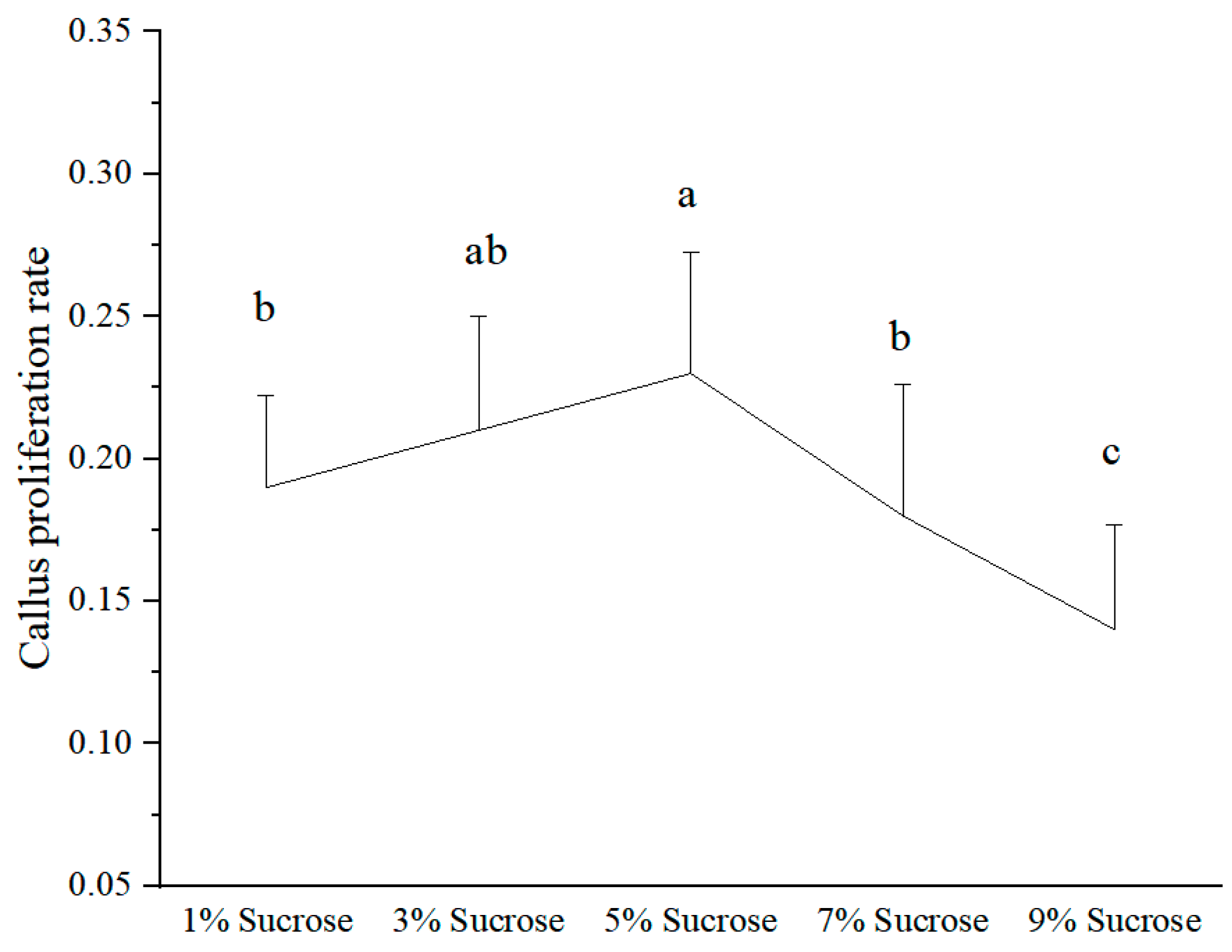
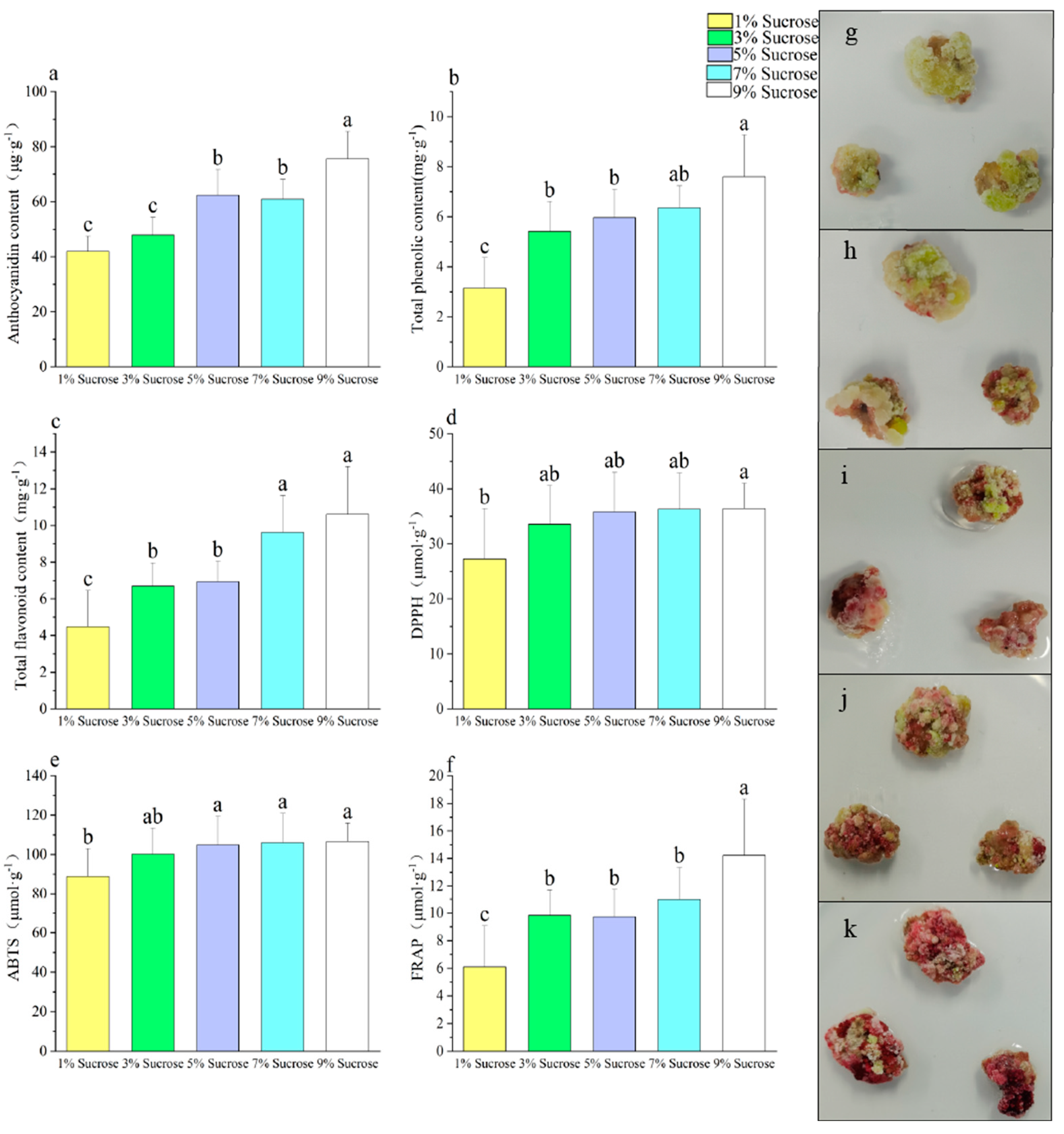
3.2. Effect of Different Sucrose Concentrations on the Callus
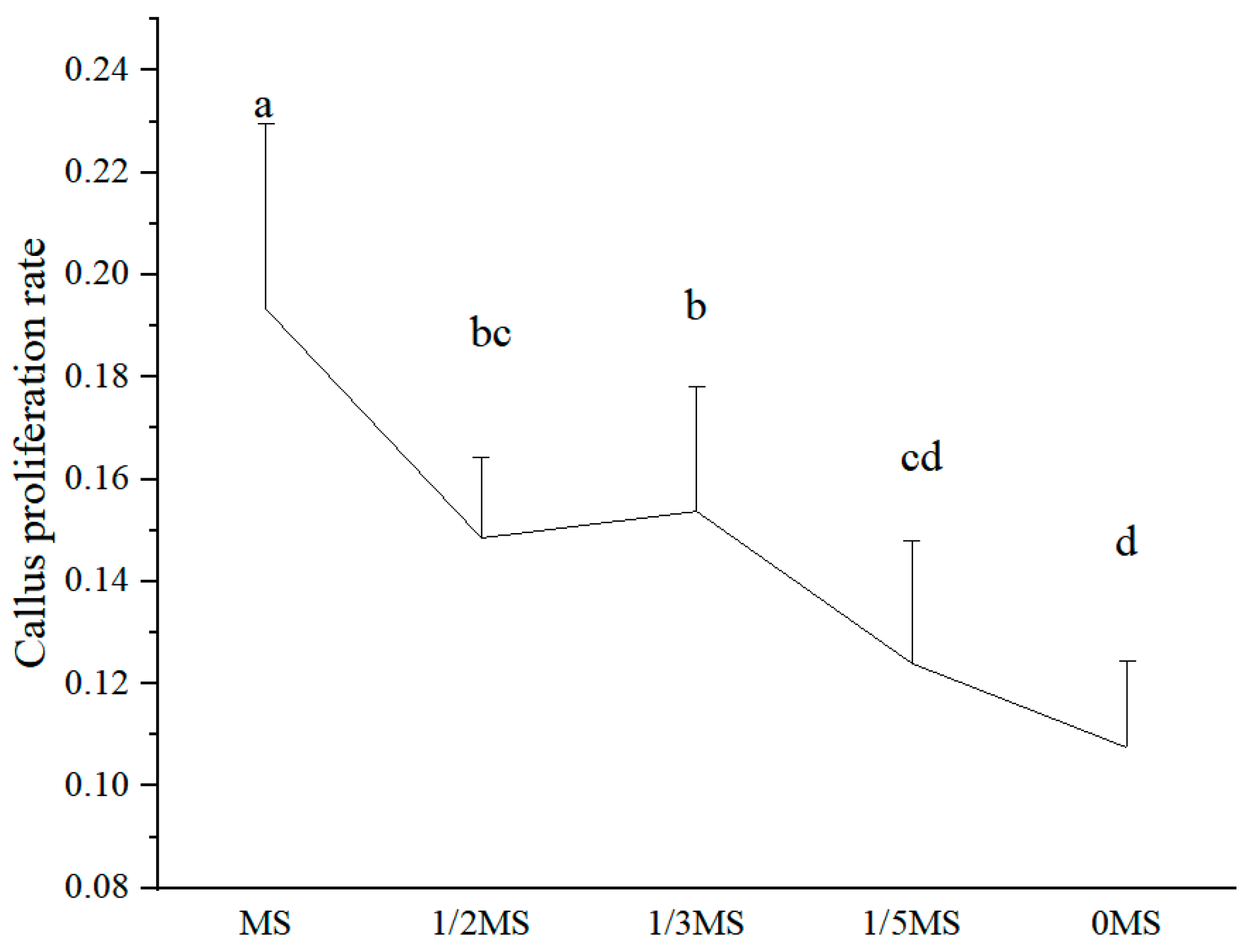
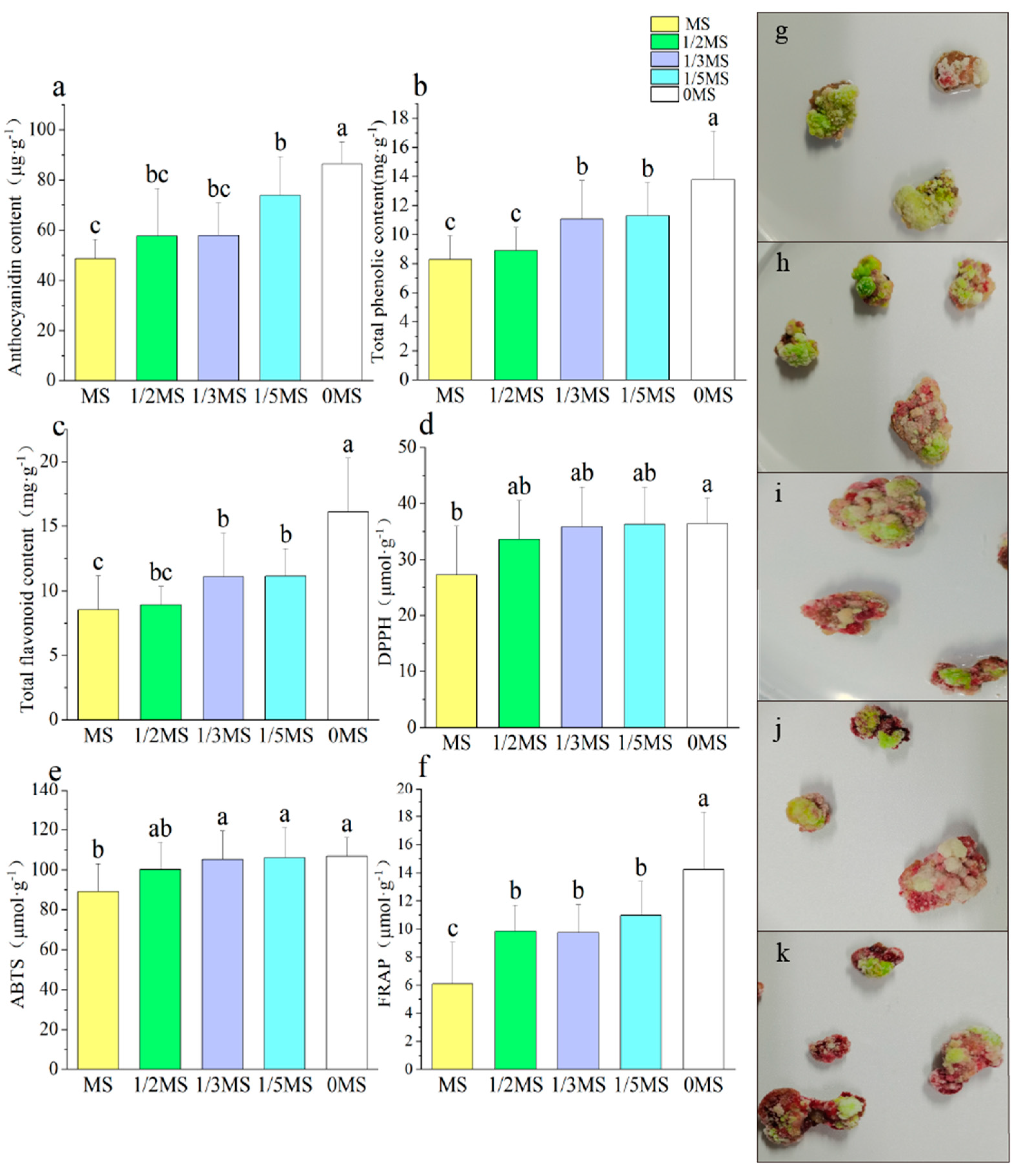
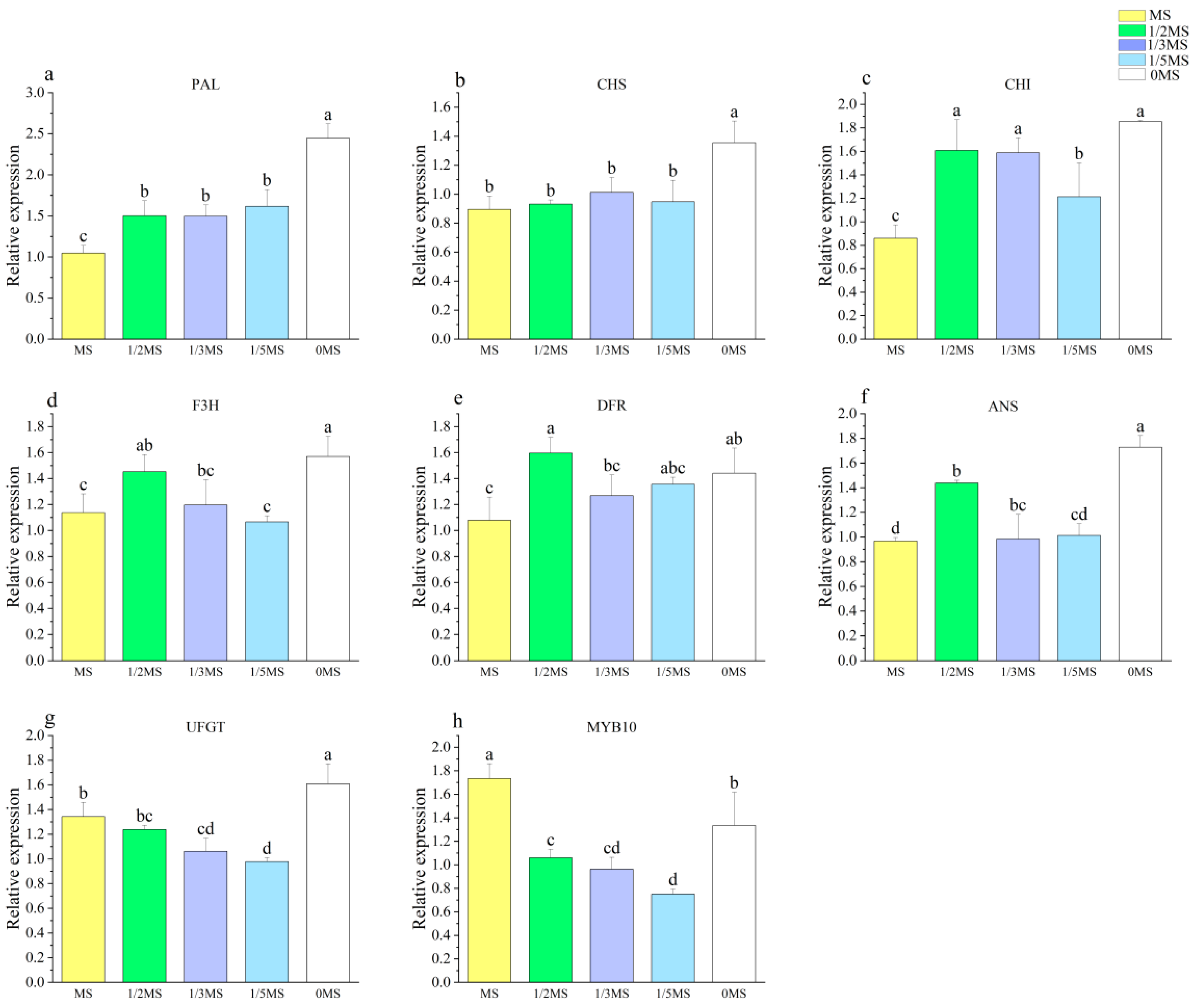
3.3. Effect of Different MS Concentrations on the Callus
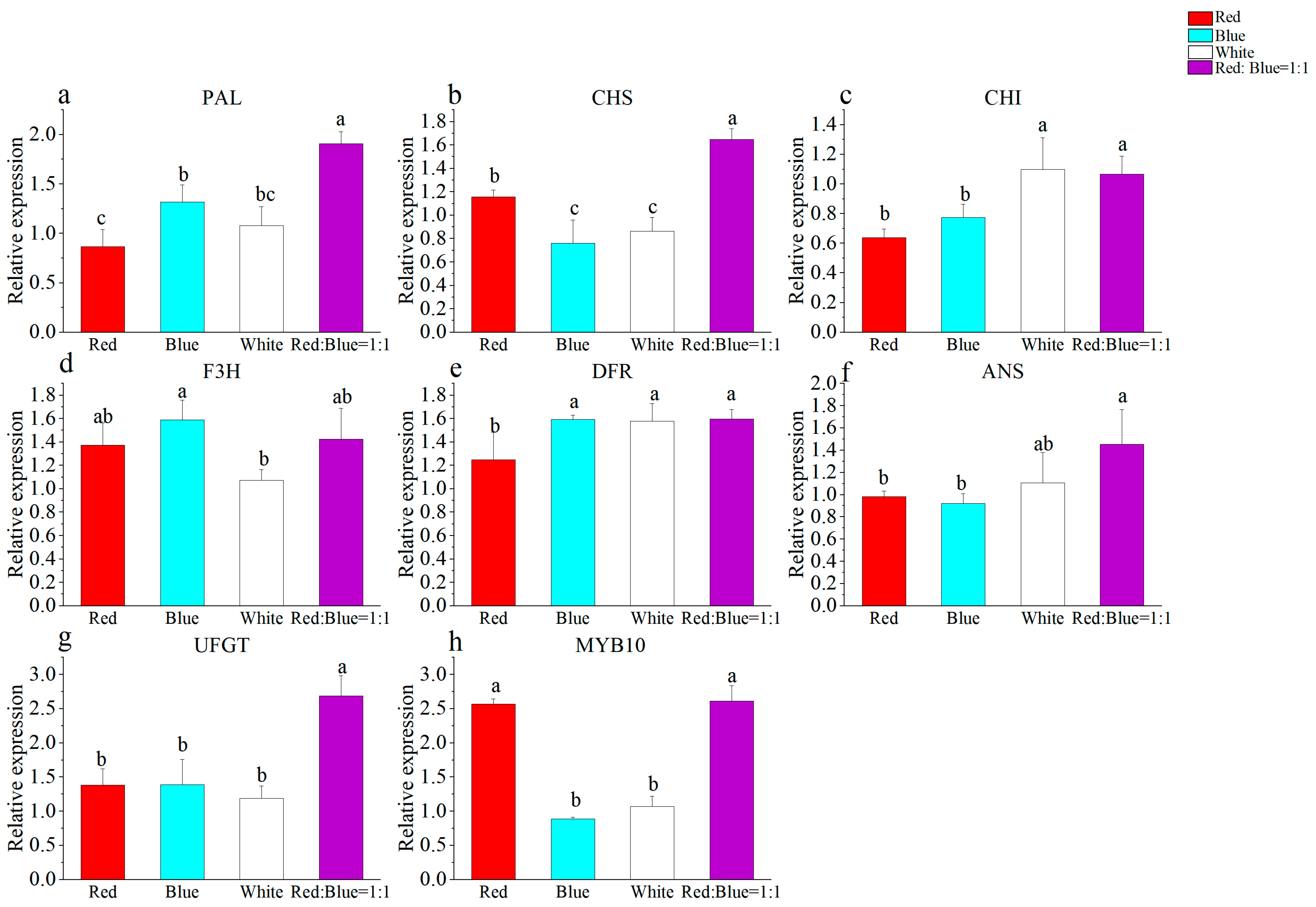
3.4. Effect of Different Light Qualities on the Callus
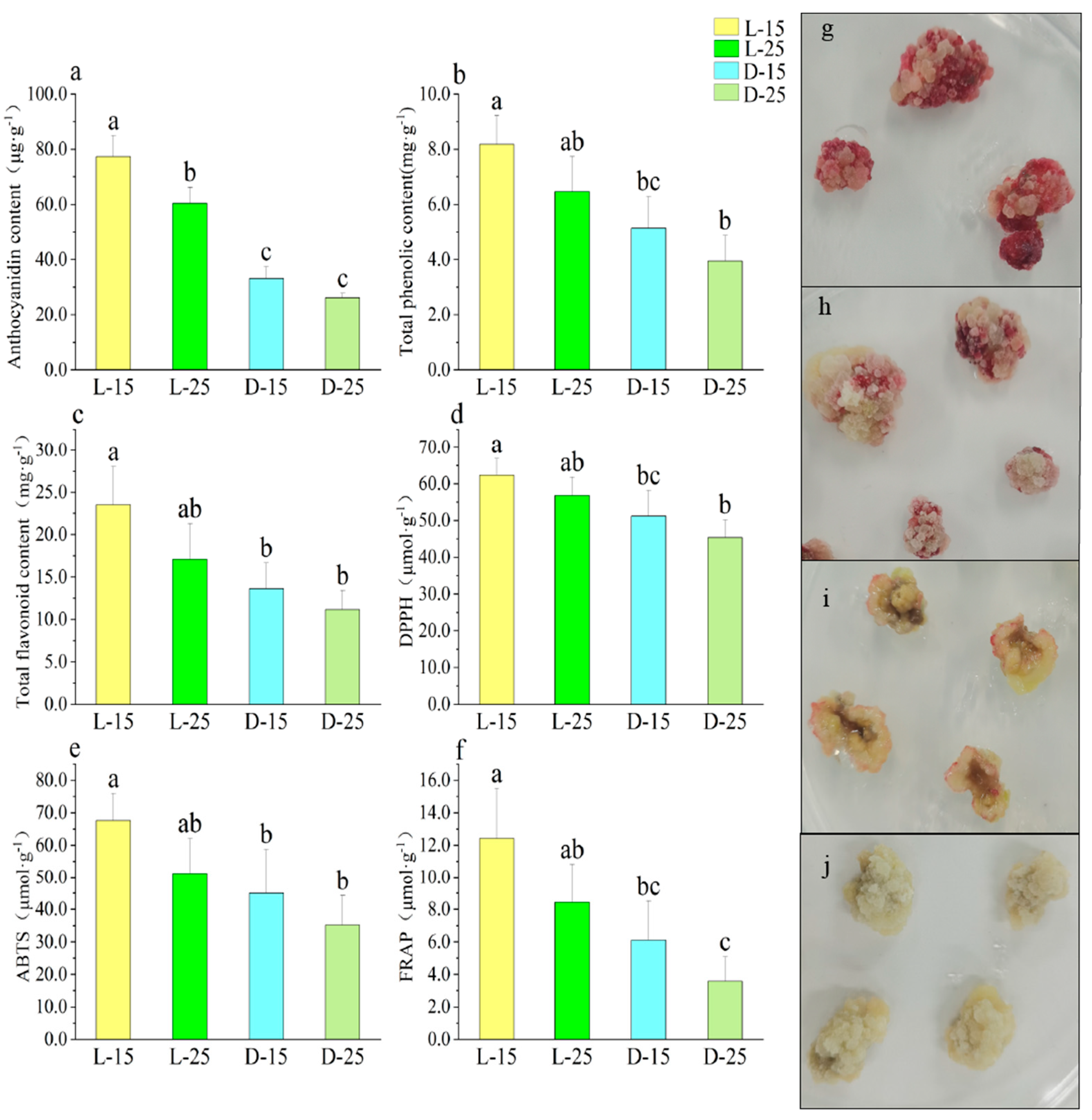
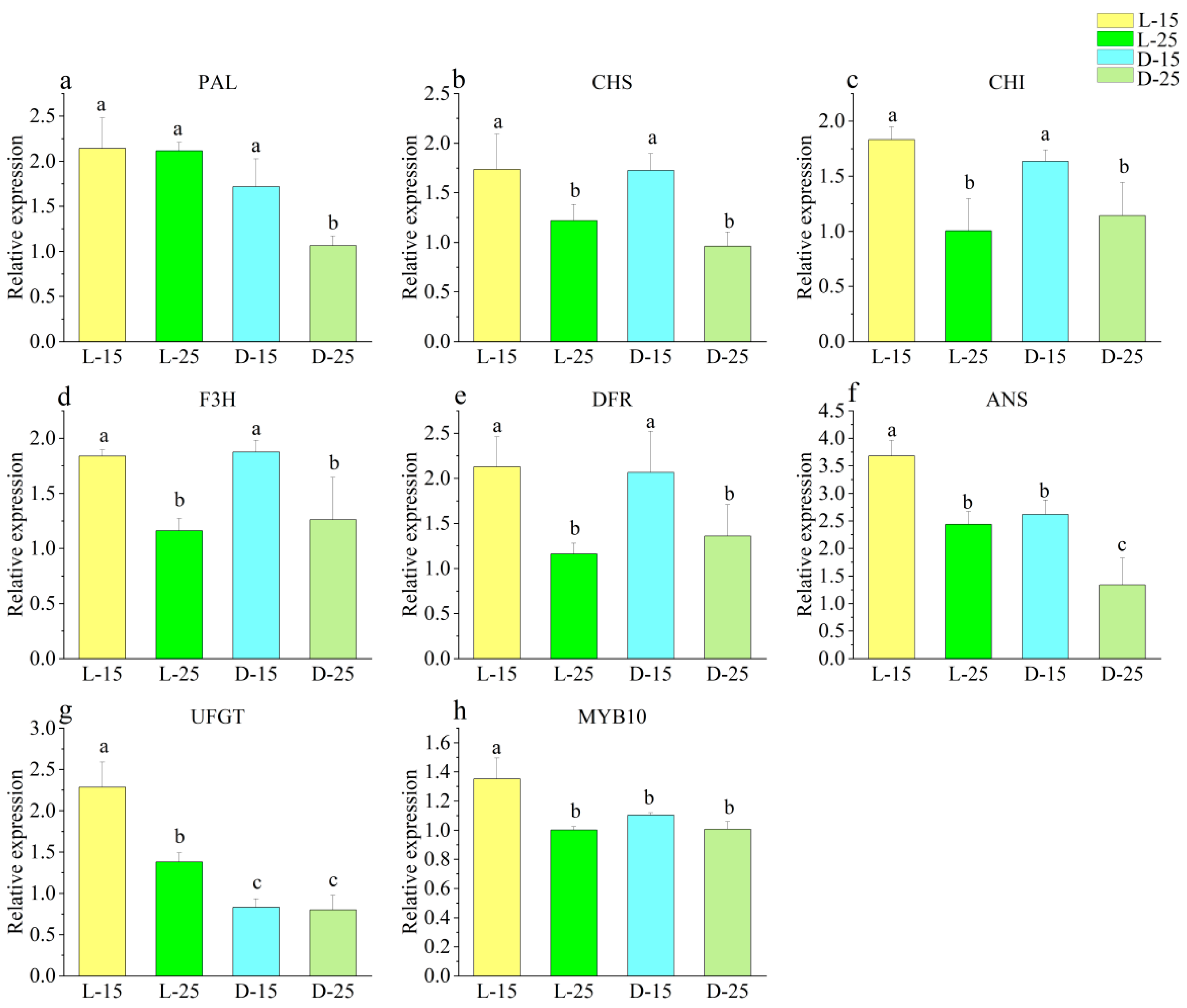
3.5. Effect of Different Temperatures on Callus under Light
3.6. Correlation Analysis of Different Treatments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


| Reagent | Catalog Number | Company | Country |
|---|---|---|---|
| NAA | N600 | Phyto Tech | United States |
| 2,4-D | D299 | Phyto Tech | United States |
| 6-BA | B800 | Phyto Tech | United States |
| MS Medium | M519 | Phyto Tech | United States |
References
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, S.; Ni, J.; Teng, Y.; Bai, S. Advances of anthocyanin synthesis regulated by plant growth regulators in fruit trees. Sci. Hortic. 2023, 307, 111476. [Google Scholar] [CrossRef]
- Sun, L.P.; Huo, J.T.; Liu, J.Y.; Yu, J.Y.; Zhou, J.L.; Sun, C.D.; Wang, Y.; Leng, F. Anthocyanins distribution, transcriptional regulation, epigenetic and post-translational modification in fruits. Food Chem. 2023, 411, 135540. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Chen, X.; Song, B.; Liu, H.; Li, J.; Wang, R.; Wu, J. Genome-wide identification of the MATE gene family and functional characterization of PbrMATE9 related to anthocyanin in pear. Hortic. Plant J. 2023. [Google Scholar] [CrossRef]
- Qu, S.S.; Li, M.M.; Wang, G.; Yu, W.T.; Zhu, S.J. Transcriptomic, proteomic and LC-MS analyses reveal anthocyanin biosynthesis during litchi pericarp browning. Sci. Hortic. 2021, 289, 110443. [Google Scholar] [CrossRef]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.B. An anthocyanin-enriched extract from Vaccinium uliginosum improves signs of skin aging in UVB-Induced photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Xu, L.; Tian, Z.; Chen, H.; Zhao, Y.; Yang, Y. Anthocyanins, Anthocyanin-Rich Berries, and Cardiovascular Risks: Systematic Review and Meta-Analysis of 44 Randomized Controlled Trials and 15 Prospective Cohort Studies. Frontiers in Nutrition, An Anthocyanin-Enriched Extract from Vaccinium Uliginosum Improves Signs of Skin Aging in UVB-Induced Photodamage. Antioxidants 2021, 8, 747884. Available online: https://www.frontiersin.org/articles/10.3389/fnut.2021.747884 (accessed on 1 January 2023).
- Li, B.; Wang, L.; Bai, W.; Chen, W.; Chen, F.; Shu, C. Biological Activity of Anthocyanins. In Anthocyanins; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Mendes, M.; Morais, R.; Calhau, C.; Pintado, M. Antimicrobial, antiadhesive and antibiofilm activity of an ethanolic, anthocyanin-rich blueberry extract purified by solid phase extraction. J. Appl. Microbiol. 2016, 121, 693–703. [Google Scholar] [CrossRef]
- Cao, X.Y.; Sun, H.L.; Wang, X.Y.; Li, W.X.; Wang, X.Q. ABA signaling mediates 5-aminolevulinic acid-induced anthocyanin biosynthesis in red pear fruits. Sci. Hortic. 2022, 304, 111290. [Google Scholar] [CrossRef]
- Du, Y.; Qu, B.; Li, R. Research progress on red pear resources and fruit coloring mechanism in China. J. Agric. Yanbian Univ. 2021, 43, 98–107. (In Chinese) [Google Scholar] [CrossRef]
- Tao, R.; Yu, W.; Gao, Y.; Ni, J.; Yin, L.; Zhang, X.; Li, H.; Wang, D.; Bai, S.; Teng, Y. Light-Induced Basic/Helix-Loop-Helix64 Enhances Anthocyanin Biosynthesis and Undergoes Constitutively Photomorphogenic1-Mediated Degradation in Pear. Plant Physiol. 2020, 4, 1684–1701. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.X. Catalogue of Fruit Germplasm Resources (First 1); Agricultural Press: Beijing, China, 1993; pp. 22–49. (In Chinese) [Google Scholar]
- Steyn, W.J.; Holcroft, D.M.; Wand, J.E.S.; Jacobs, G. Anthocyanin degradation in detached pome fruit with reference to preharvest red color loss and pigmentation patterns of blushed and fully red pears. J. Am. Soc. Hortic. Sci. 2004, 129, 13–19. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–993. [Google Scholar] [CrossRef]
- Wang, C.; Gan, Q.; Meng, F.; Yang, J.; Yan, H.; Jiang, X. Active Components and Antioxidant Activities of Four Kinds Small Berry Juices. Sci. Technol. Food Ind. 2019, 40, 71–76. [Google Scholar] [CrossRef]
- Quattrocchio, F.; Wing, J.F.; Leppen, H.; Mol, J.; Koes, R.E. Regulatory Genes Controlling Anthocyanin Pigmentation Are Functionally Conserved among Plant Species and Have Distinct Sets of Target Genes. Plant Cell 1993, 5, 1497–1512. [Google Scholar] [CrossRef]
- Winkelshirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Liu, Y.; Wang, E.; Zhang, D.; Huang, S.; Li, C.; Zhang, Y.; Chen, Z.; Zhang, Y. SlPHL1 is involved in low phosphate stress promoting anthocyanin biosynthesis by directly upregulation of genes SlF3H, SlF3′H, and SlLDOX in tomato. Plant Physiol. Biochem. 2023, 200, 107801. [Google Scholar] [CrossRef]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, I.D.; Venskutonis, P.R.; Georgiev, M.I.; Clément, C.; et al. Anthocyanins, multi-functional natural products of industrial relevance: Recent biotechnological advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef]
- María, M.; Paola, P.; Dirk, P.; Christian, G.; Lorena, J.; Perla, F.; Katy, P.; Rolando, C. Kinetics and modeling of cell growth for potential anthocyanin induction in cultures of Taraxacum officinale G.H. Weber ex Wiggers (Dandelion) in vitro. Electron. J. Biotechnol. 2018, 36, 15–23. [Google Scholar] [CrossRef]
- Lai, C.; Fan, L.; He, X.; Xie, H. Callus induction and screening of high yield proanthocyanidin cell lines. J. Plant Physiol. 2014, 50, 1683–1691. (In Chinese) [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to Total Phenolics with Phosphomolybdic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. Available online: https://10.5344/ajev.1965.16.3.144 (accessed on 3 June 2023). [CrossRef]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, C. Elucidation of the flavonoid and furocoumarin composition and radical-scavenging activity of green and ripe chinotto (Citrus myrtifolia Raf.) fruit tissues, leaves and seeds. Food Chem. 2011, 129, 1504–1512. [Google Scholar] [CrossRef]
- Almeida, M.M.B.; Sousa, P.H.M.; Arriaga, Â.M.C.; Prado, G.M.; Magalhães, C.E.C.; Maia, G.A.; Lemos, T.L.G. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res. Int. 2011, 44, 2155–2159. [Google Scholar] [CrossRef]
- Jang, H.D.; Chang, K.S.; Chang, T.C.; Hsu, C.L. Antioxidant potentials of buntan pumelo (Citrus grandis Osbeck) and its ethanolic and acetified fermentation products. Food Chem. 2010, 118, 554–558. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.W.; Wang, X.R.; Xie, X.L.; Yue, X.Y.; Tang, H.R. An alternative cetyltrimethylammonium bromide-based protocol for RNA isolation from blackberry (Rubus L.). Genet. Mol. Res. 2012, 11, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Abbasi, H.B.; Zeb, A.; Ali, G.S. Carbohydrate-induced biomass accumulation and elicitation of secondary metabolites in callus cultures of Fagonia indica. Ind. Crops Prod. 2018, 126, 168–176. [Google Scholar] [CrossRef]
- Wang, C. Factors affecting the production of secondary metabolites by plant tissue culture. Rural Econ. Sci. Technol. 2020, 31, 34+48. (In Chinese) [Google Scholar]
- Bong, F.J.; Chear, N.J.Y.; Ramanathan, S.; Mohana-Kumaran, N.; Subramaniam, S.; Chew, B.L. The development of callus and cell suspension cultures of Sabah Snake Grass (Clinacanthus nutans) for the production of flavonoids and phenolics. Biocatal. Agric. Biotechnol. 2021, 33, 101977. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Gu, K.D.; Wang, C.K.; Hu, D.G.; Hao, Y.J. How do anthocyanins paint our horticultural products? Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Kumar, G.P.; Sivakumar, S.; Govindarajan, S.; Sadasivam, V.; Manickam, V.; Mogilicherla, K.; Thiruppathi, S.K.; Narayanasamy, J. Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. SVPR-2. Biotechnol. Rep. 2015, 7, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, H.; Wang, H.; Zhan, J.; Yuan, X.; Cui, J.; Su, N. Selenium treatment promotes anthocyanin accumulation in radish sprouts (Raphanus sativus L.) by its regulation of photosynthesis and sucrose transport. Food Res. Int. 2023, 165, 112551. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Delphine, M.P.; Sonia, O.; José, G.V. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2020.564917 (accessed on 6 June 2023). [CrossRef] [PubMed]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.W.; Meddar, M.; Renaud, C.; Merlin, I.; Hilbert, G.; Delrot, S. Long-term in vitro culture of grape berries and its application to assess the effects of sugar supply on anthocyanin accumulation. J. Exp. Bot. 2014, 65, 4665–4677. [Google Scholar] [CrossRef]
- Sarmadi, M.; Karimi, N.; Palazón, J.; Ghassempour, A.; Mirjalili, M.H. The effects of salicylic acid and glucose on biochemical traits and taxane production in a Taxus baccata callus culture. Plant Physiol. Biochem. 2018, 132, 271–280. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Defensive efforts of Salix myrsinifolia plantlets I photomixotrophic culture conditions: The effect of sucrose, nitrogen and pH on the phytomass and secondary phenolic accumulation. Ecoscience 1996, 3, 297–303. [Google Scholar] [CrossRef]
- Pasqua, G.; Monacelli, B.; Mulinacci, N.; Rinaldi, S.; Giaccherini, C.; Innocenti, M.; Vinceri, F.F. The effect of growth regulators and sucrose on anthocyanin production in Camptotheca acuminata cell cultures. Plant Physiol. Biochem. 2005, 43, 293–298. [Google Scholar] [CrossRef]
- Ram, M.; Prasad, K.V.; Kaur, C.; Singh, S.K.; Arora, A.; Kumar, S. Induction of anthocyanin pigments in callus cultures of Rosa hybrida L. in response to sucrose and ammonical nitrogen levels. Plant Cell Tissue Organ Cult. 2011, 104, 171–179. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.S.; Jo, Y.D.; Kang, S.Y.; Ahn, J.W.; Kang, B.C.; Kim, J.B. Sucrose and methyl jasmonate modulate the expression of anthocyanin biosynthesis genes and increase the frequency of flower-color mutants in chrysanthemum. Sci. Hortic. 2019, 256, 108602. [Google Scholar] [CrossRef]
- Ai, T.N.; Naing, A.H.; Arun, M.; Lim, S.H.; Kim, C.K. Sucrose-induced anthocyanin accumulation in vegetative tissue of Petunia plants requires anthocyanin regulatory transcription factors. Plant Sci. 2016, 252, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260. [Google Scholar] [CrossRef]
- Gollop, R.; Even, S.; Colova-Tsolova, V.; Peri, A. Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J. Exp. Bot. 2002, 53, 13971409. [Google Scholar] [CrossRef]
- Gollop, R.; Farhi, S.; Peri, A. Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Sci. 2001, 161, 579–588. [Google Scholar] [CrossRef]
- Schiozer, A.L.; Barata, L.E.S. Stability of Natural Pigments and Dyes. Rev. Fitos 2007, 3, 6–24. Available online: https://revistafitos.far.fiocruz.br/index.php/revista-fitos/article/view/71 (accessed on 9 June 2023). [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Simões, C.; Bizarri, C.H.B.; da Silva Cordeiro, L.; Castro, T.C.; Coutada, L.C.M.; da Silva, A.J.R.; Albarello, N.; Mansur, E. Anthocyanin production in callus cultures of Cleome rosea: Modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol. Biochem. 2009, 47, 895–903. [Google Scholar] [CrossRef]
- Meng, J.X.; Gao, Y.; Han, M.L.; Liu, P.Y.; Yang, C.; Shen, T.; Li, H.H. In vitro Anthocyanin Induction and Metabolite Analysis in Malus spectabilis Leaves Under Low Nitrogen Conditions. Hortic. Plant J. 2020, 6, 284–292. [Google Scholar] [CrossRef]
- Ji, X.H.; Wang, Y.T.; Zhang, R.; Wu, S.J.; An, M.M.; Li, M.; Wang, C.Z.; Chen, X.L.; Zhang, Y.M.; Chen, X.S. Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2015, 120, 325–337. [Google Scholar] [CrossRef]
- Li, H. Modern Plant Physiology, 3rd ed.; Life World: Beijing, China, 2012; p. 2. (In Chinese) [Google Scholar]
- Li, H.; He, K.; Zhang, Z.Q.; Hu, Y. Molecular mechanism of phosphorous signaling inducing anthocyanin accumulation in Arabidopsis. Plant Physiol. Biochem. 2023, 196, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Tian, C.L.; Murch, S.J.; Saxena, P.K.; Liu, C.Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef]
- Kreuzaler, F.; Hahlbrock, K. Flavonoid glycosides from illuminated cell suspension cultures of Petroselinum hortense. Phytochemistry 1973, 12, 1149–1152. [Google Scholar] [CrossRef]
- Zhong, J.J.; Seki, T.; Kinoshita, S.I.; Yoshida, T. Effect of light irradiation on anthocyanin production by suspended culture of Perilla frutescens. Biotechnol. Bioeng. 1991, 38, 653–658. [Google Scholar] [CrossRef]
- Liu, C.Z.; Guo, C.; Wang, Y.C.; Ouyang, F. Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process. Biochem. 2002, 38, 581–585. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B Biol. 2016, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B Biol. 2016, 154, 51–56. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Zahir, A.; Hano, C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J. Photochem. Photobiol. B Biol. 2019, 190, 172–178. [Google Scholar] [CrossRef]
- Abou El-Dis, G.R.; Zavdetovna, K.L.; Nikolaevich, A.A.; Abdelazeez, W.M.A.; Arnoldovna, T.O. Influence of light on the accumulation of anthocyanins in callus culture of Vaccinium corymbosum L. cv. Sunt Blue Giant. J. Photochem. Photobiol. 2021, 8, 100058. [Google Scholar] [CrossRef]
- Nazir, M.; Ullah, M.A.; Younas, M.; Siddiquah, A.; Shah, M.; Guivarc’h, G.; Hano, C.; Abbasi, B.H. Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Plant Cell Tissue Organ Cult. 2020, 142, 107–120. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.X.; Jeong, B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B Biol. 2019, 196, 111509. [Google Scholar] [CrossRef]
- Sng, B.J.R.; Mun, B.; Mohanty, B.; Kim, M.; Phua, Z.W.; Yang, H.; Lee, D.Y.; Jang, I.C. Combination of red and blue light induces anthocyanin and other secondary metabolite biosynthesis pathways in an age-dependent manner in Batavia lettuce. Plant Sci. 2021, 310, 110977. [Google Scholar] [CrossRef]
- Jie, X.; Liu, X.; Wang, W.; Bai, J.; Li, D.; Liu, Y. Effects of Light Quality on Callus Growth Rate and Anthocyanin Synthesis of Hongyang Kiwifruit. Shanxi Agric. Sci. 2021, 49, 1166–1172. (In Chinese) [Google Scholar]
- Toguri, T.; Umemoto, N.; Kobayashi, O.; Ohtani, T. Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues, and identification of an inducible P-450 cDNA. Plant Mol. Biol. 1993, 23, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Schouten, R.E.; Tikunov, Y.; Liu, X.X.; Visser, R.G.F.; Tan, F.; Bovy, A.; Marcelis, L.F.M. Blue light increases anthocyanin content and delays fruit ripening in purple pepper fruit. Postharvest Biol. Technol. 2022, 192, 112024. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, B.; Jiang, H.; Xi, X.; Ding, J. Culture effect of different color light, temperature, and pH on grass coral callus. Jiangxi Sci. 1994, 1994, 85–89. (In Chinese) [Google Scholar]
- Wei, C.; Niu, Z.; Dou, F.; Wang, Q. Effect of test tube microenvironment on anthocyanin content in potato ‘GSAP-H’ callus. J. Gansu Agric. Univ. 2016, 51, 47–53. (In Chinese) [Google Scholar] [CrossRef]
- Narayan, M.S.; Thimmaraju, R.; Bhagyalakshmi, N. Interplay of growth regulators during solid-state and liquid-state batch cultivation of anthocyanin producing cell line of Daucus carota. Process Biochem. 2005, 40, 351–358. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Wang, Y. Artemisinin: Current state and perspectives for biotechnological production of an antimalarial drug Appl. Microb. Biotechnol. 2006, 72, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Z.; Jiang, S.; Xu, H.; Wang, Y.; Feng, S.; Chen, X. Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2016, 127, 217–227. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; You, Y.; Yang, C.; Khan, A.R.; Li, P.; Ma, F. Characterization of phenolic compounds and active anthocyanin degradation in crabapple (Malus orientalis) flowers. Hortic. Environ. Biotechnol. 2017, 58, 324–333. [Google Scholar] [CrossRef]
- Leyva, A.; Jarillo, J.A.; Salinas, J.; Martinez-Zapater, J.M. Low Temperature Induces the Accumulation of Phenylalanine Ammonia-Lyase and Chalcone Synthase mRNAs of Arabidopsis thaliana in a Light-Dependent Manner. Plant Physiol. 1995, 108, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lo Piero, A.R.; Puglisi, I.; Rapisarda, P.; Petrone, G. Anthocyanins accumulation and related gene expression in red orange fruit induced by low-temperature storage. J. Agric. Food Chem. 2005, 53, 9083–9088. [Google Scholar] [CrossRef]
- Naing, A.H.; Park, D.Y.; Park, K.I.; Kim, C.K. Differential expression of anthocyanin structural genes and transcription factors determines coloration patterns in gerbera flowers. 3 Biotech 2018, 8, 393. [Google Scholar] [CrossRef]
- Bagal, D.; Chowdhary, A.A.; Mehrotra, S.; Mishra, S.; Rathore, S.; Srivastava, V. Metabolic engineering in hairy roots: An outlook on production of plant secondary metabolites. Plant Physiol. Biochem. 2023, 201, 107847. [Google Scholar] [CrossRef] [PubMed]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Supriyono; Suhara, C.; Suastika, I.B.K.; Mahayu, W.M.; Wani, A.K. Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Koufan, M.; Belkoura, I.; Mazri, M.A.; Amarraque, A.; Essatte, A.; Elhorri, H.; Zaddoug, F.; Alaoui, T. Determination of antioxidant activity, total phenolics and fatty acids in essential oils and other extracts from callus culture, seeds and leaves of Argania spinosa (L.) Skeels. Plant Cell Tiss Organ Cult. 2020, 141, 217–227. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Q.; Lu, A.; Yang, Z.; Tan, D.; Lu, Y.; Qin, L.; He, Y. The preventive effect of secondary metabolites of Dendrobium officinale on acute alcoholic liver injury in mice. Arab. J. Chem. 2023, 16, 105138. [Google Scholar] [CrossRef]
- Liu, X.; Ma, C.; Liu, Z.; Kang, W. Natural Products: Review for Their Effects of Anti-HBV. BioMed Res. Int. 2020, 9, 3972390. [Google Scholar] [CrossRef]


| Target Gene | Primer Sequences (5′-3′) |
|---|---|
| Actin | Actin-F: CCATCCAGGCTGTTCTCTC Actin-R: GCAAGGTCCAGACGAAGG |
| PbANS | PbANS-F: TGGTAAGATTCAAGGCTATGGAAGC PbANS-R: TCACGCTTGTCCTCTGGGTATAC |
| PbCHS | PbCHS-F: ACCCAACTGTGTGCGAGTAC PbCHS-R: TGGGTGATTTTGGACTTGGGC |
| PbCHI | PbCHI-F: TCGGAGTGTACTTGGAGGAAAACG PbCHI-R: TCTCAAACGGACCTGTAACGATG |
| PbDFR | PbDFR-F: CAGGAACTGTGAACGTGGAGG PbDFR-R: GAGACGAAGTACATCCAACCAGTC |
| PbF3H | PbF3H-F:TCGCTAGAGAGTTCTTTGCTTTGC PbF3H-R: TTTCACGCCAATCTTGCACAG |
| PbPAL | PbPAL-F: ATCGCTACGCTCTCCGAAC PbPAL-R: GTGCAAGGCCTTGTTCCTC |
| PbUFGT | PbUFGT-F: ACACTCTCTTCTCGTTCTTCAGC PbUFGT-R: CATCGTACACCCTTAGGTTAGGC |
| PbMYB10 | PbMYB10-F: CAGGAAGAACAGCGAATGATGTG PbMYB10-R: GGGCTGAGGTCTTATCACATTGG |
| Different sugar source treatment | Total phenolic content (mg·g−1) | Total flavonoid content (mg·g−1) | DPPH (μmol·g−1) | ABTS (μmol·g−1) | FRAP (μmol·g−1) | Callus proliferation rate | PAL | CHS | CHI | F3H | DFR | ANS | UFGT | MYB10 |
| Anthocyanidin content (μg·g−1) | 0.889 * | 0.573 | 0.953 * | 0.987 ** | 0.946 * | 0.953 * | −0.658 | 0.932 * | 0.117 | 0.959 ** | 0.903 * | 0.973 ** | −0.586 | −0.756 |
| Different sucrose concentration treatments | Total phenolic content (mg·g−1) | Total flavonoid content (mg·g−1) | DPPH (μmol·g−1) | ABTS (μmol·g−1) | FRAP (μmol·g−1) | Callus proliferation rate | PAL | CHS | CHI | F3H | DFR | ANS | UFGT | MYB10 |
| Anthocyanidin content (μg·g−1) | 0.931 * | 0.889 * | 0.831 | 0.858 | 0.913 * | −0.521 | 0.888 * | 0.953 * | 0.917 * | 0.988 ** | 0.935 * | 0.964 ** | 0.719 | 0.642 |
| Different MS concentration treatments | Total phenolic content (mg·g−1) | Total flavonoid content (mg·g−1) | DPPH (μmol·g−1) | ABTS (μmol·g−1) | FRAP (μmol·g−1) | Callus proliferation rate | PAL | CHS | CHI | F3H | DFR | ANS | UFGT | MYB10 |
| Anthocyanidin content (μg·g−1) | 0.922 * | 0.911 * | 0.739 | 0.765 | 0.946 * | −0.948 * | 0.935 * | 0.823 | 0.597 | 0.458 | 0.447 | 0.641 | 0.344 | −0.312 |
| Different light quality treatment | Total phenolic content (mg·g−1) | Total flavonoid content (mg·g−1) | DPPH (μmol·g−1) | ABTS (μmol·g−1) | FRAP (μmol·g−1) | Callus proliferation rate | PAL | CHS | CHI | F3H | DFR | ANS | UFGT | MYB10 |
| Anthocyanidin content (μg·g−1) | 0.938 | 0.944 | 0.457 | 0.999 ** | 0.938 | 0.038 | 0.602 | 0.645 | −0.524 | 0.968 * | −0.25 | 0.766 | 0.76 | 0.665 |
| Different temperature light treatment | Total phenolic content (mg·g−1) | Total flavonoid content (mg·g−1) | DPPH (μmol·g−1) | ABTS (μmol·g−1) | FRAP (μmol·g−1) | Callus proliferation rate | PAL | CHS | CHI | F3H | DFR | ANS | UFGT | MYB10 |
| Anthocyanidin content (μg·g−1) | 0.983 * | 0.974 * | 0.977 * | 0.957 * | 0.973 * | −0.739 | 0.875 | 0.446 | 0.351 | 0.193 | 0.227 | 0.836 | 0.960 * | 0.688 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Lei, D.; Zhou, X.; Wang, H.; Lu, J.; Lin, Y.; Zhang, Y.; Wang, Y.; He, W.; Li, M.; et al. Effect of Different Culture Conditions on Anthocyanins and Related Genes in Red Pear Callus. Agronomy 2023, 13, 2032. https://doi.org/10.3390/agronomy13082032
Yao W, Lei D, Zhou X, Wang H, Lu J, Lin Y, Zhang Y, Wang Y, He W, Li M, et al. Effect of Different Culture Conditions on Anthocyanins and Related Genes in Red Pear Callus. Agronomy. 2023; 13(8):2032. https://doi.org/10.3390/agronomy13082032
Chicago/Turabian StyleYao, Wantian, Diya Lei, Xuan Zhou, Haiyan Wang, Jiayu Lu, Yuanxiu Lin, Yunting Zhang, Yan Wang, Wen He, Mengyao Li, and et al. 2023. "Effect of Different Culture Conditions on Anthocyanins and Related Genes in Red Pear Callus" Agronomy 13, no. 8: 2032. https://doi.org/10.3390/agronomy13082032
APA StyleYao, W., Lei, D., Zhou, X., Wang, H., Lu, J., Lin, Y., Zhang, Y., Wang, Y., He, W., Li, M., Chen, Q., Luo, Y., Wang, X., Tang, H., & Zhang, Y. (2023). Effect of Different Culture Conditions on Anthocyanins and Related Genes in Red Pear Callus. Agronomy, 13(8), 2032. https://doi.org/10.3390/agronomy13082032









