Morphological and Ecogeographical Diversity of the Andean Lupine (Lupinus mutabilis Sweet) in the High Andean Region of Ecuador
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Features
2.2. Genetic Material
2.3. Experimental Design
2.4. Agronomic Management
2.5. Variables Evaluated
- (a)
- plant: stem formation, procumbence resistance, plant vigor, number of pods per main axis, days to flowering, days to maturity (50% of dry pods in the main axis) and height of the plant measured at the time of harvest.
- (b)
- leaf: pubescence of the upper part and underside of the leaf.
- (c)
- flower and inflorescence: length of the peduncle of the main axis, length of the inflorescence of the main axis, color of the flower bud, and color of the wings, keel, and flower banner.
- (d)
- young pod: pubescence of the pod.
- (e)
- dry pod: pod dehiscence and number of pods in the main axis.
- (f)
- pod: length and width of the pod, and number of seeds per pod.
- (g)
- seed: seed shape, primary and secondary color of the seed, length and width of the seed, weight of 100 seeds (humidity of 12%), and yield per plant.
2.6. Morphological and Ecogeographic Agro-Biodiversity
2.7. Statistical Analysis
3. Results
3.1. Genetic Variability in the Ecuadorian Tarwi Collection
3.1.1. Quantitative Variables
3.1.2. Qualitative Variables
3.2. Multivariate Analysis
3.2.1. Principal Component Analysis
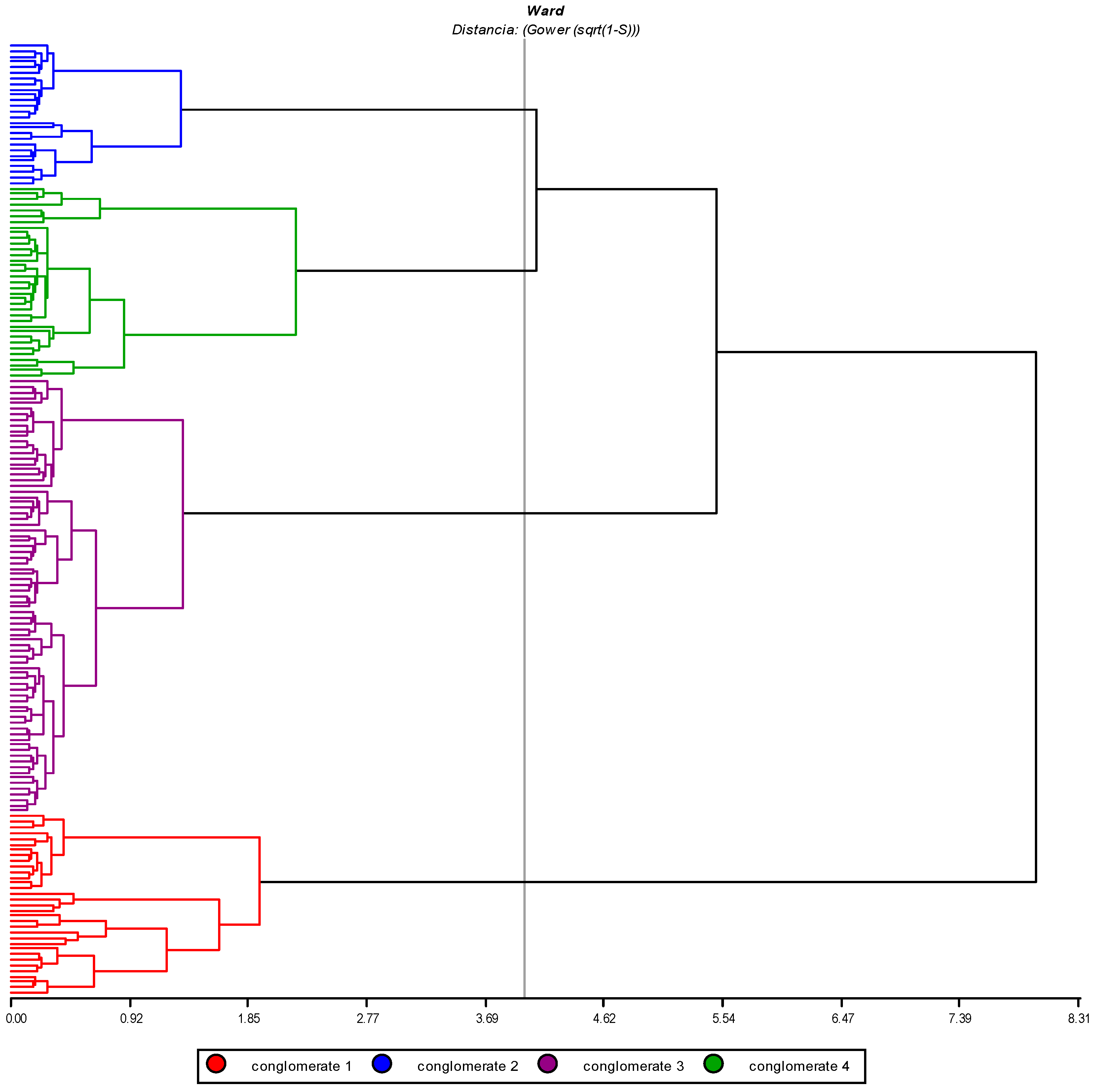
3.2.2. Group Analysis
3.2.3. Morphological Variability of Quantitative Variables at the Group Level
3.2.4. Morphological Variability of Qualitative Variables at the Group Level
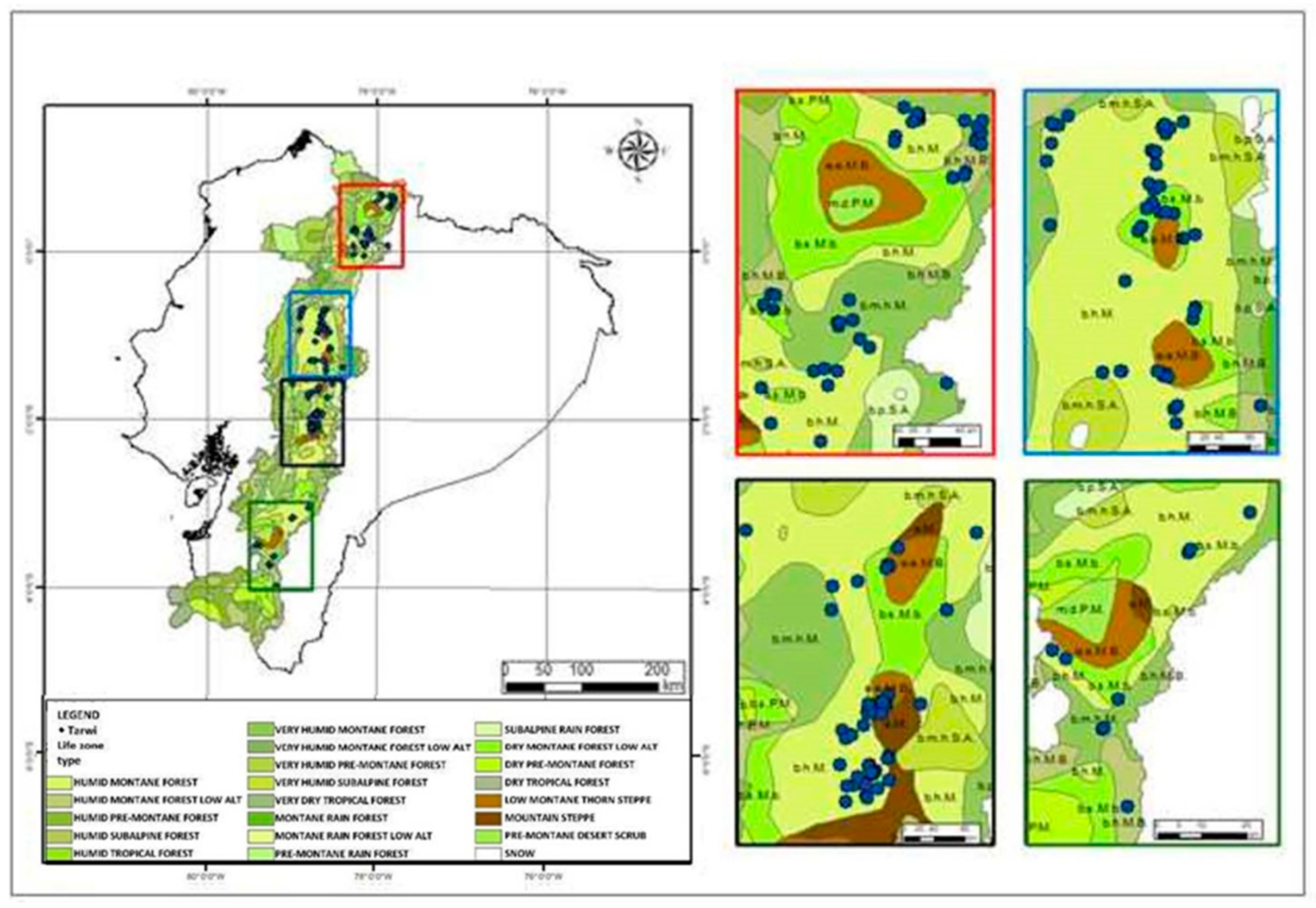
3.3. Ecogeographic Diversity Maps for Tarwi
3.4. Tarwi Phenotypic Diversity Map
4. Discussion
5. Conclusions
- Tarwi variability is represented by productivity-related characteristics such as plant size and yield, which is related to the number of pods per plant, seeds per pod and seed weight. Furthermore, although the percentage of seeds with secondary colors is low, this characteristic also suggests the presence of genetic variability, which can be explained by the degree of cross-pollination, the genetic flow and the management by farmers of their seeds.
- The present study successfully identified promising materials for genetic improvement purposes. The productive and nutritional potential of the crop are options for strengthening the food security and sovereignty of the Andean peoples. Hence, it is necessary to continue work on germplasm evaluation, crop management, traditional uses and innovation, and improving productivity levels and quality while meeting national and international demands.
- The development of research that leads to knowledge of the variability between and within a population is vital to strengthen in situ and ex situ conservation processes and promote regional strategies that encourage the consumption of lupine with its different shapes and colors.
- There is a positive correlation between morphological and ecogeographic data but there is a low association, which implies that the morphological diversity of tarwi may or may not be present in homogeneous or heterogeneous agro-ecosystems. This is also related to the fact that tarwi, being a rustic crop, can adapt to different environmental conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hondelmann, W. The lupin ancient and modern crop plant. Theor. Appl. Genet. 1984, 68, 1–9. [Google Scholar] [CrossRef]
- Mujica, A. Investigación y Producción del Tarwi (Lupinus mutabilis Sweet.) en el Perú; INIAA, PICA: Puno, Peru, 1990. [Google Scholar]
- Jacobsen, S.-E.; Mujica, A. Chapter 28. El tarwi (Lupinus mutabilis Sweet) y sus parientes silvestres. In Botánica Económica de los Andes Centrales; Moraes, R.M., Øllgaard, B., Kvist, L.P., Borchsenius, F., Balslev, H., Eds.; Universidad Mayor de San Andrés: La Paz, Bolivia, 2006; pp. 458–482. [Google Scholar]
- Caicedo, V.C.; Peralta, I.E. Zonificación potencial, sistemas de producción y procesamiento artesanal del chocho (Lupinus mutabilis Sweet) en Ecuador. Quito, Ecuador: INIAP, Estación Experimental Santa Catalina. Programa Nacional de Leguminosas. Boletín Técnico 2000, 89, 134p. Available online: https://repositorio.iniap.gob.ec/bitstream/41000/441/4/iniapscbt89.pdf (accessed on 24 March 2019).
- Blanco, G.O. Genetic variability of tarwi (Lupinus mutabilis). In Agricultural and Nutritional Aspects of Lupines, Proceedings of the 1st International Lupine Workshop, Lima-Cuzco, Peru, 12–21 April 1980; Gross, R., Bunting, E.S., Eds.; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ): Eschborn, Germany, 1982; pp. 33–49. [Google Scholar]
- Jacobsen, S.-E.; Mujica, A. Geographical distribution of the Andean lupin (Lupinus mutabilis Sweet). Plant Genet. Resour. Newsl. 2008, 155, 1–8. [Google Scholar]
- Tapia, M.E. El Tarwi, Lupino Andino. Tarwi, Tauri o Chocho (Lupinus mutabilis Sweet), 1st ed.; Corporación Gráfica Universal SAC: Lima, Peru, 2015; 108p, Available online: http://fadvamerica.org/wp-content/uploads/2017/04/TARWI-espanol.pdf (accessed on 12 February 2018).
- Mamani, P.; Calisaya, J.; Vallejos, J.; Gandarillas, A. Revalorizando el Tarwi. Una alternativa para promover la resiliencia de los sistemas productivos andinos y la mejora de la seguridad alimentaria y la economía local. In Fundación PROINPA. Informe Compendio 2011–2014; PROINPA: Cochabamba, Bolivia, 2015; pp. 12–19. Available online: https://www.proinpa.org/publico/Informe_compendio_2011_2014/tarwi.pdf (accessed on 12 February 2018).
- Tapia, M.E.; Fries, A.M. Guía de Campo de los Cultivos Andinos. FAO: ANPE-Peru. 2007. Available online: https://d1wqtxts1xzle7.cloudfront.net/55761746/Guia_de_campo_de_los_cultivos_andinos-with-cover-page-v2.pdf?Expires=1635346281&Signature=RxY1BunsygvL6wUdTPqcFq0UVsjdmKHdN2VjDOR6vOlTkZETi8z8R9pWIGAaFzuWlbLd-eGPIE60AqubsKfUnLd7pSaSHIFTsD5m4GiwWYHMHAfFSlQytbgDNdN5DZliMvhC1GR7lRH42mhR8~8mJSCWA2TvEnnQkk3TXaJKybtXW68FD1py3dlbE8ley3aPe1C0GU1C5MtCpS79V27Wf99Y7Qg8ZKO5Bj2vGJbXdWFEFfgaw72xR70BWxIvYw5nImPiYSLuPcFv77pi-2yxWgXPTjp~iPPXML1oHd8-RLm5DddIazCak16YGfsK5D-elTW~UNG7iAl8aiJxk6vo2g__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 24 March 2019).
- Aquino, V.; Camarena, F.; Julca, A.; Jiménez, J. Caracterización multivariada de fincas productoras de tarwi (Lupinus mutabilis Sweet) del Valle del Mantaro, Perú. Sci. Agropecu. 2018, 9, 269–279. [Google Scholar] [CrossRef]
- Gross, R. El cultivo y la utilización del tarwi. In Producción y Protección Vegetal; Estudio FAO: Rome, Italy, 1982; Volume 36, ISBN 9253011971. [Google Scholar]
- Aguirre, A.; Aguirre, N.; Muñoz, J. Biodiversidad de la provincia de Loja, Ecuador. Arnaldoa 2017, 24, 523–542. Available online: http://www.scielo.org.pe/pdf/arnal/v24n2/a06v24n2.pdf (accessed on 24 March 2019). [CrossRef] [Green Version]
- Lezcano Rivero, J.L. Genetica y mejoramiento de cultivos Altoandinos: Quinua, kañihua, tarwi, kiwicha, papa amarga, olluco, ashua y oca. In Programa Interinstitucional de Waru Waru. Convenio: INADE/PELT-COTESU; Convenio, INADE/PELT-COTESU, Producciones CIMA, PIWA: Puno, Peru, 1994; 459p. [Google Scholar]
- Rivera, M.; Pinzón, J.; Caicedo, C.; Murillo, A.; Mazón, N.; Peralta, E. Catálogo del banco de germoplasma del chocho (Lupinus mutabilis Sweet) y otras especies de Lupinus; INIAP: Quito, Ecuador, 1998; 111p, Available online: https://repositorio.iniap.gob.ec/bitstream/41000/511/1/iniapsci160.pdf (accessed on 12 February 2018).
- Rojas, P.M. Efecto de la Incorporación de Residuos de Cosecha de tarwi (Lupinus mutabilis Sweet) en Sistemas Marginales de Producción de Papa de Regiones Andinas Semiáridas de Cochapamba, Bolivia. Revista Latinoamericana de la Papa. 2018, Volume 22, pp. 55–65. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=7350075 (accessed on 24 March 2019).
- Solís-Neffa, V.G. Geographic patterns of morphological variation in Turnera sidoides subsp. pinnatifi (Turneraceae). Plant Syst. Evol. 2010, 284, 231–253. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Torres, E.; Iriondo, J.; López, F. Manual de Usuario Herramientas CAPFITOGEN. In Tratado Internacional de Recursos Fitogenéticos para la Alimentación y la Agricultura; FAO: Rome, Italy, 2015. [Google Scholar]
- Monteros-Altamirano, A.; Tacán, M.; Peña, G.; Tapia, C.; Paredes, N.; Lima, L. Guía Para el Manejo de los Recursos Fitogenéticos en Ecuador: Protocolos; Publicación Miscelánea No. 432; INIAP: Mejía, Ecuador, 2018; ISBN 978-9942-22-262-6. Available online: http://repositorio.iniap.gob.ec/handle/41000/4889 (accessed on 12 February 2018).
- Instituto Nacional de Estadísticas y Censos (INEC). III Censo Nacional Agropecuario. Resultados Nacionales. Ecuador. 2020. Available online: https://www.ecuadorencifras.gob.ec/documentos/web-inec/Estadisticas_agropecuarias/CNA/Tomo_CNA.pdf (accessed on 24 March 2019).
- Peralta, E.; Mazón, N.; Murillo, A.; Rivera, M.; Rodríguez, D.; Lomas, L.; Monar, C. Manual agríCola de Granos Andinos: Chocho, Quinua, Amaranto y Ataco. Cultivos, Variedades y Costos de Producción, 3rd ed.; INIAP: Quito, Ecuador, 2012; Volume 69, pp. 1–78. Available online: https://es.slideshare.net/yinath/manual-agricolagranosandinos2012 (accessed on 24 March 2019).
- Marquez, C. La siembra de chocho es más rentable. Rev. Líderes. 2016, 25. Available online: https://www.revistalideres.ec/lideres/siembra-chocho-produccion-chimborazo.html (accessed on 13 September 2018).
- Mazón, N. El chocho o Tarwi Como Recurso genéTico de la Región Andina [Online Seminar]; Interaprendizaje-IPDRS: Quito, Ecuador, 29 October 2018; Available online: https://www.sudamericarural.org/images/2018/Documentos2018/MEMORIA_CAMINOS_DEL_TARWI_14.12.18_web.pdf (accessed on 27 December 2019).
- Ortega-David, E.; Rodríguez, A.; David, A.; Zamora-Burbano, A. Caracterización de semillas de Lupinus mutabilis sembrado en los andes de Colombia. Acta Agron. 2010, 59, 111–118. Available online: https://revistas.unal.edu.co/index.php/acta_agronomica/article/view/14094 (accessed on 24 March 2019).
- Villacrés, E.; Navarrete, M.; Lucero, O.; Espín, S.; García, E. Evaluación del rendimiento, características físico-químicas y nutraceúticas del aceite de chocho (Lupinus mutabilis Sweet). Rev. Tecnol.-ESPOL 2010, 23, 57–62. Available online: https://revistas.unitru.edu.pe/index.php/scientiaagrop/article/view/2313/2200 (accessed on 13 September 2018).
- Garay, O. El Tarwi Alternative Para la Lucha Contra al Desnutrición Infantil. Serie Manual N°1. Instituto Nacional de Innovación Agraria—INIA. Perú. 2015. Available online: https://repositorio.inia.gob.pe/handle/20.500.12955/731 (accessed on 26 June 2023).
- Carhuallanqui, S.; Ccora, A.; Vilcapoma, L.; Casas, J. Caracterización del tarwi (Lupinus mutabilis) y diseño de un prototipo de desamargado para la reducción de alcaloides. J. Agric. Food Sci. 2022, 1, 53–60. Available online: https://revistas.uncp.edu.pe/index.php/jafs/article/view/1441 (accessed on 12 February 2018).
- Cowling, W.; Tarr, A. Effect of genotype and environment on seed quality in sweet narrow-leafed lupin (Lupinus angustifolius L.). Aust. J. Agric. Res. 2004, 55, 745–751. [Google Scholar] [CrossRef]
- Velásquez, J. Evaluación Agronómica y Morfológica de 283 Entradas de Lupinos (Lupinus spp.) del Banco de Germoplasma del INIAP-Ecuador. Ing. Agr. Pregrade Thesis, Facultad de Ciencias Agropecuarias, Universidad de Cuenca, Cuenca, Ecuador, 1993; 100p. [Google Scholar]
- Peralta, E. El Chocho en Ecuador. “Estado de Arte”. PRONALEG-GA; INIAP: Quito, Ecuador, 2016; 50p, Available online: http://repositorio.iniap.gob.ec/bitstream/41000/3938/1/iniapscdpCD99.pdf (accessed on 12 February 2018).
- Villacrés, E.; Peralta, I.; Cuadrado, L.; Revelo, J.; Addo, S.; Aldaz Berrones, R. Propiedades y Aplicaciones de los Alcaloides del Chocho (Lupinus mutabilis Sweet). INIAP ESPOCH SENACYT. Editorial Grafistas, Quito, Ecuador. 2009; 20p, Available online: https://repositorio.iniap.gob.ec/bitstream/41000/453/4/iniapscbt133.pdf (accessed on 24 March 2019).
- Guaytarilla, P.; Falconí, C. Selección por arquitectura de la planta y resistencia a la Antracnosis de 7 genotipos de chocho (Lupinus mutabilis). IX Congreso de Ciencia y Tecnología ESPE. 2014, pp. 63–70. Available online: http://ciencia.espe.edu.ec/wp-content/uploads/2014/04/MEMORIA-CONGRESO-CT-2014.pdf (accessed on 24 March 2019).
- González, M.; Bosland, P. Strategies for stemming genetc erosion of Capsicum germoplas, in the Americas. Diversity 1991, 7, 52–53. Available online: http://www.redalyc.org/articulo.oa?id=231125817001 (accessed on 12 February 2018).
- Peralta, E.; Mazón, N.; Murillo, A.; Villacrés, E.; Rivera, M. Catálogo de Variedades Mejoradas de Granos Andinos: Chocho, Quinua, Amaranto y Sangorache, Para la Sierra Ecuatoriana, 3rd ed.; Programa de Leguminosas y Granos Andinos. Estación Experimental Santa Catalina, INIAP: Quito, Ecuador, 2013; Volume 151, 28p, Available online: https://repositorio.iniap.gob.ec/bitstream/41000/2713/1/iniapscpm151%283%29.pdf (accessed on 13 September 2018).
- Ministerio de Agricultura, Ganadería, Acuacultura y Pesca [MAG]. Zonificación Agroecológica econóMica del Cultivo de Chocho (Lupinus mutabilis) en Ecuador Continental. Quito, Ecuador. 2014; 12p, Available online: https://fliphtml5.com/ijia/ligk/basic (accessed on 13 September 2018).
- Blanco, F. Caracterización Morfológica del Ecotipo Local de Tarwi (Lupinus mutabilis) en el Municipio de Carabuco del Departamento de la Paz. Ing. Agr. Pregade Thesis. Facultad de Agronomía. Universidad San Andrés. La Paz-Bolivia. 2011. Available online: https://repositorio.umsa.bo/bitstream/handle/123456789/10245/TD-1541.pdf?sequence=1&isAllowed=y (accessed on 12 February 2018).
- International Board for Plant Genetic Resources [IBPGR]. Descriptores de Lupinos. Roma. Available online: https://www.bioversityinternational.org/fileadmin/bioversity/publications/Web_version/103/ch1.htm (accessed on 15 May 2017).
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat. Available online: http://www.infostat.com.ar/ (accessed on 20 June 2018).
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Gower, J. A comparison of some methods of cluster analysis. Biometrics 1967, 23, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.A. The Use of Multiple Measurements in Taxonomic Problems. Ann. Eugenic. 1936, 7, 179–188. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1469-1809.1936.tb02137.x (accessed on 13 September 2018). [CrossRef]
- Franco, T.L.; Hidalgo, R. Análisis Estadístico de Datos de Caracterización Morfológica de Recursos Fitogenéticos; Instituto Internacional de Recursos Fitogenéticos (IPGRI): Cali, Colombia, 2003; Volume 8, 92p, ISBN 92-9043-543-7. Available online: https://www.bioversityinternational.org/fileadmin/_migrated/uploads/tx_news/An%C3%A1lisis_estad%C3%ADstico_de_datos_de_caracterizaci%C3%B3n_morfol%C3%B3gica_de_recursos_fitogen%C3%A9ticos_894.pdf (accessed on 12 February 2018).
- Balzarini, M.G.; González, L.; Tablada, M.; Casanoves, F.; Di Rienzo, J.A.; Robledo, C.W. Infostat Manual del Usuario; Editorial Brujas: Córdoba, Argentina, 2008; 335p, Available online: https://www.researchgate.net/profile/Fernando-Casanoves/publication/319875343_Manual_del_usuario/links/5e2ee26992851c9af7280cfa/Manual-del-usuario.pdf (accessed on 12 February 2018).
- Wilcox, A.R. Indices of qualitative variation and political measurement. Western. Polit. Quart. 1973, 26, 325–343. Available online: https://digital.library.unt.edu/ark:/67531/metadc864459/m2/1/high_res_d/4167340.pdf (accessed on 12 February 2018). [CrossRef]
- Leakey, C.L.A. Genotypic and phenotypic markers in common bean. In Genetic Resources of Phaseolus Beans; Gepts, P., Ed.; Kluwer: Dordrecht, The Netherlands, 1988; pp. 245–327. [Google Scholar] [CrossRef]
- Galek, R.G.; Zalewski, D.; Kozak, B.; Sawicka-Sienkiewicz, E. Pheno-morphological and agronomic diversity in a collection of wild and domesticated species of the genus Lupinus. Turk. J. Field Crops 2015, 20, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Martín, L.; López, R.; Haro, A. Análisis de las relaciones entre caracteres de interés agronómico en Lupinus albus L. Rev. Pastos 1985, 15, 75–85. Available online: http://polired.upm.es/index.php/pastos/article/view/497/1043 (accessed on 13 September 2018).
- Lema, M.; Lindner, R. Caracterización y evaluación de poblaciones de Lupinus hispanicus de Galicia. Congreso de mejora genética de plantas. Almería. Actas Hortic. 2002, 34, 365–369. Available online: https://digital.csic.es/bitstream/10261/163757/1/Lema_Caracterizacion_evaluacion...pdf (accessed on 20 June 2018).
- Martínez-Castillo, J.; May-Pa, F.; Zizumbo-Villareal, D.; García-Martín, P. Diversidad intraespecífica del ib (Phaseolus lunatus L.) en la agricultura tradicional de la península de Yucatán, México. In Manejo de la Diversidad de los Cultivos en los Agroecosistemas Tradicionales; Chávez-Servia, J.L., Tuxill, J., Jarvis, D.I., Eds.; Instituto Internacional de Recursos Fitogenéticos: Cali, Colombia; IPGRI: Rome Italy, 2004; pp. 26–35. ISBN 92-9043-519-4. Available online: https://www.bioversityinternational.org/fileadmin/_migrated/uploads/tx_news/Manejo_de_la_diversidad_cultivada_en_los_agroecosistemas_tradicionales___Managing_crop_diversity_in_traditional_agroecosystems_785.pdf (accessed on 24 March 2019).
- Dugje, I.; Omoigui, L.; Ekeleme, F.; Kamara, A.; Ajeigbe, H. Farmers Guide to Cowpea Production in West Africa; International Institute of Tropical Agriculture [IITA]: Ibadan, Nigeria, 2009; 19p, ISBN 978-131-332-3. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.433.2839&rep=rep1&type=pdf (accessed on 13 September 2018).
- Gross, R.; Von Baer, E.; Koch, F.; Marquard, R.; Trugo, L.; Wink, M. Chemical composition of a new variety of the Andean Lupin (Lupinus mutabilis cv. Inti) with low-alkaloid content. J. Food Compost. Anal. 1998, 1, 353–361. [Google Scholar] [CrossRef]
- Caicedo, C.; Murillo, A.; Pinzón, J.; Peralta, E.; Rivera, M. INIAP 450 Andino. Variedad de chocho (Lupinus mutabilis Sweet) FUNDACYT P-BID-206. Quito, Ecuador. Pleg. Divulg. 2010, 169, 1–65. Available online: https://repositorio.iniap.gob.ec/bitstream/41000/2584/1/iniapscpd169.pdf (accessed on 24 March 2019).
- Peralta, E.; Rivera, M.; Murillo, A.; Mazón, N.; Monar, C. INIAP 451 Guaranguito. Nueva variedad de chocho para la provincia de Bolívar. Programa Nacional de Leguminosas y Granos Andinos. Estación Experimental Santa Catalina. Quito-Ecuador. Bol. Divulg. 2010, 382, 1–4. Available online: https://repositorio.iniap.gob.ec/jspui/bitstream/41000/330/4/iniapscbd382.pdf (accessed on 24 March 2019).
- Almeida Cuastumal, J.L. Evaluación del Rendimiento de Cuatro Ecotipos de Chocho (Lupinus mutabilis) en el Centro Experimental San Francisco, en Huaca-Carchi. Ing. Agr. Pregrade Thesis. Universidad Politécnica Estatal del Carchi. Tulcán-Ecuador. 2015. Available online: http://repositorio.upec.edu.ec/bitstream/123456789/355/1/252%20Evaluaci%c3%b3n%20del%20rendimiento%20de%20cuatro%20ecotipos%20de%20chocho%20%28Lupinus%20mutabilis%29%2c%20en%20el%20Centro%20Experimental%20San%20Francisco.pdf (accessed on 20 June 2018).
- Estrella, E. El Plan de América. In Etnohistoria de los Alimentos Borígenes del Ecuador, 3rd ed.; FUNDACYT: Quito, Ecuador, 1998; 257p. [Google Scholar]
- Padilla, S. Manejo Agroforestal Andino. In Proyecto FAO-Holanda “Desarrollo Forestal Participativo de los Andes”; E. P. Centro de Impresión: Quito, Ecuador, 1995; 262p, Available online: http://www.asocam.org/sites/default/files/publicaciones/files/acac259f389f9bab78ffd1e9dc063fb0.pdf (accessed on 13 September 2018).
- Anastasova, N.; Ivanov, V.; Gevov, N.; Butnariu, M. Morphological and biological characteristics of white lupine cultivars (Lupinus albus L.). Rom. Agric. Res. 2018, 35, 109–123. Available online: https://www.incda-fundulea.ro/rar/nr35/rar35.15.pdf (accessed on 20 June 2018).
- Timmerman-Vaughan, G.; Mills, A.; Frew, T.J.; Butler, R.C.; McCallum, J.A.; Murray, S.; Whitfield, C.P.; Russell, A.C.; Wilson, D.R. Linkage Mapping of QTL for Seed Yield, Yield Components, and Developmental Traits in Pea. Crop Sci. 2005, 45, 1336–1344. [Google Scholar] [CrossRef]
- Ligarreto, G.A. Caracterización morfológica de germoplasma. Estudios de casos. Caso 2: Análisis de la variabilidad genética en frijol. In Análisis Estadístico de Datos de Caracterización Morfológica de Recursos Fitogenéticos; Franco, T., Hidalgo, R., Eds.; IPGRI: Roma, Italy, 2003; Volume 8, pp. 40–49. [Google Scholar]
- Santalla, M.; González, A.M.; Lema, M.; Rodiño, A.P.; Monteagudo, A.B.; De Ron, A.M. Improvement of large-seeded common bean cultivars under sustainable cropping systems in Spain. Euphytica 2005, 142, 85–95. [Google Scholar] [CrossRef]
- De la Cruz, N. Caracterización fenolÓgica y de Rendimiento Preliminar de Ecotipos de Tarwi (Lupinus mutabilis Sweet), Bajo Condiciones del Callejón de Haylas-Ancash. Ing. Agr. Pregade Thesis, Facultad de Agronomía. Universidad Nacional Agraria La Molina. Lima, Peru. 2018. Available online: http://repositorio.lamolina.edu.pe/handle/UNALM/3727 (accessed on 20 June 2018).
- Chirinos-Arias, M.; Jiménez, J.; Vilca-Machaca, L. Análisis de la variabilidad genética entre treinta accesiones de tarwi (Lupinus mutabilis Sweet) usando marcadores moleculares ISSR. Sci. Agropecu. 2015, 6, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and breeding of Lupinus mutabilis: An emerging protein crop. Front. Plant Sci. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aniszewski, T.; Kupari, M.H.; Leinonen, A.J. Seed number, seed size and seed diversity in Washington lupin (Lupins polyphyllus Lind). Ann. Bot. 2001, 87, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Clements, J.C.; Sweetingham, M.S.; Smith, L.; Francis, G.; Thomas, G.; Sipsas, S. Crop improvement in Lupinus mutabilis for Australian agriculture—progress and prospects. In Lupins for Health and Wealth, Proceedings of the 12th International Lupin Conference, Canterbury, New Zealand, 14–18 September 2008; Palta, J.A., Berger, J.B., Eds.; International Lupin Association: Fremantle, Australia, 2008; pp. 244–250. ISBN 0864761538. [Google Scholar]
- Ochoa-Zavala, M.; Suárez-Montes, P.; Chávez-Pesqueira, M.; López-Cobos, D.; Figueredo, C.; David, A.; Raquel, H.; Lazo, Y.; Sibile, S.; Villanueva, R.; et al. Diferencias en morfología y visitantes florales entre Lupinus multabilis y Lupinus aff. Ballianus en la microcuenca de Warmiraga, Perú. In Manejo de Biodiversidad y Evolución Dirigida por las Culturas del Nuevo Mundo. Domesticación en el Continente Americano; Appendix 3; Universidad Nacional Agraria la Molina (UNALM) del Perú: Lima, Perú, 2016; Volume 1, pp. 463–479. ISBN 978-612-4147-59-3. [Google Scholar]
- Mazón, N. El chocho o tarwi como recurso genético de la región andina [Seminario online]. In Los Caminos del tarwi y la Integración Andina: Bolivia, Perú y Ecuador; Mercado, G., Ed.; Ipdrs, HIVOS y Cipca Altiplano: La Paz, Bolivia, 2018; Available online: https://www.researchgate.net/publication/329842526_Los_caminos_del_tarwi_y_la_integracion_andina_Bolivia_Peru_y_Ecuador (accessed on 13 September 2018).





| Province | No. of Accessions in the Germplasm Bank * | No. of Accessions in the Germplasm Bank ** | Total | Percentage | Altitudinal Range (m a.s.l.) |
|---|---|---|---|---|---|
| Carchi | 8 | 14 | 22 | 12.71 | 2597–3172 |
| Imbabura | 4 | 5 | 9 | 5.20 | 2204–3057 |
| Pichincha | 7 | 4 | 11 | 6.35 | 2690–3500 |
| Cotopaxi | 12 | 20 | 32 | 18.49 | 2538–3507 |
| Tungurahua | 7 | 11 | 18 | 10.40 | 2600–3390 |
| Chimborazo | 40 | 30 | 70 | 40.46 | 2380–3600 |
| Bolívar | 1 | - | 1 | 0.57 | 2460–2837 |
| Azuay | - | 2 | 2 | 1.15 | 2450–2918 |
| Loja | 5 | 3 | 8 | 4.62 | 1900–2900 |
| Total | 84 | 89 | 173 | 100.00 |
| Qualitative Descriptors | Quantitative Descriptors | |||
|---|---|---|---|---|
| Code | Character | State | Code | Character |
| SF | Stem formation | 0. Main stem not prominent 1. Prominent main stem | PH | Plant height (main axis) (cm) |
| PR | Procumbence resistance | 3. Little 5. Medium 7. Considerable | DFF | Days to first flower |
| PV | Plant vigor | 3. Poor 5. Not vigorous 7. Vigorous | DM | Days to maturity |
| PAdL | Pubescence of adaxial side of leaflet | 0. Absent 1. Present | PL | Peduncle length (cm) |
| PAbL | Pubescence of abaxial side of leaflet | 0. Absent 1. Present | ALI | Average length of inflorescence (cm) |
| CFB | Color of flower bud | 1. White 2. Yellow 3. Orange 4. Rosa 5. Red 6. Green 7. Blue 8. Violet 9. Brown | PdL | Pod length (cm) |
| CWP | Color of wing petals | PdW | Pod width (cm) | |
| CKP | Color of keel petals | NPS | No. of pods main stem−1 | |
| CMB | Color of the marginal band on the standard petal | NSP | No. of seeds pod−1 (on main stem) | |
| PP | Pod pubescence | 0. Absent 3. Little 5. Medium 7. Abundant | SL | Seed length (cm) |
| DP | Dehiscence of pod | 0. Indehiscent 1. Dehiscent | SW | Seed width (cm) |
| SS | Seed shape | 1. Spherical 2. Flattened or lenticular spherical 3. Oval 4. Oval flattened 5. Square 6. Square flattened | W100 | Weight of 100 seeds (g plant−1) |
| PSC | Predominant seed color | 1. White 2. Yellow 3. Cream 4. Light brown 5. Black 6. Brown 7. Grey 8. Dark brown 9. Other | YGP | Yield (g plant−1) |
| SSC | Secondary seed color | |||
| DSC | Distribution of the secondary seed color | 0. Absent 1. Crescent 2. Brow-shaped 3. On flattened side 4. Dotted 5. Streaked 6. Mottled 7. Spotted crescent 8. Streaked brows 9. Absent 10. Stained 11. Dots on flattened side | ||
| Code | Character | Average | SD | CV (%) | Minimum Value | Maximum Value |
|---|---|---|---|---|---|---|
| YGP | Yield of 100 seeds (g plant−1) | 103.78 | 81.38 | 78.42 | 12.75 | 499.50 |
| NPS | No. of pods main stem−1 | 9.51 | 2.98 | 31.32 | 3.50 | 18.40 |
| PH | Plant height (main axis) (cm) | 133.50 | 27.89 | 20.89 | 61.60 | 190.00 |
| ALI | Average length of inflorescence (cm) | 18.42 | 3.62 | 19.67 | 9.00 | 29.30 |
| PL | Peduncle length (cm) | 10.37 | 1.62 | 15.60 | 4.00 | 15.75 |
| PdW | Pod width (cm) | 1.61 | 0.25 | 15.41 | 1.35 | 3.30 |
| W100 | Seed weight (g 100 seed−1) | 26.03 | 3.43 | 13.18 | 12.00 | 33.00 |
| NSP | Nr. of seeds pod−1 (on main stem) | 5.05 | 0.50 | 9.85 | 3.33 | 6.50 |
| PdL | Pod length (cm) | 9.65 | 0.91 | 9.42 | 8.21 | 17.13 |
| DM | Days to maturity | 192.73 | 9.09 | 4.72 | 178.00 | 206.00 |
| DFF | Days to first flower | 121.66 | 2.99 | 2.45 | 120.00 | 127.00 |
| SL | Seed length (cm) | 0.99 | 0.04 | 4.00 | 0.77 | 1.09 |
| SW | Seed width (cm) | 0.81 | 0.03 | 3.97 | 0.63 | 0.89 |
| Code | Character | Category | State | Overall Frequency | Relative Frequency |
|---|---|---|---|---|---|
| SF | Stem formation | 0 | Main stem not prominent | 14 | 8% |
| 1 | Prominent main stem | 159 | 92% | ||
| PV | Plant vigor | 5 | Not vigorous | 19 | 11% |
| 7 | Vigorous | 154 | 89% | ||
| PR | Procumbence resistance | 3 | Little | 91 | 52% |
| 5 | Medium | 67 | 39% | ||
| 7 | Considerable | 15 | 9% | ||
| PAdL | Pubescence of adaxial side of leaflet | 0 | Absent | 170 | 98% |
| 1 | Present | 3 | 2% | ||
| PAbL | Pubescence of abaxial side of leaflet | 0 | Absent | 148 | 86% |
| 1 | Present | 25 | 14% | ||
| CFB | Color of flower bud | 6 | Green | 173 | 100% |
| CWP | Color of wing petals | 1 | White | 4 | 2% |
| 7 | Blue | 29 | 17% | ||
| 8 | Violet | 140 | 81% | ||
| CKP | Color of keel petals | 1 | White | 173 | 100% |
| CMBS | Color of the marginal band on the standard petal | 1 | White | 4 | 2% |
| 7 | Blue | 29 | 17% | ||
| 8 | Violet | 140 | 81% |
| Code | Character | Class | State | Overall Frequency | Relative Frequency |
|---|---|---|---|---|---|
| DP | Dehiscence of pod | 0 | Indehiscent | 172 | 99% |
| 1 | Dehiscent | 1 | 1% | ||
| PP | Pod pubescence | 7 | Abundant | 173 | 100% |
| SS | Seed shape | 1 | Spherical | 3 | 2% |
| 2 | Flattened or lenticular spherical | 5 | 3% | ||
| 3 | Oval | 42 | 24% | ||
| 4 | Oval flattened | 120 | 69% | ||
| 5 | Square | 2 | 1% | ||
| 6 | Square flattened | 1 | 1% | ||
| PSC | Predominant seed color | 1 | White | 173 | 100% |
| SSC | Secondary seed color | 0 | Absent | 160 | 93% |
| 4 | Light brown | 2 | 1% | ||
| 5 | Black | 7 | 4% | ||
| 8 | Dark brown | 3 | 2% | ||
| DSC | Distribution of the secondary seed color | 9 | Absent | 160 | 93% |
| 1 | Crescent | 11 | 6% | ||
| 4 | Dotted | 2 | 1% |
| Components | Eigenvalue | Percentage of Variance | Cumulative Percentage |
|---|---|---|---|
| 1 | 4.46 | 34 | 34 |
| 2 | 2.26 | 17 | 52 |
| 3 | 1.37 | 11 | 62 |
| 4 | 1.23 | 09 | 72 |
| Variable | CV% | Group (no. Accessions) | p-Value | |||
|---|---|---|---|---|---|---|
| G1 (33) | G2 (26) | G3 (79) | G4 (35) | |||
| PL (cm) | 14.09 | 010.60 ± 0.25 b | 011.96 ± 0.29 c | 009.95 ± 0.16 a | 009.90 ± 0.25 a | 0.0001 |
| ALI (cm) | 18.52 | 018.42 ± 0.59 a | 021.42 ± 0.67 b | 017.98 ± 0.38 a | 017.35 ± 0.58 a | 0.0001 |
| PdL (cm) | 9.42 | 009.57 ± 0.16 a | 009.92 ± 0.18 a | 009.59 ± 0.10 a | 009.66 ± 0.15 a | 0.4812 |
| PW (cm) | 15.53 | 001.62 ± 0.04 a | 001.60 ± 0.05 a | 001.61 ± 0.03 a | 001.60 ± 0.04 a | 0.9825 |
| SL (cm) | 3.96 | 000.98 ± 0.01 ab | 000.99 ± 0.01 ab | 011.00 ± 4.4 b | 000.98 ± 0.01 a | 0.1009 |
| SW (cm) | 3.95 | 000.81 ± 0.01 ab | 000.81 ± 0.01 ab | 000.82 ± 3.6 × 10−3 b | 000.80 ± 0.01 a | 0.1505 |
| PH (cm) | 15.10 | 120.17 ± 3.51b | 094.12 ± 3.95 a | 147.11 ± 2.27 c | 144.59 ± 3.41 c | 0.0001 |
| NPS | 22.85 | 010.53 ± 0.38 b | 013.90 ± 0.43 c | 008.17 ± 0.24 a | 008.31 ± 0.37 a | 0.0001 |
| NSP | 9.52 | 005.04 ± 0.08 b | 004.72 ± 0.09 a | 005.11 ± 0.05 b | 005.15 ± 0.08 b | 0.0021 |
| DFF | 1.33 | 122.97 ± 0.28 b | 127.00 ± 0.32 c | 120.09 ± 0.18 a | 120.00 ± 0.27 a | 0.0001 |
| DM | 3.47 | 195.82 ± 1.16 b | 206.00 ± 1.31 c | 188.63 ± 0.73 a | 189.20 ± 1.13 a | 0.0001 |
| YGP (g plant−1) | 64.68 | 127.41 ± 11.68 b | 203.52 ± 13.16 c | 072.42 ± 7.55 a | 078.20 ± 11.35 a | 0.0001 |
| W100 (g) | 13.01 | 025.94 ± 0.59 ab | 027.58 ± 0.66 b | 025.91 ± 0.38 a | 025.26 ± 0.57 a | 0.0649 |
| Variable | Chi2 | Coef. (P) | Cramer (V) | p-Value |
|---|---|---|---|---|
| CWP | 173.00 | 0.71 | 0.58 | <0.0001 |
| CMBS | 173.00 | 0.71 | 0.58 | <0.0001 |
| SS | 113.65 | 0.63 | 0.41 | <0.0001 |
| SSC | 41.76 | 0.44 | 0.25 | <0.0001 |
| DSC | 28.77 | 0.38 | 0.24 | <0.0001 |
| Variable | DM Index | |||
|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |
| CWP | 0.18 | 0.00 | 0.00 | 0.00 |
| CMBS | 0.18 | 0.00 | 0.00 | 0.00 |
| SS | 0.36 | 0.55 | 0.00 | 0.27 |
| SSC | 0.24 | 0.00 | 0.00 | 0.23 |
| DSC | 0.27 | 0.00 | 0.00 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalampuente-Flores, D.; Mosquera-Losada, M.R.; Ron, A.M.D.; Tapia Bastidas, C.; Sørensen, M. Morphological and Ecogeographical Diversity of the Andean Lupine (Lupinus mutabilis Sweet) in the High Andean Region of Ecuador. Agronomy 2023, 13, 2064. https://doi.org/10.3390/agronomy13082064
Chalampuente-Flores D, Mosquera-Losada MR, Ron AMD, Tapia Bastidas C, Sørensen M. Morphological and Ecogeographical Diversity of the Andean Lupine (Lupinus mutabilis Sweet) in the High Andean Region of Ecuador. Agronomy. 2023; 13(8):2064. https://doi.org/10.3390/agronomy13082064
Chicago/Turabian StyleChalampuente-Flores, Doris, María Rosa Mosquera-Losada, Antonio M. De Ron, César Tapia Bastidas, and Marten Sørensen. 2023. "Morphological and Ecogeographical Diversity of the Andean Lupine (Lupinus mutabilis Sweet) in the High Andean Region of Ecuador" Agronomy 13, no. 8: 2064. https://doi.org/10.3390/agronomy13082064










