Harnessing the Eco-Friendly Potential of Asparagus racemosus Leaf Extract Fabricated Ni/Ni(OH)2 Nanoparticles for Sustainable Seed Germination and Seedling Growth of Vigna radiata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biofabrication of Ni/Ni(OH)2 NPs
2.2. Seeds Collection and Treatment
2.3. Estimation of Morphological Parameters
2.3.1. Germination
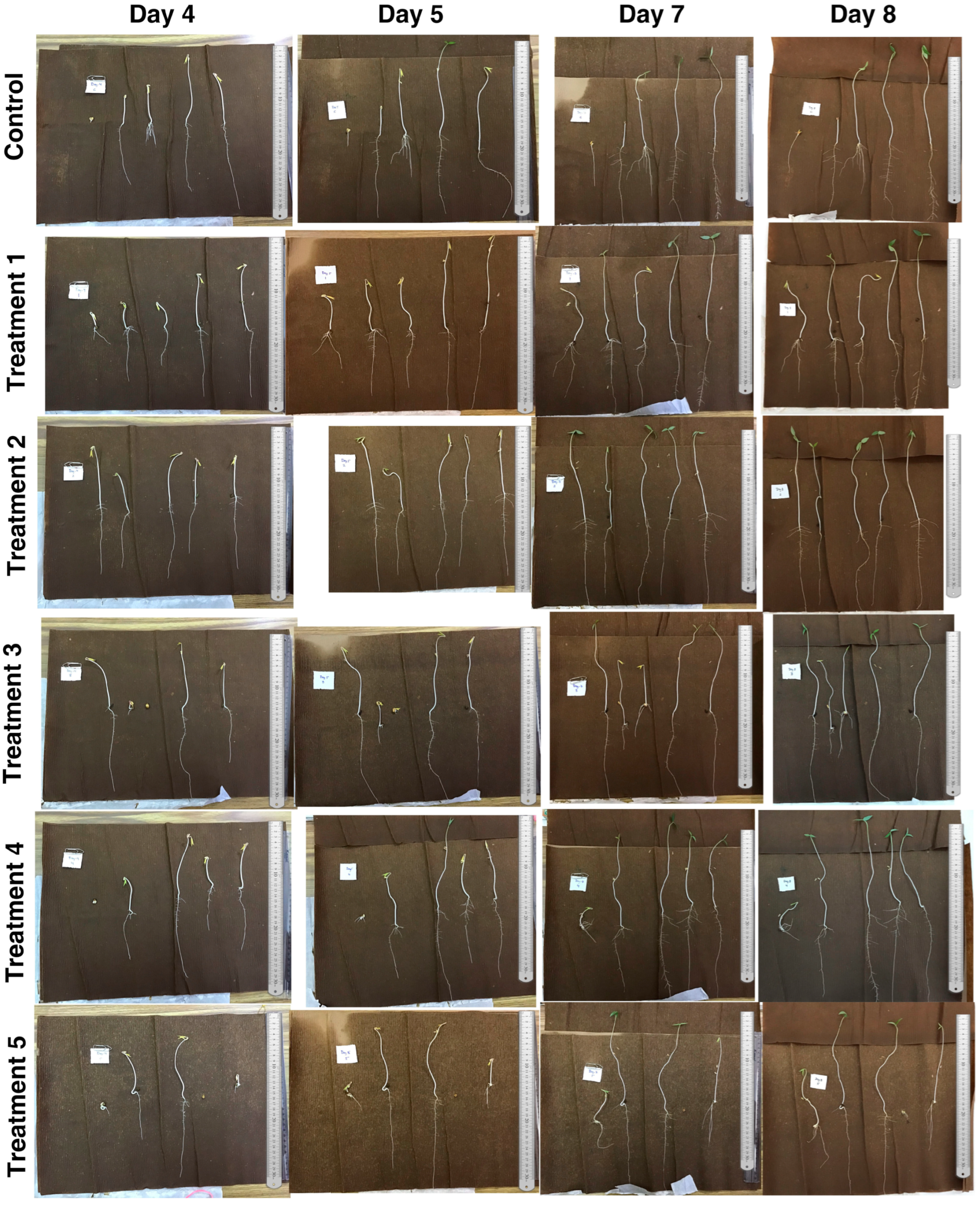
2.3.2. Germinated Seedlings Length
2.3.3. Germinated Seedlings Biomass
2.4. Estimation of Biochemical Parameters
2.4.1. Chlorophyll Estimation
2.4.2. Chlorophyll Stability Index
2.4.3. Membrane Stability Index
2.4.4. Root Ion Leakage
2.5. Statistical Analysis
3. Results and Discussion
3.1. Germination of V. radiata Seeds
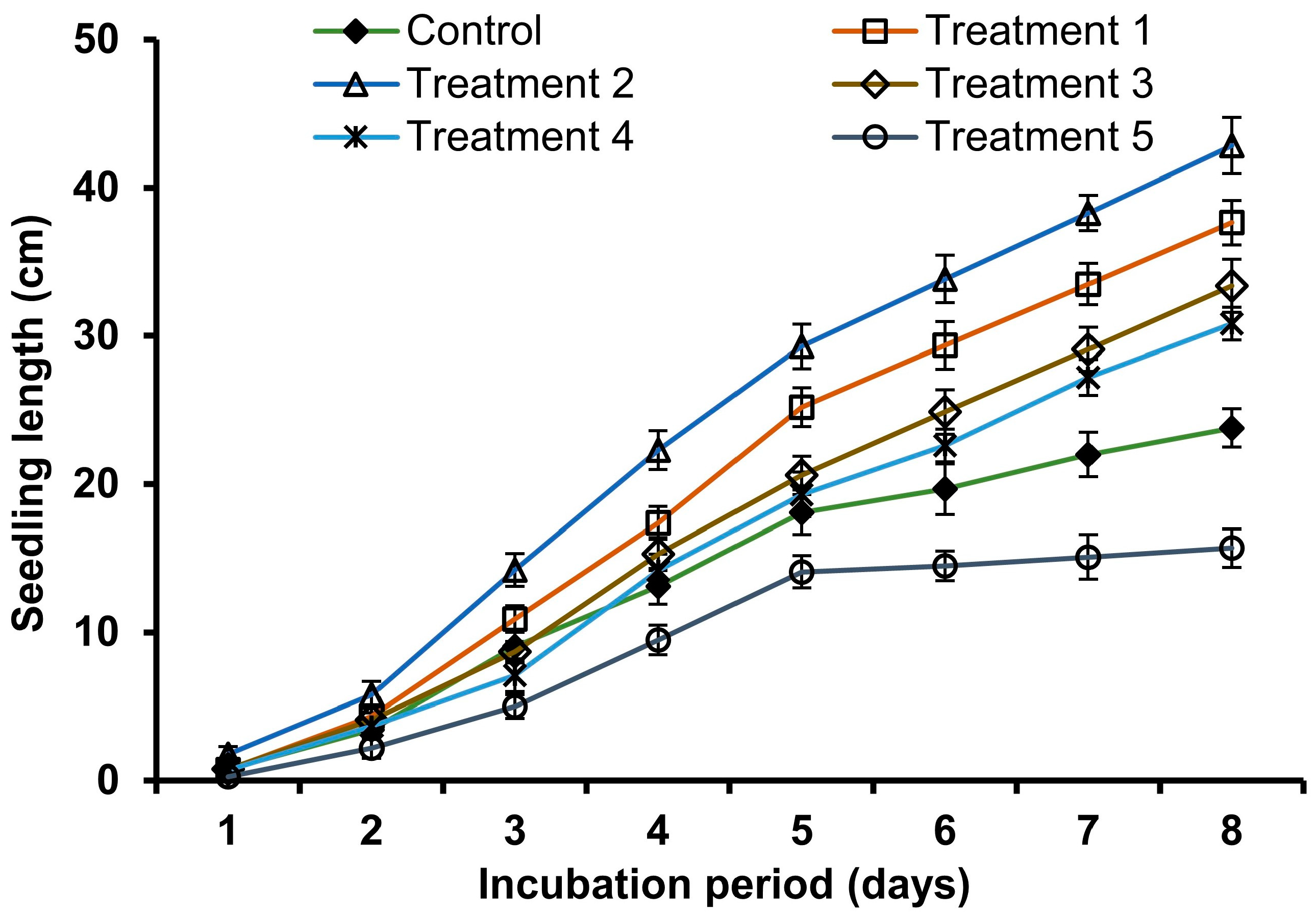
3.2. Growth Rate of V. radiata Seedlings
3.3. Biomass of V. radiata Seedlings
3.4. Chlorophyll Content in Ni/Ni(OH)2 NPs Treated V. radiata Plants
3.5. Membrane Stability Index and Root Ion Leakage of Ni/Ni(OH)2 NP-Treated V. radiata Seedlings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parisi, C.; Vigani, M.; Rodríguez-Cerezo, E. Agricultural nanotechnologies: What are the current possibilities? Nano Today 2014, 10, 124–127. [Google Scholar] [CrossRef]
- Shinde, S.; Paralikar, P.; Ingle, A.P.; Rai, M. Promotion of seed germination and seedling growth of Zea mays by magnesium hydroxide nanoparticles synthesized by the filtrate from Aspergillus niger. Arab. J. Chem. 2020, 13, 3172–3182. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.K.; Kumar, P.; Burman, U.; Joshi, P.; Agrawal, A.; Raliya, R.; Tarafdar, J.C. Magnesium and iron nanoparticles production using microorganisms and various salts. Mater. Sci. Pol. 2020, 30, 254–258. [Google Scholar] [CrossRef]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 20, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of nanoparticles in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–35. [Google Scholar] [CrossRef]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Kolbert, Z.; Szőllősi, R.; Rónavári, A.; Molnár, Á. Nanoforms of essential metals: From hormetic phytoeffects to agricultural potential. J. Exp. Bot. 2022, 73, 1825–1840. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Bhandari, G.; Dhasmana, A.; Chaudhary, P.; Gupta, S.; Gangola, S.; Gupta, A.; Rustagi, S.; Shende, S.S.; Rajput, V.D.; Minkina, T.; et al. A Perspective Review on Green Nanotechnology in Agro-Ecosystems: Opportunities for Sustainable Agricultural Practices & Environmental Remediation. Agriculture 2023, 13, 668. [Google Scholar] [CrossRef]
- Jaji, N.D.; Lee, H.L.; Hussin, M.H.; Akil, H.M.; Zakaria, M.R.; Othman, M.B.H. Advanced nickel nanoparticles technology: From synthesis to applications. Nanotech. Rev. 2020, 9, 1456–1480. [Google Scholar] [CrossRef]
- Hill, D.; Barron, A.R.; Alexander, S. Comparison of hydrophobicity and durability of functionalized aluminium oxide nanoparticle coatings with magnetite nanoparticles–links between morphology and wettability. J. Colloid Interface Sci. 2019, 555, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A review on bimetallic nickel-based catalysts for CO2 reforming of methane. Chem. Phys. Chem. 2017, 18, 3117–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Jia, Y.; He, Y.; Wang, L.; Fan, J.; Xie, H.; Yu, W. Enhanced photothermal conversion properties of magnetic nanofluids through rotating magnetic field for direct absorption solar collector. J. Colloid Interface Sci. 2019, 557, 266–275. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kaur, Y.; Jayee, B.; Chaudhary, G.R.; Umar, A. NiO nanodisks: Highly efficient visible-light driven photocatalyst, potential scaffold for seed germination of Vigna Radiata and antibacterial properties. J. Clean. Prod. 2018, 190, 563–576. [Google Scholar] [CrossRef]
- Singh, Y.; Sodhi, R.S.; Singh, P.P.; Kaushal, S. Biosynthesis of NiO nanoparticles using Spirogyra sp. cell-free extract and their potential biological applications. Mater. Adv. 2022, 3, 4991–5000. [Google Scholar] [CrossRef]
- Parveen, A.; Sonkar, S.; Yadav, T.P.; Sarangi, P.K.; Singh, A.K.; Singh, S.P.; Gupta, R. Asparagus racemosus leaf extract mediated bioconversion of nickel sulfate into nickel/nickel hydroxide nanoparticles: In vitro catalytic, antibacterial, and antioxidant activities. Biomass Convert. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef]
- Hajra, A.; Mondal, N.K. Effects of ZnO and TiO2 nanoparticles on germination, biochemical and morphoanatomical attributes of Cicer arietinum L. Energ. Ecol. Environ. 2017, 2, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Bayat, M.; Zargar, M.; Murtazova, K.M.-S.; Nakhaev, M.R.; Shkurkin, S.I. Ameliorating Seed Germination and Seedling Growth of Nano-Primed Wheat and Flax Seeds Using Seven Biogenic Metal-Based Nanoparticles. Agronomy 2022, 12, 811. [Google Scholar] [CrossRef]
- Rawat, P.S.; Kumar, R.; Ram, P.; Pandey, P. Effect of nanoparticles on wheat seed germination and seedling growth. Int. J. Agric. Biosyst. Eng. 2018, 12, 13–16. [Google Scholar]
- Bates, L.S.; Waldren, R.A.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kaloyereas, S.A. A New Method of Determining Drought Resistance. Plant Physiol. 1958, 33, 232. [Google Scholar] [CrossRef] [PubMed]
- Premachandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J. Agric. Sci. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994, 32, 594. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. J. Exp. Bot. 1995, 46, 1843–1852. [Google Scholar] [CrossRef]
- Asagba, S.O.; Apiamu, A.; Enokpe, F.E. Effects of nickel toxicity on the indices of germination and Ca2+ ATPase activity in cowpea plant (Vigna unguiculata). J. Appl. Sci. Environ. Manag. 2019, 23, 1147–1152. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Sadiq, R.; Hussain, M.; Ashraf, M.; Ahmad, M.S.A. Toxic effect of nickel (Ni) on growth and metabolism in germinating seeds of sunflower (Helianthus annuus L.). Biol. Trace Elem. Res. 2011, 143, 1695–1703. [Google Scholar] [CrossRef]
- Parlak, U.K. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS Wagening. J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Bhalerao, S.A.; Sharma, A.S.; Poojari, A.C. Toxicity of Nickel in plants. Int. J. Pure Appl. Biosci. 2015, 3, 345–355. [Google Scholar]
- Ahmad, M.S.A.; Hussain, M.; Saddiq, R.; Alvi, A.K. Mungbean: A nickel indicator, accumulator or excluder? Bull. Environ. Contam. Toxicol. 2007, 78, 319–324. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.M. Effect of varying concentration of nickel and cobalt on the plant growth and yield of chickpea. Aust. J. Basic Appl. Sci. 2010, 4, 1036–1046. [Google Scholar]
- Pandey, V.K.; Gopal, R. Nickel toxicity effects on growth and metabolism of eggplant. Int. J. Veg. Sci. 2010, 16, 351–360. [Google Scholar] [CrossRef]
- Argenta, G.; Silva, P.R.F.D.; Sangoi, L. Leaf relative chlorophyll content as an indicator parameter to predict nitrogen fertilization in maize. Ciência Rural 2004, 34, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Zvezdanović, J.; Petrović, S.; Lazarević, A. Nickel (II) interactions with chlorophylls in solution: Impact to degradation induced by UV-irradiation. Chem. Naissensis 2023, 5, 1–17. [Google Scholar] [CrossRef]
- Hong, F.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem. Res. 2005, 105, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Sharma, S.; Gadgil, K. Response of Indian mustard (Brassica juncea arawali) plants under nickel stress with special reference to nickel phytoextraction potential. EQA-Int. J. Environ. Qual. 2019, 34, 17–33. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. The mechanism of hydrogen sulfide mitigation of iron deficiency-induced chlorosis in strawberry (Fragaria × ananassa) plants. Protoplasma 2019, 256, 371–382. [Google Scholar] [CrossRef]
- Mohan, M.M.; Narayanan, S.L.; Ibrahim, S.M. Chlorophyll stability index (CSI): Its impact on salt tolerance in rice. Int. Rice Res. Notes 2000, 25, 38–39. [Google Scholar]
- Radoglou, K.; Cabral, R.; Repo, T.; Hasanagas, N.; Sutinen, M.L.; Waisel, Y. Appraisal of root leakage as a method for estimation of root viability. Plant Biosyst. 2007, 141, 443–459. [Google Scholar] [CrossRef] [Green Version]
- McKay, H.M. A review of the effect of stresses between lifting and planting on nursery stock quality and performance. New Forests 1997, 13, 369–399. [Google Scholar] [CrossRef]
- Ritchie, G.A.; Landis, T.D. Seedling quality tests: Root electrolyte leakage. In Forest Nursery Notes; PNW Region. Winter; USDA Forest Service: Washington, DC, USA, 2006; pp. 6–10. [Google Scholar]
- Hatami, M.; Ghorbanpour, M. Defense enzyme activities and biochemical variations of Pelargonium zonale in response to nanosilver application and dark storage. Turk. J. Biol. 2014, 38, 130–139. [Google Scholar] [CrossRef]
- Manna, I.; Sahoo, S.; Bandyopadhyay, M. Effect of Engineered Nickel Oxide Nanoparticle on Reactive Oxygen Species-Nitric Oxide Interplay in the Roots of Allium cepa L. Front. Plant Sci. 2021, 12, 586509. [Google Scholar] [CrossRef] [PubMed]
- Romero-Puertas, M.C.; Sandalio, L.M. Nitric oxide level is self-regulating and also regulates its ROS partners. Front. Plant Sci. 2016, 7, 316. [Google Scholar] [CrossRef] [Green Version]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Manna, I.; Bandyopadhyay, M. Engineered nickel oxide nanoparticle causes substantial physicochemical perturbation in plants. Front. Chem. 2017, 5, 92. [Google Scholar] [CrossRef] [Green Version]
- Ates, M.; Demir, V.; Arslan, Z.; Camas, M.; Celik, F. Toxicity of engineered nickel oxide and cobalt oxide nanoparticles to Artemia salina in seawater. Water Air Soil Pollut. 2016, 227, 70. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Cuypers, A.; Hendrix, S.; Amaral dos Reis, R.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef] [PubMed]
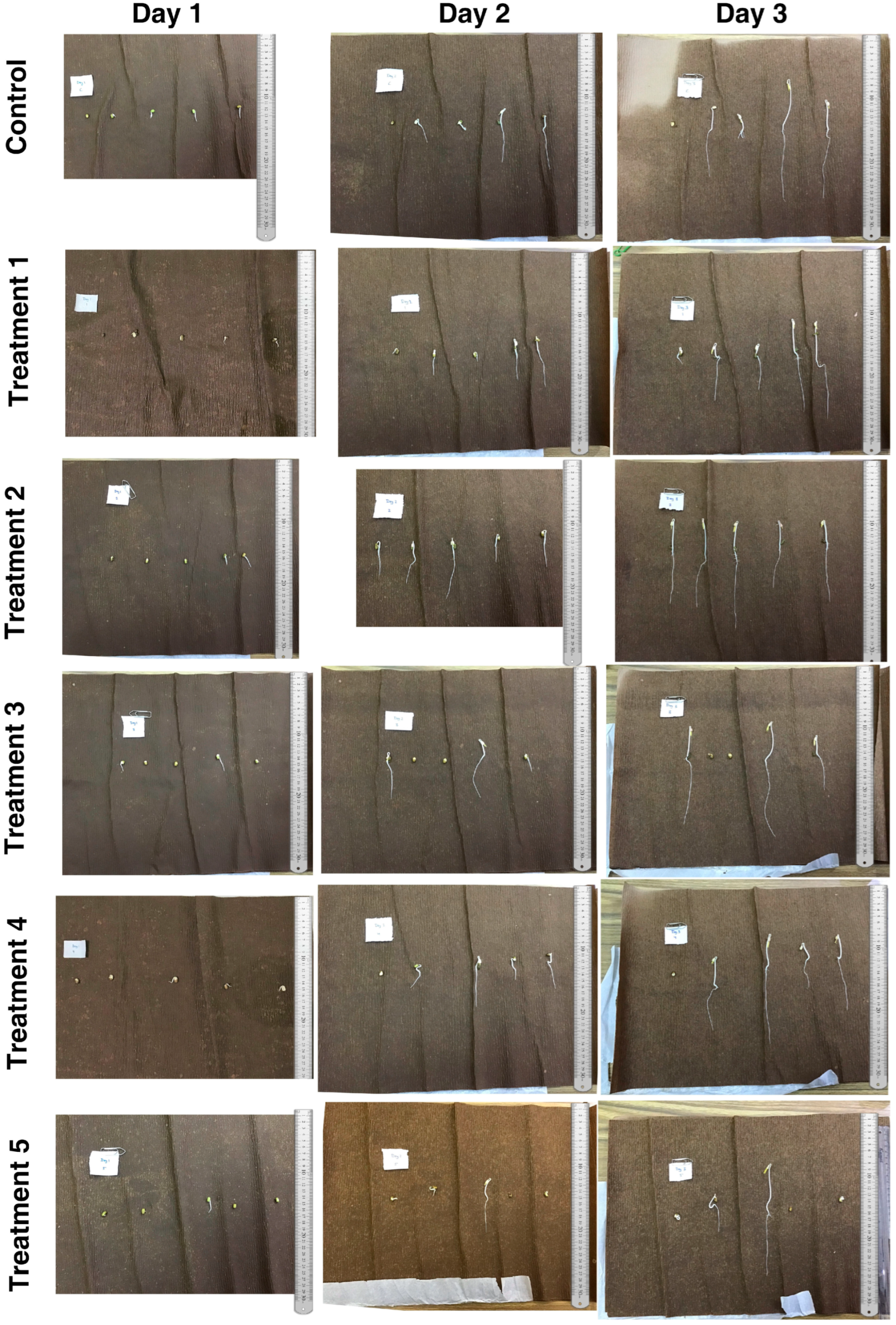
| Conditions | Germination % of Seeds | ||
|---|---|---|---|
| Day 1 (24 h) | Day 2 (48 h) | Day 3 (72 h) | |
| Control | 80 ± 5 a | 80 ± 4 b | 80 ± 2 b |
| Treatment 1 | 60 ± 2 b | 100 ± 0 a | 100 ± 0 a |
| Treatment 2 | 80 ± 3 a | 100 ± 0 a | 100 ± 0 a |
| Treatment 3 | 60 ± 2 b | 60 ± 4 c | 80 ± 2 b |
| Treatment 4 | 60 ± 3 b | 80 ± 3 b | 80 ± 4 b |
| Treatment 5 | 20 ± 2 c | 40 ± 3 d | 60 ± 2 c |
| Conditions | Fresh Weight (mg) | Dry Weight (mg) |
|---|---|---|
| Control | 345.0 ± 8.1 c | 20.1 ± 0.5 b |
| Treatment 1 | 347.4 ± 10.3 c | 21.0 ± 0.7 bc |
| Treatment 2 | 386.5 ± 12.3 d | 24.9 ± 1.0 d |
| Treatment 3 | 326.2 ± 13.6 c | 22.3 ± 0.6 c |
| Treatment 4 | 306.4 ± 11.1 b | 19.8 ± 0.7 b |
| Treatment 5 | 214.2 ± 8.5 a | 13.3 ± 0.6 a |
| Conditions | Chlorophyll a (µg g−1) | Chlorophyll b (µg g−1) | Total Chlorophyll (µg g−1) |
|---|---|---|---|
| Control | 0.61 ± 0.04 a | 0.32 ± 0.04 a | 32.4 ± 4.1 a |
| Treatment 1 | 42.12 ± 1.05 c | 1.14 ± 0.05 c | 116.4 ± 3.8 cd |
| Treatment 2 | 45.04 ± 1.18 d | 1.21 ± 0.07 cd | 122.1 ± 5.5 d |
| Treatment 3 | 41.37 ± 1.34 bc | 1.11 ± 0.05 bc | 115.5 ± 3.9 c |
| Treatment 4 | 39.21 ± 1.26 b | 1.03 ± 0.04 b | 105.3 ± 3.5 b |
| Treatment 5 | 0.61 ± 0.09 a | 0.33 ± 0.06 a | 33.0 ± 4.9 a |
| Treatment | CSI (%) | MSI (%) | RIL (%) |
|---|---|---|---|
| Control | 19.61 ± 0.86 a | 58.12 ± 2.45 a | 35.53 ± 3.23 d |
| Treatment 1 | 21.36 ± 1.11 b | 63.0 ± 3.66 bc | 29.58 ± 3.11 bc |
| Treatment 2 | 23.73 ± 1.98 c | 67.89 ± 4.65 d | 24.75 ± 2.56 a |
| Treatment 3 | 21.43 ± 0.53 bc | 62.33 ± 2.98 ab | 27.84 ± 2.71 ab |
| Treatment 4 | 20.06 ± 0.85 a | 59.37 ± 3.13 ab | 32.9 ± 3.98 d |
| Treatment 5 | 18.83 ± 0.71 a | 57.89 ± 2.91 a | 33.51 ± 3.72 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, A.; Sonkar, S.; Sarangi, P.K.; Singh, A.K.; Sahoo, U.K.; Gupta, R.; Prus, P.; Imbrea, F.; Șmuleac, L.; Pașcalău, R. Harnessing the Eco-Friendly Potential of Asparagus racemosus Leaf Extract Fabricated Ni/Ni(OH)2 Nanoparticles for Sustainable Seed Germination and Seedling Growth of Vigna radiata. Agronomy 2023, 13, 2073. https://doi.org/10.3390/agronomy13082073
Parveen A, Sonkar S, Sarangi PK, Singh AK, Sahoo UK, Gupta R, Prus P, Imbrea F, Șmuleac L, Pașcalău R. Harnessing the Eco-Friendly Potential of Asparagus racemosus Leaf Extract Fabricated Ni/Ni(OH)2 Nanoparticles for Sustainable Seed Germination and Seedling Growth of Vigna radiata. Agronomy. 2023; 13(8):2073. https://doi.org/10.3390/agronomy13082073
Chicago/Turabian StyleParveen, Ashna, Sashi Sonkar, Prakash Kumar Sarangi, Akhilesh Kumar Singh, Uttam Kumar Sahoo, Rahul Gupta, Piotr Prus, Florin Imbrea, Laura Șmuleac, and Raul Pașcalău. 2023. "Harnessing the Eco-Friendly Potential of Asparagus racemosus Leaf Extract Fabricated Ni/Ni(OH)2 Nanoparticles for Sustainable Seed Germination and Seedling Growth of Vigna radiata" Agronomy 13, no. 8: 2073. https://doi.org/10.3390/agronomy13082073







