Pneumatic Defoliation Enhances Fruit Skin Color and Anthocyanin Pigments in ‘Picnic’ Apples
Abstract
1. Introduction
2. Materials and Methods
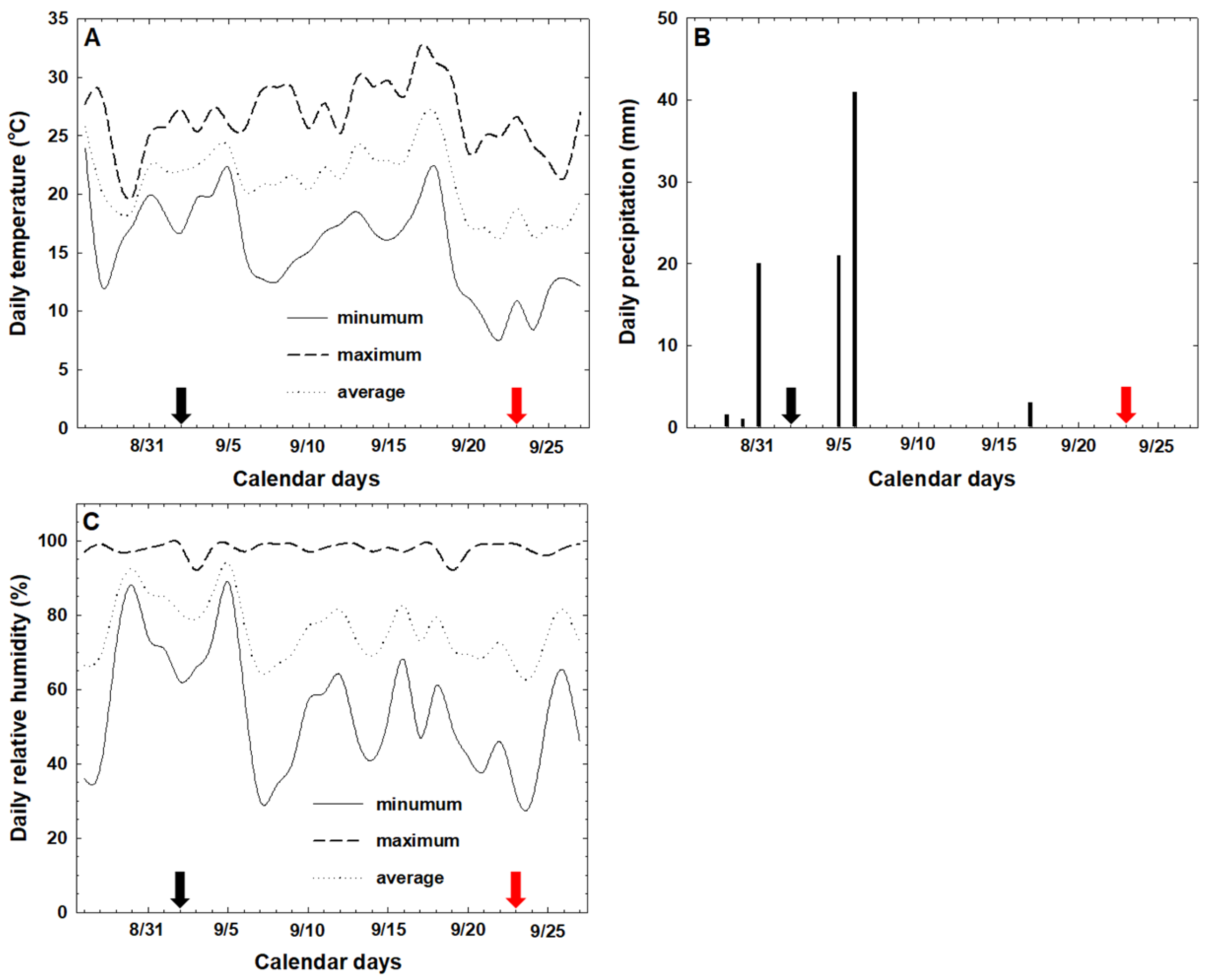
2.1. Plant Materials and Field Trial
2.2. Defoliation Treatments
2.3. Assessments of Defoliation Rate, Leaf Damage Rate, and Sunlight Availability in the Tree
2.4. Assessments of Fruit Quality Attributes
2.5. Determination of Anthocyanin Contents
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Statistical Analysis
3. Results
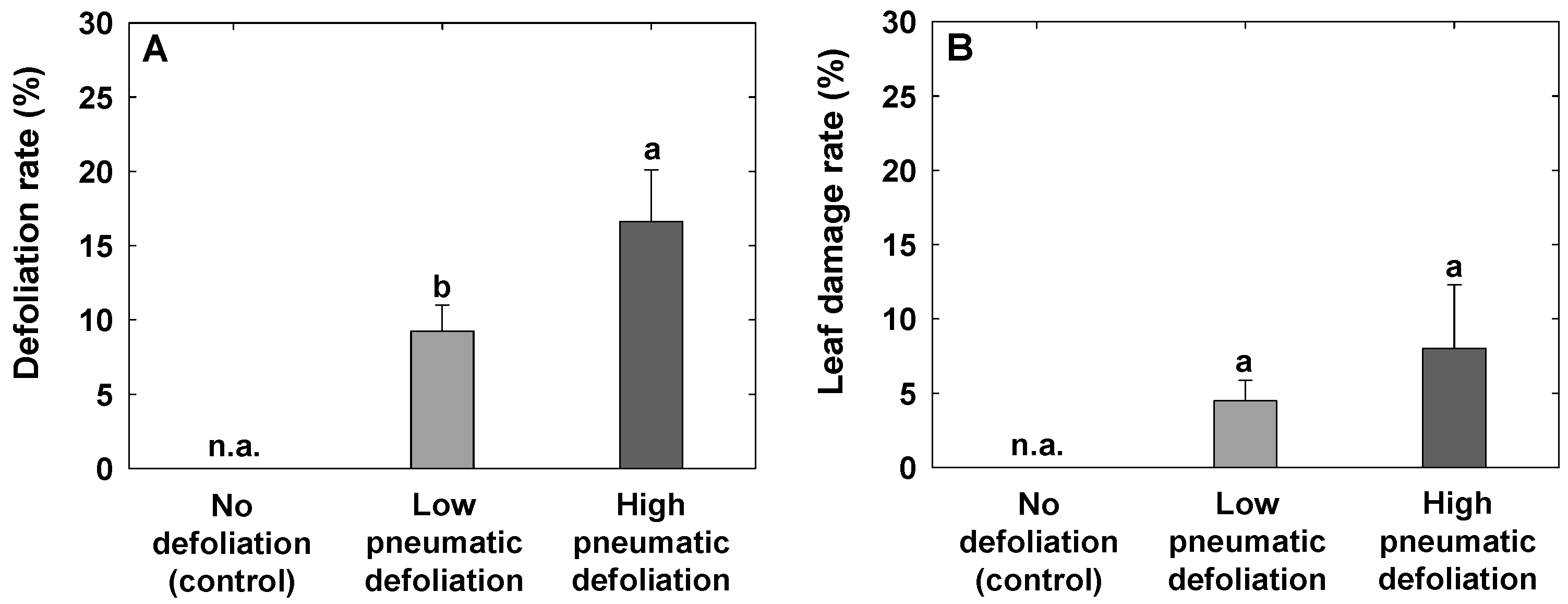
3.1. Defoliation Rate, Leaf Damage Rate, and Sunlight Availability in Trees
3.2. Fruit Quality Attributes
3.3. Fruit Skin Color
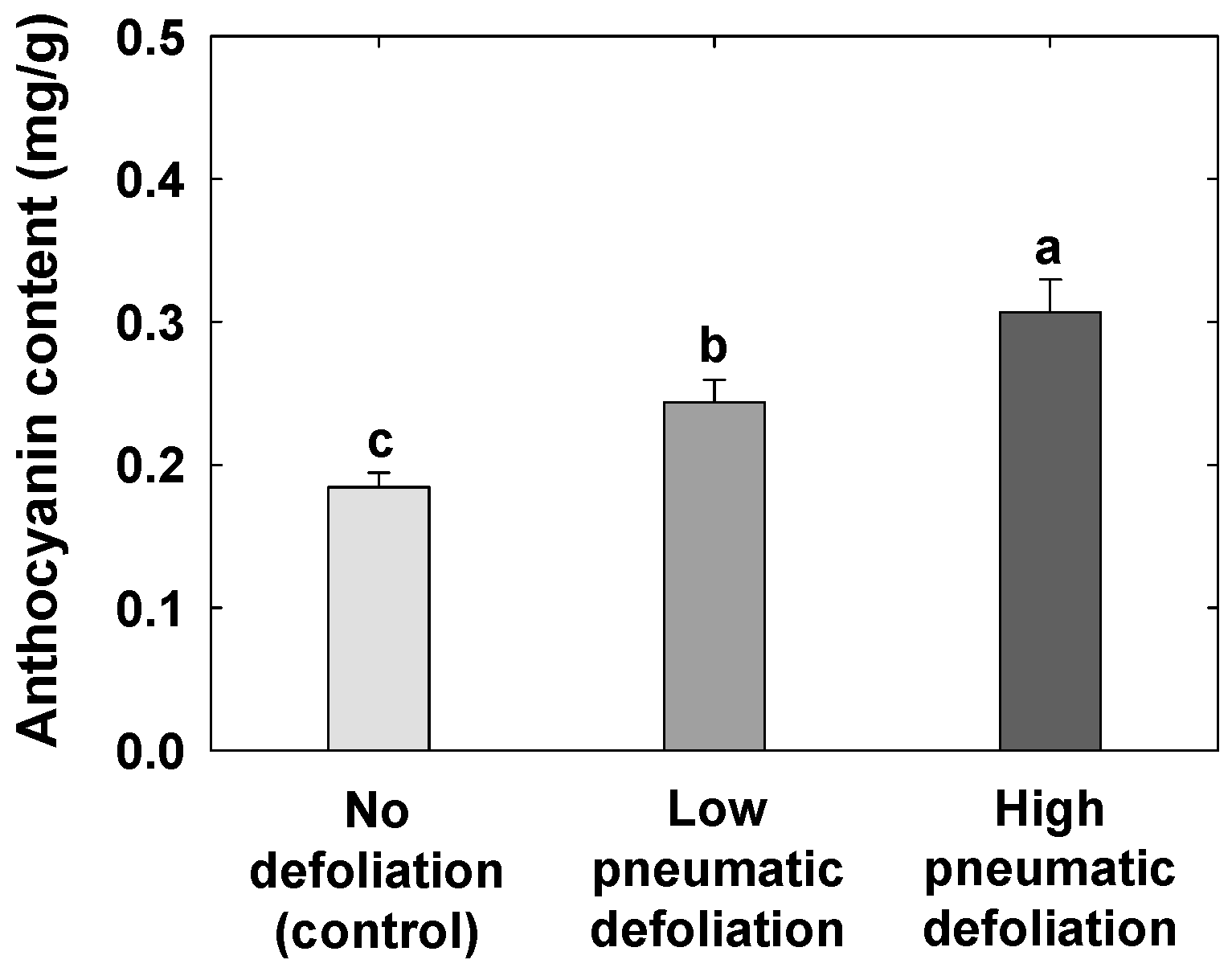
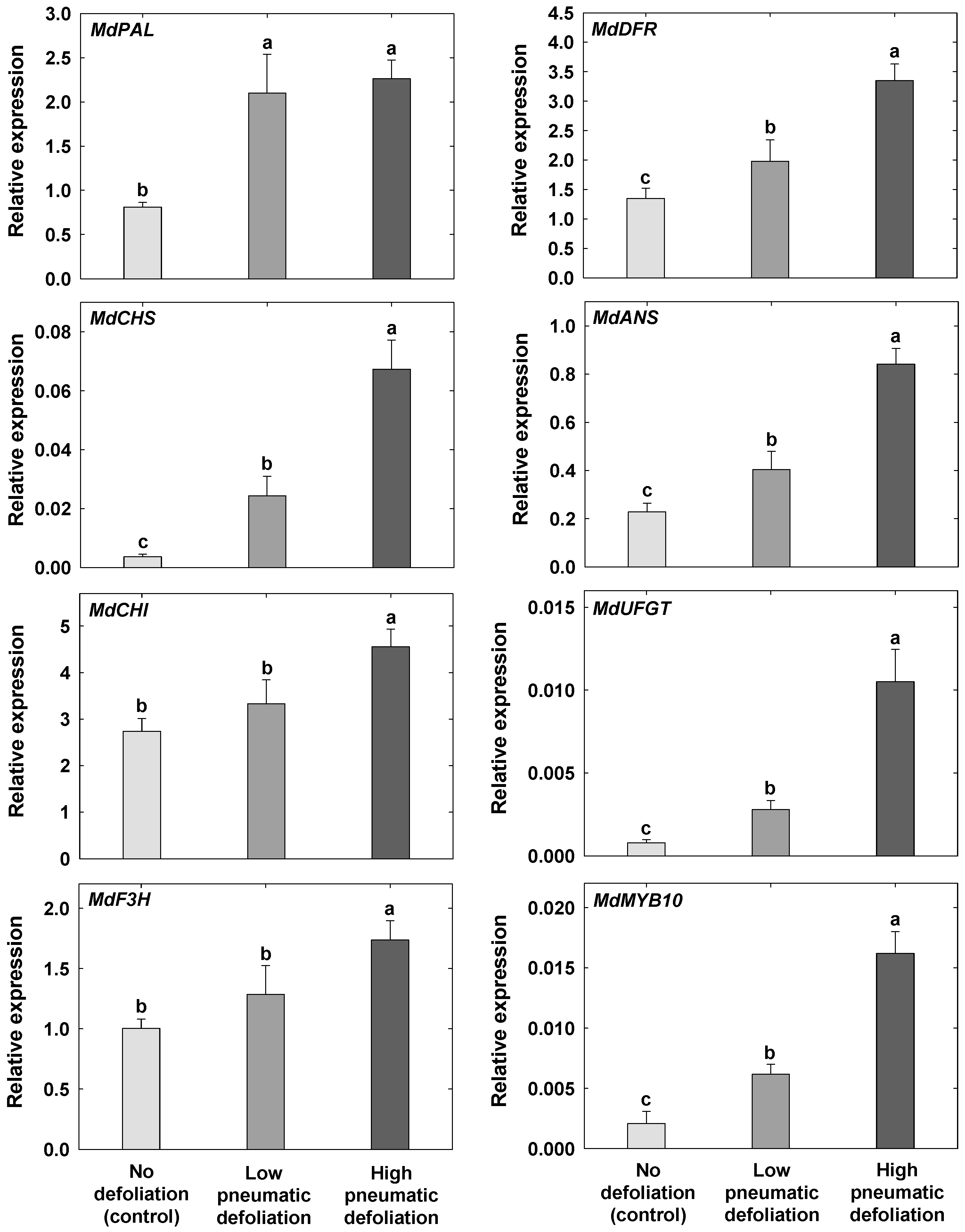
3.4. Anthocyanin Contents and Expression of Anthocyanin Synthesis-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Antúnez, L.; Ares, G.; Swaney-Stueve, M.; Jin, D.; Harker, F.R. Quality perceptions regarding external appearance of apples: Insights from experts and consumers in four countries. Postharv. Biol. Technol. 2018, 146, 99–107. [Google Scholar] [CrossRef]
- Dar, J.A.; Wani, A.A.; Ahmed, M.; Nazir, R.; Zargar, S.M.; Javaid, K. Peel colour in apple (Malus × domestica Borkh.): An economic quality parameter in fruit market. Sci. Hortic. 2019, 244, 50–60. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, S.; Zhang, Z.; Fang, H.; Xu, H.; Wang, Y.; Chen, X. Malus sieversii: The origin, flavonoid synthesis mechanism, and breeding of red-skinned and red-fleshed apples. Hortic. Res. 2018, 5, 70. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthesis genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Espley, R.V.; Hellens, R.P.; Putterill, J.; Stevenson, D.E.; Kutty-Amma, S.; Allan, A.C. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 2007, 49, 414–427. [Google Scholar] [CrossRef]
- Chagné, D.; Kirk, C.; How, N.; Whitworth, C.; Fontic, C.; Reig, G.; Sawyer, G.; Rouse, S.; Poles, L.; Gardiner, S.E.; et al. A functional genetic marker for apple red skin coloration across different environments. Tree Genet. Genomes 2016, 12, 67. [Google Scholar] [CrossRef]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ apple. HortScience 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Jing, C.; Feng, D.; Zhao, Z.; Wu, X.; Chen, X. Effect of environmental factors on skin pigmentation and taste in three apple cultivars. Acta Physiol. Plant. 2020, 42, 69. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffe, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Meng, R.; Qu, D.; Liu, Y.; Gao, Z.; Yang, H.; Shi, X.; Zhao, Z. Anthocyanin accumulation and related gene family expression in the skin of dark-grown red and non-red apples (Malus domestica Borkh.) in response to sunlight. Sci. Hortic. 2015, 189, 66–73. [Google Scholar] [CrossRef]
- Do, V.G.; Kim, S.; Lee, Y.; Kweon, H.J. Effect of reflected sunlight on differential expression of anthocyanin synthesis-related genes in young apple fruit. Int. J. Fruit Sci. 2021, 21, 440–455. [Google Scholar]
- Do, V.G.; Lee, Y.; Kweon, H.; Kim, S. Light induces carotenoid biosynthesis-related gene expression, accumulation of pigment content, and expression of the small heat shock protein in apple fruit. Int. J. Mol. Sci. 2022, 23, 6153. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J.O.; Kim, K.R. Effects of defoliation treatments during maturation on fruit quality of ‘Fuji’ apples. J. Korean Soc. Hortic. Sci. 2000, 41, 383–386. [Google Scholar]
- Matsumoto, K.; Fujita, T.; Sato, S.; Chun, J.P. Comparison of the effects of early and conventional defoliation on fruit growth, quality and skin color development in ‘Fuji’ apples. Hortic. Sci. Technol. 2017, 35, 410–417. [Google Scholar]
- Overbeck, V.; Schmitz-Eiberger, M.; Blanke, M. Reflective mulch enhances ripening and health compounds in apple fruit. J. Sci. Food Agric. 2013, 93, 2575–2579. [Google Scholar] [CrossRef]
- Yoo, J.; Kwon, J.G.; Kang, I.K. Effects of reflective film treatments on fruit quality attributes and peel color variables in ‘Fuji’ apple fruit. Korean J. Agric. Sci. 2022, 49, 521–529. [Google Scholar]
- Yim, Y.J.; Jang, J.Y.; Lee, H.C. Effect of optically active ABA and its synthetic intermediate STC4771 on defoliation and fruit color in ‘Fuji’ apple trees. J. Korean Soc. Hortic. Sci. 2000, 41, 53–55. [Google Scholar]
- Lee, C.H.; Seo, S.H.; Kwon, O.J.; Park, M.; Kim, W.C.; Kang, S.J. Functional characterization of a chemical defoliant that activates fruit cluster leaf defoliation in ‘Fuji’ apple trees. Appl. Biol. Chem. 2016, 59, 711–720. [Google Scholar] [CrossRef]
- Lim, H.K.; Shin, H.; Son, I.C.; Oh, Y.; Kim, K.; Han, H.; Oh, S.; Kim, D. Defoliation and fruit coloration in ‘Fuji’/M.9 apple affected by Cu-EDTA and Fe-EDTA foliar spray. Hortic. Sci. Technol. 2019, 37, 448–454. [Google Scholar]
- Lim, H.K.; Shin, H.; Son, I.C.; Oh, Y.; Kim, K.; Oh, S.I.; Oh, S.; Kim, D. Leaf thinning and fruit quality of ‘Hongro’/M.9 apple trees by foliar application of Cu-EDTA and Fe-EDTA. Korean J. Plant Res. 2019, 32, 677–682. [Google Scholar]
- Andergassen, C.; Pichler, D. Pneumatic defoliation of apple trees for colour improvement. Acta Hortic. 2022, 1346, 391–398. [Google Scholar] [CrossRef]
- Kwon, S.I.; Park, J.T.; Lee, J.W.; Kim, M.J.; Kim, J.H. ‘Picnic’, a new mid-season apple cultivar with medium size and good taste. Korean J. Hortic. Sci. Technol. 2015, 33, 784–788. [Google Scholar] [CrossRef]
- Lee, E.H.; Cho, E.B.; Lee, J.Y.; Bae, J.H.; Lee, E.C.; Yoo, J.; Kang, I.K.; Cho, Y.J. Functional properties of newly bred Picnic apple (Malus pumila Mill.). J. Appl. Biol. Chem. 2019, 62, 187–193. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I.K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in ‘Hwangok’ and ‘Picnic’ apples during cold storage. Postharv. Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Yoo, J.; Kwon, J.G.; Kang, I.K. Prediction of fruit maturity with starch pattern index in Korean apple cultivars. Hortic. Sci. Technol. 2023, 41, 27–35. [Google Scholar]
- Yoo, J.; Win, N.M.; Kang, I.K. Changes in the environmental conditions of an ‘Arisoo’ apple orchard with a shading net system. Korean J. Agric. Sci. 2022, 49, 561–570. [Google Scholar]
- Kim, K.O.; Yoo, J.; Lee, J.; Win, N.M.; Ryu, S.; Han, J.S.; Jung, H.Y.; Choung, M.G.; Kwon, Y.D.; Lee, D.H.; et al. Effects of 1-methylcyclopropene (1-MCP) and polyethylene (PE) film liner treatments on the fruit quality of cold-stored ‘Gamhong’ apples. Hortic. Environ. Biotechnol. 2018, 59, 51–57. [Google Scholar] [CrossRef]
- Win, N.M.; Lee, D.; Park, J.; Song, Y.Y.; Cho, Y.S.; Lee, Y.; Park, M.Y.; Kweon, H.K.; Kang, I.K.; Nam, J.C. Effects of bloom thinning with lime sulfur on fruit set, yield, and fruit quality attributes of ‘RubyS’ apples. Hortic. Sci. Technol. 2022, 40, 253–260. [Google Scholar]
- Fawbush, F.; Nock, J.F.; Watkins, C.B. Antioxidant contents and activity of 1-methylcyclopropene (1-MCP)-treated ‘Empire’ apples in air and controlled atmosphere storage. Postharv. Biol. Technol. 2009, 52, 30–37. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food. Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Ouyanga, K.; Li, J.; Huang, H.; Que, Q.; Li, P.; Chen, X. A simple method for RNA isolation from various tissues of the tree Neolamarckia cadamba. Biotechnol. Equip. 2014, 28, 1008–1013. [Google Scholar] [CrossRef]
- Zhou, Z.; Cong, P.; Tian, Y.; Zhu, Y. Using RNA-seq data to select reference genes for normalizing gene expression in apple roots. PLoS ONE 2017, 12, e0185288. [Google Scholar] [CrossRef]
- Lone, P.M.; Nazar, R.; Singh, S.; Khan, N.A. Effects of timing of defoliation on nitrogen assimilation and associated changes in ethylene biosynthesis in mustard (Brassica juncea). Biologia 2008, 63, 207–210. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, M.; Li, Q.; Liu, X.; Song, H.; Peng, X.; Wang, H.; Yang, N.; Fan, P.; Wang, R.; et al. Effects of defoliation modalities on plant growth, leaf traits, and carbohydrate allocation in Amorpha fruticose L. and Robinia pseudoacacia L. seedlings. Ann. For. Sci. 2020, 77, 53. [Google Scholar] [CrossRef]
- Iqbal, N.; Masood, A.; Khan, N.A. Analyzing the significance of defoliation in growth, photosynthetic compensation and source-sink relations. Photosynthetica 2012, 50, 161–170. [Google Scholar] [CrossRef]
- Lavely, E. Using Pneumatic Defoliation to Improve Fruit Color and Quality in Apple: A Follow-Up from the International Tree Fruit Association Annual Conference. 2022. Available online: https://www.canr.msu.edu/news/using-pneumatic-defoliation-to-improve-fruit-color-and-quality-in-apple (accessed on 17 March 2022).
- Anten, N.P.R.; Ackerly, D.D. Canopy-level photosynthetic compensation after defoliation in a tropical understorey palm. Funct. Ecol. 2001, 15, 252–262. [Google Scholar]
- Loescher, W.H.; McCamant, T.; Keller, J.D. Carbohydrate reserves, translocation, and storage in woody plant roots. HortScience 1990, 25, 274–281. [Google Scholar] [CrossRef]
- Jakopič, J.; Fajt, N.; Štampar, F.; Veberič, R. Effect of crop load on fruit quality of ‘Fuji’ apple. Acta Aliment. 2013, 42, 318–327. [Google Scholar] [CrossRef]
- Srisook, W.; Lim, C.K.; Oh, E.U.; Yi, K.; Kumarihami, H.M.P.C.; Kim, S.C.; Park, K.S.; Song, K.J. Defoliation time influences vine regrowth, off-season flowering, and fruit quality in ‘Jecy Gold’ kiwifruit vines. Hortic. Environ. Biotechnol. 2016, 57, 219–224. [Google Scholar] [CrossRef]
- Racsko, J.; Schrader, L.E. Sunburn of apple fruit: Historical background, recent advances and future perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar]
- Tsao, R.; Yang, R.; Young, J.C.; Zhu, H. Polyphenolic profiles in eight apple cultivars using high performance liquid chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef]
- Feng, F.; Kotoda, N.; Ma, F.; Cheng, L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]



| Defoliation Treatments | Fruit Weight (g) | Flesh Firmness (N) | SSC (%) | TA (%) | SPI (1–8) | Sunburn (%) |
|---|---|---|---|---|---|---|
| No defoliation (control) | 199.60 ± 3.66 z a y | 71.61 ± 1.50 b | 13.94 ± 0.18 a | 0.44 ± 0.01 a | 5.60 ± 0.48 a | 1.00 ± 0.26 a |
| Low pneumatic defoliation (0.7 bar) | 205.68 ± 3.54 a | 75.32 ± 0.64 a | 14.30 ± 0.14 a | 0.43 ± 0.01 a | 5.80 ± 0.12 a | 1.50 ± 0.34 a |
| High pneumatic defoliation (0.9 bar) | 198.19 ± 2.86 a | 74.30 ± 0.92 ab | 14.27 ± 0.18 a | 0.41 ± 0.01 a | 5.60 ± 0.10 a | 1.70 ± 0.49 a |
| Defoliation Treatments | Red-Colored Area (%) | L* | a* | b* |
|---|---|---|---|---|
| No defoliation (control) | 57.78 ± 1.69 z c y | 47.99 ± 1.54 a | 22.49 ± 1.28 c | 15.98 ± 0.44 a |
| Low pneumatic defoliation (0.7 bar) | 67.22 ± 2.65 b | 42.98 ± 0.44 b | 24.99 ± 0.79 b | 14.24 ± 0.79 b |
| High pneumatic defoliation (0.9 bar) | 78.33 ± 1.86 a | 39.72 ± 0.91 c | 28.56 ± 0.34 a | 13.09 ± 0.47 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Win, N.M.; Lee, Y.; Kim, S.; Do, V.G.; Cho, Y.S.; Kang, I.-K.; Yang, S.; Park, J. Pneumatic Defoliation Enhances Fruit Skin Color and Anthocyanin Pigments in ‘Picnic’ Apples. Agronomy 2023, 13, 2078. https://doi.org/10.3390/agronomy13082078
Win NM, Lee Y, Kim S, Do VG, Cho YS, Kang I-K, Yang S, Park J. Pneumatic Defoliation Enhances Fruit Skin Color and Anthocyanin Pigments in ‘Picnic’ Apples. Agronomy. 2023; 13(8):2078. https://doi.org/10.3390/agronomy13082078
Chicago/Turabian StyleWin, Nay Myo, Youngsuk Lee, Seonae Kim, Van Giap Do, Young Sik Cho, In-Kyu Kang, Sangjin Yang, and Juhyeon Park. 2023. "Pneumatic Defoliation Enhances Fruit Skin Color and Anthocyanin Pigments in ‘Picnic’ Apples" Agronomy 13, no. 8: 2078. https://doi.org/10.3390/agronomy13082078
APA StyleWin, N. M., Lee, Y., Kim, S., Do, V. G., Cho, Y. S., Kang, I.-K., Yang, S., & Park, J. (2023). Pneumatic Defoliation Enhances Fruit Skin Color and Anthocyanin Pigments in ‘Picnic’ Apples. Agronomy, 13(8), 2078. https://doi.org/10.3390/agronomy13082078









