Organic Material Addition Optimizes Soil Structure by Enhancing Copiotrophic Bacterial Abundances of Nitrogen Cycling Microorganisms in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Site
2.2. Experiment Design
2.3. Soil Sampling and Analyses
2.4. DNA Extraction and Quantitative PCR
2.5. Processing of Illumina MiSeq Sequencing Data
2.6. Statistical Analysis
3. Results
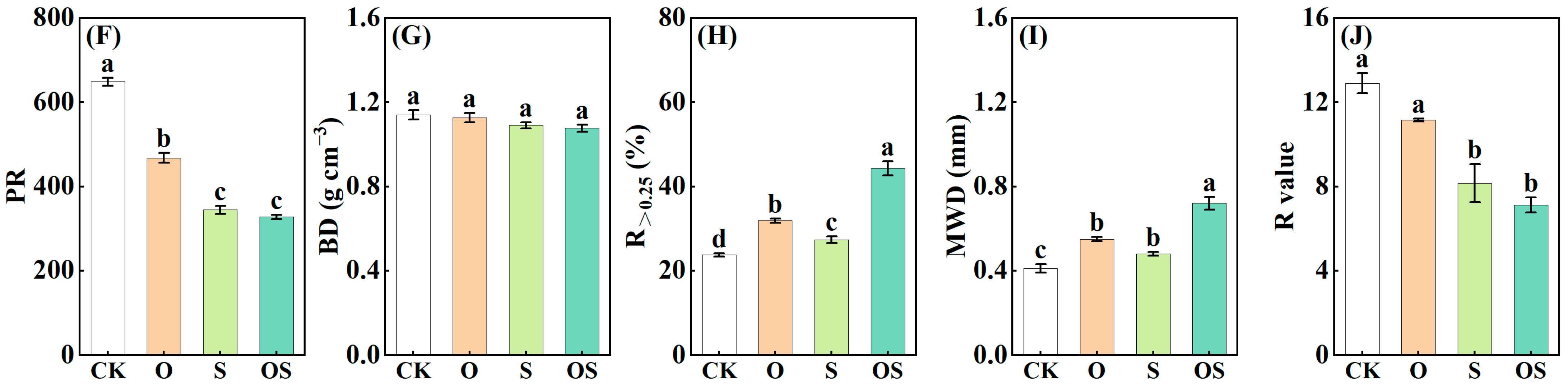
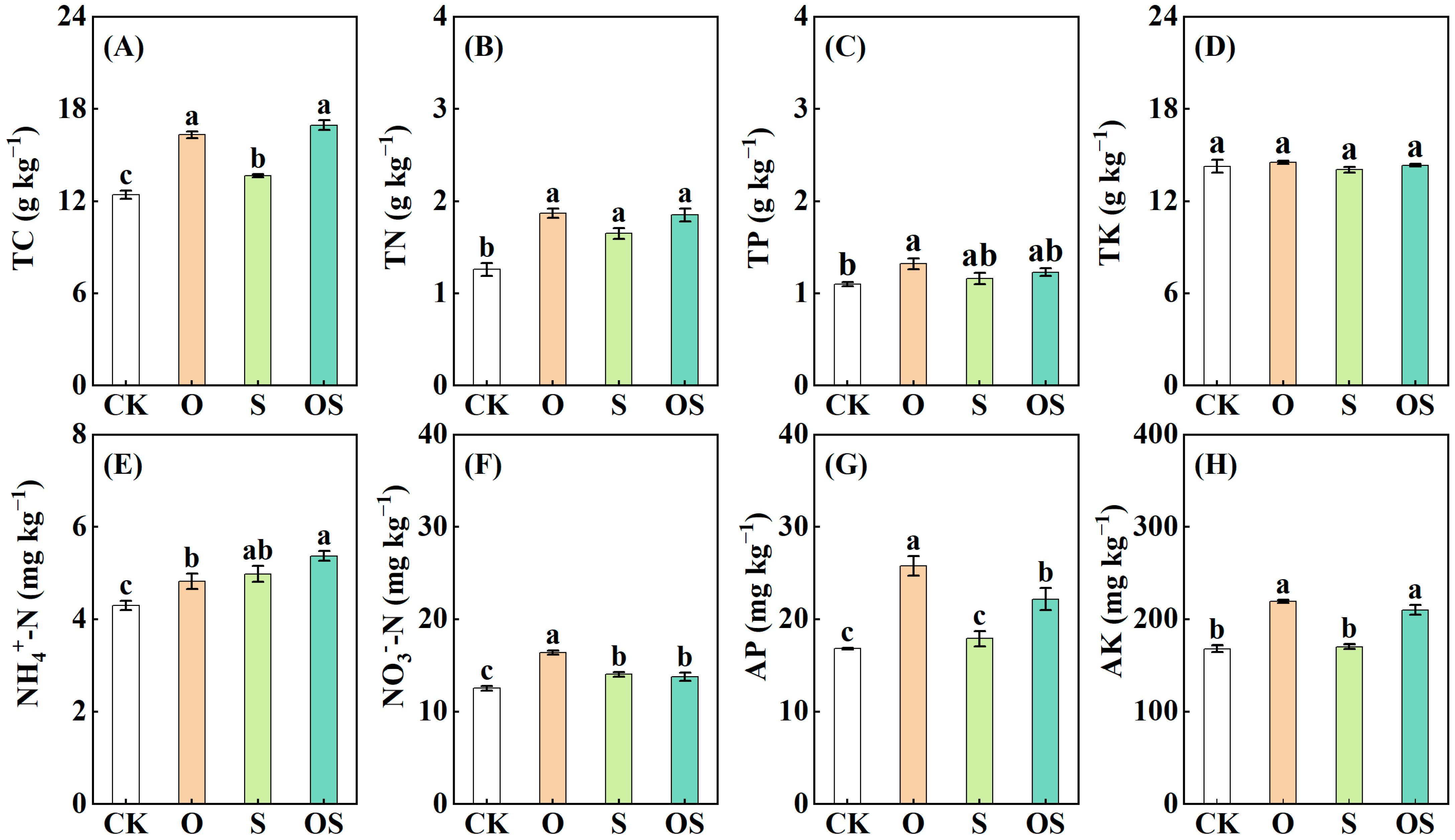
3.1. Soil Physicochemical Properties
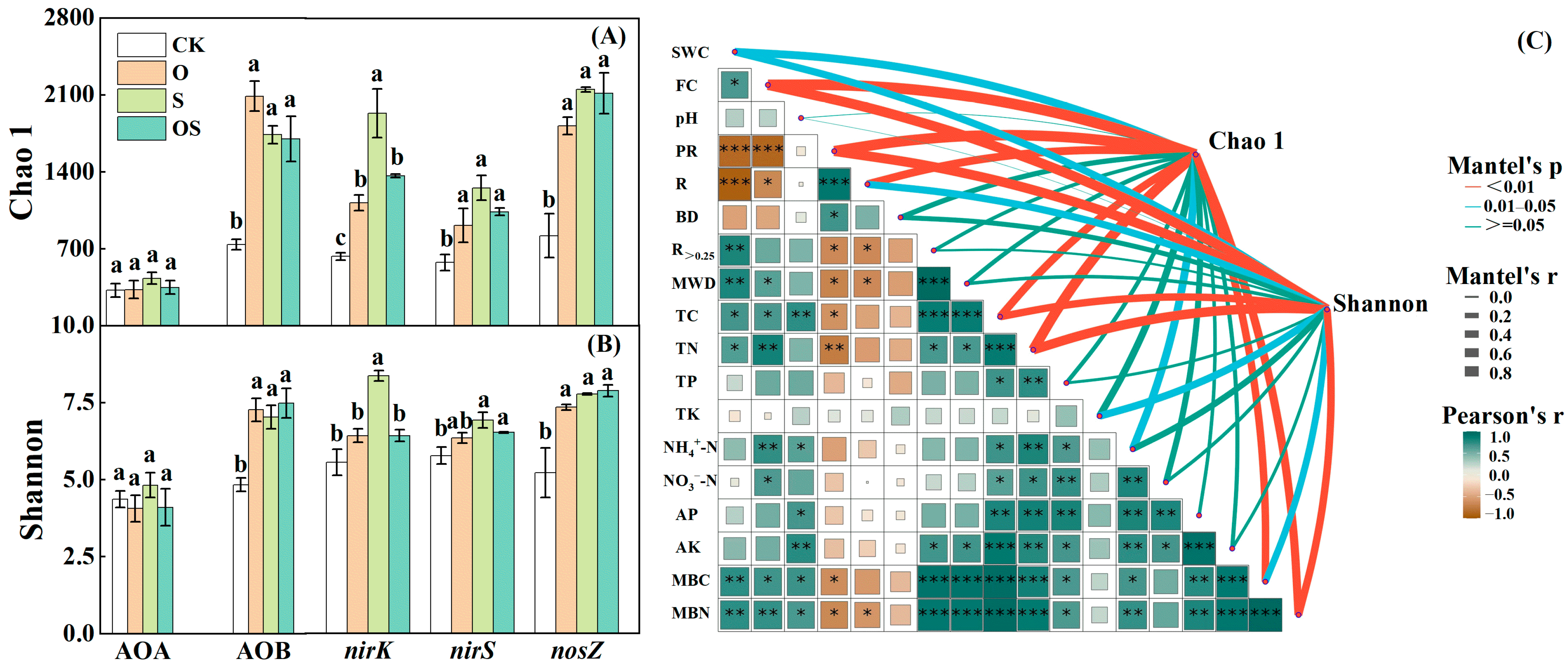
3.2. The Abundance of the Microbial Community
3.3. The Alpha Diversity Index of Microorganisms
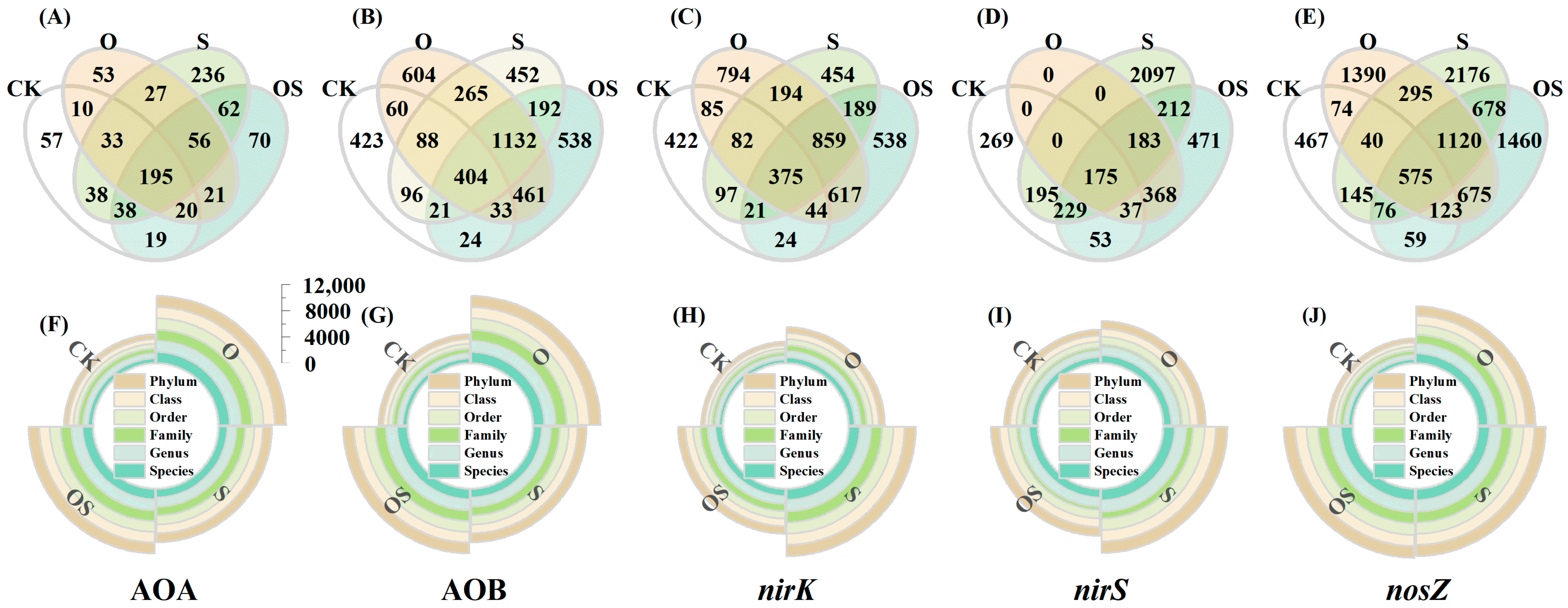
3.4. The Richness of OTUs
3.5. The Microbial Community Composition
4. Discussion
4.1. Nitrification and Denitrification Microbial Abundances and Soil Properties
4.2. Diversity and Structure of the Nitrifying and Denitrifying Microbial Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asai, H.; Saito, K.; Kawamura, K. Application of a Bayesian approach to quantify the impact of nitrogen fertilizer on upland rich yield in sub-Saharan Africa. Field Crops Res. 2021, 272, 108284. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Yue, Y.; Li, C.F.; Wang, Y.J.; Zhang, H.Y.; Ren, H.; Gong, X.W.; Jiang, Y.; Qi, H. Revolutionizing maize crop productivity: The winning combination of zigzag planting and deep nitrogen fertilizer for maximum yield through root-shoot ratio management. Agronomy 2023, 13, 1307. [Google Scholar] [CrossRef]
- Xu, X.; Schaeffer, S.; Sun, Z.; Wang, J.K. Carbon stabilization in aggregate fractions responds to straw input levels under varied soil fertility levels. Soil Tillage Res. 2020, 199, 104593. [Google Scholar] [CrossRef]
- Guan, S.; Liu, S.J.; Liu, R.Y.; Zhang, J.J.; Ren, J.; Cai, H.G.; Lin, X.X. Soil organic carbon associated with aggregate-size and density fractions in a Mollisol amended with charred and uncharred maize straw. J. Integr. Agric. 2019, 18, 1496–1507. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Sui, P.X.; Lian, H.L.; Li, Y.N.; Xu, H.R.; Zhang, H.Y.; Xu, Y.Y.; Gong, X.W.; Qi, H.; Jiang, Y. Tillage with straw incorporation reduces the optimal nitrogen rate for maize production by affecting crop uptake, utility efficiency, and the soil balance of nitrogen. Land Degrad. Dev. 2023, 34, 2825–2837. [Google Scholar] [CrossRef]
- Tang, Q.; Ti, C.P.; Xia, L.L.; Xia, Y.Q.; Wei, Z.J.; Yan, X.Y. Ecosystem services of partial organic substitution for chemical fertilizer in a peri-urban zone in China. J. Clean. Prod. 2019, 224, 779–788. [Google Scholar] [CrossRef]
- Pan, J.X.; Shang, Y.W.; Zhang, W.J.; Chen, X.P.; Cui, Z.L. Improving soil quality for higher grain yield in Chinese wheat and maize production. Land Degrad. Dev. 2020, 31, 1125–1137. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Y.L.; Tian, Y.H.; Ceng, K.; Zhao, M.; Zhao, M.; Yin, B. Increasing yield and N use efficiency with organic fertilizer in Chinese intensive rice cropping systems. Field Crops Res. 2018, 227, 102–109. [Google Scholar] [CrossRef]
- Lai, Z.L.; Fan, J.L.; Yang, R.; Xu, X.Y.; Liu, L.J.; Li, S.E.; Zhang, F.C.; Li, Z.J. Interactive effect of plant denisity and nitrogen rate on grain yield, economic benefit, water productivity and nitrogen use efficiency of drip-fertigated maize in northwest China. Agric. Water Manag. 2022, 263, 107453. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; He, P.; Zhou, W. Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol. Biochem 2013, 57, 30–42. [Google Scholar] [CrossRef]
- Purkhold, U.; Pommerening-Röser, A.; Juretschko, S.; Schmid, M.C.; Koops, H.P.; Wagner, M. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: Implications for molecular diversity surveys. Appl. Environ. Microbiol. 2000, 66, 5368–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.C.; Zhang, L.; Lu, Q.; Raza, W.; Huang, Q.W.; Shen, Q.R. Ammonia oxidizer abundance in paddy soil profile with different fertilizer regimes. Appl. Soil Ecol. 2014, 84, 38–44. [Google Scholar] [CrossRef]
- Yang, Y.D.; Ren, Y.F.; Wang, X.Q.; Hu, Y.G.; Wang, Z.M.; Zeng, Z.H. Ammonia-oxidizing archaea and bacteria responding differently to fertilizer type and irrigation frequency as revealed by Illumina Miseq sequencing. J. Soils Sediments 2018, 18, 1029–1040. [Google Scholar] [CrossRef]
- Yin, C.; Fan, F.L.; Song, A.L.; Li, Z.J.; Yu, W.T.; Liang, Y.C. Different denitrification potential of aquic brown soil in Northeast China under inorganic and organic fertilization accompanied by distinct changes of nirS- and nirK-denitrifying bacterial community. Eur. J. Soil Biol. 2014, 65, 47–65. [Google Scholar] [CrossRef]
- Yin, C.; Fan, F.L.; Song, A.L.; Cui, P.Y.; Li, T.Q.; Liang, Y.C. Denitrification potential under different fertilization regimes is closely coupled with changes in the denitrifying community in a black soil. Appl. Microbiol. Biotechnol. 2015, 99, 5719–5729. [Google Scholar] [CrossRef]
- Shi, Y.L.; Liu, X.R.; Zhang, Q.W.; Li, Y.C. Contrasting effects of biochar- and organic fertilizer- amendment on community compositions of nitrifiers and denitrifiers in a wheat-maize rotation system. Appl. Soil Ecol. 2022, 171, 104320. [Google Scholar] [CrossRef]
- Han, B.; Mo, L.Y.; Fang, Y.T.; Di, H.J.; Wang, J.T.; Shen, J.P.; Zhang, L.M. Rates and microbial communities of denitrification and anammox accorss coastal tidal flat lands and inland paddy soils in East China. Appl. Soil Ecol. 2021, 157, 103768. [Google Scholar] [CrossRef]
- Wang, W.Y.; Hou, Y.T.; Pan, W.H.; Vinay, N.; Mo, F.; Liao, Y.C.; Wen, X.X. Continuous application of conservation tillage affects in situ N2O emissions and nitrogen cycling gene abundances following nitrogen fertilization. Soil Biol. Biochem. 2021, 157, 108239. [Google Scholar] [CrossRef]
- Prosser, J.I.; Nicol, G.W. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012, 20, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Tao, R.; Wakelin, S.A.; Liang, Y.; Chu, G. Response of ammonia-oxidizing archaea and bacteria in calcareous soil to mineral and organic fertilizer application and their relative contribution to nitrification. Soil Biol. Biochem. 2017, 114, 20–30. [Google Scholar] [CrossRef]
- Muema, E.K.; Cadisch, G.; Rasche, F. Soil texture modulates the response of ammonia-oxidizing prokaryotes to biochemical quality of organic inputs in tropical agricultural soils. Soil Biol. Biochem. 2016, 100, 218–228. [Google Scholar] [CrossRef]
- Tao, R.; Wakelin, S.A.; Liang, Y.; Hu, B.; Chu, G. Nitrous oxide emission and denitrifier communities in drip-irrigated calcareous soil as affected by chemical and organic fertilizers. Sci. Total Environ. 2018, 612, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Fan, F.; Yin, C.; Song, A.; Huang, P.; Tang, Y.; Zhu, P.; Peng, C.; Li, T.; Wakelin, S.; et al. Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol. Biochem. 2016, 93, 131–141. [Google Scholar] [CrossRef]
- Walker, C.B.; Torre, J.R.; Klotz, M.G.; Urakawa, H.; Pinel, N.; Arp, D.J.; Brochier-Armanet, C.; Chain, P.S.G.; Chan, P.P.; Gollabgir, A.; et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. USA 2010, 107, 8818–8823. [Google Scholar] [CrossRef]
- He, Z.Y.; Sun, A.Q.; Jiao, X.Y.; Ge, A.H.; Hu, H.W.; Jin, S.S.; Liu, X.; Lin, Y.X.; He, J.Z. Fertilization has a greater effect than rhizosphere on community strctures of comammox Nitrospira in an alkaline agricultural soil. Appl. Soil Ecol. 2022, 175, 104456. [Google Scholar] [CrossRef]
- Huang, R.; Wang, Y.Y.; Liu, J.; Li, J.C.; Xu, G.X.; Luo, M.; Xu, C.; Ci, E.; Gao, M. Variation in N2O emission and N2O related microbial functional genes in straw- and biochar-amended and non-amended soils. Appl. Soil Ecol. 2019, 137, 57–68. [Google Scholar] [CrossRef]
- Pereg, L.; Morugán-Coronado, A.; McMillan, M.; García-Orenes, F. Restoration of nitrogen cycling community in grapevine soil by a decade of organic fertilization. Soil Tillage Res. 2018, 179, 11–19. [Google Scholar] [CrossRef]
- Sun, R.B.; Guo, X.S.; Wang, D.Z.; Chu, H.Y. Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl. Soil Ecol. 2015, 95, 171–178. [Google Scholar] [CrossRef]
- Miller, M.N.; Zebarth, B.J.; Dandie, C.E.; Burton, D.L.; Goyer, C.; Trevors, J.T. Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Biol. Biochem. 2008, 40, 2553–2563. [Google Scholar] [CrossRef]
- Mei, N.; Yang, B.; Tian, P.; Jiang, Y.; Sui, P.X.; Sun, D.Q.; Zhang, Z.P.; Qi, H. Using a modified soil quality index to evaluate densely tilled soils with different yields in Northeast China. Environ. Sci. Pollut. Res. 2019, 26, 13867–13877. [Google Scholar] [CrossRef]
- Sui, P.X.; Tian, P.; Lian, H.L.; Wang, Z.Y.; Ma, Z.Q.; Qi, H.; Mei, N.; Sun, Y.; Wang, Y.Y.; Su, Y.H.; et al. Straw incorporation management affects maize grain yield through regulating nitrogen uptake, water use efficiency, and root distribution. Agronomy 2020, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Flint, L.E.; Flint, A.L. Porosity. In Methods of Soil Analysis. Part 4. Physical Methods; Dane, J.H., Top, G.C., Eds.; SSSA Special Publication: Madison, WI, USA, 2002; pp. 241–253. [Google Scholar]
- Lian, H.L.; Wang, Z.Y.; Li, Y.N.; Xu, H.R.; Zhang, H.Y.; Gong, X.W.; Qi, H.; Jiang, Y. Straw strip return increases soil organic carbon sequestration by optimizing organic and humus carbon in aggregates of mollisols in northeast china. Agronomy 2022, 12, 784. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, H.J.; Yao, X.D.; Zeng, W.J.; Wang, W. Latitudinal and depth patterns of soil microbial biomass carbon, nitrogen, and phosphorus in grasslands of an agro-pastoral ecotone. Land Degrad. Dev. 2021, 32, 3833–3846. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, Y.M.; Zhao, J.F.; Zhou, J.Y.; Abbas, F. Effects of planting structure on soil water-stable aggregates, microbial biomass and enzyme activity in a catchment of Loess Plateau terraces, China. Appl. Soil Ecol. 2020, 159, 103819. [Google Scholar] [CrossRef]
- Guo, H.B.; Ji, B.Y.; Wang, Q.F.; Zhao, Y.L.; Mu, X.Y.; Xue, Z.W.; Li, C.H.; Zhao, Z.J. Effects of deep tillage and straw returning on soil physical properties and grain yield of different soil texture. J. Henan Agric. Univ. 2014, 48, 505–511. [Google Scholar]
- Lin, Y.X.; Ding, W.X.; Liu, D.Y.; He, T.H.; Yoo, G.; Yuan, J.; Chen, Z.M.; Fan, J.L. Wheat straw-derived biochar amendment stimulated N2O emissions from rice paddy soils by regulating the amoA genes of ammonia-oxidizing bacteria. SBB 2017, 113, 89–98. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Z.C.; Cao, X.C.; Zhu, S.C.; Zhang, X.; Wu, L.H. Responses of rice production, milled rice quality and soil properties to various nitrogen inputs and rice straw incorporation under continuous plastic film mulching cultivation. Field Crops Res. 2014, 155, 164–171. [Google Scholar] [CrossRef]
- Shang, Q.; Ling, N.; Feng, X.M.; Yang, X.X.; Wu, P.P.; Zou, J.W.; Shen, Q.R.; Guo, S.W. Soil fertility and its significance to crop productivity and sustainability in typical agroecosystem: A summary of long-term fertilizer experiments in China. Plant Soil 2014, 381, 13–23. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; Jose, R.T.; Stahl, D. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef]
- Stahl, D.A.; Torre, J.R.D.L. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 2012, 66, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, D.T.; Prosser, J.I.; Nicol, G.W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011, 5, 1067–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerou, M.; Offre, P.; Valledor, L.; Abby, S.S.; Melcher, M.; Nagler, M.; Weckwerth, W.; Schleper, C. Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proc. Natl. Acad. Sci. USA 2016, 113, 7937–7946. [Google Scholar] [CrossRef] [PubMed]
- Hink, L.; Nicol, G.W.; Prosser, J.I. Archaea produce lower yields of N2O than bacteria during aerobic ammonia oxidation in soil. Environ. Microbiol. 2017, 19, 4829–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicol, G.W.; Schleper, C. Ammonia-oxididing crenarchaeota: Important players in the nitrogen cycle? Environ. Microbiol. 2006, 14, 207–212. [Google Scholar] [CrossRef]
- Aigle, A.; Prosser, J.I.; Gubry-Rangin, C. The application of high-throughput sequencing technology to analysis of amoA phylogeny and environmental niche specialisation of terrestrial bacterial ammonia-oxidisers. Environ. Microbiol. 2019, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Gubry-Rangin, C.; Nicol, G.W.; Prosser, J.I. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol. Ecol. 2010, 74, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, H.; Shen, J.; He, J. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, C.A.; Wakelin, S.A.; Fillery, I.R.P.; Roper, M.M. Factors affecting ammonia-oxidising microorganisms and potential nitrification rates in southern Australian agricultural soils. Soil Res. 2013, 51, 240–252. [Google Scholar] [CrossRef]
- Celik, I.; Ortas, I.; Kilic, S. Effects of compost, mycorrhiza, manure and fertilizer on some physical properties of a Chromoxerert soil. Soil Tillage Res. 2004, 78, 59–67. [Google Scholar] [CrossRef]
- Pu, X.Z.; Zhang, G.J.; Zhang, P.P.; Liu, Y.J.; Zhang, W.F. Effects of straw management, inorganic fertiliser, and manure amendment on soil microbial properties, nutrient availability, and root growth in a drip-irrigated cotton field. Crop Pasture Sci. 2016, 67, 1297–1308. [Google Scholar] [CrossRef]
- Lognoul, M.; Theodorakopoulos, N.; Hiel, M.P.; Regaert, D.; Broux, F.; Heinesch, B.; Bodson, B.; Vandenbol, M. Impact of tillage on greenhouse gas emissions by an agricultural crop and dynamics of N2O fluxes: Insights from automated closed chamber measurements. Soil Tillage Res. 2017, 167, 80–89. [Google Scholar] [CrossRef]
- Petersen, D.G.; Blazewicz, S.J.; Firestone, M.; Herman, D.J.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Hallins, S.; Jones, C.M.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.M.; Hallin, S. Ecological and evolutionary factors underlying global and local assembly of denitrifier communities. ISME J. 2010, 4, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.Y.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. Effect of nitregen fertilization on the abundance of nitrogen cycling genes in agricultural soil: A meta-analysis of field studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Núñez, E.V.; Valenzuela-Encinas, C.; Alcántara-Hernández, R.J.; Navarro-Noya, Y.E.; Luna-Guido, M.; Marsch, R.; Dendooven, L. Modifications of bacterial populations in anthracene contaminated soil. Appl. Soil Ecol. 2012, 61, 113–126. [Google Scholar] [CrossRef]
- Jennings, A.A. Worldwide regulatory guidance values for surface soil exposure to carcinogenic or mutagenic polycyclic aromatic hydrocarbons. J. Environ. Manag. 2012, 110, 82–102. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Wang, X.L.; Zhang, S.Q.; Palta, J.A.; Chen, Y.L. Influence of spatial arrangement in maize-soybean intercropping on root growth and water use efficiency. Plant Soil 2017, 415, 131–144. [Google Scholar] [CrossRef]
- Yao, H.; Campbell, C.D.; Chapman, S.J.; Freitag, T.E.; Nicol, G.W.; Singh, B.K. Multi-factorial drivers of ammonia oxidizer communities: Evidence from a national soil survey. Environ. Microbiol. 2013, 15, 2545–2556. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.Y.; Yang, S.; Wang, Z.R.; Feng, X.; Liu, H.Y.; Jiang, Y. Variations in soil bacterial taxonomic profiles and putative functions in response to straw incorporation combined with N fertilization during the maize growing season—ScienceDirect. Agric. Ecosyst. Environ. 2019, 283, 106578. [Google Scholar] [CrossRef]
- Singleton, D.R.; Dickey, A.N.; Scholl, E.H.; Wright, F.A.; Aitken, M.D. Complete genome sequence of a bacterium representing a deep uncultivated lineage within the gammaproteobacteria associated with the degradation of polycyclic aromatic hydrocarbons. Genome Announc. 2016, 4, e01086-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De, L.; Laura, D.B.; Domínguez-Mendoza, C.A.; Navarro-Noya, Y.E.; Marco, L.G.; Luc, D. Soil Salinity Controls Relative Abundance of Specific Bacterial Groups Involved in the Decomposition of Maize Plant Residues. Front. Ecol. Evol. 2018, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Ishii, S.; Ashida, N.; Otsuka, S.; Senoo, K. Isolation of oligotrophic denitrifiers carrying previously uncharacterized functional gene sequences. Appl. Environ. Microbiol. 2011, 77, 338–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrissey, E.H.; Mau, R.L.; Schwartz, E.; Caporaso, J.G.; Dijkstra, P.; Van Gestel, N. Phylogenetic organization of bacterial activity. ESME J. 2016, 10, 2336–2340. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, Z.W.; Yang, S.; Li, X.B.; Top Eva, M.; Wang, R.Z.; Zhang, Y.G.; Cai, J.P.; Yao, F.; Han, X.G.; et al. Responses of Soil Bacterial Communities to Nitrogen Deposition and Precipitation Increment Are Closely Linked with Aboveground Community Variation. Microbiol. Ecol. 2016, 71, 974–989. [Google Scholar] [CrossRef]
- Andrew, J.H.; Harris, R.F. R-and K-selection and Microbial Ecology. In Advances in Microbial Ecology; Springer: Boston, MA, USA, 1986; pp. 99–147. [Google Scholar]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Gong, X.; Zheng, Y.; Tian, P.; Jiang, Y.; Zhang, H.; Qi, H. Organic Material Addition Optimizes Soil Structure by Enhancing Copiotrophic Bacterial Abundances of Nitrogen Cycling Microorganisms in Northeast China. Agronomy 2023, 13, 2108. https://doi.org/10.3390/agronomy13082108
Yue Y, Gong X, Zheng Y, Tian P, Jiang Y, Zhang H, Qi H. Organic Material Addition Optimizes Soil Structure by Enhancing Copiotrophic Bacterial Abundances of Nitrogen Cycling Microorganisms in Northeast China. Agronomy. 2023; 13(8):2108. https://doi.org/10.3390/agronomy13082108
Chicago/Turabian StyleYue, Yang, Xiangwei Gong, Yongzhao Zheng, Ping Tian, Ying Jiang, Hongyu Zhang, and Hua Qi. 2023. "Organic Material Addition Optimizes Soil Structure by Enhancing Copiotrophic Bacterial Abundances of Nitrogen Cycling Microorganisms in Northeast China" Agronomy 13, no. 8: 2108. https://doi.org/10.3390/agronomy13082108
APA StyleYue, Y., Gong, X., Zheng, Y., Tian, P., Jiang, Y., Zhang, H., & Qi, H. (2023). Organic Material Addition Optimizes Soil Structure by Enhancing Copiotrophic Bacterial Abundances of Nitrogen Cycling Microorganisms in Northeast China. Agronomy, 13(8), 2108. https://doi.org/10.3390/agronomy13082108





