The Electrical Conductivity and Drying Method Changed the Secondary Metabolite Content and Photoprotective Effects of Hydroponically Cultivated Agastache rugosa Kuntze
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Parameters and Yield
2.3. Preparation of Dried Samples and Their Extracts
2.4. Antioxidative Properties
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Cell Culture
2.7. Cell Viability
2.8. ROS Reduction in UV-B Irradiated Cells
2.9. Statistical Analysis
3. Results
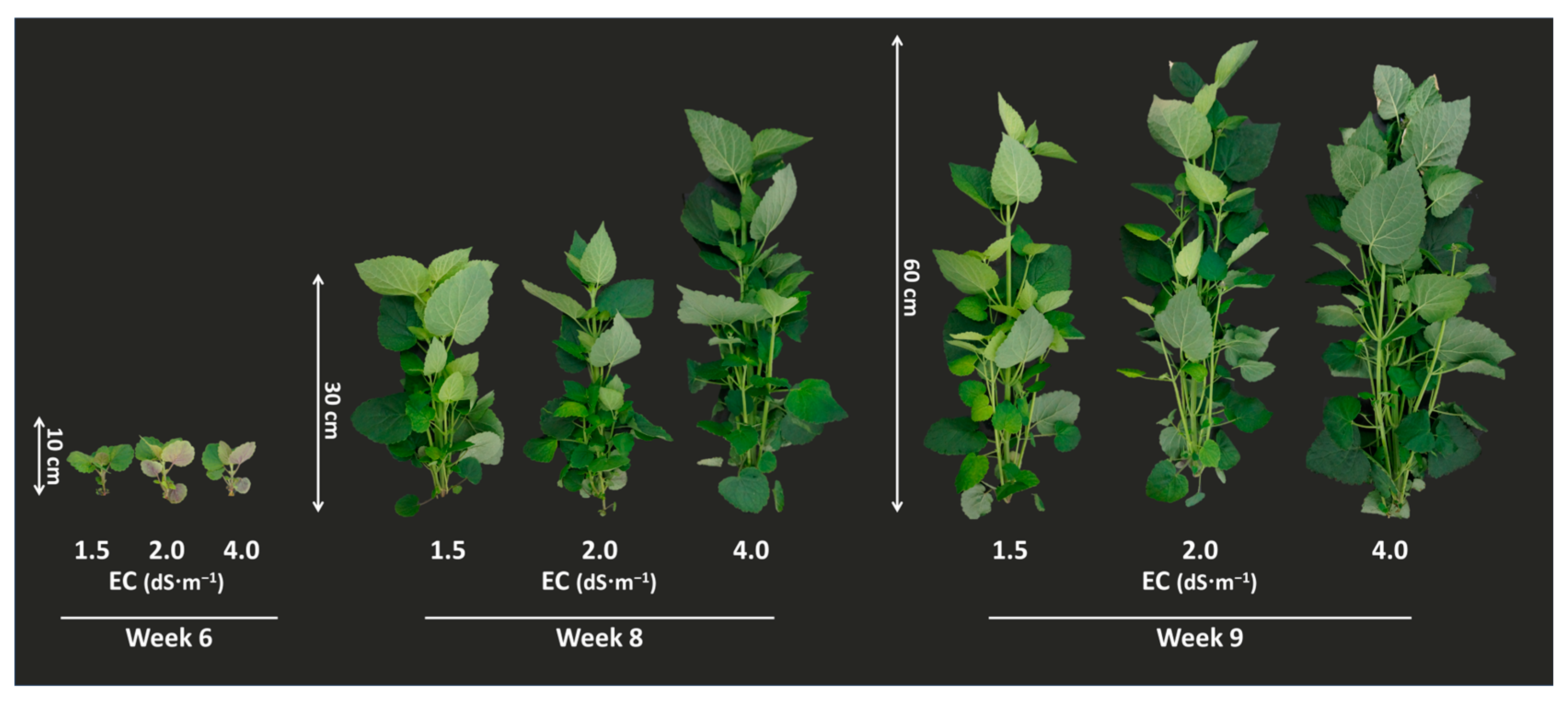
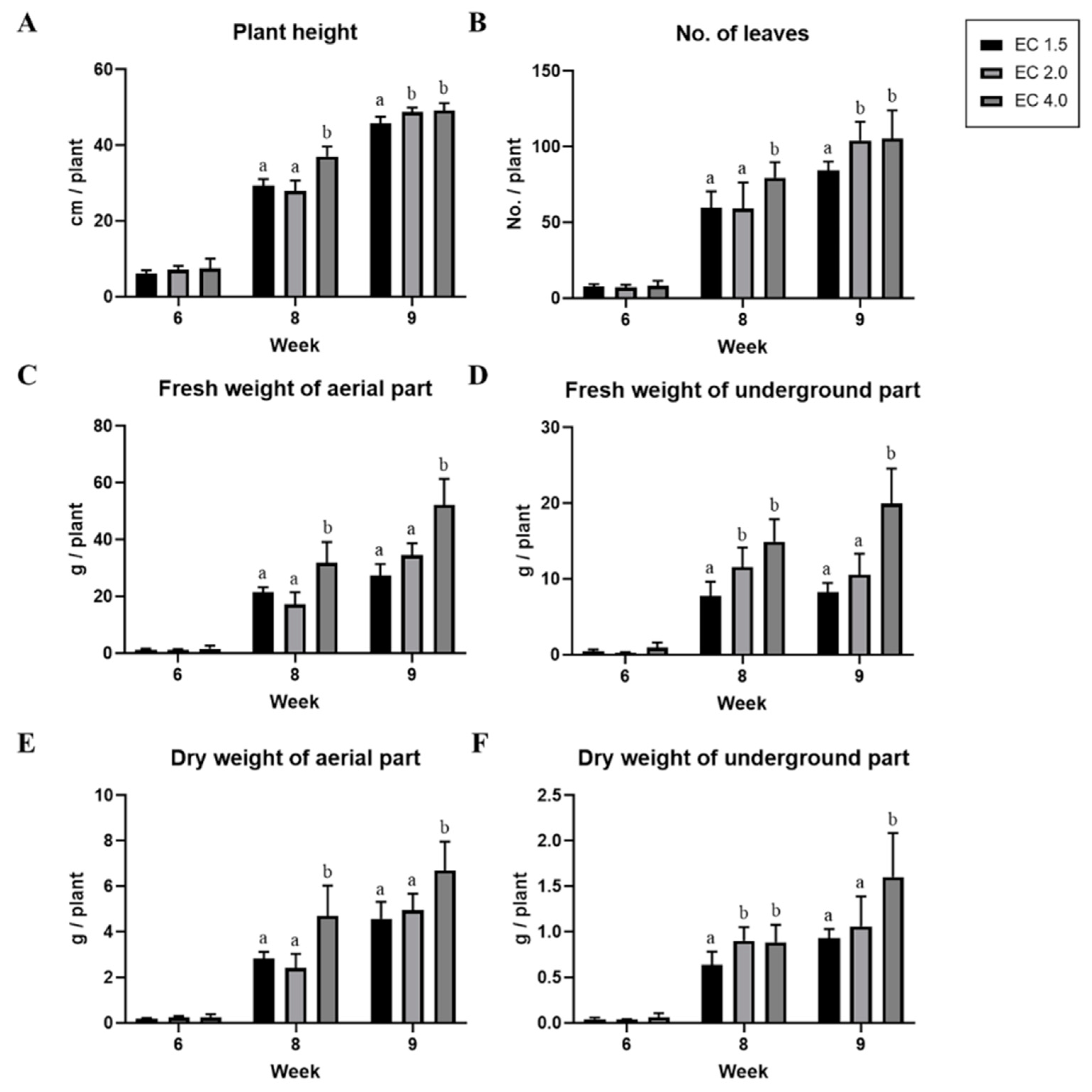
3.1. Effect of EC Condition on Growth Parameters and A. rugosa Yield
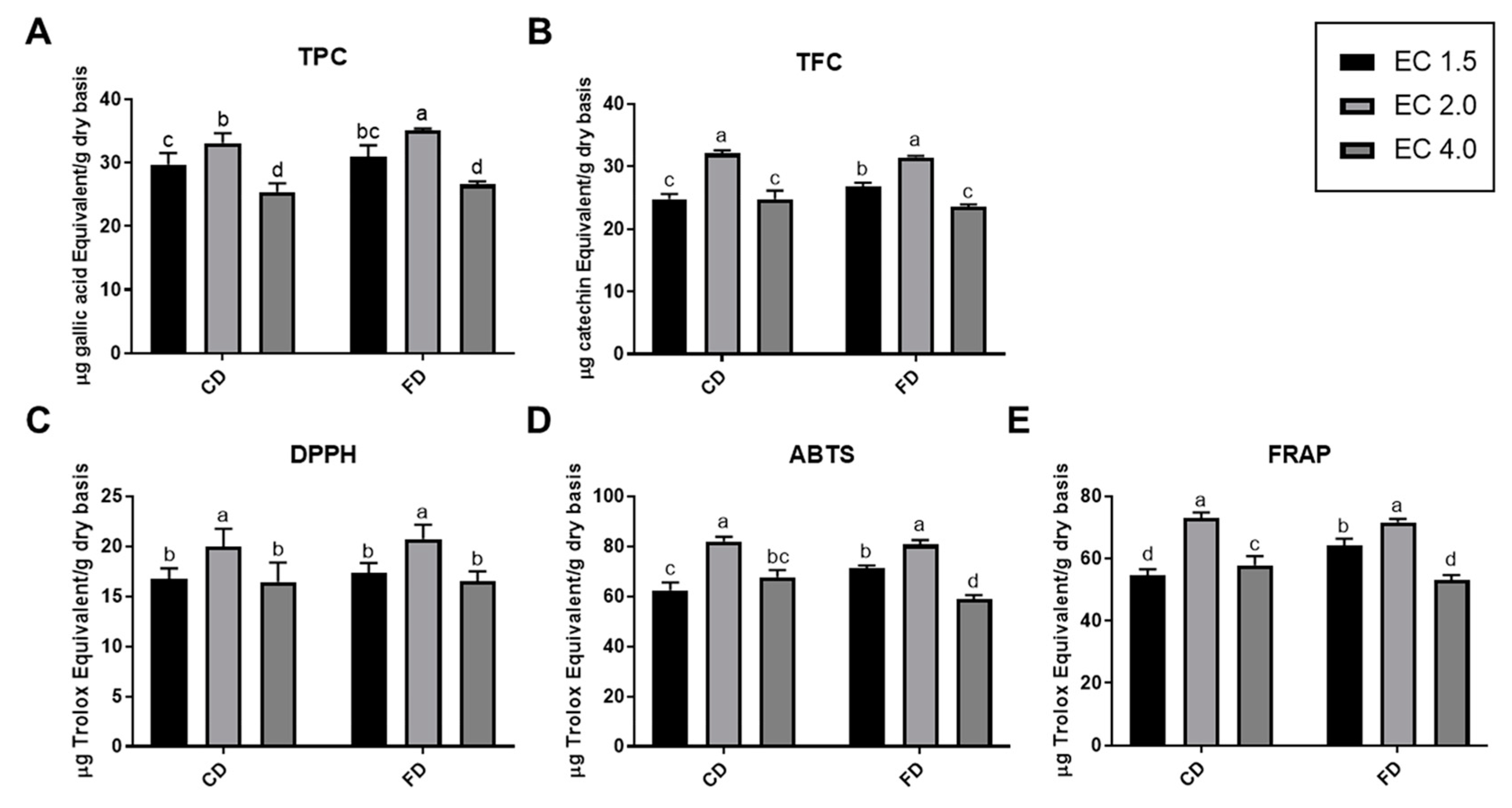
3.2. EC Conditions Varied the A. rugosa Samples’ Antioxidative Properties
3.3. The Drying Method Changed RA, Tilianin, and Acacetin Content in Dried A. rugosa
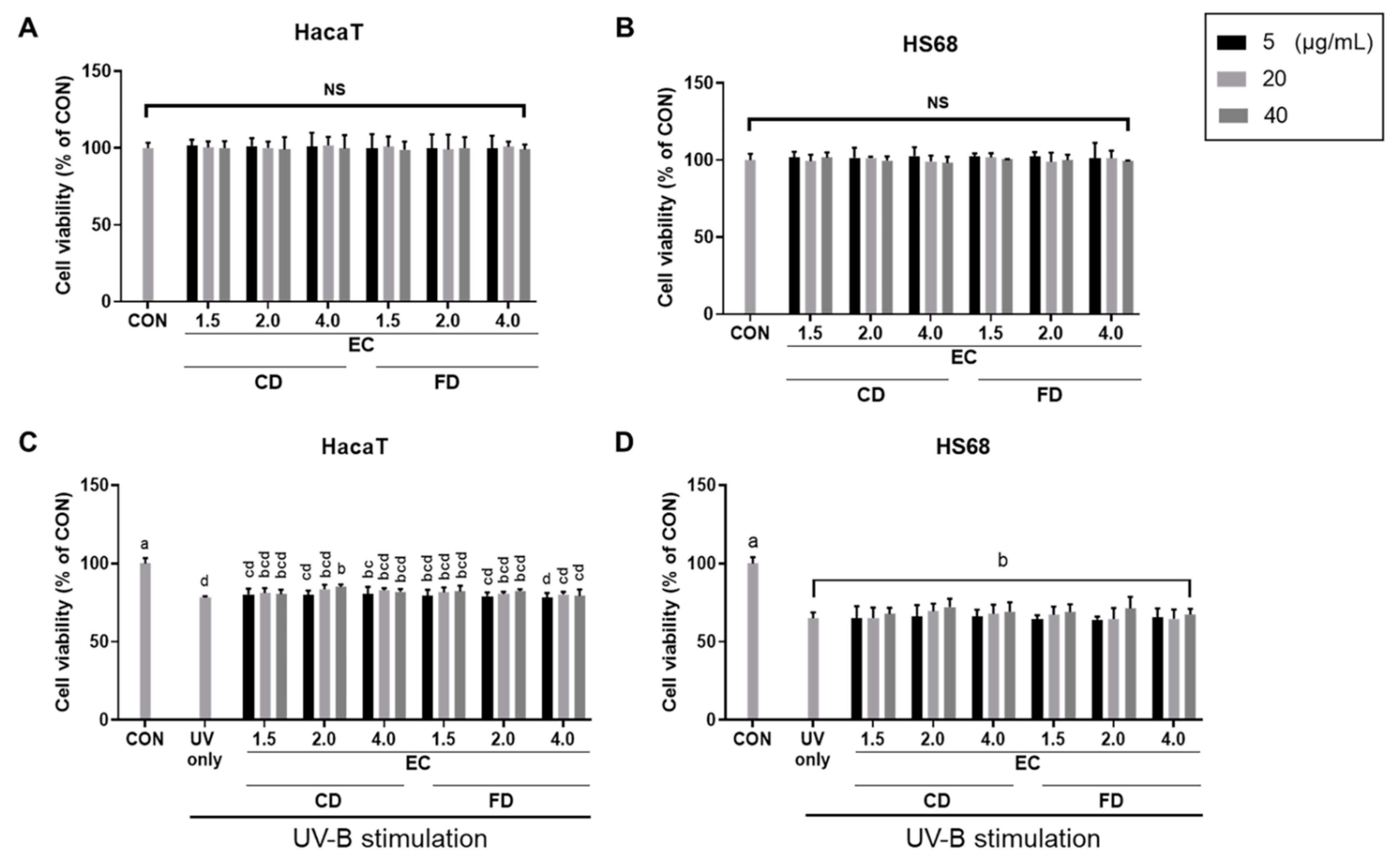
3.4. EC Conditions and Drying Methods Affect A. rugosa’s Photoprotective Effects
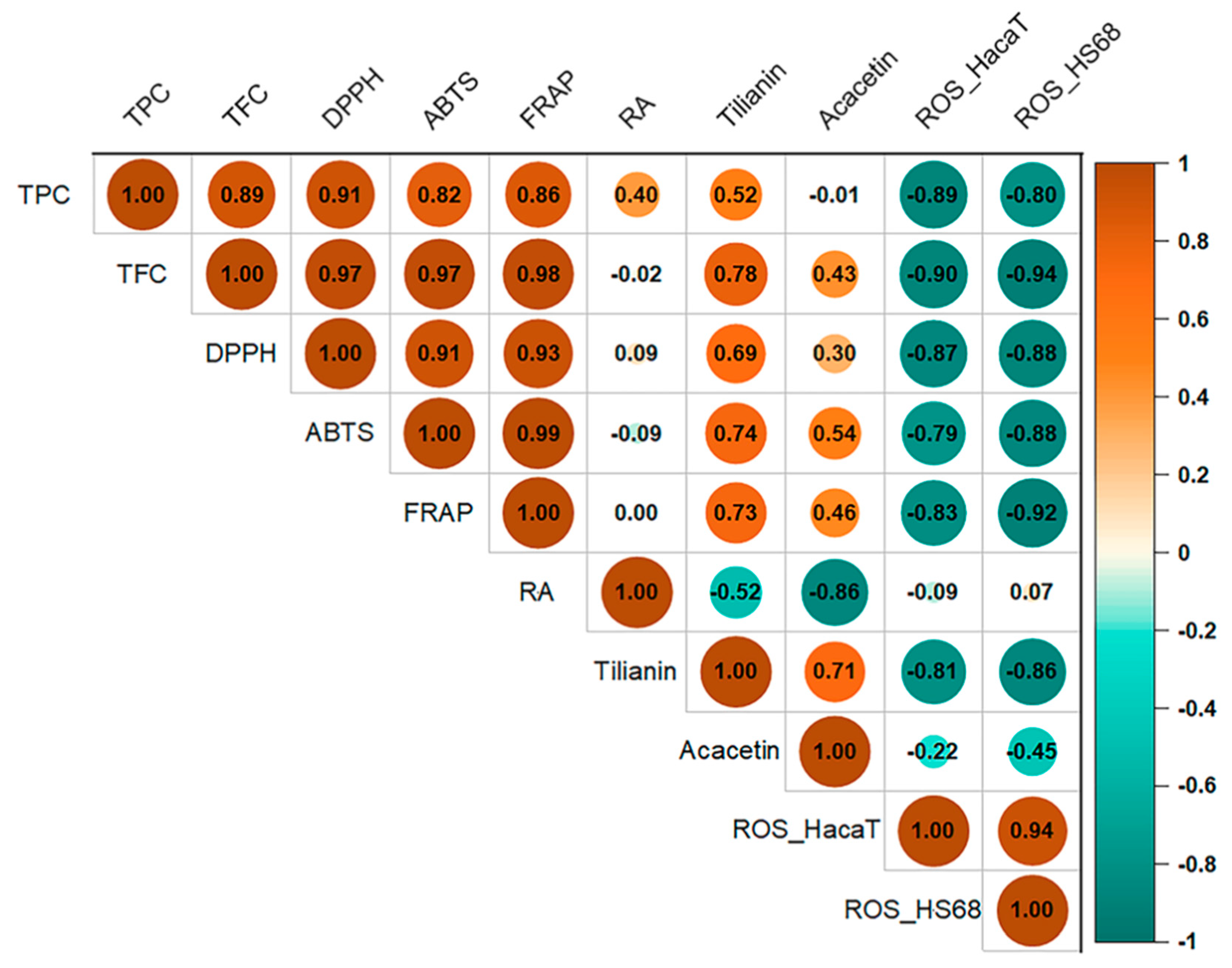
3.5. Correlations between A. rugosa Characteristics and PCA Plots
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Xu, H.; Kim, Y.K.; Jin, X.; Lee, S.Y.; Park, S.U. Rosmarinic acid biosynthesis in callus and cell cultures of Agastache rugosa Kuntze. J. Med. Plants Res. 2008, 2, 237–241. [Google Scholar]
- Desta, K.T.; Kim, G.S.; Kim, Y.H.; Lee, W.S.; Lee, S.J.; Jin, J.S.; Abd El-Aty, A.; Shin, H.C.; Shim, J.H.; Shin, S.C. The polyphenolic profiles and antioxidant effects of Agastache rugosa Kuntze (Banga) flower, leaf, stem and root. Biomed. Chromatogr. 2016, 30, 225–231. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.; Lee, J.-H.; Park, S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, H.-W.; Huang, Y.-H.; Kwon, H.-S.; Jin, C.D.; Kim, K.; Lim, C.-J. Attenuating properties of Agastache rugosa leaf extract against ultraviolet-B-induced photoaging via up-regulating glutathione and superoxide dismutase in a human keratinocyte cell line. J. Photochem. Photobiol. B Biol. 2016, 163, 170–176. [Google Scholar] [CrossRef]
- Hong, J.-J.; Choi, J.-H.; Oh, S.-R.; Lee, H.-K.; Park, J.-H.; Lee, K.-Y.; Kim, J.-J.; Jeong, T.-S.; Oh, G.T. Inhibition of cytokine-induced vascular cell adhesion molecule-1 expression; possible mechanism for anti-atherogenic effect of Agastache rugosa. FEBS Lett. 2001, 495, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Haiyan, G.; Lijuan, H.; Shaoyu, L.; Chen, Z.; Ashraf, M.A. Antimicrobial, antibiofilm and antitumor activities of essential oil of Agastache rugosa from Xinjiang, China. Saudi J. Biol. Sci. 2016, 23, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, H.-K.; Shin, C.-G.; Huh, H. HIV integrase inhibitory activity of Agastache rugosa. Arch. Pharmacal Res. 1999, 22, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.-J.; Chung, M.J.; Dawson, K.; Rodriguez, R.L.; Houng, S.-J.; Cho, S.-Y.; Jeun, J.; Kim, J.-Y.; Kim, K.H.; Park, K.W. Nutrigenomic analysis of hypolipidemic effects of Agastache rugosa essential oils in HepG2 cells and C57BL/6 mice. Food Sci. Biotechnol. 2010, 19, 219–227. [Google Scholar] [CrossRef]
- Oh, H.M.; Kang, Y.J.; Lee, Y.S.; Park, M.K.; Kim, S.H.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Protein kinase G-dependent heme oxygenase-1 induction by Agastache rugosa leaf extract protects RAW264. 7 cells from hydrogen peroxide-induced injury. J. Ethnopharmacol. 2006, 103, 229–235. [Google Scholar] [CrossRef]
- Hong, S.; Cha, K.H.; Son, Y.J.; Kim, S.M.; Choi, J.-H.; Yoo, G.; Nho, C.W. Agastache rugosa ethanol extract suppresses bone loss via induction of osteoblast differentiation with alteration of gut microbiota. Phytomedicine 2021, 84, 153517. [Google Scholar] [CrossRef]
- Shin, S.; Kang, C.A. Antifungal activity of the essential oil of Agastache rugosa Kuntze and its synergism with ketoconazole. Lett. Appl. Microbiol. 2003, 36, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.-S.; Kim, C.; Hwang, J.-K. Agastache rugosa Kuntze Attenuates UVB-Induced Photoaging in Hairless Mice through the Regulation of MAPK/AP-1 and TGF-β Smad Pathways. J. Microbiol. Biotechnol. 2019, 29, 1349–1360. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, H.-W.; Ryu, I.W.; Huang, Y.-H.; Park, M.; Chi, Y.M.; Lim, C.-J. Anti-Inflammatory, Barrier-Protective, and Antiwrinkle Properties of Agastache rugosa Kuntze in Human Epidermal Keratinocytes. BioMed Res. Int. 2020, 2020, 1759067. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Lee, Y.; Huang, Y.-H.; Lim, H.-W.; Jang, K.; Kim, D.-D.; Lim, C.-J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kim, C.; Kim, M.-B.; Hwang, J.-K. Anti-photoaging effect of Korean mint (Agastache rugosa Kuntze) extract on UVB-irradiated human dermal fibroblasts. Prev. Nutr. Food Sci. 2019, 24, 442. [Google Scholar] [CrossRef] [PubMed]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Jeong, E.H.; Yang, H.; Kim, J.-E.; Lee, K.W. Safflower Seed Oil and Its Active Compound Acacetin Inhibit UVB-Induced Skin Photoaging. J. Microbiol. Biotechnol. 2020, 30, 1567–1573. [Google Scholar] [CrossRef]
- Mu, J.; Chen, H.; Ye, M.; Zhang, X.; Ma, H. Acacetin resists UVA photoaging by mediating the SIRT3/ROS/MAPKs pathway. J. Cell. Mol. Med. 2022, 26, 4624–4628. [Google Scholar] [CrossRef]
- Park, W.T.; Arasu, M.V.; Al-Dhabi, N.A.; Yeo, S.K.; Jeon, J.; Park, J.S.; Lee, S.Y.; Park, S.U. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agastache rugosa cell culture. Molecules 2016, 21, 426. [Google Scholar] [CrossRef]
- Zielińska, S.; Dryś, A.; Piątczak, E.; Kolniak-Ostek, J.; Podgórska, M.; Oszmiański, J.; Matkowski, A. Effect of LED illumination and amino acid supplementation on phenolic compounds profile in Agastache rugosa in vitro cultures. Phytochem. Lett. 2019, 31, 12–19. [Google Scholar] [CrossRef]
- Zielińska, S.; Piątczak, E.; Kalemba, D.; Matkowski, A. Influence of plant growth regulators on volatiles produced by in vitro grown shoots of Agastache rugosa (Fischer & C.A.Meyer) O. Kuntze. Plant Cell Tissue Organ Cult. PCTOC 2011, 107, 161. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, J.H.; Baek, H.H.; Lee, H.J. Volatile flavour compounds in suspension culture of Agastache rugosa Kuntze (Korean mint). J. Sci. Food Agric. 2001, 81, 569–575. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Wu, K.; Pan, L.; Tang, Z.-H. Comparative analysis of metabolite profiles from Panax herbs in specific tissues and cultivation conditions reveals the strategy of accumulation. J. Pharm. Biomed. Anal. 2020, 188, 113368. [Google Scholar] [CrossRef] [PubMed]
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Son, Y.-J.; Choi, S.Y.; Hwang, I.-K.; Nho, C.W.; Kim, S.H. Could Defatted Mealworm (Tenebrio molitor) and Mealworm Oil Be Used as Food Ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Li, Y.L.; Stanghellini, C. Analysis of the effect of EC and potential transpiration on vegetative growth of tomato. Sci. Hortic. 2001, 89, 9–21. [Google Scholar] [CrossRef]
- Auerswald, H.; Schwarz, D.; Kornelson, C.; Krumbein, A.; Brückner, B. Sensory analysis, sugar and acid content of tomato at different EC values of the nutrient solution. Sci. Hortic. 1999, 82, 227–242. [Google Scholar] [CrossRef]
- Wu, M.; Kubota, C. Effects of high electrical conductivity of nutrient solution and its application timing on lycopene, chlorophyll and sugar concentrations of hydroponic tomatoes during ripening. Sci. Hortic. 2008, 116, 122–129. [Google Scholar] [CrossRef]
- Degl’Innocenti, E.; Hafsi, C.; Guidi, L.; Navari-Izzo, F. The effect of salinity on photosynthetic activity in potassium-deficient barley species. J. Plant Physiol. 2009, 166, 1968–1981. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the electrical conductivity of a nutrient solution for plant growth and bioactive compounds of Agastache rugosa in a plant factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef]
- Hoekenga, O.A. Using metabolomics to estimate unintended effects in transgenic crop plants: Problems, promises, and opportunities. J. Biomol. Tech. JBT 2008, 19, 159. [Google Scholar]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s wort. Plant Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Q.; Liu, L.; Liao, L.; Zhu, Z. Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J. Med. Plants Res. 2011, 5, 1749–1755. [Google Scholar]
- Stewart, A.J.; Chapman, W.; Jenkins, G.I.; Graham, I.; Martin, T.; Crozier, A. The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant Cell Environ. 2001, 24, 1189–1197. [Google Scholar] [CrossRef]
- Tuan, P.A.; Park, W.T.; Xu, H.; Park, N.I.; Park, S.U. Accumulation of Tilianin and Rosmarinic Acid and Expression of Phenylpropanoid Biosynthetic Genes in Agastache rugosa. J. Agric. Food Chem. 2012, 60, 5945–5951. [Google Scholar] [CrossRef]
- Akagi, T.; Ikegami, A.; Suzuki, Y.; Yoshida, J.; Yamada, M.; Sato, A.; Yonemori, K. Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit. Planta 2009, 230, 899–915. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel Drying Techniques for the Food Industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Park, C.; Chung, Y.S.; Park, S.U. The Effect of Different Drying Methods on Primary and Secondary Metabolites in Korean Mint Flower. Agronomy 2021, 11, 698. [Google Scholar] [CrossRef]
- Das, A.; Raychaudhuri, U.; Chakraborty, R. Effect of freeze drying and oven drying on antioxidant properties of fresh wheatgrass. Int. J. Food Sci. Nutr. 2012, 63, 718–721. [Google Scholar] [CrossRef]
- Najjaa, H.; Chekki, R.; Elfalleh, W.; Tlili, H.; Jaballah, S.; Bouzouita, N. Freeze-dried, oven-dried, and microencapsulation of essential oil from Allium sativum as potential preservative agents of minced meat. Food Sci. Nutr. 2020, 8, 1995–2003. [Google Scholar] [CrossRef]
- Song, J.-H.; Kim, M.-J.; Kwon, H.-D.; Park, I.-H. Antimicrobial activity and components of extracts from Agastache rugosa during growth period. Prev. Nutr. Food Sci. 2001, 6, 10–15. [Google Scholar]
- Lee, T.H.; Park, S.; Yoo, G.; Jang, C.; Kim, M.-h.; Kim, S.H.; Kim, S.Y. Demethyleugenol β-glucopyranoside isolated from Agastache rugosa decreases melanin synthesis via down-regulation of MITF and SOX9. J. Agric. Food Chem. 2016, 64, 7733–7742. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin—A simple flavone exhibiting diverse pharmacological activities. Phytochem. Lett. 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Akanda, M.R.; Uddin, M.N.; Kim, I.-S.; Ahn, D.; Tae, H.-J.; Park, B.-Y. The biological and pharmacological roles of polyphenol flavonoid tilianin. Eur. J. Pharmacol. 2019, 842, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Ryu, H.W.; Baek, S.C.; Kang, M.-G.; Park, D.; Han, H.-Y.; An, J.H.; Oh, S.-R.; Kim, H. Potent inhibitions of monoamine oxidase A and B by acacetin and its 7-O-(6-O-malonylglucoside) derivative from Agastache rugosa. Int. J. Biol. Macromol. 2017, 104, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Cao, W.; Yue, R.; Yuan, Y.; Guo, X.; Qin, D.; Xing, J.; Wang, X. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J. Pharmacol. Sci. 2019, 139, 352–360. [Google Scholar] [CrossRef] [PubMed]


| Sample | Rosmarinic Acid (mg/g, DW) | Tilianin (mg/g, DW) | Acacetin (mg/g, DW) |
|---|---|---|---|
| CD_1.5 (1) | 3.20 ± 0.83 b (2) | 3.83 ± 1.23 a | 0.02 ± 0.05 a |
| CD_2.0 | 0.61 ± 0.23 a | 9.10 ± 1.22 b | 0.98 ± 0.46 b |
| CD_4.0 | 0.09 ± 0.01 a | 4.01 ± 3.13 a | 0.87 ± 0.72 b |
| FD_1.5 | 4.50 ± 0.61 c | 3.30 ± 2.10 a | 0.20 ± 0.10 a |
| FD_2.0 | 4.18 ± 1.22 c | 4.86 ± 2.57 a | 0.29 ± 0.21 a |
| FD_4.0 | 3.00 ± 0.37 b | 3.17 ± 2.37 a | 0.12 ± 0.06 a |
| Sample Condition | Sample Concentration (μg/mL) | ROS Content (Fold) | |
|---|---|---|---|
| Drying Method | EC | ||
| Control cell (UV-B only) | - | 34.29 ± 1.95 a | |
| Cold air drying | 1.5 | 5 | 32.39 ± 2.20 abc |
| 20 | 31.38 ± 1.62 abcde | ||
| 40 | 28.55 ± 1.74 efg | ||
| 2.0 | 5 | 31.83 ± 0.83 abcd | |
| 20 | 28.48 ± 1.60 efg | ||
| 40 | 26.68 ± 1.78 g | ||
| 4.0 | 5 | 32.34 ± 1.17 abc | |
| 20 | 31.28 ± 2.07 bcde | ||
| 40 | 29.85 ± 2.06 cdef | ||
| Freeze drying | 1.5 | 5 | 33.52 ± 1.46 ab |
| 20 | 31.42 ± 2.36 abcde | ||
| 40 | 28.53 ± 2.02 efg | ||
| 2.0 | 5 | 32.57 ± 1.85 abc | |
| 20 | 29.89 ± 1.49 cdef | ||
| 40 | 27.57 ± 2.02 fg | ||
| 4.0 | 5 | 33.50 ± 2.06 ab | |
| 20 | 31.84 ± 1.55 abcd | ||
| 40 | 29.11 ± 1.49 defg | ||
| Sample Condition | Sample Concentration (μg/mL) | ROS Content (Fold) | |
|---|---|---|---|
| Drying Method | EC | ||
| Control cell (UV-B only) | - | 30.22 ± 1.44 a | |
| Cold air drying | 1.5 | 5 | 29.69 ± 1.75 abc |
| 20 | 28.44 ± 1.98 abcde | ||
| 40 | 28.81 ± 2.67 abcd | ||
| 2.0 | 5 | 28.54 ± 0.98 abcde | |
| 20 | 26.37 ± 1.46 ef | ||
| 40 | 25.67 ± 2.07 f | ||
| 4.0 | 5 | 30.49 ± 0.31 a | |
| 20 | 28.99 ± 1.39 abcd | ||
| 40 | 28.96 ± 0.83 abcd | ||
| Freeze drying | 1.5 | 5 | 29.13 ± 0.79 abcd |
| 20 | 28.56 ± 0.34 abcde | ||
| 40 | 27.80 ± 0.76 bcdef | ||
| 2.0 | 5 | 30.35 ± 1.26 a | |
| 20 | 27.45 ± 0.79 cdef | ||
| 40 | 26.96 ± 0.74 def | ||
| 4.0 | 5 | 29.81 ± 0.56 ab | |
| 20 | 28.39 ± 1.18 abcde | ||
| 40 | 28.53 ± 1.78 abcde | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, Y.-J.; Park, J.-E.; Nho, C.W. The Electrical Conductivity and Drying Method Changed the Secondary Metabolite Content and Photoprotective Effects of Hydroponically Cultivated Agastache rugosa Kuntze. Agronomy 2023, 13, 2165. https://doi.org/10.3390/agronomy13082165
Son Y-J, Park J-E, Nho CW. The Electrical Conductivity and Drying Method Changed the Secondary Metabolite Content and Photoprotective Effects of Hydroponically Cultivated Agastache rugosa Kuntze. Agronomy. 2023; 13(8):2165. https://doi.org/10.3390/agronomy13082165
Chicago/Turabian StyleSon, Yang-Ju, Jai-Eok Park, and Chu Won Nho. 2023. "The Electrical Conductivity and Drying Method Changed the Secondary Metabolite Content and Photoprotective Effects of Hydroponically Cultivated Agastache rugosa Kuntze" Agronomy 13, no. 8: 2165. https://doi.org/10.3390/agronomy13082165
APA StyleSon, Y.-J., Park, J.-E., & Nho, C. W. (2023). The Electrical Conductivity and Drying Method Changed the Secondary Metabolite Content and Photoprotective Effects of Hydroponically Cultivated Agastache rugosa Kuntze. Agronomy, 13(8), 2165. https://doi.org/10.3390/agronomy13082165






