Abstract
The steadfast propensity to global warming has had a severe impact on overall viticulture. Given the observed increase in growing season temperatures in Europe (+1.7 °C from 1950 to 2004), between 2000 and 2049, it is assumed that temperatures for major wine regions will increase on average by about +0.42 °C per decade and will generally increase by +2.04 °C. Phenolic compound development is affected by environmental parameters such as ultraviolet (UV) radiation, sunlight, maximum and minimum temperatures, and grapevine water status. Proanthocyanidins, flavan-3-ol monomers, and other pigmented polymers are impacted by soil management and canopy handling strategies, as well as obtaining a microclimate around the developing bunch. This review, after a necessary summary of the synthesis of phenolic compounds in the berry (flavonoids and non-flavonoids) to let the lector delve into the topic, describes the impact of climate change and therefore of environmental factors on their accumulation and storage throughout ripening and harvesting. For example, high berry temperatures can reduce the total concentrations of skin anthocyanin; a 35 °C temperature entirely obstructed anthocyanin synthesis, and instead quercetin 3-glucoside could be enhanced with exposure to solar radiation. In addition, increments via water deficit in the relative abundance of methoxylated anthocyanins were also found. The vineyard management strategies to mitigate the degradation of phenolic compounds and preserve their concentration are also further discussed. Finally, it is believed that it is necessary today to establish an elastic and variable approach towards the single wine year, moving away from the concept of product standardization.
1. Introduction
In climatology, global warming or climate change indicates the alteration in the earth’s climate that developed from the end of the 19th century to the beginning of the 20th century and is still ongoing [1]; it is generally represented by inflation in the global average temperature and associated atmospheric phenomena (e.g., drought [2], heatwaves [3], increase in extreme phenomena linked to the water cycle such as floods, desertification [4], melting ice, rising ocean levels [5], and changes to atmospheric circulation patterns with storms, hurricanes, and phenomena that are more intense such as cyclones [6]). On one hand, the anthropical increase in the amount of greenhouse gases (GHGs) such as ozone (O3), water vapor (H2O), methane (CH4), carbon dioxide (CO2), chlorofluorocarbons (CFCs), and nitrous oxide (N2O) in the atmosphere and, on the other, the natural variation in the incident solar radiation due to the Milankovitch cycle, have led to these dangerous phenomena [7,8,9]. By the end of the 21st century, the global temperatures under different scenarios (i.e., global climate models characterized by the representative concentration pathways and a set of alternative plausible trajectories of future global development (SSPs)) are projected to increment by +1.18 °C/100 years. (SSP1 2.6), +3.22 °C/100 yearrs. (SSP2 4.5), +5.50 °C/100 years. (SSP3 7.0), and +7.20 °C/100 years. (SSP5 8.5), with greater warming oriented over the Northern hemisphere’s high latitudes and feebler warming over the Southern hemisphere [10].
The steadfast propensity to global warming has had a severe impact on overall viticulture [11]. Given the observed increase in growing season temperatures in Europe (+1.7 °C from 1950 to 2004), between 2000 and 2049, it is assumed that temperatures for major wine regions will increase on average by about +0.42 °C per decade and will generally increase by +2.04 °C [12,13]. Districts usually entertained as highly desirable for grapevine growing can now show an excess of heat load when confronted with the needs of the currently grown selective breeding [14]. In these areas, global warming could randomize the appropriateness of wine growing and synchronously lead to a switch in grape cultivars’ cultivation, creating extensive damage to wineries subject to production regulations [15].
The typical microclimate of the area is fundamental for characterizing the particular style of wine produced in very specific areas [16]. Obtaining the complete and balanced ripening of the bunch is essential for determining the best time for harvesting and this is highly influenced by climatic variability which poses new challenges for winemakers from year to year [17]. Several models have predicted that all assessed grape phenological phases will undergo anticipation that will be significant for the véraison and the maturity stages (until 15 days before) with respect to the flowering phase [18]. All this can translate into a series of problems in the berry, such as the presence of unbalanced qualitative parameters [19]. In fact, the worsening of drought can lead to an excess of the sugar content of the berry and a 40% reduction in production [20]. Owing to a loss in membrane stability of Oenococcus oeni cells, caused by high alcohol content, malolactic fermentation can negatively be triggered (i.e., undesirable sensory alteration and lag in wine stabilization) [21,22]. In addition, an excess of total soluble solids leads to the formation of undesirable fermentation by-products (e.g., acetic acid and glycerol) with up-regulating glycolytic and pentose phosphate pathway genes [23].
Phenolic compound development is affected by genetic factors as well as environmental parameters such as ultraviolet (UV) radiation, sunlight, maximum and minimum temperatures, and grapevine water status [24,25,26]. The berry composition (i.e., total soluble solids, proanthocyanidins, flavan-3-ol monomers, and pigmented polymers; [27]) is impacted by cultivation practices (soil management and canopy handling strategies) and the existing microclimate around the developing vines [28,29,30].
This review, after a summary of the synthesis of phenolic compounds in the berry (flavonoids and non-flavonoids), describes the impact of climate change and therefore of environmental elements (such as temperature, water, solar radiation, and UV-B light) on the berries accumulation and storage throughout ripening and harvesting. The soil and canopy management techniques achieved in the field to mitigate the degradation of phenolic compounds and preserve their concentration are also further discussed.
2. Phenolic Compounds Classification
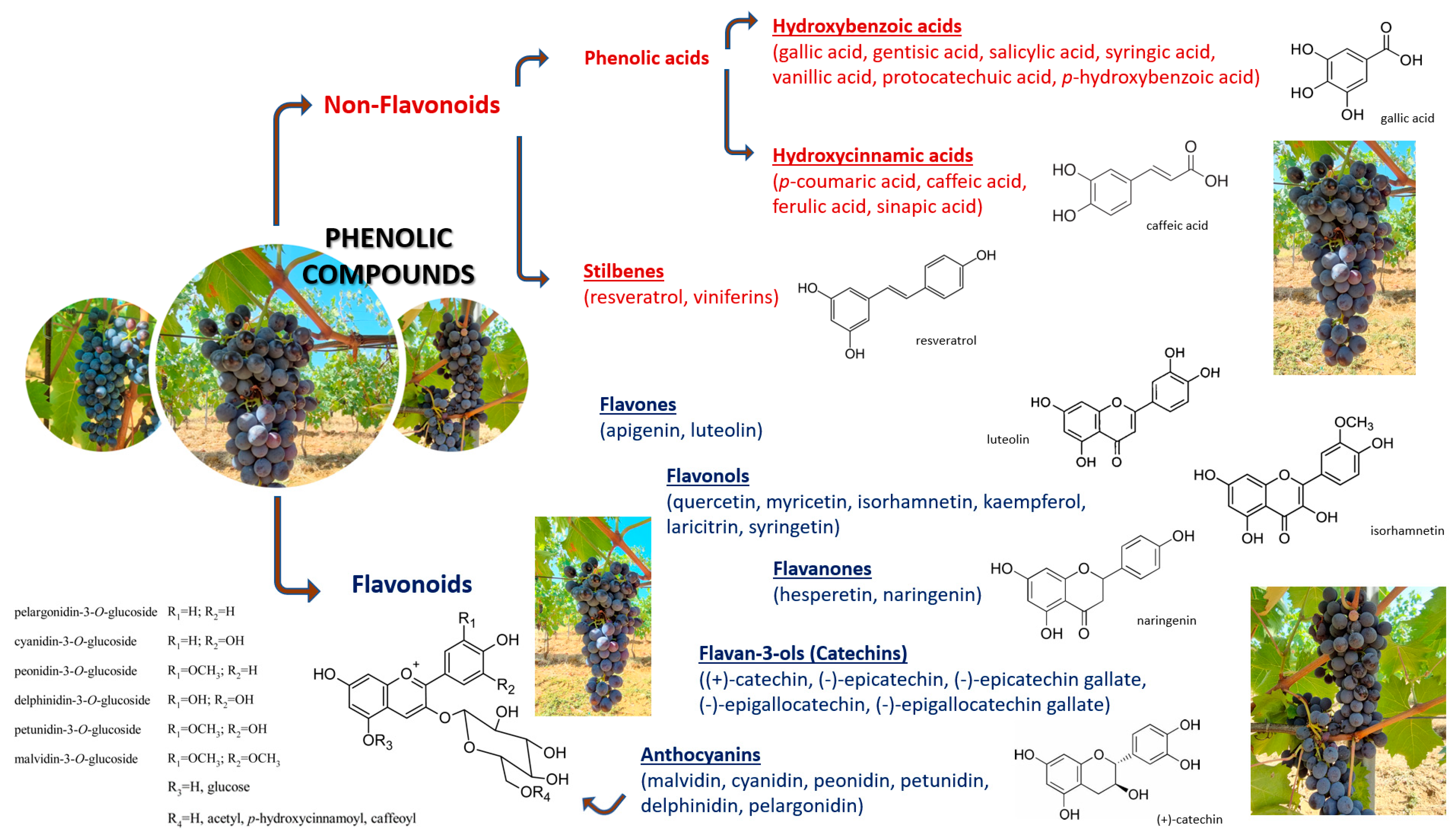
All phenolic compounds (PhCs), a large group of the secondary metabolites, hold at least one aromatic ring with one hydroxyl group (−OH functional group composed of one oxygen atom covalently bonded to one hydrogen atom) in their structure [31]. In plant organs or vegetable tissues such as fruits, leaves, seeds, stems, and roots there are more than 8000 PhCs, with huge structural variability [32]. In general, plant phenolic compounds are judged to have a key task as plant defenses (antioxidants, UV filters, etc.) when environmental stresses, pathogen contagion, or herbivore attacks can lead to strengthened development of free radicals and other oxidative species in several parts of the plant [24,26].
Grapevine berry phenolics cooperate with cultivar organoleptic features and skin color; in addition, they have a role as protection against pathogens and environmental damage [33]. There is no failing to understand that wine is a hugely complex product and that PhCs exhibit an essential role in its final taste and sensory properties [34]. Each group can be present in free or conjugated forms, with disparate hydroxylation levels and substitutions [35]. Briefly, anthocyanins are chiefly present in skins while flavan-3-ols and condensed tannins are principally present in seeds and skins; these PhCs are the most plentiful in berries. Anthocyanins’ profile can be exploited as an analytical tool for variety authenticity certification; they are responsible for the color of red wine [36]. Flavan-3-oils (catechins) and condensed tannins are involved in color stabilization and astringency [31]. Moreover, flavonols and hydroxycinnamic acids are mostly known for behaving as co-pigments [37].
The wide chemical diversity of polyphenols in berries (Vitis vinifera L.) can be explained because each group can be attendant in their free or conjugated forms, differing by their hydroxylation level and by the substitution of the hydroxy groups (i.e., glycosylation, methylation, or acylation) and even establishing adducts between them (for example condensed tannins; phenolic acids with anthocyanins) [38,39].
PhCs are usually pigeonholed into two master groups: flavonoids and nonflavonoids (Figure 1) [40].
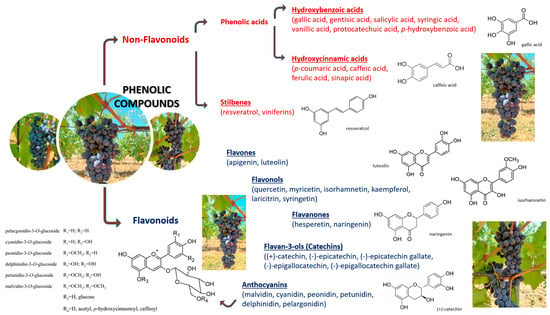
Figure 1.
Classification of phenolic compounds [41,42,43,44].
Flavonoids, with a C6-C3-C6 structure [45], encompass a phenyl benzopyran skeleton [46]: two phenyl rings joined through a heterocyclic pyran ring; based on divergences in the pyran ring, they can be divided into families (i.e., major flavonoid types: flavanones, flavonols, flavones, anthocyanidins, and catechins [47]). In each group, individual compounds differ in their layout of hydroxylation and methylation of two phenyl rings [48].
Well-known examples from the flavanones class (also called 2,3-dihydroxyflavones) include hesperetin, eriodictyol, and naringenin [49]. In addition, taxifolin, eriodictyol-glucoside, and taxifolin-pentoside were identified in grape skins by HPLC–DAD–ESI-MSn [50]. The flavanone structure is characterized by a benzopyranone core substituted at the C2 position with possible substitution on the aryl backbone of the benzopyranone core [51]. They lack the double bond between carbons 2 and 3 in the C-ring of the flavonoid skeleton, which is present in flavones and flavonols. Flavanones are generally glycosylated by glucoside or disaccharide at the 7 position to give flavanone glycosides [52]. In summary, the following eight flavanones have been documented in Vitis vinifera: taxifolin, taxifolin-O-pentoside, taxifolin-3-O-glucoside, taxifolin-3-O-rhamnoside, hesperetin, eriodictyol-7-O-glucoside, naringenin, and naringenin-7-O-glucoside [53,54]. They are the direct precursors of the vast majority of flavonoids and are synthesized from the amino acid, phenylalanine [55].
Flavonols are characterized by a 3-hydroxyflavone backbone. In the grape’s berry are synthesized kaempferol, quercetin, myricetin, and their methylated forms isorhamnetin, laricitrin, and syringetin [43]. They are normally found as glucosides, galactosides, rhamnosides, and glucuronides [26]. In fact, in grapes, they only exist as 3-O-glycosides, while the corresponding free aglycones can be detected in wines together with the 3-O-glycosides, after acid hydrolysis that develops during winemaking and aging [56]. Flavonols, synthesized only in the skin, grasp the high point amount per berry a few weeks after véraison [57]. Kaempferol and quercetin type are present in both white and red grapes, and it was seen that myricetin and isorhamnetin type account for only red grapes (Vitis vinifera L.) [58]. Nevertheless, the presence of small amounts of isorhamnetin in white berries’ skins was recently noted [59], and myricetin was found in Vitis rotundifolia white berries [60].
Flavones represent the simplest category of the flavonoids class and consist of 4H-chromen-4-one bearing a phenyl substituent at position 2 [52]. The known examples from this class of flavonoids include luteolin and apigenin [61]. Apigenin presents a hydrogen bond between 5-OH and the 4-keto groups. In the luteolin molecule, all the most stable conformations are characterized by a hydrogen bond in the ortho-dihydroxy functionality and by another similar interaction as in apigenin [62,63]. Regarding luteolin accumulation in the skin of the berry, the Merlot grape cultivar exhibits a high luteolin concentration, with a fluctuation of 63.65–73.41 μg/g skin [64]. Subject matters of apigenin and luteolin both showed one accumulation apogee during grapevine berry development. Apigenin aglycone reached the highest level 80–90 days following full bloom; after, its content briskly declined (undetectable in ripe fruit). For luteolin, the accumulation began after véraison, followed by a peak at 95 days subsequent to full bloom; after, it remained at an elevated level in ripe fruit (slight shifts in its aglycone content were noted) [65].
Anthocyanins (C6-C3-C6 carbon skeleton) are synthesized in the epidermal cells (i.e., cytosol) with proanthocyanidins in the skin’s hypodermal stratum and garnered in the cellular vacuole [33]. Anthocyanins are glycosylated (mainly at the C3 position) analogs of anthocyanidins, both being based on the basic structure of the flavylium ion (2-phenyl-benzopyrylium chromophore) [66]. Anthocyanidins, not bringing the sugar part, present a semi-planar framework with a dihedral angle (<10°) between the B ring and the benzopyrylium [67]. As a rule, anthocyanidin glycosides are 3-monoglycosides and 3,5-diglycosides; notwithstanding xylose, arabinose, rhamnose, galactose, and rutinose could have some sugars attached, glucose is the most usual [68]. They represent the flavonoids’ group most embroiled in berry pigmentation (i.e., berries’ skin; red, purple, and blue colors) [69]. Odds in either the number/position of hydroxyl and/or methoxy groups and the differences in the structure/position of sugars stuck to the benzopyrilium skeleton cause a multitude of these compounds [70]. The berry anthocyanidins are peonidin, cyanidin, pelargonidin, malvidin, petunidin, and delphinidin [71,72]. Adopting spectrophotometric or HPLC methods, the anthocyanin layout and profile were analyzed by many authors in order to typify the cultivars or fix the grape’s origin [73]. Vitis vinifera L. cultivars usually show 3-mono-glucoside, 3-acetyl glucoside, and 3-p-coumarylglucoside (and to a lesser extent caffeoyl glucoside [74]) derivatives of the aglycones delphinidin, peonidin, petunidin, cyanidin, and malvidin [75]. Anthocyanins storage in berries’ skin begins at the véraison stage and it peaks at a maximum round at the harvest stage [76]. Some works portray a drop in total anthocyanin compounds just before vintage or during the over-ripening phase [77,78,79].
Flavan-3-ols, located in both the grape skin and the seed [80,81], are characterized by a 2-phenyl-3,4-dihydro-2H-chromene skeleton that is hydroxylated at position 3 of ring C [82]. Favan-3-ols have two chiral centers at positions 2 and 3 creating chiral diastereomers with four possible configurations: two enantiomers for each epimer [83]. Nowadays, the chemical structures of 11 monomeric flavan-3-ols are reported in grape seed:
- Most abundant; the monomeric flavan-3-ols (+)-catechin, (−)-epicatechin and (−)-epicatechin 3-O-gallate [80].
- Less abundant; the monomers (+)-catechin-3-O-gallate, (+)-gallocatechin, (−)-epigallocatechin, (+)-gallocatechin-3-O-gallate, (−)-epigallocatechin-3-O-gallate, and (−)-epigallocatechin-3-O-vanillate [84].
- The two preeminent glycosylated flavan-3-ols; (+)-catechin-4′-O-β-glucoside and (+)-catechin-7-O-β-glucoside; in fact, flavan-3-ols are found as sugar-linked molecules too, notwithstanding only as subordinate components in grape seed extracts [85].
According to some authors, there are 14 patterns of flavan-3-ol monoglycosides consisting of 4 aglycone units (i.e., (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, and (−)-epicatechin gallate), [86], but their structures were not settled. Briefly, grape seed flavan-3-ols are detected as monomers, dimers, oligomers (3 to 10 units), and polymers (more than 10 units = condensed tannins) [83]. The condensation of (+)-catechin or (−)-epicatechin units generates procyanidins (grape seeds) (known as proanthocyanidins or condensed polyphenols [87]), and gallocatechins generate prodelphinidins (grape skins) [88].
Nonflavonoid (distinguished by a simple C6 backbone) are tracked down in grapes in low concentrations (with the exception of hydroxycinnamic acids) [89,90]. They are classified into two families [91]: phenolic acids and stilbenes. Phenolic acids can be subdivided into two principal groups: hydroxybenzoic acids (C6-C1) and hydroxycinnamic acids (C6-C3) [92].
Several kinds of hydroxybenzoic acids were identified in grapes such as protocatechuic, gallic, syringic, and p-hydroxybenzoic acids [93]. Gallic acid is the precursor of hydrolyzable tannins [94]; the main steps of its transformation via 1-O-galloylglucose into a large range of more elaborate galloylglucoses and ellagitannins were studied [95]. In grapevine berries, gallic acid mainly accumulates as galloylated flavan-3-ols [96].
Grapevine berry mesocarp and exocarp tissues contain both gallic acids and the tartaric acid esters of coumaric, ferulic, and caffeic acid (such as caftaric acid), as well as the glucose esters of p-coumaric and ferulic acid [97]. Glucose esters have a role as activated intermediates in other phenolic compounds’ biosynthesis [98]. In fact, hydroxycinnamic acids are found in the form of tartaric esters [99]. At the level of the pulp, caffeoyl tartaric acid mainly prevails, while coumaroyl tartaric acid prevails in the skin. Caffeic, p-coumaric, and ferulic acids are present primarily as trans isomers, although traces of cis isomers were detected [33]. Their synthesis continues up to the green phase, then the contents of these acids undergo a slow decrease (e.g., an increase in the volume of the berry, degradation reactions, and their use as intermediates in the synthesis of other polyphenols) which continues until harvesting [100].
Stilbenes can be found within the berry as aglycones and mono glucosides; the stilbenes glucosylation protects them from enzymatic oxidation by polyphenol oxidases (hereby enhancing their half-life) [101].
3. Phenolic Compounds Biosynthesis
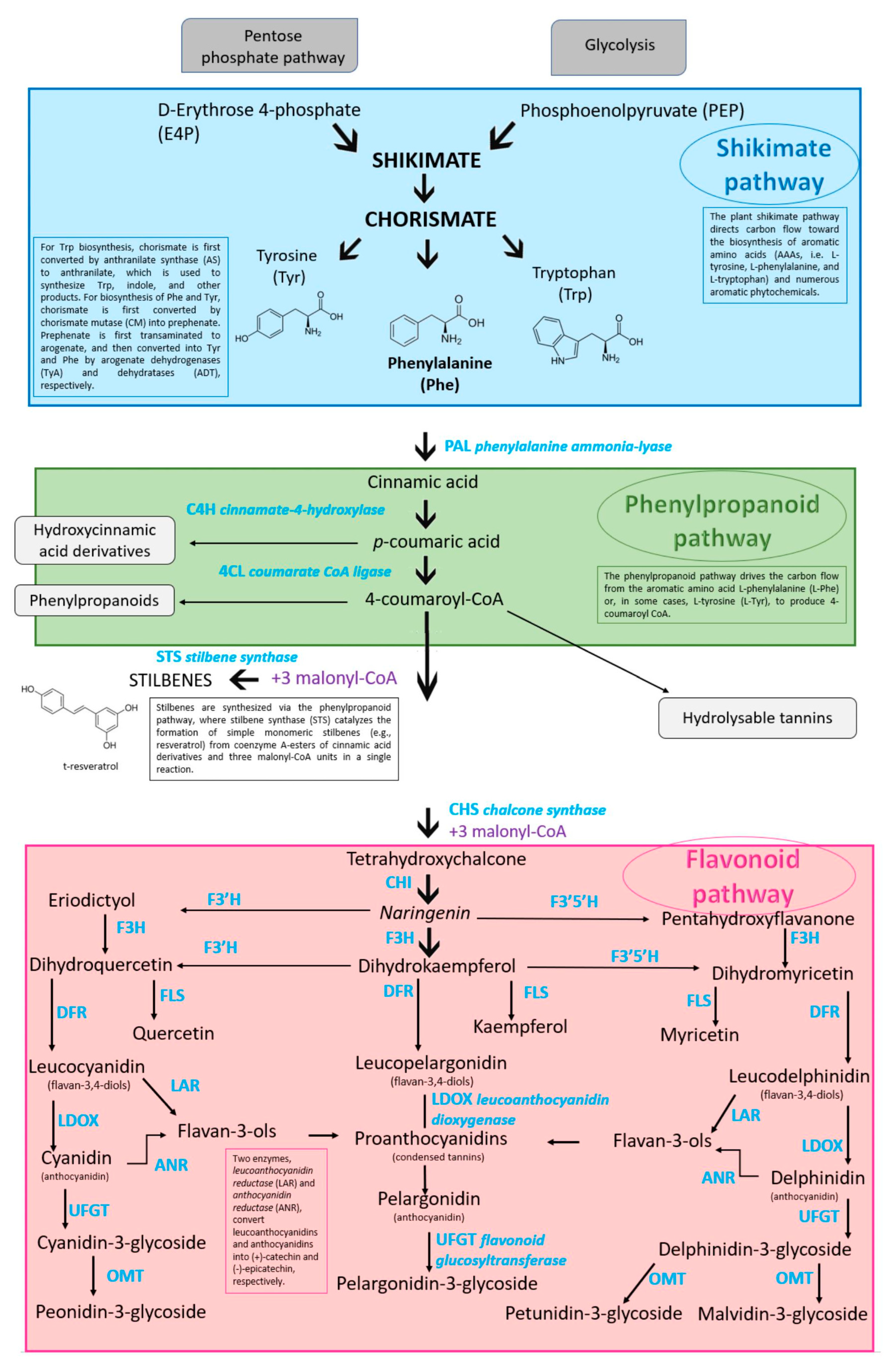
The biosynthetic pathways of phenolic compounds are schematically shown in Figure 2. In addition, the principal enzymes and intermediates driving the formation of phenols are shown in the same Figure. Nevertheless, more extensive discussions are supplied by other authors [54,102,103].
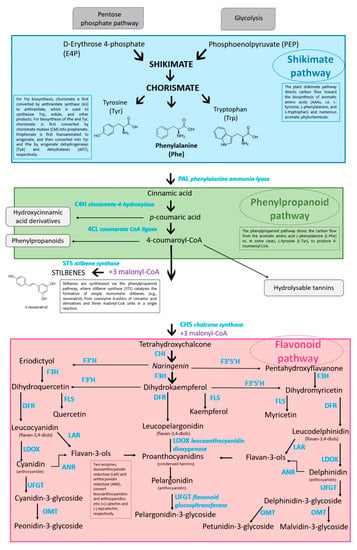
Figure 2.
Biosynthetic pathways of grapevine berry secondary compounds. Phenylalanine ammonia-lyase (PAL); cinnamate-4-hydroxylase (C4H); 4-coumaroyl: CoA-ligase (4CL); stilbene synthase (STS); chalcone synthase (CHS); chalcone isomerase (CHI); flavonoid 3′-hydroxylase (F3′H); flavonoid 3′,5′-hydroxylase (F3′5′H); flavanone-3-hydroxylase (F3H); flavonol synthase (FLS); dihydroflavonol reductase (DFR); leucoanthocyanidin reductase (LAR); anthocyanidin reductase (ANR); leucoanthocyanidin dioxygenase (LDOX); dihydroflavonol 4-reductase (DFR); flavonoid glucosyltransferase (UFGT); O-methyltransferase (OMT) (adapted from [33,104,105,106]).
The aromatic amino acid phenylalanine (C9H11NO2) is biosynthesized via the shikimate pathway that joins carbohydrate metabolism with the biosynthesis of secondary metabolites; all phenolic compounds are synthesized from this amino acid [43] (or from tyrosine, such as resveratrol [107]). The rate-limiting enzyme [108] of phenylpropanoid biosynthesis is the phenylalanine ammonia-lyase (PAL) which orders the flux through this pathway. Phe is converted to p-coumaroyl-CoA by PAL, C4H, and 4CL. The entry of p-coumaroyl-CoA (C30H42N7O18P3S) into the flavonoid biosynthesis pathway depicts the beginning of the synthesis of specific flavonoids; in fact, two major categories of compounds can be engendered (i.e., flavonoids by the CHS, chalcone synthase or naringenin-chalcone synthase enzyme and stilbenes by the STS, stilbene synthase enzyme) [109]. Flavonoid compounds arise from 4,2′,4′,6′ tetrahydroxychalcone (naringenin chalcone; C15H12O5). The dihydroflavonols DHK (dihydrokaempferol or aromodendrin), DHQ (dihydroquercetin or taxifolin), and DHM (dihydromyricetin or ampelopsin) are, respectively, changed into the flavonols kaempferol (C15H10O6), quercetin (C15H10O7), and myricetin (C15H10O8) by the flavonol synthase enzyme (FLS) [48]. The flavonoid pathway leads to the synthesis of different classes of metabolites such as flavan-3-ols, flavonols, procyanidins, and anthocyanidins. DFR (dihydroflavonol reductase), a NADPH-dependent reductase, is the significant enzyme in the anthocyanidin and proanthocyanidin pathway by forming a hydroxyl group at position C4 of ring C [110]. The flavan-3,4-ols (leucoanthocyanidins or flavan-diols) derive from reduction in dihydroflavonols [111] catalyzed by dihydroflavonol reductase; leucopelargonidin is generated from DHK, leucocyanidin is generated from DHQ, and leucodelphinidin is generated from DHM [112]. They are anthocyanidin and proanthocyanidin precursors [113]. The leucoanthocyanidin (leucocyanidin, leucopelargonidin, and leucodelphinidin) are colorless; during their pathway with the catalysis of ANS (anthocyanidin synthase), also called leucoanthocyanidin dioxygenase; LDOX), they are turned into the following colored anthocyanidins: cyanidin, pelargonidin, and delphinidin [113,114]. UFGT (UDP-glucose flavonoid 3-glucosyltransferase) turns into cyanidin-3-glucoside (C21H21O11; known as chrysanthemin), pelargonidin-3-glucoside (C21H21O10; known as callistephin), and delphinidin-3-glucoside (C21H21O12; known as myrtillin) the unstable anthocyanidins [115].
4. Climate Change and Climate Elements: Effects on the Phenolic Compounds of Grapes
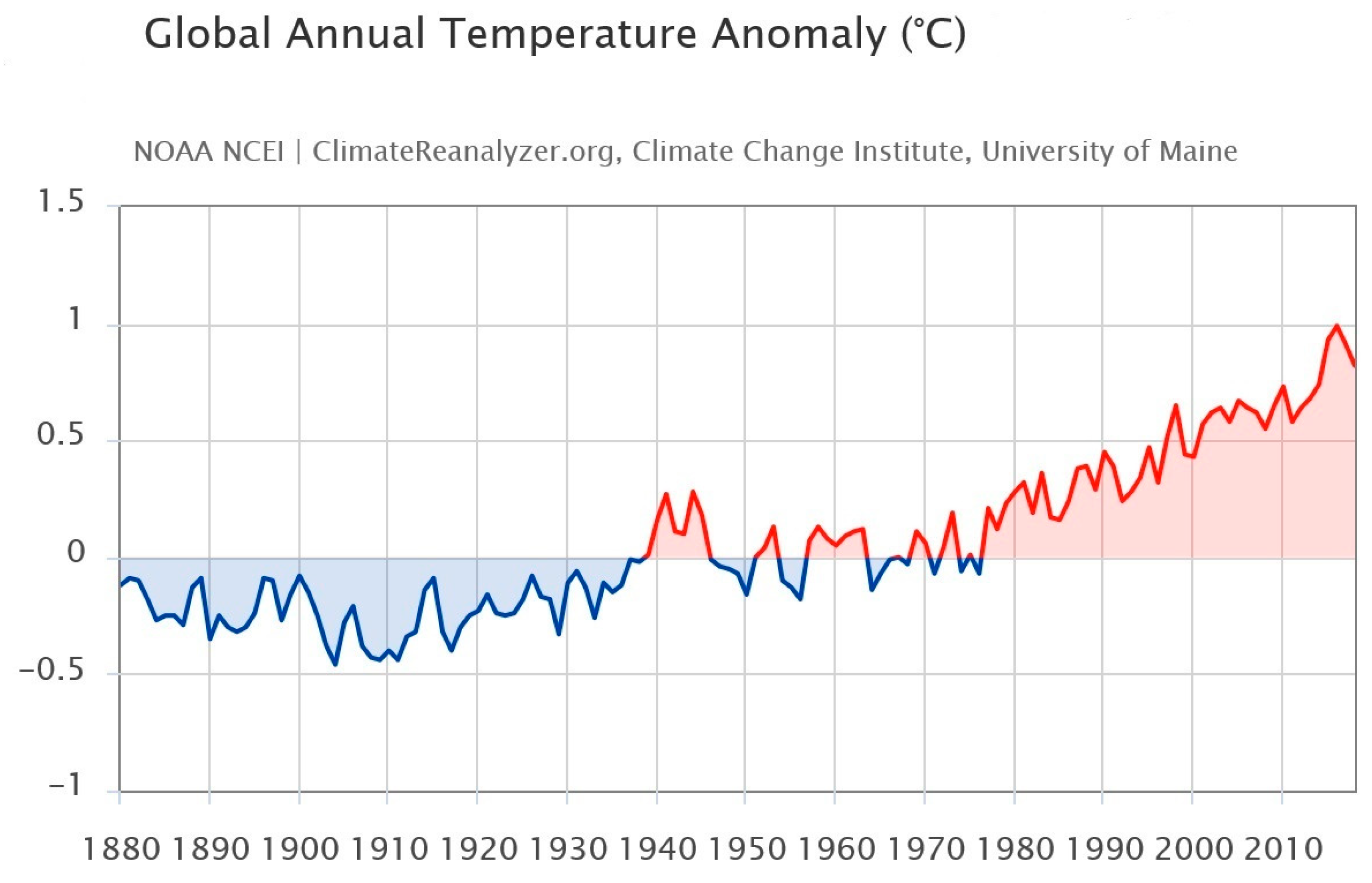
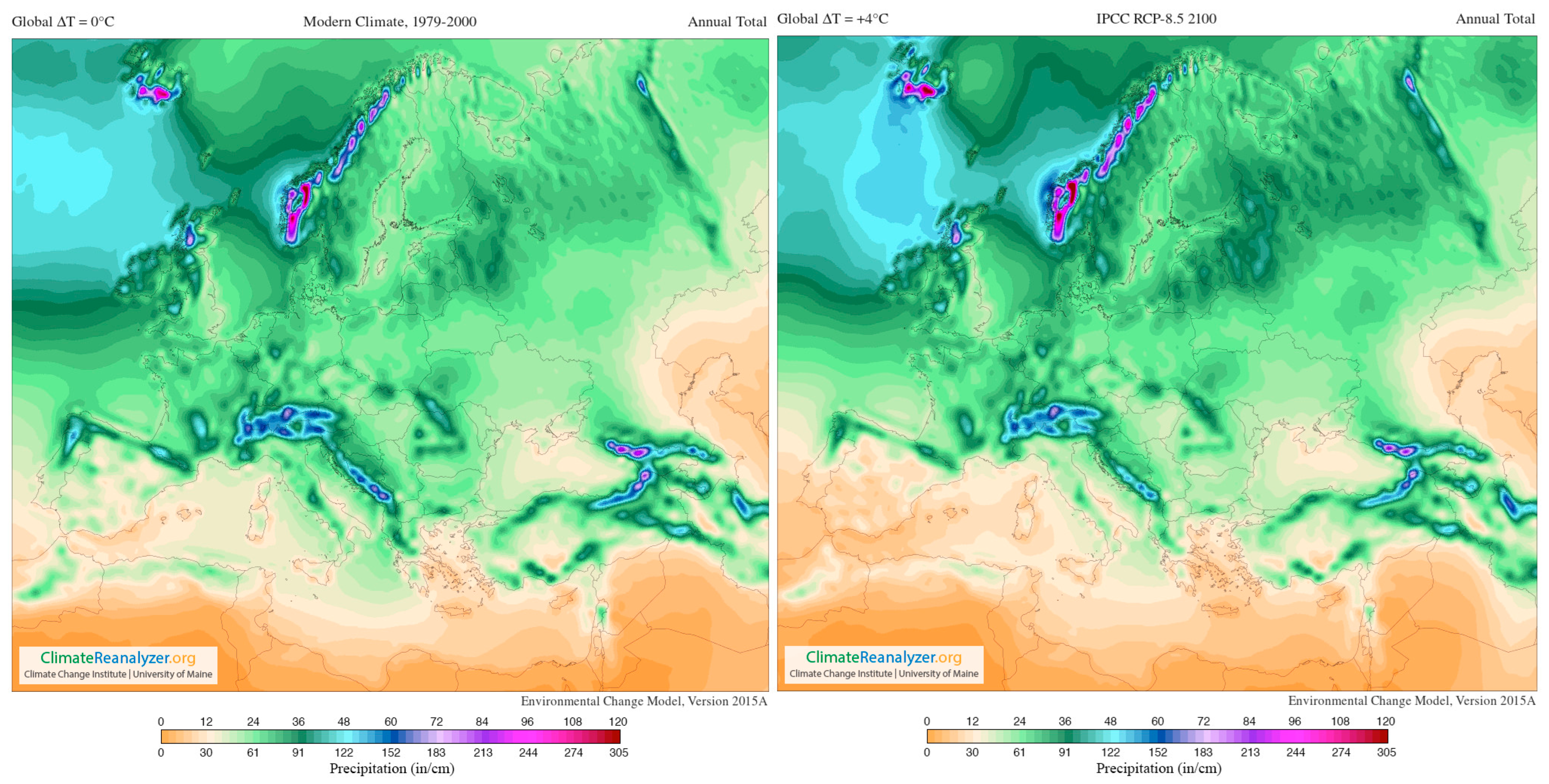
Global warming alludes to long-term variations in the climate of Earth affected by warmth imbalances from natural origin (e.g., volcanic, solar, or internal dynamics) and anthropogenic sources (e.g., greenhouse gas ejections and soil management) [116,117,118]. Below is an image to highlight and underline how the global temperature is undergoing a change (Figure 3).
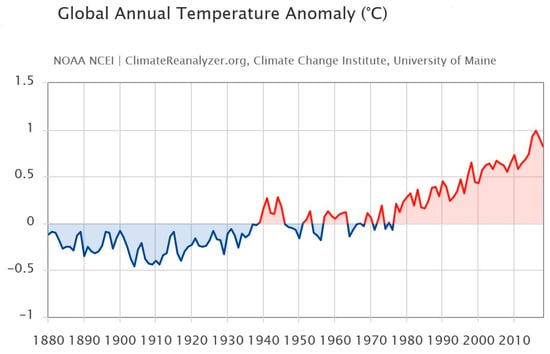
Figure 3.
Images from Birkel, S.D. ‘Explore Climate Change’, Climate Reanalyzer (https://ClimateReanalyzer.org), Climate Change Institute, University of Maine, USA. Accessed on 22 June 2023. Global annual temperature anomaly (°C) 1901–2000 baseline [119].
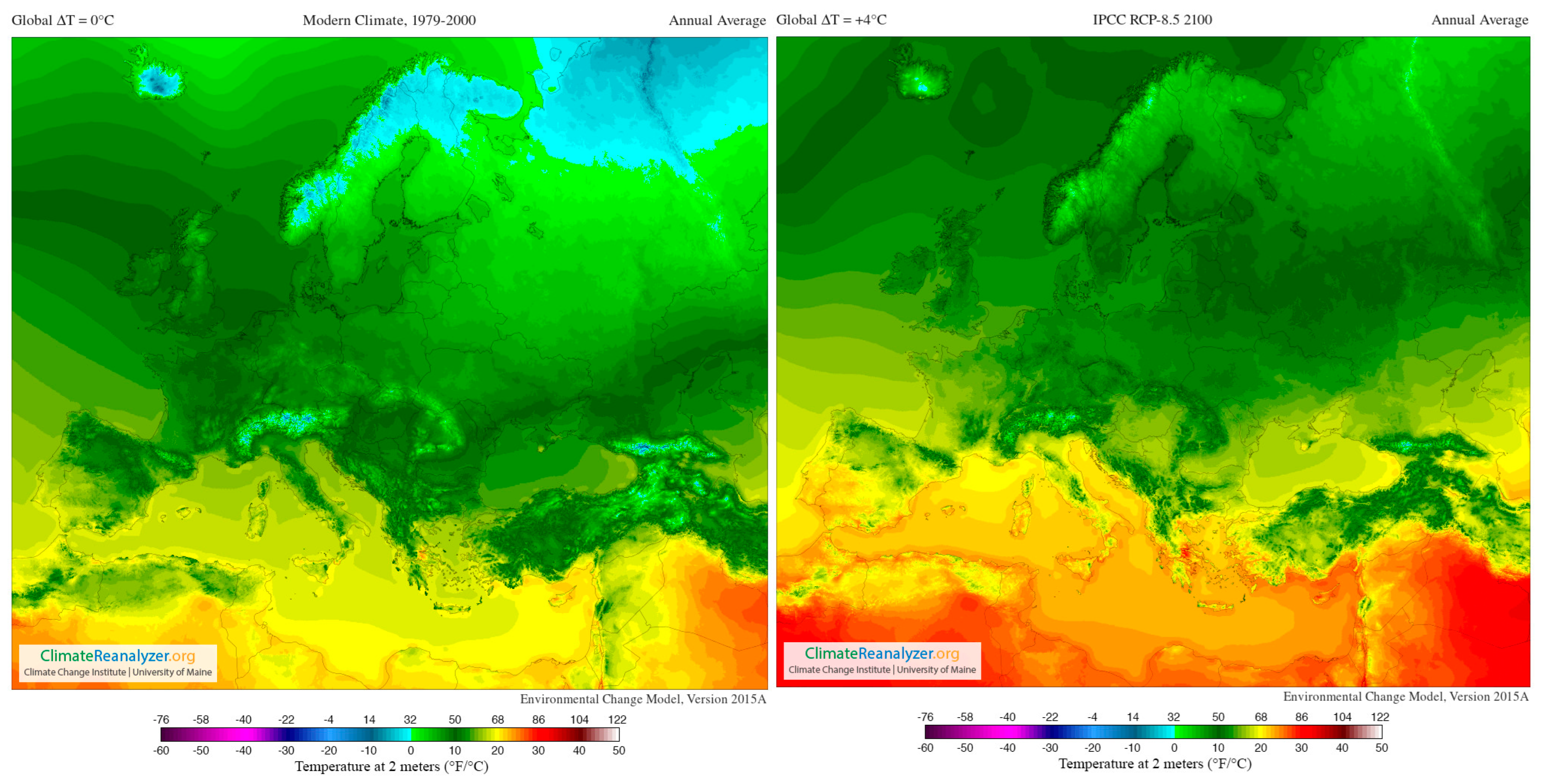
In addition, Figure 4 supplies a selection of template maps and time-series performing modifications in temperature and precipitation over the past century and how they are predicted to shift in the future (RPC 8.5) [120].
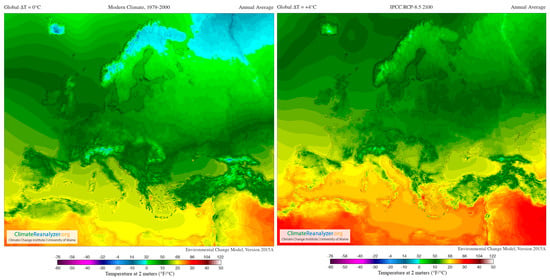
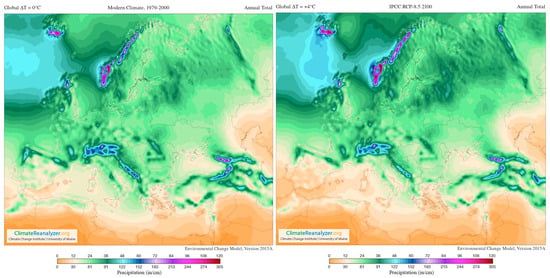
Figure 4.
Images from Birkel, S.D. ‘Environmental Change Model’, Climate Reanalyzer (https://ClimateReanalyzer.org), Climate Change Institute, University of Maine, USA. Accessed on 22 June 2023. In the Environmental Change Model (ECM), solutions are calculated from gridded inputs of monthly temperature and precipitation using a degree day solver and biome rubric. Boundary conditions for a given experiment are derived by blending reanalysis (modern climate) and general circulation model (future climate) climatologies. The ratio of reanalysis to the future climate depends on a user-selected global temperature departure value, ∆T. For the modern climate, ∆T = 0 °C; for 2100, ∆T = +4 °C (European model) [119].
The most important viticultural regions in Europe are situated equatorward top at the 37–50° N parallels [121]; as seen in the figures, these areas are significantly affected by climate change (present and future). In the short-term, the climate markedly governs the whole ripening development process of grapes, because it demands appropriate temperatures, radiation intensities, as well as specific standards of water availability throughout the growth and maturation cycle, ultimately influencing berry characteristics [121,122].
4.1. Water
During the last decennium, in a lot of viticultural areas, pressure on water resources increased owing to global warming being associated with drought conditions. So, the interest in water use optimization to guarantee crop sustainability is urging researchers to introduce new approaches for reducing water losses (e.g., partial root-zone drying and regulated deficit irrigation) [123].
A recent RNA-sequencing analysis work carried out on white grapes reported that water deficit can affect the berries’ composition [124]. In particular, chalcone synthases, two chalcone isomerases, one flavonoid-3′5′-hydroxylase, two flavanone-3-hydroxylases, one dihydroflavonol reductase were up-regulated by deficit irrigation (−1.5 MPa ΨStem of vines at 67 days after anthesis). Water deficit significantly promoted the expression of flavonol synthase (a key enzyme for flavonol production). Leucoanthocyanidin reductase (VviLAR1) and anthocyanidin reductase (as well-known regulators of the flavan-3-ols and proanthocyanidin biosynthesis) were also up-regulated by water deficit.
Anthocyanins’ hydroxylation and methoxylation degrees in berries were altered by environmental markers, such as the water status [125]. With partial rootzone drying (PRD) management, a relative reduction in the proportion of methoxylated anthocyanins in grape berries was found [126], owing to the enhancement in endogenous abscisic acid levels [127]. Dihydroxylated and non-acylated forms have high antioxidant capacity, whereas trihydroxylated and methoxylated are the most oxidized anthocyanins [128]. Shellie and Bowen [129] noted increases in monomeric anthocyanins under water deficit, but possibly by an indirect effect such as a reduction in the size of the berry; (i.e., skin-to-pulp ratio increased). Increments by water deficit in the relative abundance of methoxylated anthocyanins were also found (most oxidized forms) [125]. In this experiment, the procyanidin epicatechin gallate, and the flavonols quercetin and quercetin-3-glucoside declined; however, total low molecular weight phenols were not significantly reduced by water deficit. Several authors observed an increase in total anthocyanins in grapes of plants subjected to water stress [130,131]. However, it was recently observed that an excess of water stress (associated with heatwaves; 35 °C) led to the reduction in extractable anthocyanins in the must and to an increase in quercetin-3-O-glucoside and quercetin-3-O-glucuronide [132,133]. An interesting experiment showed two types of berry responses to drought stress: (I) indirect and positive action on phenolic compounds concentration owing to berry size reduction; (II) direct effect on biosynthesis that can be positive or negative depending on the phenolic compound type, application period, and severity of drought [134]. Briefly, flavonols’ biosynthesis was higher for medium water stress (early water deficit between anthesis and véraison) and strong water stress (between véraison and harvest maturity); biosynthesis of flavan-3-ols was reduced by the early water deficit between anthesis and véraison; and biosynthesis of proanthocyanins and anthocyanins increased only for late significant water deficit between véraison and harvest maturity. In each case, the drought stress enhanced the degree of tannin polymerization.
Berries skin with an intermediate deficit (40% of field capacity) showed a higher content of trisubstituted anthocyanins [135]. Structural genes of the phenylpropanoid pathway which encode flavonoid 3′ (F3′H) and 3′,5′-hydroxylases (F3′5′H) explain the biosynthesis of cyanidin-based and delphinidin-based anthocyanin pigments. The alternative accumulation of trisubstituted rather than disubstituted anthocyanins is attributed to a fork in the pathway and is joined to the competitive activity of F3′H and F3′5′H [136]. The elevated amount of trisubstituted anthocyanins (major color stability in red wines [57]) promotes greater resistance to oxidative damage [137]. Guidoni et al. [138] found that 3′-substituted anthocyanin biosynthesis was probably more strongly influenced by environmental conditions (and cultural practices) than was 3′,5′-substituted ones. In fact, the peonidin-3-glucoside/malvidin-3-glucoside ratio differed on average from 1.9 during the hot and rainy season to 1.2 in the cooler and very dry season. Regulated deficit irrigation (RDI) normally refers to all irrigation strategies that take vines at some degree of water deficit for a prescribed part of the season [139]. According to these results, in Tempranillo grapevines, a step-by-step upgrade in water deficit as summer progressed (ideally from −0.2 to −0.9 MPa) (that implied a greater water deficit between véraison and harvest when anthocyanin synthesis happens) encouraged the synthesis of malvidin (i.e., the main tri-hydroxylated anthocyanin), compared with water stress caused in the berry herbaceous stage growth (RTD_1), or even with RDI_2 that included an additional stress period shortly after véraison. On the contrary, RDI water management resulted in a higher proportion of non-acylated delphinidine and petunidine. In contrast, some results showed that an altered grapevine water status would impact the skin flavonoids’ biosynthesis only slightly, and its effect would not depend that much on the vine water status, but on the seasons [140,141]; additionally, in a recent work, no evidence that the water stress level caused significant differences in monomeric flavan-3-ol compounds was found [142].
A recent work [135] showed the pre-véraison water deficit effects on vines grafted on Mgt 101-14 and 1103 Paulsen rootstocks. From the obtained data, the presence of VIT_18s0001g03430 (VvFLS gene; it encodes a flavonol synthase) transcript was enhanced in severe deficit (25% of field capacity). A study [143] where irrigation was applied every week (when Ψpd reached −0.4 MPa; moderate water deficit) indicated no significant key metabolites modified (phenolic acids, stilbenoid DP1 and DP2, flavonols, flavan-3-ols, and di-OH anthocyanins and tri-OH anthocyanins) by treatments.
Considering the presence of contrasting works regarding the effects of water deficit alone, a more in-depth investigation into potted plants (controlled environment) with multiple cultivars and multiple rootstocks to which different levels of stress can be applied without other variables would be desirable. Quantitative, metabolomic, and transcriptomic analyzes during bunch ripening could provide answers to doubts regarding water scarcity and its repercussions on quality.
4.2. Temperature
The Intergovernmental Panel on Climate Change has denoted the ineluctable increase in worldwide temperature and has recognized climate change as an important menace to the overall food supply [144]. Environmental temperature is a climate element that influences phenological phases and causes the timing of ripening and harvest [145].
In general, an increased temperature in the plant will increase its rate of metabolic processes with an associated rise in metabolite accumulation [146]. However, the following statement would need to be corrected from a climate change perspective for the reasons discussed below. Merlot berries’ extreme temperatures exposure for short periods in ripening (blew forced air across the bunches; to a target temperature 2 × ∆Tr degrees above the average; where ∆Tr represented the temperature difference between the sun bunches average and the shade bunches average) altered the anthocyanins’ partitioning between acylated and non-acylated and between dihydroxylated and trihydroxylated forms in the biosynthetic pathway. Briefly in this trial, with ten combinations, the exposure hours varied from 2 to 200 and the arbitrary thresholds were >30 °C, >35 °C, and >40 °C. Higher berry temperatures led to a higher concentration of malvidin-based anthocyanins, driven chiefly by growth in the acylated derivatives (i.e., malvidin 3-coumaroyl-glucoside). At berry temperatures tantamount to those of shaded ones, exposure to solar radiation reduced the acylated forms of the five base anthocyanins and augmented the proportion of dihydroxylated anthocyanins [147]. Other authors found that temperature did not influence the accumulation of malvidin 3-glucoside in Merlot [148] or in Pinot noir [149].
qRT-PCR analysis proved that temperature (and light conditions) affected the anthocyanin composition in the skin through the regulation of the flavonoid biosynthesis pathway genes (VlMYBA1-3, VlMYBA1-2, and VlMYBA2) [150]. These results suggested that low temperature (and light) had a synergistic incidence on gene expression in the flavonoid biosynthesis pathway; high temperature (>34 °C) (or dark treatment) severely suppressed anthocyanin accumulation. The authors affirmed that the skin anthocyanin composition in the 35 °C (and dark) treatment could not be defined (the content was too low). In 15 °C (and light), 40.2% peonidin and 28.1% malvidin derivatives were found; in addition, malvidin derivatives’ levels decreased (about 17.5%) in 15 °C (and dark), and peonidin (30.7%) and malvidin (7.3%) derivatives decreased in 35 °C (and light) treatment.
Peonidin-3-monoglucoside was the prevailing anthocyanin form in 15 °C (and light) treatment, but its percentage dwindled in two treatments: 15 °C (and dark; 5.1%) and 35 °C (and light; 4.8%). Moreover, the peonidin-3-p-coumarylglucoside-5-glucoside augmented in 15 °C (and dark; 24.0%) and 35 °C (and light; 14.0%) treatments. Moreover, chemical composition changes were also correlated with warmer seasons, as indicated by the rising formation of malvidin, petunidin, and delphinidin coumaroyl derivatives [146].
In sunlit growth chambers (rotating and stationary phytotron rooms), optimal requirements for anthocyanin accumulation (Cardinal, Pinot noir, and Tokay grapevines) came about when bunches were set out to cool nights (15 °C) and temperate days (25 °C) during maturation; this deep coloration was not found in clusters ripened at 35 °C day combined with 15 °C night, 35 °C day combined with 25 °C night, and 15 °C day combined with 25 °C night temperatures. Moreover, a day temperature of 35 °C entirely obstructed anthocyanin synthesis in Tokay grapes, regardless of night temperatures [151]. In agreement with the results just stated, high temperatures (35 °C) cut down the total anthocyanin content (Cabernet Sauvignon cultivar) with respect to the control berries (25 °C), with the exception of malvidin-3-glucoside, malvidin-3-acetylglucoside, and malvidin-3-p-coumaroylglucoside. This reduction resulted from factors such as anthocyanin degradation and from the inhibition of mRNA transcription of the anthocyanin biosynthetic genes (qRT-PCR and LC-MS analysis of 13C-labelled anthocyanin were carried out) [152].
The temperature effects on proanthocyanidin biosynthesis are understood to a small degree. A recent study on Cabernet Sauvignon grape berries cultured in vitro showed that the proanthocyanidin content was higher in berries (skin + seeds) cultured at a low temperature compared with a high one. In addition, the elevated temperatures forbade the expression level of anthocyanidin reductase and leucoanthocyanidin reductase-1 (key genes involved in proanthocyanidin biosynthesis; ANR and LAR-1) [153].
The consequence of temperature on tannins is not yet well known; one study on Merlot berries showed that high temperature during berry development heightened the start of skin tannin storage, however this effect was not evident at véraison [154]. Meanwhile, another trial on high temperature during berry ripening did not survey any interaction on skin tannins by vintage in Sangiovese [155]. Maybe, as stated by Gouot et al. [156], a deficiency of knowledge of the accurate galloylation process limits gene expression investigations to genes involved in the phenylpropanoid pathway. It was hypothesized that temperature could affect tannin biosynthesis and galloylation in young green berries, owing to modifications in the regulation of transcripts coding for glucose-gallic acid-glucosyltransferase [19].
In a recent study, a rise in galloylated flavan-3-ol subunits (berries skin) and galloylation percentage skin tannins was noted two weeks after the end of the treatment (heated bunches max 45 °C) [157].
Considering these results, in view of climate change, it would be interesting to monitor the evolution of the phenolic component during heatwaves (before, during, and immediately after the phenomenon). Furthermore, more studies would be desirable to understand the changes in the hydroxycinnamic compounds (there are still scant studies in this regard).
4.3. Solar Radiation and UV-B Light
Sunlight is chiefly comprised of infrared wavelengths but also contains 8–9% ultraviolet light (UV). UV light can be subdivided into three types: UV-A (315 to 400 nm; not absorbed by the O3 layer); UV-B (280 to 315 nm; its intensity on Earth’s surface depends on the O3 layer thickness); and UV-C (100 to 280 nm; completely absorbed by the O3 layer) [158].
Shaded fruit showed lower flavonol glucosides at vintage and during berry development in many cultivars such as Cabernet Sauvignon, Shiraz [159], and Merlot [146,160]. In fact, it was suggested [161] that the flavonol profile is a reliable indicator to estimate canopy architecture and grapes’ exposure to solar radiation. Blancquaert et al. [162] suggested that UV-B radiation plays a notable role in the photo-protection of the berry against light exposure. In their experiment, the light quantity and quality were, respectively, manipulated in the bunch zone with leaf removal and by installing ultraviolet B-suppression sheets within the bunch zone. Moreover, treatments showed only a marginal effect on skin flavan-3-ol synthesis and no impact on seed tannin. In relation to the hydroxycinnamic acid, hydroxybenzoic acid, and stilbene content, no significant correlations were found with ambient sunlight intensity [158]. So, these authors suggested that in the ripening of berries, flavonols operate as UV screening compounds, defending plant tissue from light damage (i.e., higher flavonol glucosides). In fact, light influences flavonol synthase (VvFLS) expression, a significant flavonol structural gene [163], and influences a transcriptional regulator of flavonoid synthesis (VvMYBF1) [164]. Nevertheless, UV-B is also known to up-regulate genes encoding phenylalanine ammonia-lyase and naringenin–chalcone synthase [165]. At BBCH 73 stage (groat-sized berries), responsiveness to UV increased, as detected by the induction of class III chitinase, phenylalanine ammonia-lyase, and stilbene synthase expression, together with accumulation of resveratrol [166]. Flavan-3-ols and proanthocyanidins in berry skins were affected by sun exposure. In fact, as demonstrated by [163], shadow consistently decreased the molar ratio of the tri-hydroxylated gallocatechins to di-hydroxylated catechins and decreased the mean degree of polymerization. Meanwhile, light exposure increased the proanthocyanidins galloylation rate in berries after the removal of shading.
As concerns the results of skin anthocyanin, data highlighted a complex, combined effect of solar radiation + berry temperature; in particular, there is a synergistic effect with quite moderate berry temperatures and an antagonistic effect at elevated temperature extremes. An association of low light summarized to high berry temperature dropped the total concentrations of skin anthocyanin. Instead, quercetin 3-glucoside (flavonol-glycoside) enhanced with exposure to solar radiation [147]. These results were confirmed by other authors [132,133]; high quantities were found of quercetin-3-O-glucoside and quercetin-3-O-glucuronide in stressed grapes. Vijay and Vadewki [167], in their experiment on Cabernet Sauvignon grapes, explained the benefit of uncovered clusters to solar light; in treated clusters (leaf removal, cluster thinning, and shoot thinning) higher concentrations of flavan-3-ols (i.e., catechin and epicatechin) and flavonols (i.e., quercetin and myricetin) were found (quercetin accumulation could be a light solar-dependent process). Another recent study adopting high UV-B radiation (Vitroflex 395 filter and UV-B lamps) on Tempranillo grapes showed an increase in quercetin and kaempferol contents correlated to the expression of flavonol synthase and chalcone synthase genes (VvFLS4 and VvCHS1), and an increase in hydroxycinnamic acids at the pea-size stage [168]. On the contrary, in UV-exposed grape skins, hydroxybenzoic acids showed no significant alterations [169]. In the group of non-flavonoid phenolic acids, Singleton et al. [170] hypothesized that the trans-configuration of coutaric and caftaric acids was a natural phenomenon and the cis-form was the UV-induced isomerization product.
In light of all these interesting works, it is deemed necessary to further investigate the effects of the interaction between light radiation and ambient temperature.
Another possible consideration is to carry out in-depth studies on the effects of solar radiation on the bunch, focused on the individual cultivars and on the organoleptic effects on the wines. In fact, in the light of recent studies [171], increases in the must of quercetin (Sangiovese variety) could lead to the impoverishment of the finished product (precipitated by the hydrolysis of glycosides; i.e., by the supersaturation of aglycones) [172].
5. Management Tips: How Can the Winegrower Corroborate the Grapevine Resilience?
The strategies discussed below are integrated into a respectful and conservative approach to the vineyard ecosystem. These soil or canopy managements are included in the narrative as they are considered natural resources that can increase the resilience of the plant with a view to preserving the finished product for the consumer. The use of these substances defends the plant from abiotic stress and their application is compliant with organic agriculture. The arbitrariness of use means that they can be applied preventatively and occasionally only during critical years [173]. Furthermore, the emphasis to be placed on these products is the fact of corroborating the system and not distorting the plant’s eco-physiological functions to arrive at a “standardized product”, but rather of supporting and creating adaptation to abiotic stress.
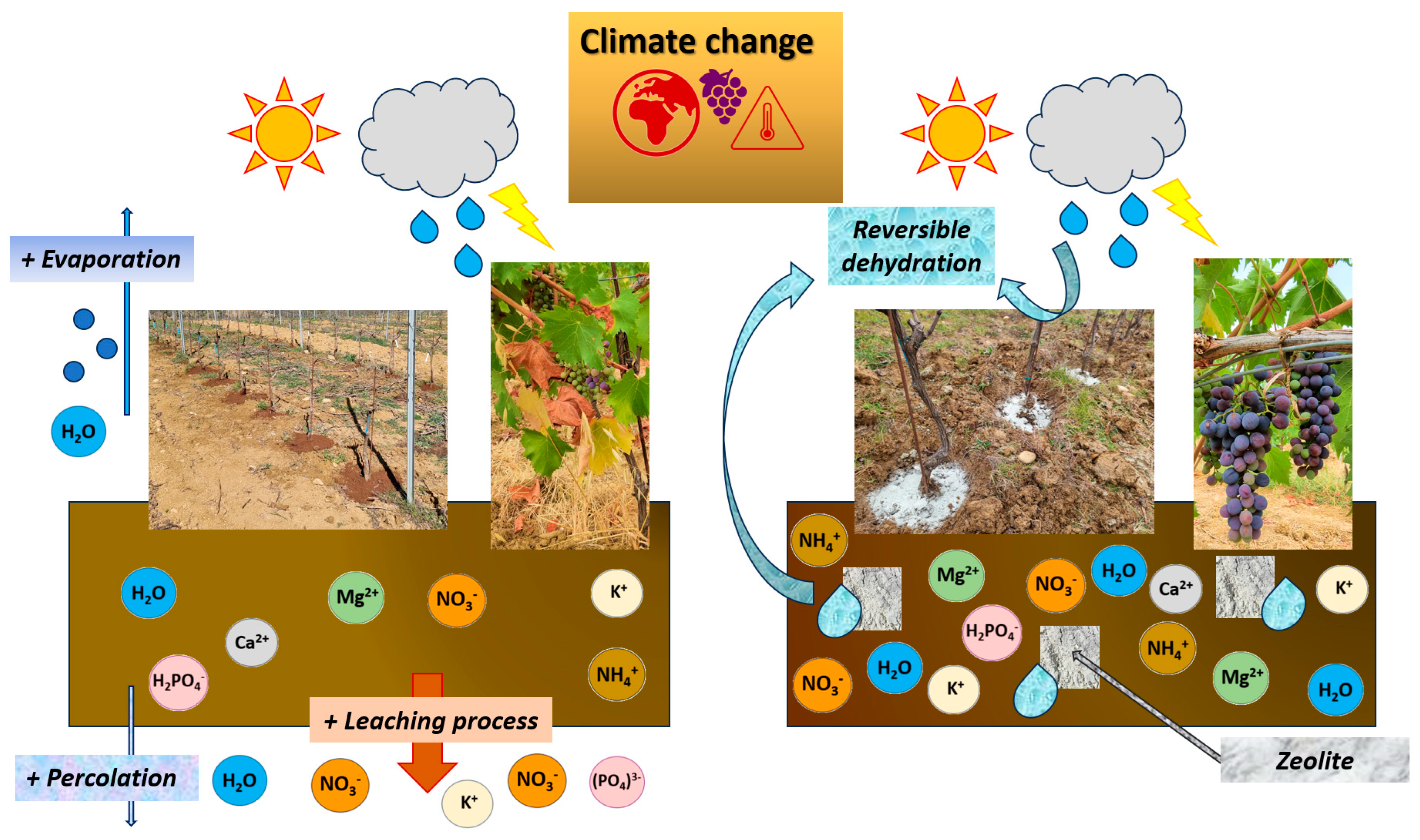
5.1. Zeolite
Zeolites are porous aluminosilicates characterized by a crystalline frame that comprehends an interconnected chambers/channels system. Their geometrical parameters are notable characteristics responsible for their adsorption skills. Consequently, zeolites can function as a sorbent for environmental pollutants, a water reservoir (reversible dehydration), and nutrients for plants (cations + anions) [174] (Figure 5).
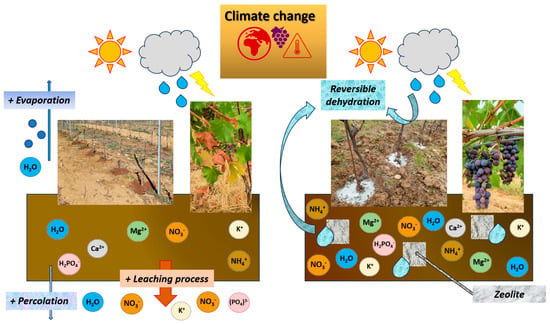
Figure 5.
Effects of zeolite on vineyard soil that mitigate the effects of climate change (adapted from [174]).
Owing to their rare properties, these aluminosilicates have become more and more trendy in recent years and found functional applications in viticulture [175]. Among natural zeolites, clinoptilolite represents the most commonly applied in agriculture and environmental defenses [176].
In a recent study [177], it was seen that canopy treatments of chabasite-rich zeolite (15 kg/L ha−1) increased the content of phenolic compounds in the grapes, as well as providing simultaneous control of gray mold (Botrytis cinerea; Botryotinia fuckeliana) and Lobesia botrana (Denis and Schiffermüller). This probably happened owing to its ability to be an inert mineral able to reduce canopy surface temperatures as well as being radiation-reflecting. Other authors found an increase in extractable anthocyanins together with a reduction in quercetin forms in plants treated with soil applications of clinoptilolite [132,133]. This could be attributable to the ability of zeolites to mitigate water stress and allow for balanced ripening in extremely dry years. However, there are still too few studies on the use of this ductile mineral on grapevines; only recently has research been turning to its possible uses to tackle climate change. Given its abundance in nature and given its ease of application, this corroborant product could represent a valid vineyard management strategy in critical years.
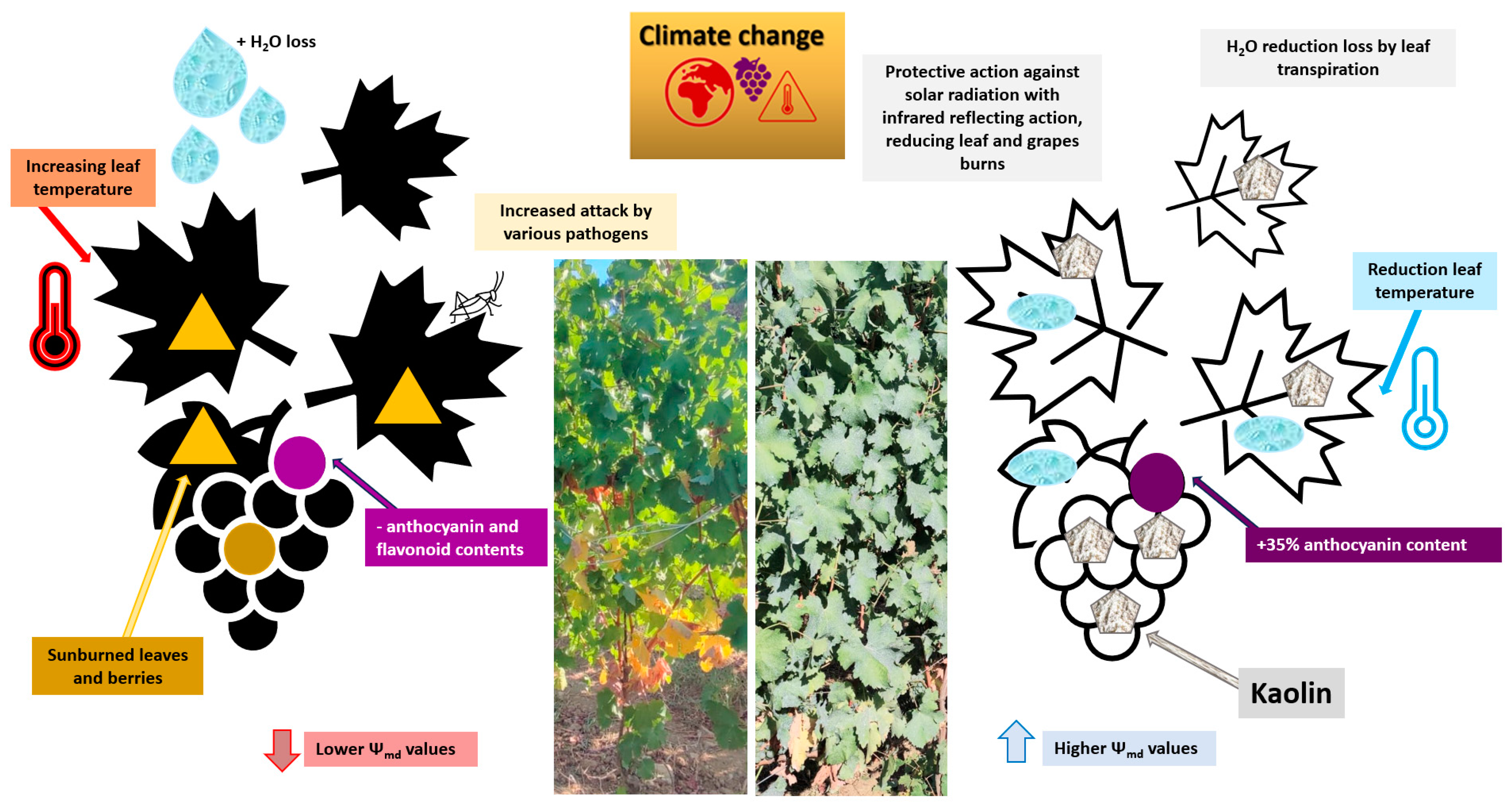
5.2. Kaolin
Al2Si2O5(OH)4 (i.e., kaolin) is a mineral rock consisting predominantly of white kaolinite that is inert, harmless, and is skillfully squanders in water. Kaolin’s force, once sprayed, is correlated to the white safeguarding particle film that gathers on the grapevine leaf’s surface; this particle film is able to augment excess radiation reflection, by reducing heat load accumulation damage and UV solar detriments [178].
As shown in Figure 6, it was found that kaolin enhanced the total monomeric anthocyanins amount in Malbec [179] and Meili berries [180], reducing their sensibility to temperature and solar exposure.
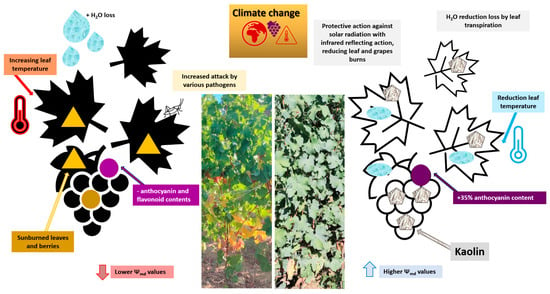
Figure 6.
Effects of kaolin on grapevines (Vitis vinifera L.) to mitigate the effects of climate change [181,182].
A recent study confirmed that kaolin treatments induced an increase in total phenols (+13.0%), flavonoids (+10.5%), tannins (+27.5%), and ortho-diphenols (+30.0%) confronted with the control treatment [183]. Bernardo et al. [184] confirmed that ortho-diphenols, flavonoids, and tannins were ameliorated in kaolin-treated plants.
All of these experiments highlighted the salient role of weather conditions in triggering the accumulation of phenolic processes. Moreover, they showed that kaolin-treated vines exhibited a major response to oxidative stress markers by enhancing secondary metabolite storage in hot harvests. The employment of kaolin supported different cultivars reinforcing their performance in alleviating severe drought, high temperature, and sunburned stress impacts.
5.3. Seaweed Extract
Biostimulant products are recognized as formulated biological substances that ameliorate the productivity of plants as a result of the novel or emergent properties of the complex of constituents, plant growth regulators, and/or plant safeguarding complexes; they are auxiliary tools to improve vine nutrition and to develop vine resilience to environmental stress [185]. Among the biostimulants’ group achieved from seaweeds, as shown in Table 1, Ascophyllum nodosum extract is the most considered by authors [186].

Table 1.
Effects of Ascophyllum nodosum on grapevine berries (Vitis vinifera L.).
Ascophyllum nodosum can be a sustainable management tool to lessen the short-period shock of severe temperatures and strong light irradiance in grapevines; in fact, it was found that it was able to encourage a prompt photosynthetic recovery after drought stress [184]. In addition to this, numerous studies have highlighted its positive effect on the phenolic composition of berries [189,192,194].
6. Conclusions
It is believed that techniques to allay the effects of climate change on grapevine clusters and phenolic compounds should be taken from a “single case” perspective. It is not possible to standardize and approve treatments or agronomic techniques for each different case in different environments (for example, different cultivars, different legislations, and obligations). In order to intensify the resilience of the plant and therefore to maintain or implement its performance in terms of secondary metabolites, it is necessary to evaluate the “single vineyard case”. In fact, by connecting to the concept of terroir (which can be defined as that well-defined area where the natural, physical, and chemical conditions, the geographical area, and the climate allow the achievement of a fixed wine) it is necessary today to establish an elastic and variable approach towards the single wine year, moving away from the concept of product standardization. In fact, a different approach and characterization of the finished wine by the winegrowers and the consumers are desirable, or rather a tolerant point of view of a product susceptible to change based on the vintage (color, aromatic intensity, tannic persistence, etc.). Considering the product in its entirety (climatic changes and adaptations) and therefore in its specific peculiarities of the single vintage could lead to an evolution of techniques and approaches towards these fascinating ecosystems. On the other hand, becoming stuck in the spasmodic search for maintaining the same organoleptic characteristics of wine (different vintages) could lead to a loss of innovation and a loss of new organoleptic perceptions.
Author Contributions
Conceptualization and writing—original draft preparation, E.C.; supervision, A.E. and G.B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Mendeleo—Inst. of the Faculty of Genetics of Horticulture Mendel University of Brno, Lednice, Czech Republic for the hospitality and the beauty of the working environment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Hari, V.; Rakovec, O.; Markonis, Y.; Hanel, M.; Kumar, R. Increased future occurrences of the exceptional 2018–2019 Central European drought under global warming. Sci. Rep. 2020, 10, 12207. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hui, P.; Xue, D.; Tang, J. Future projection of heat waves over China under global warming within the CORDEX-EA-II project. Clim. Dyn. 2019, 53, 957–973. [Google Scholar] [CrossRef]
- Haj-Amor, Z.; Araya, T.; Kim, D.-G.; Bouri, S.; Lee, J.; Ghiloufi, W.; Yang, Y.; Kang, H.; Jhariya, M.K.; Banerjee, A.; et al. Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: A review. Sci. Total. Environ. 2022, 843, 156946. [Google Scholar] [CrossRef] [PubMed]
- Ruebsam, W.; Mayer, B.; Schwark, L. Cryosphere carbon dynamics control early Toarcian global warming and sea level evolution. Glob. Planet. Chang. 2019, 172, 440–453. [Google Scholar] [CrossRef]
- Yamada, Y.; Kodama, C.; Satoh, M.; Sugi, M.; Roberts, M.J.; Mizuta, R.; Noda, A.T.; Nasuno, T.; Nakano, M.; Vidale, P.L. Evaluation of the contribution of tropical cyclone seeds to changes in tropical cyclone frequency due to global warming in high-resolution multi-model ensemble simulations. Prog. Earth Planet. Sci. 2021, 8, 11. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar]
- Caccamo, M.T.; Magazù, S. On the breaking of the Milankovitch cycles triggered by temperature increase: The stochastic resonance response. Climate 2021, 9, 67. [Google Scholar] [CrossRef]
- Cheng, H.; Li, H.; Sha, L.; Sinha, A.; Shi, Z.; Yin, Q.; Lu, Z.; Zhao, D.; Cai, Y.; Hu, Y.; et al. Milankovitch theory and monsoon. Innovation 2022, 3, 100338. [Google Scholar] [CrossRef]
- Fan, X.; Duan, Q.; Shen, C.; Wu, Y.; Xing, C. Global surface air temperatures in CMIP6: Historical performance and future changes. Environ. Res. Lett. 2020, 15, 104056. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; de Toda, F.M. Strategies in vineyard establishment to face global warming in viticulture: A mini review. J. Sci. Food Agric. 2021, 101, 1261–1269. [Google Scholar] [CrossRef]
- Schultz, H. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effects. Aust. J. Grape Wine Res. 2000, 6, 2–12. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Moriondo, M.; Jones, G.V.; Bois, B.; Dibari, C.; Ferrise, R.; Trombi, G.; Bindi, M. Projected shifts of wine regions in response to climate change. Clim. Chang. 2013, 119, 825–839. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Badr, G.; Hoogenboom, G.; Abouali, M.; Moyer, M.; Keller, M. Analysis of several bioclimatic indices for viticultural zoning in the Pacific Northwest. Clim. Res. 2018, 76, 203–223. [Google Scholar] [CrossRef]
- Hunter, J.J.; Volschenk, C.G.; Mania, E.; Castro, A.V.; Booyse, M.; Guidoni, S.; Pisciotta, A.; Di Lorenzo, R.; Novello, V.; Zorer, R. Grapevine row orientation mediated temporal and cumulative microclimatic effects on grape berry temperature and composition. Agric. For. Meteorol. 2021, 310, 108660. [Google Scholar] [CrossRef]
- Ramos, M.C.; de Toda, F.M. Variability in the potential effects of climate change on phenology and on grape composition of Tempranillo in three zones of the Rioja DOCa (Spain). Eur. J. Agron. 2020, 115, 126014. [Google Scholar] [CrossRef]
- Rienth, M.; Torregrosa, L.; Sarah, G.; Ardisson, M.; Brillouet, J.-M.; Romieu, C. Temperature desynchronizes sugar and organic acid metabolism in ripening grapevine fruits and remodels their transcriptome. BMC Plant Biol. 2016, 16, 164. [Google Scholar] [CrossRef]
- Jones, G.V.; Davis, R.E. Climate Influences on Grapevine Phenology, Grape Composition, and Wine Production and Quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar] [CrossRef]
- da Silveira, M.G.; Romão, M.V.S.; Loureiro-Dias, M.C.; Rombouts, F.M.; Abee, T. Flow Cytometric Assessment of Membrane Integrity of Ethanol-Stressed Oenococcus oeni Cells. Appl. Environ. Microbiol. 2002, 68, 6087–6093. [Google Scholar] [CrossRef]
- Erasmus, D.J.; van der Merwe, G.K.; van Vuuren, H.J. Genome-wide expression analyses: Metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res. 2003, 3, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Pigeau, G.M.; Inglis, D.L. Upregulation of ALD3 and GPD1 in Saccharomyces cerevisiae during Icewine fermentation. J. Appl. Microbiol. 2005, 99, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Kennedy, J.A. Plant Metabolism and the Environment: Implications for Managing Phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef] [PubMed]
- de Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Bavaresco, L.; Falginella, L.; Gonçalves, M.I.V.Z.; Di Gaspero, G.; Gerós, H.; Delrot, S. Phenolics in grape berry and key antioxidants. Biochem. Grape Berry 2012, 22, 89–110. [Google Scholar]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot noir) and wine proanthocyanidins. J. Agric. Food Chem. 2005, 53, 5798–5808. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Effects of abiotic factors on phenolic compounds in the Grape Nerry—A review. S. Afr. J. Enol. Vitic. 2019, 40, 1–14. [Google Scholar]
- Abad, J.; Diana, M.; Gonzaga, S.L.; Félix, C.J.; Ana, S. Under-vine cover crops: Impact on weed development, yield and grape composition: This article is published in cooperation with the XIIIth International Terroir Congress November 17–18 2020, Adelaide, Australia. Guest editors: Cassandra Collins and Roberta De Bei. OENO One 2020, 54, 975–983. [Google Scholar]
- Buesa, I.; Mirás-Avalos, J.M.; De Paz, J.M.; Visconti, F.; Sanz, F.; Yeves, A.; Guerra, D.; Intrigliolo, D.S. Soil management in semi-arid vineyards: Combined effects of organic mulching and no-tillage under different water regimes. Eur. J. Agron. 2020, 123, 126198. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- Laura, A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 253–271. [Google Scholar]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Intech Open: London, UK, 2020; pp. 1–27. [Google Scholar]
- Waterhouse, A.L. Wine phenolics. Ann. New York Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Palade, L.M.; Popa, M.E. Polyphenol Fingerprinting Approaches in Wine Traceability and Authenticity: Assessment and Implications of Red Wines. Beverages 2018, 4, 75. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, V.-C.; Paun, N.; Ionete, R.-E. The Evolution of Polyphenols from Grapes to Wines. In Grapes Wines—Advances in Production, Processing, Analysis and Valorization; Intech Open: London, UK, 2018. [Google Scholar]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Merken, H.M.; Beecher, G.R. Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones. J. Chromatogr. A 2000, 897, 177–184. [Google Scholar] [CrossRef]
- Fang, F.; Li, J.-M.; Zhang, P.; Tang, K.; Wang, W.; Pan, Q.-H.; Huang, W.-D. Effects of grape variety, harvest date, fermentation vessel and wine ageing on flavonoid concentration in red wines. Food Res. Int. 2008, 41, 53–60. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Zhuge, Y.; Zhang, M.; Liu, C.; Jia, H.; Fang, J. Integrative Analyses of Metabolomes and Transcriptomes Provide Insights into Flavonoid Variation in Grape Berries. J. Agric. Food Chem. 2021, 69, 12354–12367. [Google Scholar] [CrossRef]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventós, R.M. Phenolic Compounds: Chemistry and Occurrence in Fruits and Vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 53–88. [Google Scholar] [CrossRef]
- Rauter, A.P.; Herold, B.J.; Horton, D.; Moss, G.; Schomburg, I.; Hellwich, K.H.; Ennis, M. Nomenclature of Flavonoids. International Union of Pure and Applied Chemistry. 2013. Available online: https://iupac.qmul.ac.uk/flavonoid/ (accessed on 22 June 2023).
- Baranowski, R.; Kabut, J.; Baranowska, I. Analysis of Mixture of Catechins, Flavones, Flavanones, Flavonols, and Anthocyanidins by RP-HPLC. Anal. Lett. 2004, 37, 157–165. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Lu, Y.; Santos, S.A.; Silvestre, A.J.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC–DAD–ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Nibbs, A.E.; Scheidt, K.A. Asymmetric Methods for the Synthesis of Flavanones, Chromanones, and Azaflavanones. Eur. J. Org. Chem. 2011, 2012, 449–462. [Google Scholar] [CrossRef]
- Braidot, E.; Zancani, M.; Petrussa, E.; Peresson, C.; Bertolini, A.; Patui, S.; Macrì, F.; Vianello, A. Transport and accumulation of flavonoids in grapevine (Vitis vinifera L.). Plant Signal. Behav. 2008, 3, 626–632. [Google Scholar] [CrossRef]
- Handoussa, H.; Hanafi, R.; El-Khatib, A.; Linscheid, M.; Mahran, L.; Ayoub, N. Computer-assisted HPLC method development using DryLab for determination of major phenolic components in Corchorus olitorius and Vitis vinifera by using HPLC-PDA-ESI-TOF-MSn. Res. Rev. J. Bot. Sci. 2017, 6, 9–16. [Google Scholar]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Fowler, Z.L.; Koffas, M.A.G. Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.; Martínez, M.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 2012, 130, 9–19. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and Flavone Intakes in US Health Professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Leopoldini, M.; Pitarch, I.P.; Russo, N.; Toscano, M. Structure, Conformation, and Electronic Properties of Apigenin, Luteolin, and Taxifolin Antioxidants. A First Principle Theoretical Study. J. Phys. Chem. A 2004, 108, 92–96. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2020, 307, 125518. [Google Scholar] [CrossRef]
- Fang, F.; Tang, K.; Huang, W.-D. Changes of flavonol synthase and flavonol contents during grape berry development. Eur. Food Res. Technol. 2013, 237, 529–540. [Google Scholar] [CrossRef]
- Ayvaz, H.; Cabaroglu, T.; Akyildiz, A.; Pala, C.U.; Temizkan, R.; Ağçam, E.; Ayvaz, Z.; Durazzo, A.; Lucarini, M.; Direito, R.; et al. Anthocyanins: Metabolic Digestion, Bioavailability, Therapeutic Effects, Current Pharmaceutical/Industrial Use, and Innovation Potential. Antioxidants 2022, 12, 48. [Google Scholar] [CrossRef]
- Calzolari, A.; Varsano, D.; Ruini, A.; Catellani, A.; Tel-Vered, R.; Yildiz, H.B.; Ovits, O.; Willner, I. Optoelectronic Properties of Natural Cyanin Dyes. J. Phys. Chem. A 2009, 113, 8801–8810. [Google Scholar] [CrossRef]
- Sinopoli, A.; Calogero, G.; Bartolotta, A. Computational aspects of anthocyanidins and anthocyanins: A review. Food Chem. 2019, 297, 124898. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, E.; Shangguan, L.; Korir, N.K.; Sun, X.; Bilkish, N.; Zhang, Y.; Han, J.; Song, C.; Cheng, Z.-M.; Fang, J. Fruit skin color and the role of anthocyanin. Acta Physiol. Plant. 2013, 35, 2879–2890. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Oliveira Brett, A.M. Redox Behavior of Anthocyanins Present in Vitis vinifera L. Electroanalysis 2007, 19, 1779–1786. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Núñez, V.; Monagas, M.; Gomez-Cordovés, M.; Bartolomé, B. Vitis vinifera L. cv. Graciano grapes characterized by its anthocyanin profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
- Jeong, S.T.; Goto-Yamamoto, N.; Hashizume, K.; Esaka, M.J.P.S. Expression of the flavonoid 3′-hydroxylase and flavonoid 3′, 5′-hydroxylase genes and flavonoid composition in grape (Vitis vinifera). Plant Sci. 2006, 170, 61–69. [Google Scholar] [CrossRef]
- Boss, P.; Davies, C.; Robinson, S.P. Anthocyanin composition and anthocyanin pathway gene expression in grapevine sports differing in berry skin colour. Aust. J. Grape Wine Res. 1996, 2, 163–170. [Google Scholar] [CrossRef]
- Fournand, D.; Vicens, A.; Sidhoum, L.; Souquet, J.-M.; Moutounet, M.; Cheynier, V. Accumulation and Extractability of Grape Skin Tannins and Anthocyanins at Different Advanced Physiological Stages. J. Agric. Food Chem. 2006, 54, 7331–7338. [Google Scholar] [CrossRef]
- Roggero, J.P.; Coen, S.; Ragonnet, B. High Performance Liquid Chromatography Survey on Changes in Pigment Content in Ripening Grapes of Syrah. An Approach to Anthocyanin Metabolism. Am. J. Enol. Vitic. 1986, 37, 77–83. [Google Scholar] [CrossRef]
- González-San José, M.L.; Barron, L.J.R.; Díez, C. Evolution of anthocyanins during maturation of tempranillo grape variety (Vitis vinifera) using polynomial regression models. J. Sci. Food Agric. 1990, 51, 337–343. [Google Scholar] [CrossRef]
- Ryan, J.-M.; Revilla, E. Anthocyanin Composition of Cabernet sauvignon and Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Grosskopf, E.; Sadgrove, N.J.; Simmonds, M.S. Chemical Diversity of Flavan-3-Ols in Grape Seeds: Modulating Factors and Quality Requirements. Plants 2022, 11, 809. [Google Scholar] [CrossRef]
- Ma, W.; Waffo-Téguo, P.; Jourdes, M.; Li, H.; Teissedre, P.L. First evidence of epicatechin vanillate in grape seed and red wine. Food Chem. 2018, 259, 304–310. [Google Scholar] [CrossRef]
- Zerbib, M.; Mazauric, J.-P.; Meudec, E.; Le Guernevé, C.; Lepak, A.; Nidetzky, B.; Cheynier, V.; Terrier, N.; Saucier, C. New flavanol O-glycosides in grape and wine. Food Chem. 2018, 266, 441–448. [Google Scholar] [CrossRef]
- Delcambre, A.; Saucier, C. Identification of new flavan-3-ol monoglycosides by UHPLC-ESI-Q-TOF in grapes and wine. J. Mass. Spectrom. 2012, 47, 727–736. [Google Scholar] [CrossRef]
- Rohr, G.; Meier, B.; Sticher, O. Analysis of procyanidins. Stud. Nat. Prod. Chem. 2000, 21, 497–570. [Google Scholar] [CrossRef]
- Escribano Bailón, M.T.; Guerra, M.T.; Rivas Gonzalo, J.C.; Santos Buelga, C. Proanthocyanidins in Skins from Different Grape Varieties; Universidad de Salamanca (USAL): Salamanca, Spain, 1995. [Google Scholar]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality. Global Science Books: Isleworth, UK, 2007. [Google Scholar]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. Int. J. Mol. Sci. 2012, 13, 3492–3510. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Silva, L.R.; Queiroz, M. Bioactive compounds of red grapes from Dão region (Portugal): Evaluation of phenolic and organic profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Ossipov, V.; Salminen, J.-P.; Ossipova, S.; Haukioja, E.; Pihlaja, K. Gallic acid and hydrolysable tannins are formed in birch leaves from an intermediate compound of the shikimate pathway. Biochem. Syst. Ecol. 2003, 31, 3–16. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Roslin, T.; Karonen, M.; Sinkkonen, J.; Pihlaja, K.; Pulkkinen, P. Seasonal Variation in the Content of Hydrolyzable Tannins, Flavonoid Glycosides, and Proanthocyanidins in Oak Leaves. J. Chem. Ecol. 2004, 30, 1693–1711. [Google Scholar] [CrossRef]
- Bontpart, T.; Marlin, T.; Vialet, S.; Guiraud, J.-L.; Pinasseau, L.; Meudec, E.; Sommerer, N.; Cheynier, V.; Terrier, N. Two shikimate dehydrogenases, VvSDH3 and VvSDH4, are involved in gallic acid biosynthesis in grapevine. J. Exp. Bot. 2016, 67, 3537–3550. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated Knowledge About the Presence of Phenolic Compounds in Wine. Crit. Rev. Food Sci. Nutr. 2005, 45, 85–118. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 115–124. [Google Scholar]
- Winter, M.; Herrmann, K. Esters and glucosides of hydroxycinnamic acids in vegetables. J. Agric. Food Chem. 1986, 34, 616–620. [Google Scholar] [CrossRef]
- Ong, B.Y.; Nagel, C.W. Hydroxycinnamic acid-tartaric acid ester content in mature grapes and during the maturation of White Riesling grapes. Am. J. Enol. Vitic. 1978, 29, 277–281. [Google Scholar] [CrossRef]
- Hall, D.; De Luca, V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of Concord grape (Vitis labrusca). Plant J. 2007, 49, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Corso, M.; Perreau, F.; Mouille, G.; Lepiniec, L. Specialized phenolic compounds in seeds: Structures, functions, and regulations. Plant Sci. 2020, 296, 110471. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; Dos Santos, W.D.; Constantin, R.P.; De Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, R.; de Oliveira, M.V.V.; Kleven, B.; Maeda, H.A. The entry reaction of the plant shikimate pathway is subjected to highly complex metabolite-mediated regulation. Plant Cell 2021, 33, 671–696. [Google Scholar] [CrossRef] [PubMed]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Jeandet, P.; Clément, C.; Cordelier, S. Regulation of resveratrol biosynthesis in grapevine: New approaches for disease resistance? J. Exp. Bot. 2019, 70, 375–378. [Google Scholar] [CrossRef]
- Zhao, T.; Li, R.; Yao, W.; Wang, Y.; Zhang, C.; Li, Y. Genome-wide identification and characterisation of phenylalanine ammonia-lyase gene family in grapevine. J. Hortic. Sci. Biotechnol. 2021, 96, 456–468. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signal. Mol. 2019, 157–168. [Google Scholar] [CrossRef]
- LaFountain, A.M.; Yuan, Y. Repressors of anthocyanin biosynthesis. New Phytol. 2021, 231, 933–949. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, T.; Zhang, Z.-W.; Meng, J. Reduction of Dihydrokaempferol by Vitis vinfera Dihydroflavonol 4-Reductase to Produce Orange Pelargonidin-Type Anthocyanins. J. Agric. Food Chem. 2018, 66, 3524–3532. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.H.; Kim, C.K. Roles of R2R3-MYB transcription factors in transcriptional regulation of anthocyanin biosynthesis in horticultural plants. Plant Mol. Biol. 2018, 98, 1–18. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Che, X.-N.; Pan, Q.-H.; Li, X.-X.; Duan, C.-Q. Transcriptional activation of flavan-3-ols biosynthesis in grape berries by UV irradiation depending on developmental stage. Plant Sci. 2013, 208, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Letcher, T.M. Why do we have global warming? In Managing Global Warming; Academic Press: Cambridge, MA, USA, 2019; pp. 3–15. [Google Scholar]
- Mikhaylov, A.; Moiseev, N.; Aleshin, K.; Burkhardt, T. Global climate change and greenhouse effect. Entrep. Sustain. Issues 2020, 7, 2897. [Google Scholar] [CrossRef]
- Sippel, S.; Meinshausen, N.; Fischer, E.M.; Székely, E.; Knutti, R. Climate change now detectable from any single day of weather at global scale. Nat. Clim. Chang. 2020, 10, 35–41. [Google Scholar] [CrossRef]
- Climate Reanalyzer. Available online: https://ClimateReanalyzer.org (accessed on 22 June 2023).
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Change 2011, 109, 33–57. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the Potential Climate Change Impacts and Adaptation Options for European Viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.; Molina, D.I.; Vivaldi, G.; García-Esparza, M.; Lizama, V.; Álvarez, I. Effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: I. Water relations, vine performance and grape composition. Agric. Water Manag. 2021, 248, 106772. [Google Scholar] [CrossRef]
- Romero, P.; Dodd, I.C.; Martinez-Cutillas, A. Contrasting physiological effects of partial root zone drying in field-grown grapevine (Vitis vinifera L. cv. Monastrell) according to total soil water availability. J. Exp. Bot. 2012, 63, 4071–4083. [Google Scholar] [CrossRef] [PubMed]
- Savoi, S.; Wong, D.C.J.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef]
- Alonso, R.; Berli, F.J.; Fontana, A.; Piccoli, P.; Bottini, R. Malbec grape (Vitis vinifera L.) responses to the environment: Berry phenolics as influenced by solar UV-B, water deficit and sprayed abscisic acid. Plant Physiol. Biochem. 2016, 109, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.; Dry, P.; Loveys, B. Influence of partial rootzone drying on the composition and accumulation of anthocyanins in grape berries (Vitis vinifera cv. Cabernet sauvignon). Aust. J. Grape Wine Res. 2008, 14, 91–103. [Google Scholar] [CrossRef]
- Stoll, M.; Loveys, B.; Dry, P. Hormonal changes induced by partial rootzone drying of irrigated grapevine. J. Exp. Bot. 2000, 51, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Shellie, K.C.; Bowen, P. Isohydrodynamic behavior in deficit-irrigated Cabernet sauvignon and Malbec and its relationship between yield and berry composition. Irrig. Sci. 2013, 32, 87–97. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; DI Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Manzi, D.; Masini, C.M.; Doni, S.; Mattii, G.B. Sustainable Soil Management: Effects of Clinoptilolite and Organic Compost Soil Application on Eco-Physiology, Quercitin, and Hydroxylated, Methoxylated Anthocyanins on Vitis vinifera. Plants 2023, 12, 708. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Manzi, D.; Peruzzi, E.; Mattii, G. Effects of Zeowine and compost on leaf functionality and berry composition in Sangiovese grapevines. J. Agric. Sci. 2023, 1–16. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of pre-and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Zombardo, A.; Mica, E.; Puccioni, S.; Perria, R.; Valentini, P.; Mattii, G.B.; Cattivelli, L.; Storchi, P. Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation. Agronomy 2020, 10, 680. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Di Gaspero, G.; Marconi, R.; Nonis, A.; Peterlunger, E.; Paillard, S.; Adam-Blondon, A.F.; Testolin, R. Colour variation in red grapevines (Vitis vinifera L.): Genomic organisation, expression of flavonoid 3′-hydroxylase, flavonoid 3′,5′-hydroxylase genes and related metabolite profiling of red cyanidin-/blue delphinidin-based anthocyanins in berry skin. BMC Genom. 2006, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Guidoni, S.; Ferrandino, A.; Novello, V. Effects of Seasonal and Agronomical Practices on Skin Anthocyanin Profile of Nebbiolo Grapes. Am. J. Enol. Vitic. 2008, 59, 22–29. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Regulated deficit irrigation effects on growth, yield, grape quality and individual anthocyanin composition in Vitis vinifera L. cv. ‘Tempranillo’. Agric. Water Manag. 2011, 98, 1171–1179. [Google Scholar] [CrossRef]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.-P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, Genetic, and Molecular Causes of Variation in Grape Berry Weight and Composition: A Review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef]
- Cooley, N.M.; Clingeleffer, P.R.; Walker, R.R. Effect of water deficits and season on berry development and composition of Cabernet sauvignon (Vitis vinifera L.) grown in a hot climate. Aust. J. Grape Wine Res. 2017, 23, 260–272. [Google Scholar] [CrossRef]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Romero, E.; Gómez-Alonso, S.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on the Phenolic Compounds of ‘Merlot’ Grapes in a Semi-Arid Mediterranean Climate. Horticulturae 2021, 7, 161. [Google Scholar] [CrossRef]
- Cabral, I.L.; Teixeira, A.; Lanoue, A.; Unlubayir, M.; Munsch, T.; Valente, J.; Alves, F.; da Costa, P.L.; Rogerson, F.S.; Carvalho, S.M.P.; et al. Impact of Deficit Irrigation on Grapevine cv. ‘Touriga Nacional’ during Three Seasons in Douro Region: An Agronomical and Metabolomics Approach. Plants 2022, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga-Arriazu, M.; Gomès, E.; Morales, F.; Irigoyen, J.J.; Pascual, I.; Hilbert, G. High Temperature and Elevated Carbon Dioxide Modify Berry Composition of Different Clones of Grapevine (Vitis vinifera L.) cv. Tempranillo. Front. Plant Sci. 2020, 11, 603687. [Google Scholar] [CrossRef]
- Parker, A.K.; de Cortázar-Atauri, I.G.; Gény, L.; Spring, J.L.; Destrac, A.; Schultz, H.; Molitor, D.; Lacombe, T.; Graca, A.; Monamy, C.; et al. Temperature-based grapevine sugar ripeness modelling for a wide range of Vitis vinifera L. cultivars. Agric. For. Meteorol. 2020, 285, 107902. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Pereira, G.E.; Gaudillere, J.-P.; Pieri, P.; Hilbert, G.; Maucourt, M.; Deborde, C.; Moing, A.; Rolin, D. Microclimate Influence on Mineral and Metabolic Profiles of Grape Berries. J. Agric. Food Chem. 2006, 54, 6765–6775. [Google Scholar] [CrossRef]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot noir) anthocyanins. 2. Anthocyanins and pigmented polymers in wine. J. Agric. Food Chem. 2007, 55, 6585–6595. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Torres, R.E. Effect of Controlled Day and Night Temperatures on Grape Coloration. Am. J. Enol. Vitic. 1972, 23, 71–77. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Poudel, P.R.; Koyama, K.; Goto-Yamamoto, N. Evaluating the influence of temperature on proanthocyanidin biosynthesis in developing grape berries (Vitis vinifera L. ). Mol. Biol. Rep. 2020, 47, 3501–3510. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Gambetta, G.A.; Matthews, M.A.; Kennedy, J.A. Impact of diurnal temperature variation on grape berry development, proanthocyanidin accumulation, and the expression of flavonoid pathway genes. J. Exp. Bot. 2012, 63, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.; Dal Santo, S.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole Plant Temperature Manipulation Affects Flavonoid Metabolism and the Transcriptome of Grapevine Berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape berry flavonoids: A review of their biochemical responses to high and extreme high temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef] [PubMed]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Impact of short temperature exposure of Vitis vinifera L. cv. Shiraz grapevine bunches on berry development, primary metabolism and tannin accumulation. Environ. Exp. Bot. 2019, 168, 103866. [Google Scholar] [CrossRef]
- Turóczy, Z.; Olejníčková, J.; Sotoláf, R. Effect of ambient sunlight intensity on the temporal phenolic profiles of Vitis vinifera L. Cv. chardonnay during the ripening season-a field study. S. Afr. J. Enol. Vitic. 2017, 38, 94–102. [Google Scholar]
- Haselgrove, L.; Botting, D.; van Heeswijck, R.; Høj, P.B.; Dry, P.R.; Ford, C. Canopy microclimate and berry composition: The effect of bunch exposure on the phenolic composition of Vitis vinifera L. cv. Shiraz grape berries. Aust. J. Grape Wine Res. 2000, 6, 141–149. [Google Scholar]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 533, 171–181. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol Profile Is a Reliable Indicator to Assess Canopy Architecture and the Exposure of Red Wine Grapes to Solar Radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-Da-Silva, J.M.; Deloire, A.J. Grape Flavonoid Evolution and Composition Under Altered Light and Temperature Conditions in Cabernet sauvignon (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light quality affects flavonoid biosynthesis in young berries of Cabernet sauvignon grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The Grapevine R2R3-MYB Transcription Factor VvMYBF1 Regulates Flavonol Synthesis in Developing Grape Berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-Viana, L.; Silva, M.F.; Bressan-Smith, R.; Cavagnaro, J.B.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar] [PubMed]
- Petit, A.-N.; Baillieul, F.; Vaillant-Gaveau, N.; Jacquens, L.; Conreux, A.; Jeandet, P.; Clément, C.; Fontaine, F. Low responsiveness of grapevine flowers and berries at fruit set to UV-C irradiation. J. Exp. Bot. 2009, 60, 1155–1162. [Google Scholar] [CrossRef]
- Vijay, P.; Vadewki, R. Canopy management control practices on fruit composition of wine grape cultivars grown in semi-arid tropical region of India. Int. J. Enol. Vitic. 2014, 1, 59–69. [Google Scholar]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Ranieri, A.; Castagna, A.; Martínez-Abaigar, J.; Núñez-Olivera, E. Secondary metabolites and related genes in Vitis vinifera L. cv. Tempranillo grapes as influenced by ultraviolet radiation and berry development. Physiol. Plant 2021, 173, 709–724. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Phenolic characteristics acquired by berry skins of Vitis vinifera cv. Tempranillo in response to close-to-ambient solar ultraviolet radiation are mostly reflected in the resulting wines. J. Sci. Food Agric. 2020, 100, 401–409. [Google Scholar]
- Singleton, V.L.; Timberlake, C.F.; Lea, A.G.H. The phenolic cinnamates of white grapes and wine. J. Sci. Food Agric. 1978, 29, 403–410. [Google Scholar] [CrossRef]
- Vendramin, V.; Pizzinato, D.; Sparrow, C.; Pagni, D.; Cascella, F.; Carapelli, C.; Vincenzi, S. Prevention of quercetin precipitation in red wines: A promising enzymatic solution. OENO One 2022, 56, 41–51. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Forino, M.; Blaiotta, G.; Moine, V.; Moio, L. New insights into the formation of precipitates of quercetin in Sangiovese wines. J. Food Sci. Technol. 2020, 57, 2602–2611. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The use of zeolites as an addition to fertilisers—A review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Manjaiah, K.M.; Mukhopadhyay, R.; Paul, R.; Datta, S.C.; Kumararaja, P.; Sarkar, B. Clay minerals and zeolites for environmentally sustainable agriculture. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–329. [Google Scholar]
- Szerement, J.; Szatanik-Kloc, A.; Jarosz, R.; Bajda, T.; Mierzwa-Hersztek, M. Contemporary applications of natural and synthetic zeolites from fly ash in agriculture and environmental protection. J. Clean. Prod. 2021, 311, 127461. [Google Scholar] [CrossRef]
- Calzarano, F.; Valentini, G.; Arfelli, G.; Seghetti, L.; Manetta, A.C.; Metruccio, E.G.; Di Marco, S. Activity of Italian natural chabasite-rich zeolitites against grey mould, sour rot and grapevine moth, and effects on grape and wine composition. Phytopathol. Mediterr. 2019, 58, 307–322. [Google Scholar]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Shellie, K.C.; King, B.A. Kaolin Particle Film and Water Deficit Influence Malbec Leaf and Berry Temperature, Pigments, and Photosynthesis. Am. J. Enol. Vitic. 2013, 64, 223–230. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, T.; Han, X.; Guan, L.; Zhang, L.; Wang, H.; Li, H. Kaolin Particle Film Affects Grapevine Berry Quality in cv. Meili in Humid Climate Conditions. HortScience 2020, 55, 1987–2000. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing Kaolin and Pinolene to Improve Sustainable Grapevine Production during Drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef]
- Teker, T. A study of kaolin effects on grapevine physiology and its ability to protect grape clusters from sunburn damage. Sci. Hortic. 2023, 311, 111824. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Matos, C.; Malheiro, A.; Flores, R.; Alves, S.; Costa, C.; Rocha, S.; Correia, C.; Luzio, A.; et al. Overview of Kaolin Outcomes from Vine to Wine: Cerceal White Variety Case Study. Agronomy 2020, 10, 1422. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Barros, A.; Pitarch-Bielsa, M.; Gómez-Cadenas, A.; Moutinho-Pereira, J. Kaolin impacts on hormonal balance, polyphenolic composition and oenological parameters in red grapevine berries during ripening. J. Berry Res. 2021, 11, 465–479. [Google Scholar] [CrossRef]
- Tombesi, S.; Frioni, T.; Sabbatini, P.; Poni, S.; Palliotti, A. Ascophyllum nodosum extract improves leaf thermoregulation by reducing stomatal sensitivity to VPD in Vitis vinifera L. J. Appl. Phycol. 2021, 33, 1293–1304. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Quaglia, M.; Calderini, O.; Moretti, C.; Poni, S.; Gatti, M.; Moncalvo, A.; Sabbatini, P.; Berrios, J.G.; et al. Metabolic and transcriptional changes associated with the use of Ascophyllum nodosum extracts as tools to improve the quality of wine grapes (Vitis vinifera cv. Sangiovese) and their tolerance to biotic stress. J. Sci. Food Agric. 2019, 99, 6350–6363. [Google Scholar] [PubMed]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef]
- Taskos, D.; Stamatiadis, S.; Yvin, J.-C.; Jamois, F. Effects of an Ascophyllum nodosum (L.) Le. Jol. extract on grapevine yield and berry composition of a Merlot vineyard. Sci. Hortic. 2019, 250, 27–32. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Martínez-Lapuente, L.; Costa, B.S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Phenolic composition of Tempranillo Blanco (Vitis vinifera L.) grapes and wines after biostimulation via a foliar seaweed application. J. Sci. Food Agric. 2020, 100, 825–835. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Storchi, P.; Mattii, G.B. Eco-physiological traits and phenylpropanoid profiling on potted Vitis vinifera L. cv Pinot noir subjected to Ascophyllum nodosum treatments under post-veraison low water availability. Appl. Sci. 2020, 10, 4473. [Google Scholar]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Ayestarán, B.; González-Lázaro, M.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of seaweed foliar application to Tempranillo grapevines on grape and wine phenolic compounds over two vintages. Food Chem. 2021, 345, 128843. [Google Scholar]
- Pessenti, I.L.; Ayub, R.A.; Marcon Filho, J.L.; Clasen, F.C.; Rombaldi, C.V.; Botelho, R.V. Influence of abscisic acid, Ascophyllum nodosum and Aloe vera on the phenolic composition and color of grape berry and wine of ‘Cabernet sauvignon’ variety. Cienc. Tec. Vitivinic. 2022, 37, 1–12. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).