Abstract
Field experiments were conducted to investigate the effects of K application on the nitrogen and potassium utilization efficiency and yield of foxtail millet (Setaria italica L.). The experiment was performed with a completely randomized design with two millet cultivars (Jingu 21 and Zhangza 10) and five K2O rates (0, 60, 120, 180, and 240 kg/hm2) in 2020 and 2021. We found that K promoted K and N absorption; significantly increased dry matter (DM), N, and K accumulation in millet organs; caused dry matter accumulation to peak earlier; and increased the DM accumulation rate. In addition, K accumulation preceded that of DM or N. Relative to the crop cycle, most K (61.07%) accumulated at booting, whereas N accumulated mostly (33.86%) during grain filling. N absorption efficiency increased by 31.87%, and the apparent and agronomic utilization rate of K fertilizer remained high, increasing millet yield, which peaked at a K rate of 180 kg/hm2 for both cultivars, by 29.91% and 31.51% in Jingu 21 and Zhangza 10, respectively, relative to untreated controls. Stepwise regression and path analysis showed that the leaf and spike K accumulation, stem N accumulation, and stem DM were the main factors affecting yield, with DM having the greatest direct effect, followed by leaf K accumulation. The K concentration (0.77–3.04%) in Zhangza 10 was higher than that in Jingu 21 (0.69–2.91%) in untreated plants. Under the same K application rate, N and K accumulation and the harvest index were higher for Zhangza 10 than those for Jingu 21, and the nutrient utilization ability was greater for Zhangza 10. The results demonstrated that rational K fertilizer application can increase K concentration and accumulation in leaves, promote N metabolism and accumulation, increase N and K utilization efficiency, and improve DM accumulation and millet yield.
1. Introduction
Potassium is an essential macronutrient in crop plants and the primary cation in the cytoplasm. Indeed, K plays a vital role in crop growth and development, as it is involved in various functions, including enzyme activation, maintenance of membrane electric potential, osmoregulation, cell expansion, and plant responses to abiotic stress [1,2]. Furthermore, K is required for optimizing N metabolism, photosynthetic performance, and photosynthate transport [3,4], thus, exerting a great influence on the determination of crop yield and quality.
Average soil reserves of K are generally large, but approximately 90–98% of total soil K is unavailable [5]. In contrast to a relatively high and stable K+ concentration (~100 mM) in the cytoplasm to maintain metabolic homeostasis, the bio-available K+ levels, including exchangeable K and water-soluble K, in most natural soils are limited and account for 1–2% of total soil K [6,7]. The increase in the multiple-crop index and the promotion of high-yield and quality varieties leads to a large amount of soil potassium depletion during crop harvest, and it is difficult to obtain enough potassium from the soil during the crop growth and development stage [8]. Large areas of agricultural land in the world are deficient in K (e.g., 2/3 of the wheatbelt in southern Australia and the Mekong River Delta in Vietnam), with the absorption of agricultural products and leaching (especially in sandy soils) contributing to the lowering of the K content in the soil [9,10]. The potassium deficiency in arable fields has restricted the sustainable development of agricultural production [11]. In the case of unbalanced fertilization, a lower K application can lead to significant depletion of available soil K reserves, resulting in a decline in soil fertility and an ongoing downward trend of negative K balance [5,12]. Soil K deficiency hinders normal crop plant development, reduces the absorption and utilization of water and nutrients, and affects the synthesis and transportation of photosynthates by affecting the diffusion of CO2 in chloroplasts, phloem-loading, and the long-distance transport of photoassimilates, ultimately decreasing crop yield [13,14]. Potassium is closely related to the root absorption of and K+ and the coordinated transportation of the phloem sap [15,16]. Further, K significantly affects the synthesis and degradation of amino acids and proteins during N metabolism [2], which, in K-deficient plants, is severely inhibited. Insufficient K not only affects its own absorption and utilization but limits the absorption of other nutrients and reduces fertilizer utilization rates as well [17]. In contrast, rational fertilization to improve crop nutrient-use efficiency is an effective measure for reducing agricultural production costs and improving crop yield and product quality. Clarifying the K nutritional characteristics of foxtail millet is of practical significance as a premise for the rational application of K fertilizers.
Nutrient absorption is the physiological basis of dry matter accumulation, while optimizing the process of dry matter accumulation is the material basis for improving crop yield [18]. Quantitative analysis of the dynamics of dry matter and nutrient accumulation in crops is beneficial for implementing effective real-time nutrient control measures and obtaining high yields. K-efficient genotypes have a higher acquisition of K from soil (uptake efficiency) and/or higher dry matter production per unit of K taken up (utilization efficiency); moreover, genotypes efficient in K uptake may have a larger surface area of contact between the roots and soil and increased uptake at the root–soil interface to maintain a larger diffusive gradient towards the roots [19]. For example, Wu et al. [20] showed that fertilization significantly improved the dry matter accumulation and yield of winter wheat and that there was a highly significant positive correlation between them. In turn, Gu et al. [21] found that the dry matter accumulation in millet before flowering accounted for 55–60% of the total accumulation, and the contribution rate to spikes was 8–10%. Further, K can reportedly increase dry matter accumulation by increasing the maximum dry matter accumulation rate in rice and promoting dry matter transport in the stem and sheath to increase yield, whereas N mainly increases dry matter accumulation by prolonging the duration of rapid dry matter accumulation [22]. Potassium application increases the proportion of post-anthesis dry matter accumulation and the extent of dry matter transport to the ear. Additionally, it promotes the transport of pre-flowering stored nutrients to the grains and has a great effect on N transport, which increases the contribution of nutrient transport to the developing grains at the filling stage [23]. Furthermore, an appropriate K application rate can increase the nutrient transport from vegetative organs to reproductive organs, transport efficiency, and the grain nutrient ratio [24].
Previous studies have focused primarily on the characteristics of dry matter accumulation and transport before and after anthesis under different K supply conditions [25]. However, based on the entire growth period, few studies have explored the mechanisms of K-mediated N accumulation, the distribution of dry matter and nutrients among plant organs, and their contribution to yield components. In this study, we explored the dynamic changes in dry matter, N, and K accumulation in various organs of millet plants under different K fertilizer application rates using two contrasting foxtail millet varieties for the response to K fertilizer application. Our findings provide a theoretical basis for the rational application of K fertilizer and more efficient cultivation of foxtail millet.
2. Materials and Methods
2.1. Experimental Location
Field experiments were conducted from May to October 2020 and 2021, at the Experimental Station of the Shanxi Agricultural University (Shenfeng Village, Taigu County, Jinzhong City, Shanxi Province, China; 37° 25′ 24.26″ N, 112° 34′ 42.34″ E; 804 m elevation). Average temperature and rainfall were calculated every 15 days from 13 May to 9 October in both years (Figure 1). Seeds were sown on 13 and 18 May, and plants were harvested on 6 and 3 October, 2020 and 2021, respectively. The average temperature and rainfall over the whole millet growth were 21.3 and 22.2 °C and 419.1 and 331.8 mm in 2020 and 2021, respectively. Rainfall from 15 September to 9 October 2021 (harvest) accounted for 17.4% of the total rainfall during the cropping season.
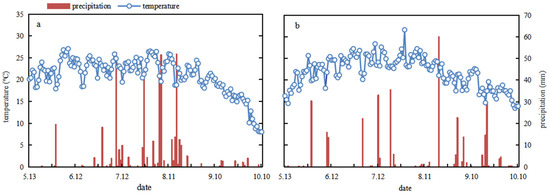
Figure 1.
Temperature and precipitation during the millet growing period in (a) 2020 and (b) 2021.
2.2. Experimental Design
The experiment was performed in a completely randomized design with three replicates. The study site had a temperate continental climate. The soil was loam (carbonate brown soil), and the physicochemical characteristics of the 0–20 cm topsoil layer were as follows: pH, 7.63; organic matter content, 16.93 g·kg−1; total N, 0.81 g·kg−1; alkali-hydrolyzed N, 51.84 mg·kg−1; available K, 98.75 mg·kg−1; and available P, 22.37 mg·kg−1. The treatments included five K (K2O) fertilization rates: 0 (K0) as control, 60 (K60), 120 (K120), 180 (K180), and 240 (K240) kg/hm2 of K2O. Nitrogen (150 kg/hm2 of N) and phosphorus (90 kg/hm2 of P2O5) fertilizers were applied. Fertilizers used were urea (42% N), superphosphate (12% P2O5), and K sulfate (50% K2O). All fertilizers were broadcasted before sowing. Jingu 21 (K-sensitive) and Zhangza 10 (K-insensitive) foxtail millet cultivars were used in this study. Experimental units were comprised of square 25-m2 plots. Millet seeds were mechanized and sown by drilling about 4 cm deep and with 35 cm between the rows. Seedlings were thinned out at the three-leaf stage to maintain a plant distance of 8 cm and 12 cm for Jingu 21 and Zhangza 10, respectively. Weed control was performed by hand during the millet growing period, without artificial irrigation and pest control.
2.3. Biomass
After sowing, six representative plants were sampled from each experimental plot every 27 days in 2020 and every 17 days in 2021. Plant height and stem diameter were measured. Then, the plant samples were separated into different parts, including the roots, stems, leaves, and panicles. All samples were oven-dried at 105 °C for 30 min and then to a constant weight at 85 °C to determine the dry weight.
2.4. Plant N and K Concentration
Three 0.2-g dry powdered samples per treatment were digested with a mixture of concentrated H2SO4 and H2O2. Then, the K concentration was determined using atomic absorption spectrometry (ZEEnit 700P, Jena, Germany) after dilution, and the N concentration was determined using a Discrete Chemistry Analyzer (Smartchem 200, AMS, Roma, Italy).
2.5. Enzyme Extraction and Analysis
Millet leaves were homogenized at 4 °C with extraction buffer (50 mmol·L−1 Tris-HCl (pH 8.0), 2 mmol·L−1 MgSO4, 2 mmol·L−1 DTT, 0.4 mol·L−1 sucrose); the homogenates were centrifuged at 15,000× g at 4 °C for 20 min and the supernatant was used for glutamine synthase (GSs) activity assays according to Seabra et al. [26]. Reactions were started by the addition of protein extracts (0.7 mL) to an assay mixture (1.6 mL) composed of 100 mmol·L−1 Tris (pH 7.4), 80 mmol·L−1 Mg2+, 20 mmol·L−1 glutamate, 20 mmol·L−1 cysteine, 2 mmol·L−1 EDTA, and 80 mmol·L−1 hydroxylamine at the same time, plus 0.7 mL of 20 mmol·L−1 ATP. The reaction proceeded at 37 °C for 30 min and was stopped by the addition of l mL color agent composed of 0.2 mmol·L−1 trichloroacetic acid, 0.37 mmol·L−1 FeCl3, and 0.6 mol·L−1 HCl. The γ-glutamyl hydroxamate concentration was determined spectrophotometrically (Mapada UV1800) at 540 nm. The GS-specific activity was determined as the μmol of γ-glutamyl hydroxamate produced per hour per mg of protein. The protein concentration was measured via the Coomassie dye-binding assay using BSA as a standard.
For measuring the activity of nitrate reductase (NR), 1.0 g of fresh leaf tissue was incubated in 100 mM potassium phosphate buffer (pH 7.5) containing KNO3 (200 mM) for 30 min in the dark at 30 °C. Thereafter, a 1 mL aliquot was taken and 2 mL sulphanilamide (1%) and 2 mL 1-naphthylethylene diamine dihydrochloride (0.2%) were added. Absorbance was recorded at 520 nm [27].
2.6. Yield
All plants within three 2-m2 subplots randomly selected within each experimental plot were hand-sampled at maturity. All spikes in each subplot were harvested and dried for one week until the grains’ water content was about 13% and then threshed to determine yield.
2.7. Calculation Formulae for Relevant Parameters
A logistic equation was used to fit the dynamics of dry matter accumulation in millet shoots. The logistic curve model was expressed as follows: W = W0/(1 + ae−bt), according to Hu et al. [28], where W stands for dry matter accumulation, W0 is the potential maximum accumulation, a is the retardation coefficient of dry matter accumulation, b is the growth rate, and t is the number of days after sowing. The first derivative of the logistic equation was used to obtain the dry matter accumulation rate.
Characteristic parameters were calculated as follows: The third derivative of the logistic equation can be used to calculate three key time points of dry matter accumulation in millet: start point of the rapid growth period T1 = (lna − 1.317)/b; peak period Tmax = (lna)/b; and end point of the rapid growth period T2 = (lna + 1.317)/b.
K (N) accumulation value (KAV, NAV, kg) = shoot dry matter weight × shoot K (N) concentration.
K (N) absorption efficiency (KAE, NAE, kg/kg) = shoot absorption at the mature stage/K (N) application rate.
K (N) harvest index (KHI, NHI, %) = KAV, NAV in grain/KAV, NAV in shoots × 100.
The apparent utilization rate of K fertilizer (AURK, %) = (KAV in plants receiving K application − KAV in plants receiving no K application)/K application rate × 100.
Agronomic efficiency of K fertilizer (AEK, kg/kg) = (grain yield receiving K application − grain yield receiving no K application)/K application rate.
2.8. Statistical Analysis
All figures were drawn using Excel 2010. SPSS 25.0 was used to analyze the differences among the independent samples t-test, analysis of variance (ANOVA), and multiple comparisons (Duncan) (p = 0.05). Logistic model fitting, stepwise regression, and path analyses were performed using SPSS software. Stepwise regression and path analyses were performed to clarify the effects of dry matter and K and N accumulation on grain yield.
3. Results
3.1. Potassium Promoted Dry Matter Accumulation in Foxtail Millet
The dry matter accumulation and the accumulation rate in millet shoots treated with different K fertilizer application rates showed similar dynamics with the time-course (Figure 2). Potassium significantly increased the accumulation and accumulation rate of dry matter during the entire growth period; however, there was no significant difference between the K120 and K180 treatments. As growth proceeded, dry matter accumulation in shoots followed a sigmoidal pattern. In each treatment, the biomass increased rapidly in the early stages, and differences among treatments were small. Subsequently, at the late growth stage, dry matter accumulation was slow and tended to be stable; however, the difference among treatments increased. Compared to the K0 treatment, at maturity, the dry matter accumulation of Jingu 21 and Zhangza 10 under the K180 treatment increased significantly by 19.53% and 17.00%, respectively. In turn, the rate of dry matter accumulation initially increased and then decreased, showing a single peak, and finally tending to zero. The rate peaked during the booting-to-filling stage when the dry matter accumulation rate in the K120 and K180 treatments increased significantly.
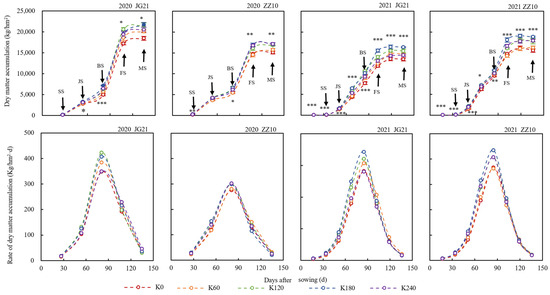
Figure 2.
Time-course of dry matter accumulation and accumulation rate in the aboveground millet Jingu 21 (JG21) and Zhangza 10 (ZZ10); *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively. Potassium applications include five levels: 0 (K0) as control and 60 (K60), 120 (K120), 180 (K180), and 240 (K240) kg/hm2 of K2O. SS: seedling stage; JS: jointing stage; BS: booting stage; FS: filling stage; MS: and maturity stage.
The aboveground millet biomass was fitted using a logistic model, and the dynamic change model for dry matter accumulation for each treatment was obtained (Table 1). The R2 between the measured and predicted values of the fitting equation for each treatment of Zhang Za 10 in 2020 reached a significant level, while the rest reached a highly significant level, indicating that the logistic equation simulates the dynamics of dry matter accumulation accurately. Thus, the maximum potential accumulation (W0) of Jingu 21 in the two years reached a maximum under the K180 treatment, which significantly increased by 15.92% (2020) and 21.50% (2021) compared with the K0 treatment. Zhangza 10 reached its maximum under the K120 (2020) and K180 (2021) treatments, significantly increasing by 11.70% (2020) and 18.36% (2021) compared with the K0 treatment. Compared with the K0 treatment, the peak period (T1) for dry matter accumulation occurred 2.22 and 4.08 d earlier in Jingu 21 and Zhangza 10, respectively, under the K180 treatment. Further, although the optimal K application rate advanced the rapid dry matter-accumulation period in the two varieties, it had no significant effect on the duration of rapid dry matter accumulation (ΔT). Specifically, the rapid dry matter-accumulation period occurred earlier in Zhangza 10 than in Jingu 21, and ΔT was longer in the former than in the latter. Potassium increased the maximum dry matter-accumulation rate (Vmax) in both cultivars. Compared with the K0 treatment, the Vmax of Jingu 21 and Zhangza 10 increased by 0.17–21.47% (with an average increase of 11.45%) and 1.64–28.63% (with an average increase of 8.40%), respectively, under the K60–K240 treatments.

Table 1.
The logistic model and character value of dry matter accumulation in the aboveground millet plant body.
Further analysis of the dry matter accumulation dynamics in various organs of millet showed that root and grain dry matter increased rapidly and then tended to be stable during the growth process in both Jingu 21 and Zhangza 10, whereas, in the stems and leaves, it reached a maximum at the early filling stage and then showed a decreasing trend (Figure 3). Potassium significantly increased the dry matter accumulation in various millet organs, particularly after the rapid dry matter accumulation period (Supplementary Table S1). Specifically, compared to the K0 treatment, the dry matter accumulation in stems, leaves, and grains increased by 19.34, 19.31, and 19.87%, respectively, in plants of Jingu 21 under the K180 treatment. Meanwhile, the corresponding increases in plants of the cultivar Zhangza 10 were 23.13, 17.76, and 13.88%, respectively. As for grain, the dry matter accumulation was 9217–10,835 kg/hm2 in 2020 and 5072–6197 kg/hm2 in 2021, in the spikes of Jingu 21. The major difference between the two years may be partly due to more rainfall in early 2020, and higher soil moisture usually means greater K availability. Increasing soil moisture increases K’s movement to plant roots and enhances availability, allowing the millet to absorb more K in a diffused absorption manner. In contrast, the grain filling in the spikes of Jingu 21 was influenced by the continuous rain during the late filling stage in 2021,whereas the rapid grain filling in the spikes of Zhangza10 occurred earlier than the continuous rain period, which had little influence on dry ma atter accumulation in this case.
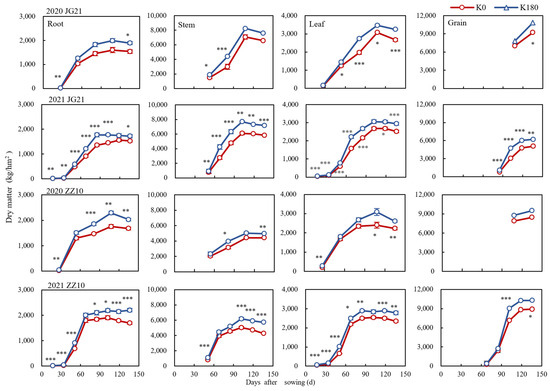
Figure 3.
Time-course of the dry matter accumulation in different organs of millet; *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively. Potassium applications include K0 and K180.
3.2. Potassium Application Increased K and N Concentrations in Foxtail Millet
Potassium fertilization significantly increased the K concentration in various millet organs (Figure 4 and Supplementary Table S2). In particular, compared with the K0 treatment, K180 increased the K concentration by 54.24% and 43.69% in the Jingu 21 leaves and grains, respectively, and by 65.26% and 48.34%, respectively, in Zhangza 10. The effect of the K concentration on the leaves was greater than that on the grains. The whole-plant K concentration accounted for 0.41–2.00% of the dry weight of millet plants. Further, under K0, the shoot K concentration in Zhangza 10 was 0.77–3.04%, which was slightly higher than that in Jingu 21 (0.69–2.91%). As for the root K concentration, this showed an approximate “M”-shaped change trend (2021) with growth, while decreasing continuously in 2020 due to the long sampling interval. The potassium concentration in the stems first decreased and then increased as the growth proceeded and reached the lowest level at the grain-filling stage. Potassium was translocated gradually to stems at the late grain-filling stage, which led to its allocation mainly in the stems at maturity and accounted for 60.79–71.97% of the total plant K concentration. In turn, the leaf K concentration increased continuously during the first month after sowing and then decreased continuously. Lastly, the grain K concentration was highest at the early heading stage. As grain filling proceeded, the K concentration first decreased rapidly, then slowly, and finally decreased to 0.13–0.23%.
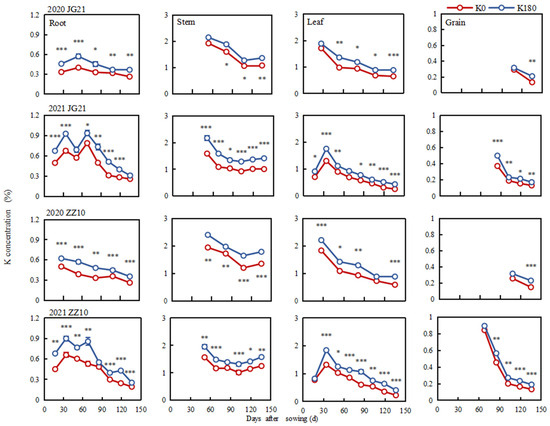
Figure 4.
Potassium concentration time-course in different millet organs. *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively.
Nitrogen is mainly concentrated in the leaves and stems during the vegetative growth stage and was gradually translocated to the spikes during reproductive growth. The nitrogen concentration accounted for 0.57–2.87% of the total millet plant dry weight (Figure 5). The root N concentration showed a trend of increase-decrease-increase. Meanwhile, the stem N concentration decreased sharply and then tended to be stable at 0.13–0.38% in the stems of mature plants. In turn, the leaf N concentration peaked at 2.79–3.31% one month after sowing and then decreased continuously. Finally, the grain N concentration initially decreased and then increased, reaching 1.36–1.92% at maturity, when it accounted for 41.23–61.94% of the whole plant N content. The potassium application increased N concentration in different millet organs (Supplementary Table S3). Thus, in the roots, it significantly increased N concentration, particularly before jointing. Furthermore, compared to the K0 treatment, the root N concentrations in plants of Jingu 21 and Zhangza 10 grown under the K180 treatment increased by 64.29% and 39.01%, respectively, which was higher than the N increase in the leaves of the two varieties, i.e., 12.42% and 8.36%, respectively, during the same period. At the grain-filling stage, the N concentration in the stems and leaves of Jingu 21 significantly increased by 77.08% and 22.26%, respectively, while that of Zhangza 10 increased by 115.42% and 34.18%, respectively, under the K180 treatment, compared to the K0 treatment. Further, compared with the K0 treatment, the N concentration of Jingu 21 and Zhangza 10 at maturity significantly increased by 17.81% and 21.57%, respectively, under the K180 treatment. These results indicate that K fertilizer application seemingly promoted root N-uptake and transport to other plant organs, thereby promoting an increase in grain N concentration.
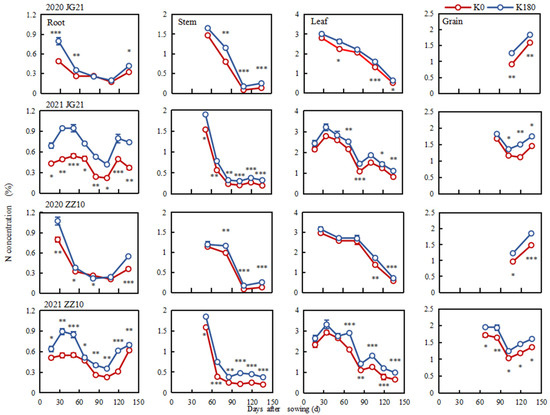
Figure 5.
Nitrogen concentration dynamics of different millet organs. *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively.
3.3. Potassium Fertilization Improved K and N Accumulation in Foxtail Millet Plants
The application of K fertilizer significantly increased K accumulation in shoot organs and lead to growth in millet plants (Figure 6 and Figure 7). There were significant differences in the dynamic changes of K accumulation in the various organs of millet plants during the entire growth period (Figure 6). Thus, the K accumulation in the roots and leaves first increased and then decreased, peaking at the booting stage. In turn, the K accumulation in stems gradually increased, whereas that in the grains first increased and then decreased with grain filling progression. In contrast, the K accumulation was highest in the stems and was significantly higher than that in the leaves, grains, or roots, in which case (i.e., roots) it was the lowest among all the plant organs studied.
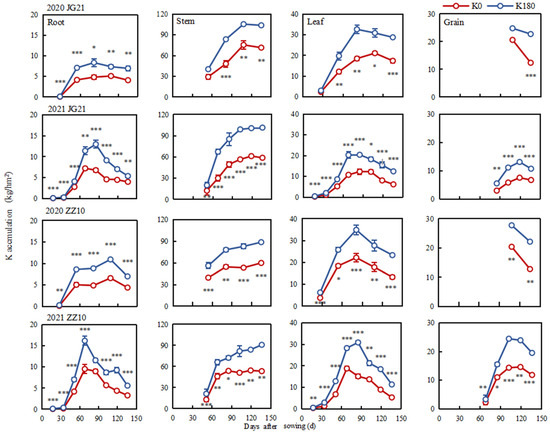
Figure 6.
Time-course of potassium accumulation in different millet organs. *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively.
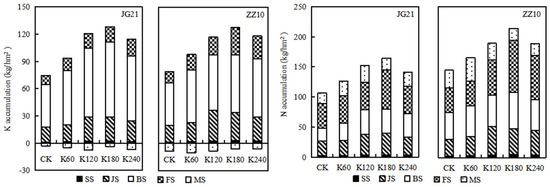
Figure 7.
Effect of K application on aboveground K and N accumulation in millet at different growth stages. SS: seedling stage; JS: joining stage; BS: booting stage; FS: filling stage; and MS: maturity stage.
Potassium fertilization significantly promoted K accumulation in various millet organs but did not change the regulatory pattern of such accumulation (Supplementary Table S4). Rapid K accumulation occurred mainly at the booting stage, accounting for 61.07% of the highest accumulation during the entire growth period. This is important because adequate K accumulation lays the foundation for reproductive growth. Compared with the K0 treatment, the K accumulation in Jinju 21 and Zhangza 10 treated with K60–K240 increased the most during grain filling, at 10.79–17.00 and 13.05–35.18 kg/hm2, respectively (Figure 7). Treatments K60, K120, K180, and K240 increased K accumulation at maturity by 18.69, 50.93, 63.98, and 46.84%, respectively, compared to the K0 treatment. Specifically, the K in grains increased by 1.67–10.37 kg/hm2 and 2.70–9.37 kg/hm2 in Jingu 21 and Zhangza 10, respectively (Figure 6 and Supplementary Table S5).
Potassium fertilizer application significantly increased N accumulation at each growth stage and in the various millet organs studied herein (Figure 7 and Figure 8). Nitrogen accumulation in the shoots increased continuously and occurred mainly during the grain-filling stage. Thus, N accumulation at the seedling, jointing, booting, grain filling, and maturity stages accounted for 2.31, 21.31, 26.48, 33.86, and 16.03% of the total accumulation, respectively. Compared with K0, treatments K60–K240 increased N accumulation in Jingu 21 at grain filling (0.21–31.45 kg/hm2) and in Zhangza 10 at booting (7.91–19.88 kg/hm2). Further, the N accumulation in the roots, stems, and leaves of millet plants generally followed a bimodal curve, increasing in the grains with grain-filling progression (Figure 8). Specifically, the N accumulation in the grains was highest at maturity and in the order grain > leaf > stem > root. Potassium fertilization promoted N accumulation in millet (Figure 8 and Supplementary Table S6). Nitrogen accumulation increased by 47.81% and 55.16%, respectively, in the leaves of Jingu 21 and Zhangza 10 plants grown under the K180 treatment at grain filling, while at maturity, it increased by 71.77% and 69.23%, respectively, in the grains of Jingu 21 and Zhangza 10, relative to the K0 treatment, which laid the foundation for yield formation.
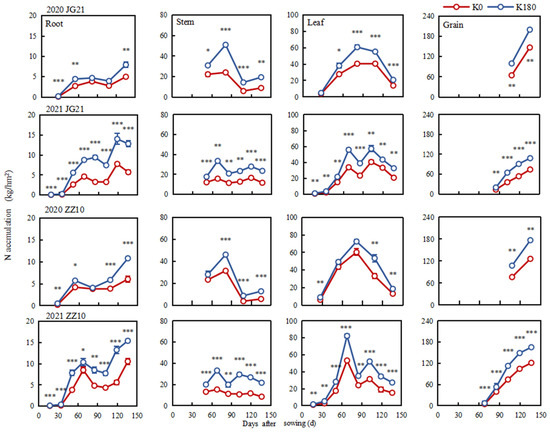
Figure 8.
Nitrogen accumulation time-course in different millet organs. *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively.
Nitrogen and K accumulation in millet shoots were not synchronized under K fertilization, and a net K loss was observed concomitant with a net N accumulation in the reproductive growth stage. With the progression of the growth process, the K accumulation in the shoot increased at first and then tended to stabilize or slightly decrease, likely due to the loss, leaching, and transfer of K with dry matter redistribution in the late growth stage. Potassium fertilization promoted N accumulation, and K was greater in the middle and late growth stages (late booting and maturity) than in the early growth stages (seedling and early booting). The accumulation of K and N first increased and then decreased with increasing K application rate, and reached a maximum under the K180 treatment, but the promotive effect of excessive K application on nutrient accumulation was not significant (Figure 7).
3.4. Potassium Improves the Activity of Enzymes Related to N Metabolism in Millet
Nitrate reductase (NR) and glutamine synthase (GS) are closely related to the conversion and absorption of . Nitrate reductase activity at the filling stage was higher than that at the booting stage; however, no significant difference in GS activity was observed (Figure 9). Potassium fertilization increased the activities of NR and GS in the leaves of Jingu 21 and Zhangza 10 at the booting and grain-filling stages. However, excessive K application did not further improve their activities, and there was no significant difference in NR activity among the K120, K180, and K240 treatments at grain filling. Compared with the K0 treatment, GS activity increased by 8.52–31.52% and 12.70–29.46%, respectively, in Jingu 21 and Zhangza 10 at the booting stage. Similarly, at the grain-filling stage, the corresponding activities in Jingu 21 and Zhangza 10 increased by 2.37–26.84% and 24.97–49.35%, respectively.
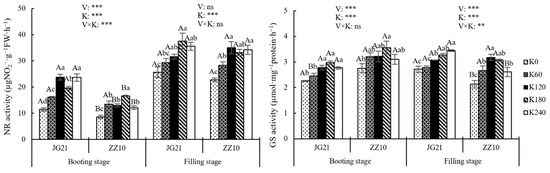
Figure 9.
Effects of potassium application rate on NR and GS activity of foxtail millet plants (2021). Different lowercase letters of the same variety show significant differences among different K rates at the 5% level, and different uppercase letters of the same K rates show significant differences among different varieties at the 5% level. ns, not significant; *, **, and *** indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, respectively. NR, nitrate reductase; GS, glutamine synthase.
3.5. Potassium Application Improves Millet Nutrient Utilization Efficiency and Grain Yield
Potassium fertilization significantly increased millet yield. Compared with untreated plants, grain yield increased by 4.03–37.84% and 4.65–31.56% for Jingu 21 and Zhangza 10, respectively (Table 2). The yield variation in Jingu 21 was greater than that observed for Zhangza 10 and was more responsive to K fertilizer application. Additionally, the K absorption efficiency decreased gradually with increasing application rate, and there were significant differences among treatments. In turn, the N absorption efficiency first increased and then decreased, reaching a peak in the K180 treatment; however, there was no significant difference between the K120 and K180 treatments in Jingu 21, and there was no significant difference between the K120, K180, and K240 treatments in Zhangza 10. Under the same K application rate, the K and N AE values were higher for Jingu 21 than those for Zhangza 10, while the K and N HI were opposite. These results indicate that Jingu 21 (K-sensitive) was more responsive to K than Zhangza 10 (K-insensitive). The foxtail millet KHI increased in 2020 and decreased in 2021, likely due to the influence of continuous rain on the transfer of photosynthates and K from the stems and leaves to the grains in the late-filling period of 2021. The apparent utilization rate of K fertilizer reflects the K utilization efficiency of millet plants, while the agronomic efficiency of K fertilizer reflects the response of grain yield to K fertilizer. Both efficiencies first increased and then decreased with increasing K rate. The apparent utilization rate of K fertilizer in Jingu 21 and Zhangza 10 reached a maximum under the K120 treatment, which indicated that K fertilizer application at a rate of 120 kg/hm2 effectively met the growth demand of millet plants. Meanwhile, the agronomic efficiency of K fertilizer reached a maximum under the K180 treatment, which indicated that K fertilizer application at a rate of 180 kg/hm2 effectively improved and achieved maximum yield. The apparent utilization rate of K fertilizer in Jingu 21 ranged from 4.99% to 26.82% and that in Zhangza 10 ranged from 3.49% to 14.57%.

Table 2.
Nutrient utilization efficiency of foxtail millet under different K application rates.
3.6. Effects of N and K Accumulation, and DM Accumulation on Millet Yield
Leaf photosynthates are the main source of material for plant growth and development. Correlation analyses were performed between the leaf N and K concentration, N metabolism-related enzyme activities, dry matter, N and K accumulation, and millet yield (Table 3). This analysis demonstrated a significant positive correlation between the yield and K accumulation, N accumulation, leaf N concentration, and N metabolism. Additionally, the leaf K concentration significantly correlated with dry matter, K accumulation, N accumulation, N concentration, and N metabolism-related enzyme activity. These findings indicate that reasonable K fertilization promotes N metabolism and accumulation by increasing the K concentration and accumulation in millet leaves, whereby, the DM accumulates concomitantly with a grain yield increase.

Table 3.
Correlation analysis of the yield, dry matter, N and K accumulation, leaf N concentration, NR, GS, and leaf K concentration.
To further explore the effects of DM, N, and N accumulation in different organs during grain filling on yield, a stepwise regression analysis was performed. Stem K accumulation (X1), leaf K accumulation (X2), grain K accumulation (X3), stem N accumulation (X4), leaf N accumulation (X5), grain N accumulation (X6), stem DM (X7), leaf DM (X8), and spike DM (X9) were set as independent variables, and millet yield (Y) was set as the dependent variable. The following multilinear equation and fit values were obtained:
(R = 0.883, F = 104.207, p = 0.0001)
This indicated that the above indexes were the main factors affecting the formation of millet yield. Path analysis further clarified the regulatory effects of the indices determined by the stepwise regression analysis of yield (Table 4). The results showed that stem DM had the greatest direct effect on yield, followed by leaf K accumulation, whereas stem N accumulation had a negative effect on yield. In addition, the indirect effect of grain K accumulation on yield through leaf K accumulation was significant.

Table 4.
Path coefficients of yield and dry matter, N and K accumulation in stem, leaf, and grain.
4. Discussion
4.1. Potassium Fertilization Promoted Dry Matter Accumulation
Potassium can promote leaf expansion and chlorophyll synthesis, improve chloroplast structure, regulate stomatal morphology, and increase the CO2 assimilation rate to improve leaf photosynthesis [29]. Dry matter is the final product of photosynthesis in plants. Further, DM accumulation in grains is mainly due to the transport and distribution of assimilates produced and stored in vegetative organs pre-anthesis to the grains formed post-anthesis [30]. Consequently, DM accumulation and partitioning are important factors affecting grain yield formation [31]. Soil fertility can significantly regulate the accumulation and partitioning of dry matter in plants [32]. Specifically, under- and over-fertilization reportedly have a significant effect on crop yield, with a reduced DM partitioning ratio of vegetative organs, and a reduced material basis for yield formation under conditions of low fertility [33,34]. In contrast, excessively high fertilization causes vegetative organs to grow, resulting in a decrease in the proportion of DM partitioning to the seeds [35]. Conversely, rational K application promotes photosynthate transport to reproductive organs [36]. Potassium application increased the maximum potential accumulation of dry matter and K in cotton and extended the duration of the maximum accumulation rate of dry matter in low-K-sensitive cotton [28]. Similarly, the K application increased DM accumulation by increasing the maximum DM accumulation rate in rice. When the K application rate was lower than 180 kg/hm2, the DM transport of rice increased with increasing K application rate [22]. In turn, Li et al. [37] found that K mainly affects the DM accumulation of millet before jointing and at the late growth stage, which might improve the DM transport rate of millet and increase DM accumulation at the late growth stage, especially at the grain formation stage. In this study, the period of rapid DM accumulation ranged from the booting to grain filling stage. Potassium application increased the potential maximum DM accumulation in millet and caused an earlier peak period of DM accumulation (Table 1). Potassium application increased the DM accumulation rate in millet and then increased DM accumulation, which was related to the improvement of the leaf photosynthetic rate and the coordination of sink–source relationships to promote photosynthate accumulation [38].
4.2. Potassium Application Improves N and K Utilization Efficiency
Crop yield is based on biomass, and biomass accumulation is based on nutrient absorption. Potassium application can improve crop nutrient absorption and coordinate the accumulation and utilization of N and P fertilizers [39]. Further, K application significantly increased the amount and rate of K accumulation at different growth stages in rice and significantly increased DM and K accumulation in various organs of rice, but the peak period of K accumulation was delayed, and the duration of the rapid accumulation period was shortened [40]. In maize, K absorption is mainly concentrated in the early growth stage and gradually decreases during grain filling; furthermore, the amount of nutrient accumulation in the late growth stage largely determines the grain yield [23]. Potassium application rates have a significant influence on straw K absorption, and the ratio of wheat kernel and straw K absorption to total K absorption increases with increasing K application rate [41]. In this study, the K accumulation in millet stems at maturity accounted for 62.87–78.05% of whole-plant K accumulation, which means excess K absorption (Figure 7), whereas the absorption capacity of grains was limited; this phenomenon has also been observed in wheat [42]. Hou et al. [43] found that the higher the amount of K application in rice, the higher the N uptake and N use efficiencies, but there was no significant effect on the NHI. The increase in K accumulation in the stem reduces the crop KHI, and the stem should be returned to the field after rotting or overfeeding to ensure the balance of K nutrition in the field. Potassium-efficient genotypes had a strong tolerance to low K, and their K+ redistribution, dry matter distribution, and KHI were significantly higher than that of low-efficiency genotypes when the K supply was insufficient [44,45]. In this study, Zhangza 10 maintained a high K concentration without K application and greater roots (Supplementary Table S2; Figure 3), with a higher NHI and KHI (Table 2), indicating Zhangza 10 is the cultivar with more K absorptive ability. The KAE and NAE of Jingu 21 were higher than that of Zhangza 10, and at the same time, the KUE (yield per unit crop K content, g Y g−1 Kcrop; White et al. [46]) of Jingu 21 was higher (Table 2, Supplementary Table S4), indicting Jingu 21 is the cultivar with more K utilization efficiency. Nutrient absorption efficiency is an important index for characterizing plant nutrient-absorption ability, and rational fertilization can significantly improve grain nutrient-utilization efficiency [47]. However, once the amount of K applied exceeds the crop requirement, K use efficiency will decline [48]. Crop K absorption efficiency gradually decreases with an increase in K application rate [49], while the appropriate K fertilizer improves root characteristics [50], promotes N absorption and metabolism, increases leaf N concentration [2] and N transfer to ears, and increases N and P absorption efficiency [23]. In this study, K application promoted the absorption of N by millet, enhanced the activities of NR and GS, and increased the proportion of N accumulated in millet roots and stems (Figure 9 and Supplementary Table S7). With the increase in the K application rate, the absorption and utilization rate of K fertilizer and agronomic efficiency of K fertilizer first increased and then decreased, reaching a maximum under the K120 and K180 treatments, respectively (Table 2).
4.3. Effects of N and K Accumulation and Dry Matter Accumulation on Yield
Adequate nutrient supply is the key to high crop yields. Rational fertilization accelerates the accumulation of nutrients and DM and coordinates source–sink relationships, which is conducive to yield formation [51]. In turn, nutrient accumulation in crops is the basis of biomass accumulation and crop yield formation [52]. Furthermore, there is a time difference between them and there is no synchronization; the rapid K accumulation period in rice occurs 23.8 d earlier than the rapid DM accumulation period [53]. In this study, the rapid K accumulation period (i.e., booting stage) occurred earlier than the rapid DM and N accumulation period (i.e., grain filling stage) and the K net loss during the reproductive growth stage (Table 2 and Figure 7). Ning et al. [54] found that the K net loss in maize after silking was greater and the net increase was smaller, which led to a net loss of K at the whole-plant level during reproductive growth. Nitrogen accumulation in cotton is positively correlated with K accumulation [2]. Consistently, N and N metabolism are the most important physiological factors affecting rice yield [55]. Rational fertilization can prolong the wheat filling period and increase grain weight and yield [56]. The correlation analysis performed herein showed that K accumulation in the leaves and stems of millet promoted NR and GS activities and N accumulation, while concomitantly increasing the early-stage DM accumulation in millet, especially in stems, thereby increasing millet yield (Table 3 and Table 4).
4.4. Conclusions
The rational application of K fertilizer promoted the absorption of K and N. Additionally, it significantly increased N and K accumulation and improved the N and K utilization efficiency in millet. Rapid K accumulation laid the foundation for DM accumulation while simultaneously improving the activity of N metabolism-related enzymes, which caused a large amount of N to accumulate at the grain-filling stage and enhanced yield. Stem DM and leaf K accumulation had a direct effect on millet yield (Figure 10). Jingu 21 is a K-utilization efficiency cultivar with higher KAE and KUE, and Zhangza 10 is a K-absorptive ability cultivar with greater roots and higher K concentration without K application. K120 effectively met the growth demand of foxtail millet plants, with the maximum apparent utilization rate of K fertilizer (AURK) and K180 achieved maximum yield, with the maximum agronomic efficiency of K fertilizer (AEK).
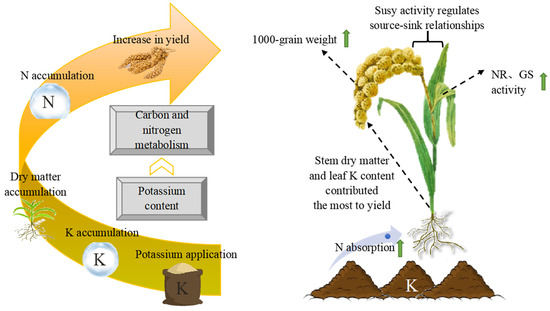
Figure 10.
Schematic diagram illustrating the increase in millet yield upon potassium application. Susy, sucrose synthase; NR, nitrate reductase; and GS, glutamine synthase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13092200/s1, Table S1: Time-course of dry matter accumulation in the aboveground millet. Table S2: Time-course of potassium concentration in different organs of millet. Table S3: Time-course of nitrogen concentration in different organs of millet. Table S4: Time-course of the distribution proportion of potassium accumulation in different organs of millet. Table S5: Time-course of potassium accumulation in different organs of millet. Table S6: Time-course of nitrogen accumulation in different organs of millet. Table S7: Time-course of the distribution proportion of nitrogen accumulation in different organs of millet.
Author Contributions
Conceptualization, Y.W., X.Y. (Xiangyang Yuan) and S.D.; writing-original draft preparation, Y.L. and M.Y.; data curation, Q.H. and X.Y. (Xiangjun Yu); formal analysis, M.H. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2021YFD1901101-2); China Agriculture Research System of MOF and MARA (CARS-06-14.5-A28); Science and Technology Major Special Plan “Unveiled” project in Shanxi Province, and the earmarked fund for Modern Agro-industry Technology Research System in Shanxi Province.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Hu, W.; Zhao, W.Q.; Yang, J.S.; Oosterhuis Derrick, M.; Loka Dimitra, A.; Zhou, Z.G. Relationship between potassium fertilization and nitrogen metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll during the boll development stage. Plant Physiol. Bioch. 2016, 101, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita, F.M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Xu, X.X.; Wang, F.; Xing, Y.; Liu, J.Q.; Lv, M.X.; Meng, H.; Du, X.; Zhu, Z.L.; Ge, S.F.; Jiang, Y.M. Appropriate and Constant Potassium Supply Promotes the Growth of M9T337 Apple Rootstocks by Regulating Endogenous Hormones and Carbon and Nitrogen Metabolism. Front. Plant Sci. 2022, 13, 827478. [Google Scholar] [CrossRef] [PubMed]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture–Status and perspectives. J. Plant Physiol. 2014, 2014, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef]
- Li, K.-L.; Tang, R.-J.; Wang, C.; Luan, S. Potassium nutrient status drives posttranslational regulation of a low-K response network in Arabidopsis. Nat. Commun. 2023, 14, 360. [Google Scholar] [CrossRef]
- Wang, X.W.; Hu, W.H.; Ning, X.L.; Wei, W.W.; Tang, Y.J.; Gu, Y. Effects of potassium fertilizer and straw on maize yield, potassium utilization efficiency and soil potassium balance. Arch. Agron. Soil Sci. 2022, 69, 679–692. [Google Scholar] [CrossRef]
- Pal, Y.; Gilkes, R.J.; Wong, M.T.F. Soil factors affecting the availability of potassium to plants for Western Australian soils: A glasshouse study. Soil Res. 2001, 39, 611–625. [Google Scholar] [CrossRef]
- Hoa, N.M.; Janssen, B.H.; Oenema, O.; Dobermann, A. Potassium budgets in rice cropping systems with annual flooding in the Mekong River Delta. Better Crops Plant Food. 2006, 90, 25–29. [Google Scholar]
- Yang, X.E.; Liu, J.X.; Wang, W.M.; Ye, Z.Q.; Luo, A.C. Potassium Internal Use Efficiency Relative to Growth Vigor, Potassium Distribution, and Carbohydrate Allocation in Rice Genotypes. J. Plant Nutr. 2004, 27, 837–852. [Google Scholar] [CrossRef]
- DU, Q.; Zhao, X.-H.; Xia, L.; Jiang, C.-J.; Wang, X.-G.; Han, Y.; Wang, J.; Yu, H.-Q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef]
- Ghulam, H.A.; Javaid, A.; Rafiq, A.; Moazzam, J.; Muhammad Anwar-ul-Haq Shafaqat, A.; Muhammad, I. Potassium application mitigates salt stress differently at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul. 2015, 76, 111–125. [Google Scholar]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Rufty, T.W.; Jackson, W.A.; Raper, C.D. Nitrate Reduction in Roots as Affected by the Presence of Potassium and by Flux of Nitrate through the Roots. Plant Physiol. 1981, 68, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M.; Romero, L. Relationship between potassium fertilisation and nitrate assimilation in leaves and fruits of cucumber (Cucumis sativus) plants. Ann. Appl. Biol. 2002, 140, 241–245. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, A.; Xin, X.; Yang, W.; Zhang, J.; Ding, S. Tillage and residue management for long-term wheat-maize cropping in the North China Plain: I. Crop yield and integrated soil fertility index. Field Crop. Res. 2018, 221, 157–165. [Google Scholar] [CrossRef]
- Zhiipao, R.R.; Pooniya, V.; Biswakarma, N.; Kumar, D.; Shivay, Y.S.; Dass, A.; Mukri, G.; Lakhena, K.K.; Pandey, R.K.; Bhatia, A.; et al. Timely sown maize hybrids improve the post-anthesis dry matter accumulation, nutrient acquisition and crop productivity. Sci. Rep. 2023, 13, 1688. [Google Scholar] [CrossRef]
- Rengel, Z.; Damon, P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef]
- Wu, G.L.; Guo, L.Y.; Cui, Z.Y.; Li, Y.; Yin, Y.P.; Wang, Z.L.; Jiang, G.M. Differential effects of nitrogen managements on nitrogen, dry matter accumulation and transportation in late-sowing winter wheat. Actaecologicasinica 2012, 32, 5128–5137. [Google Scholar]
- Gu, S.L.; Ma, J.P. Accumulation and distribution rule of dry materials and its contribution to foxtail millet yield. Acta Agric. Boreali Sin. 2002, 2, 30–35. [Google Scholar]
- Yang, L.S.; Zhang, Y.T.; Yang, L.Q.; Xie, J.; Yang, M.; Zhang, Y.Q.; Shi, X.J. Effects of different nitrogen and potassium rates on dry matter accumulation, transport and yield of rice. Soil Fert. Sci. China 2019, 4, 89–95. [Google Scholar]
- Song, J.; Wang, S.X.; Li, L.; Huang, J.L.; Zhao, B.; Zhang, J.W.; Ren, B.Z.; Liu, P. Effects of potassium application rate on NPK uptake and utilization and grain yield in summer maize (Zea mays L.). Acta. Agron. Sin. 2023, 49, 539–551. [Google Scholar]
- Sharma, S.; Kaur, G.; Singh, P.; Alamri, S.; Kumar, R.; Siddiqui, M.H. Nitrogen and potassium application effects on productivity, profitability and nutrient use efficiency of irrigated wheat (Triticum aestivum L.). PLoS ONE 2022, 17, e0264210. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhao, K.; Ma, J.; Huang, L.; Zhuang, H. Post-Anthesis Nitrogen Dynamic Models and Characteristics of Rice Combined with Sowing Date and Nitrogen Application Rate. Sustainability 2022, 14, 4956. [Google Scholar] [CrossRef]
- Seabra, A.R.; Silva, L.S.; Carvalho, H.G. Novel aspects of glutamine synthetase (GS) regulation revealed by a detailed expression analysis of the entire GS gene family of Medicago truncatula under different physiological conditions. BMC Plant Biol. 2013, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, N.; Yang, J.S.; Meng, Y.L.; Wang, Y.H.; Chen, B.L.; Zhao, W.Q.; Oosterhuis Derrick, M.; Zhou, Z.G. Potassium (K) supply affects K accumulation and photosynthetic physiology in two cotton (Gossypium hirsutum L.) cultivars with different K sensitivities. Field Crops Res. 2016, 196, 51–63. [Google Scholar] [CrossRef]
- Hu, W.; Lu, Z.; Gu, H.; Ye, X.; Li, X.; Cong, R.; Ren, T.; Lu, J. Potassium availability influences the mesophyll structure to coordinate the conductance of CO2 and H2O during leaf expansion. Plant Cell Environ. 2022, 45, 2987–3000. [Google Scholar] [CrossRef]
- Wang, D.R.; Wolfrum, E.J.; Virk, P.; Ismail, A.; Greenberg, A.J.; McCouch, S.R. Robust phenotyping strategies for evaluation of stem non-structural carbohydrates (NSC) in rice. J. Exp. Bot. 2016, 67, 6125–6138. [Google Scholar] [CrossRef]
- Qi, W.-Z.; Liu, H.-H.; Liu, P.; Dong, S.-T.; Zhao, B.-Q.; So, H.B.; Li, G.; Liu, H.-D.; Zhang, J.-W.; Zhao, B. Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 2012, 38, 54–63. [Google Scholar] [CrossRef]
- Fan, P.; Ming, B.; Evers, J.B.; Li, Y.; Li, S.; Xie, R.; Anten, N.P. Nitrogen availability determines the vertical patterns of accumulation, partitioning, and reallocation of dry matter and nitrogen in maize. Field Crop. Res. 2023, 297, 108927. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Mu, X.; Zhao, G.; Gao, P.; Sun, W. Effects of Different Fertilization Regimes on Crop Yield and Soil Water Use Efficiency of Millet and Soybean. Sustainability 2020, 12, 4125. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Li, G.; Liu, G.; Geissen, V.; Ritsema, C.J.; Xue, S. Impact of nitrogen addition on plant-soil-enzyme C–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar] [CrossRef]
- Bahrami, M.; Talebnejad, R.; Sepaskhah, A.R.; Bazile, D. Irrigation Regimes and Nitrogen Rates as the Contributing Factors in Quinoa Yield to Increase Water and Nitrogen Efficiencies. Plants 2022, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hua, H.; Eneji, A.E.; Li, Z.; Duan, L.; Tian, X. Genotypic variations in photosynthetic and physiological adjustment to potassium deficiency in cotton (Gossypium hirsutum). J. Photochem. Photobiol. B Biol. 2012, 110, 1–8. [Google Scholar] [CrossRef]
- Li, Y.H.; Cao, M.L.; Du, H.L.; Guo, P.Y.; Zhang, H.Y.; Guo, M.J.; Yuan, X.Y. Effects of fertilization location and amount on dry matter accumulation, translocation and yield of hybrid millet. Sci. Agric. Sin. 2019, 52, 4177–4190. [Google Scholar]
- Li, Y.; Yin, M.; Li, L.; Zheng, J.; Yuan, X.; Wen, Y. Optimized potassium application rate increases foxtail millet grain yield by improving photosynthetic carbohydrate metabolism. Front. Plant Sci. 2022, 13, 1044065. [Google Scholar] [CrossRef]
- Singh, P.; Agrawal, V.K.; Singh, Y.V. Effect of potassium and FYM on growth parameters, yield and mineral composition of wheat (Triticum aestivum L.) in alluvial soil. J. Pharmacogn. Phytochem. 2019, 8, 24–27. [Google Scholar]
- Ye, T.; Xue, X.; Lu, J.; Hou, W.; Ren, T.; Cong, R.; Li, X. Yield and potassium uptake of rice as affected by potassium rate in the middle reaches of the Yangtze River, China. Agron. J. 2020, 112, 1318–1329. [Google Scholar] [CrossRef]
- Rawal, N.; Pande, K.R.; Shrestha, R.; Vista, S.P. Nutrient Concentration and Its Uptake in Various Stages of Wheat (Triticum aestivum L.) as Influenced by Nitrogen, Phosphorus, and Potassium Fertilization. Commun. Soil Sci. Plant Anal. 2023, 54, 1151–1166. [Google Scholar] [CrossRef]
- Zhan, A.; Zou, C.; Ye, Y.; Liu, Z.; Cui, Z.; Chen, X. Estimating on-farm wheat yield response to potassium and potassium uptake requirement in China. Field Crop. Res. 2016, 191, 13–19. [Google Scholar] [CrossRef]
- Hou, W.; Xue, X.; Li, X.; Khan, M.R.; Yan, J.; Ren, T.; Cong, R.; Lu, J. Interactive effects of nitrogen and potassium on: Grain yield, nitrogen uptake and nitrogen use efficiency of rice in low potassium fertility soil in China. Field Crop. Res. 2019, 236, 14–23. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Tu, B.; Li, Y.; Liu, X.; Zhang, Q.; Herbert, S.J. Dry matter partitioning and K distribution of vegetable soybean genotypes with higher potassium efficiency. Arch. Agron. Soil Sci. 2019, 66, 717–729. [Google Scholar] [CrossRef]
- Damon, P.M.; Rengel, Z. Wheat genotypes differ in potassium efficiency under glasshouse and field conditions. Aust. J. Agric. Res. 2007, 58, 816–825. [Google Scholar] [CrossRef]
- White, P.J.; Bell, M.J.; Djalovic, I.; Hinsinger, P.; Rengel, Z. Potassium use efficiency of plants. In Improving Potassium Recommendations for Agricultural Crops; Murrell, T.S., Mikkelsen, R.L., Sulewski, G., Norton, R., Thompson, M.L., Eds.; Springer: Cham, Switzerland; Singapore, 2021. [Google Scholar]
- Chuan, L.; He, P.; Jin, J.; Li, S.; Grant, C.; Xu, X.; Qiu, S.; Zhao, S.; Zhou, W. Estimating nutrient uptake requirements for wheat in China. Field Crop. Res. 2013, 146, 96–104. [Google Scholar] [CrossRef]
- He, B.; Xue, C.; Sun, Z.; Ji, Q.; Wei, J.; Ma, W. Effect of Different Long-Term Potassium Dosages on Crop Yield and Potassium Use Efficiency in the Maize–Wheat Rotation System. Agronomy 2022, 12, 2565. [Google Scholar] [CrossRef]
- Kumar, S.; Dhar, S.; Kumar, A.; Kumar, D. Yield and nutrient uptake of maize (Zea mays)-wheat (Triticum aestivum) cropping system as influenced by integrated potassium management. Indian J. Agron. 2016, 60, 511–515. [Google Scholar]
- Du, M.; Zhang, W.Z.; Gao, J.P.; Liu, M.Q.; Zhou, Y.; He, D.W.; Zhao, Y.Z.; Liu, S.M. Improvement of root characteristics due to nitrogen, phosphorus, and potassium interactions increases rice (Oryza sativa L.) yield and nitrogen use efficiency. Agronomy 2021, 12, 23. [Google Scholar] [CrossRef]
- Li, Z.L.; Liu, Z.G.; Zhang, M.; Li, C.L.; Li YC, C.; Wan, Y.S.; Martin, C.G. Long-term effects of controlled-released potassium chloride on soil available potassium, nutrient absorption and yield of maize plants. Soil Tillage Res. 2020, 196, 104438. [Google Scholar] [CrossRef]
- Latifmanesh, H.; Deng, A.; Nawaz, M.M.; Li, L.; Chen, Z.; Zheng, Y.; Wang, P.; Song, Z.; Zhang, J.; Zheng, C.; et al. Integrative impacts of rotational tillage on wheat yield and dry matter accumulation under corn-wheat cropping system. Soil Tillage Res. 2018, 184, 100–108. [Google Scholar] [CrossRef]
- Xue, X.X.; Li, X.K. Effects of potassium application levels on the characteristics of dry matter accumulation and potassium uptake in rice. Acta Agric. Univ. Jiangxiensis 2018, 40, 905–913. [Google Scholar]
- Ning, P.; Li, S.; Yu, P.; Zhang, Y.; Li, C. Post-silking accumulation and partitioning of dry matter, nitrogen, phosphorus and potassium in maize varieties differing in leaf longevity. Field Crop. Res. 2013, 144, 19–27. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, Q.; Song, Z.; Yin, Y.; Wang, G.; Li, Y. Increasing the yield of drip-irrigated rice by improving photosynthetic performance and enhancing nitrogen metabolism through optimizing water and nitrogen management. Front. Plant Sci. 2023, 14, 1075625. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tian, Z.; Qi, Z.; Han, J.; Zhao, X.; Xue, J. Effect of Nitrogen Application Rate on Grain Yield, Dry Matter and Nitrogen Accumulation and Remobilization in a Winter Wheat-Fresh Maize Cropping System. J. Food Nutr. Res.-Slov. 2023, 11, 176–184. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).