Effect of Rural Black-Gray Water Treatment by Subsurface Wastewater Infiltration System on Soil Environment of Vegetable Crop Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Area
2.2. Experimental Design
2.3. SWIS Setup
2.4. Analytical Methods
2.4.1. Sample Collection
2.4.2. Testing Methods
2.4.3. Health Risk Assessment
2.5. Microbial Community Analyses
2.5.1. DNA Extraction and PCR Amplification
2.5.2. Microbial Diversity Analysis
3. Results and Discussion
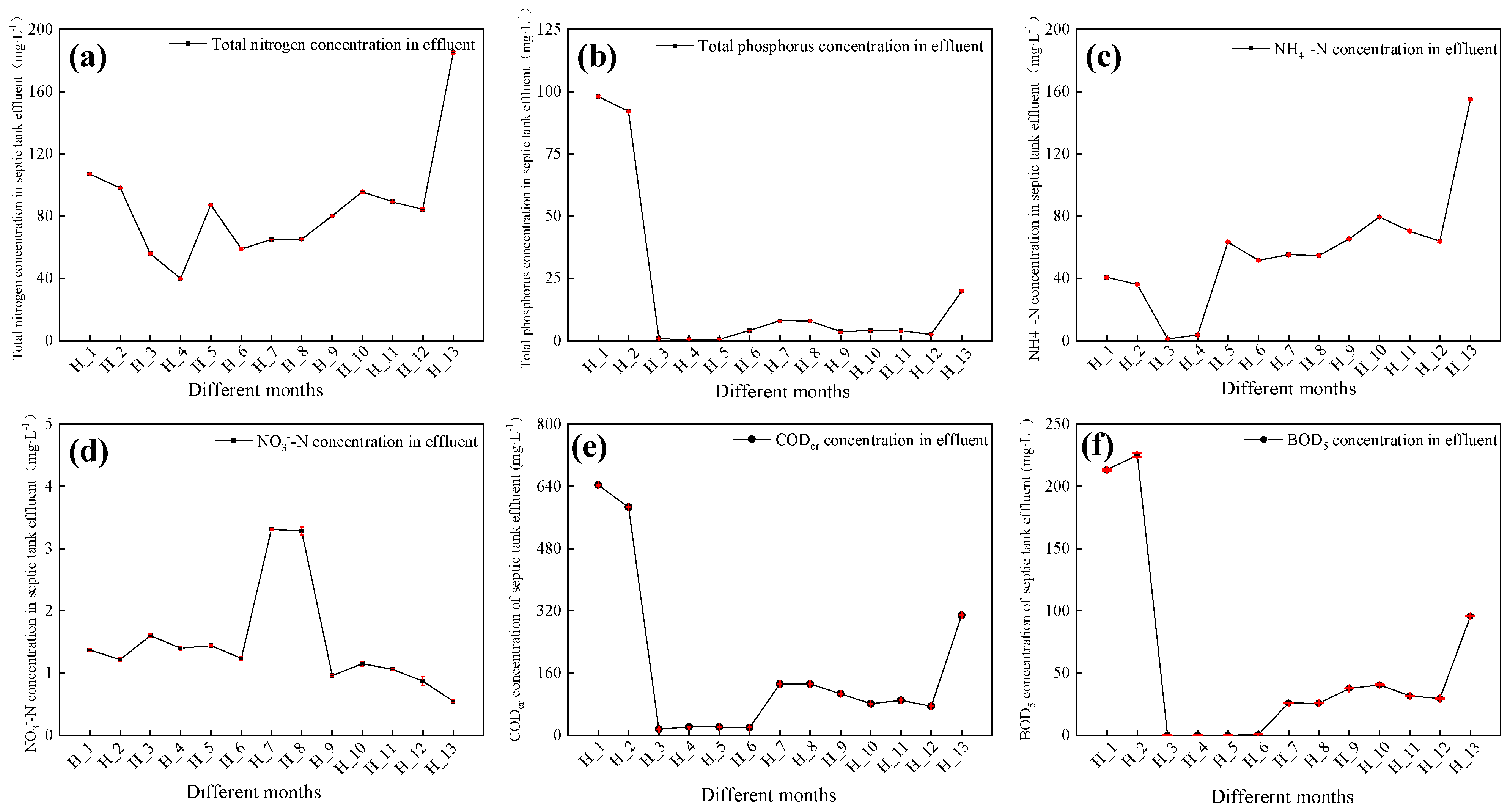
3.1. Characteristics of SPT Effluent Quality
3.1.1. Physicochemical Indexes
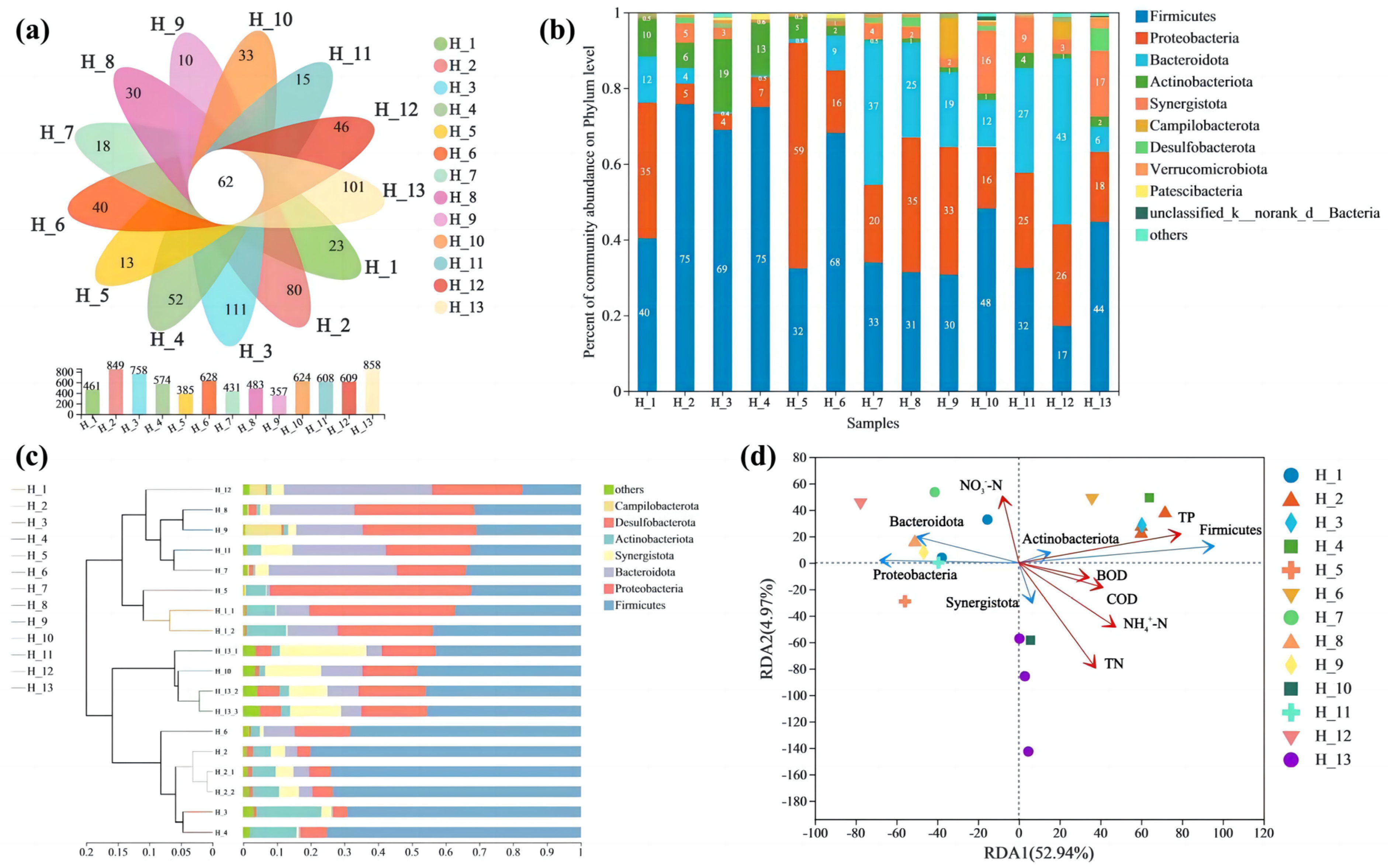
3.1.2. Microbial Community
3.2. Effects of SWIS Treatment of Rural Black-Grey Water on the Vegetable Garden Soil Environment
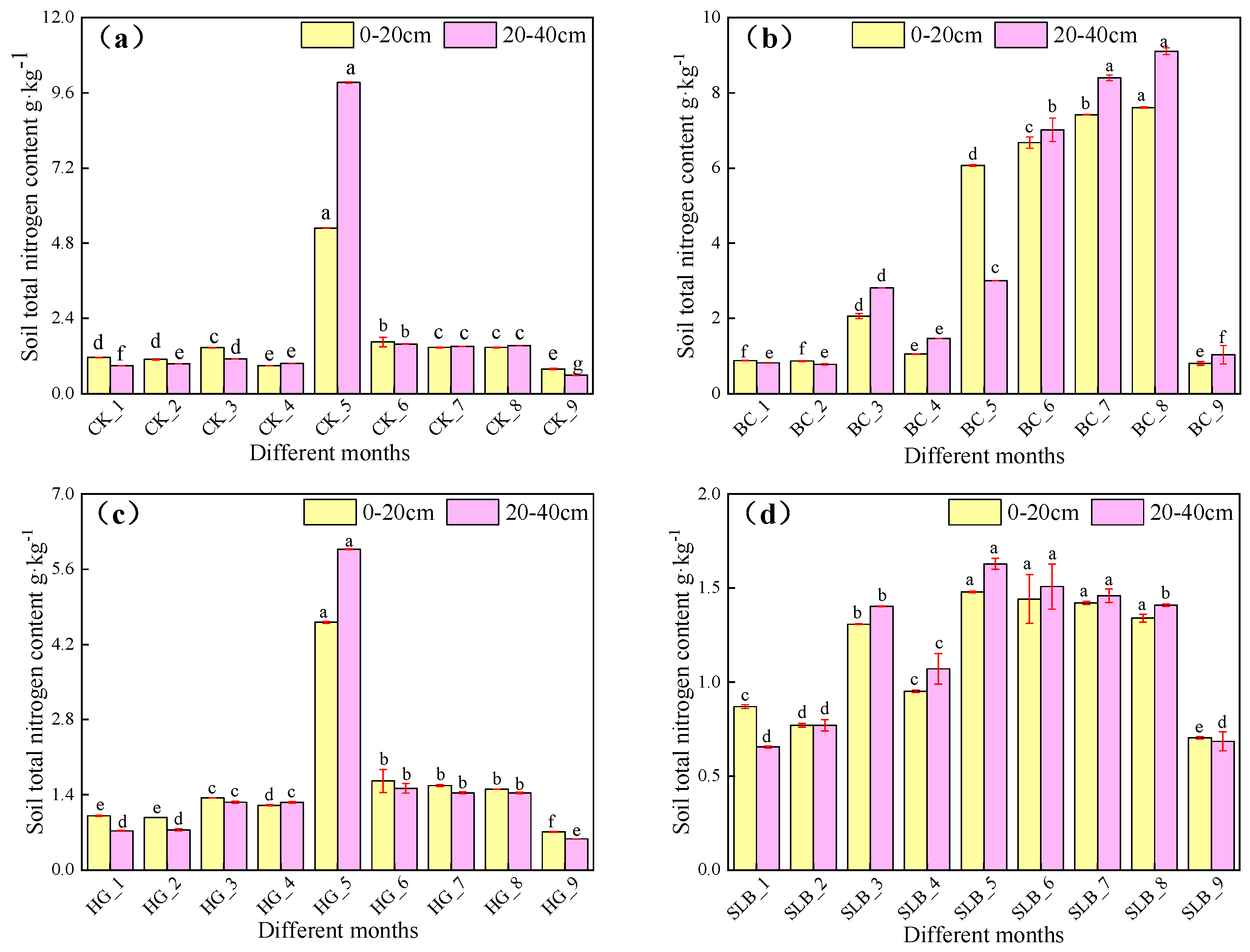
3.2.1. Soil Physicochemical Properties
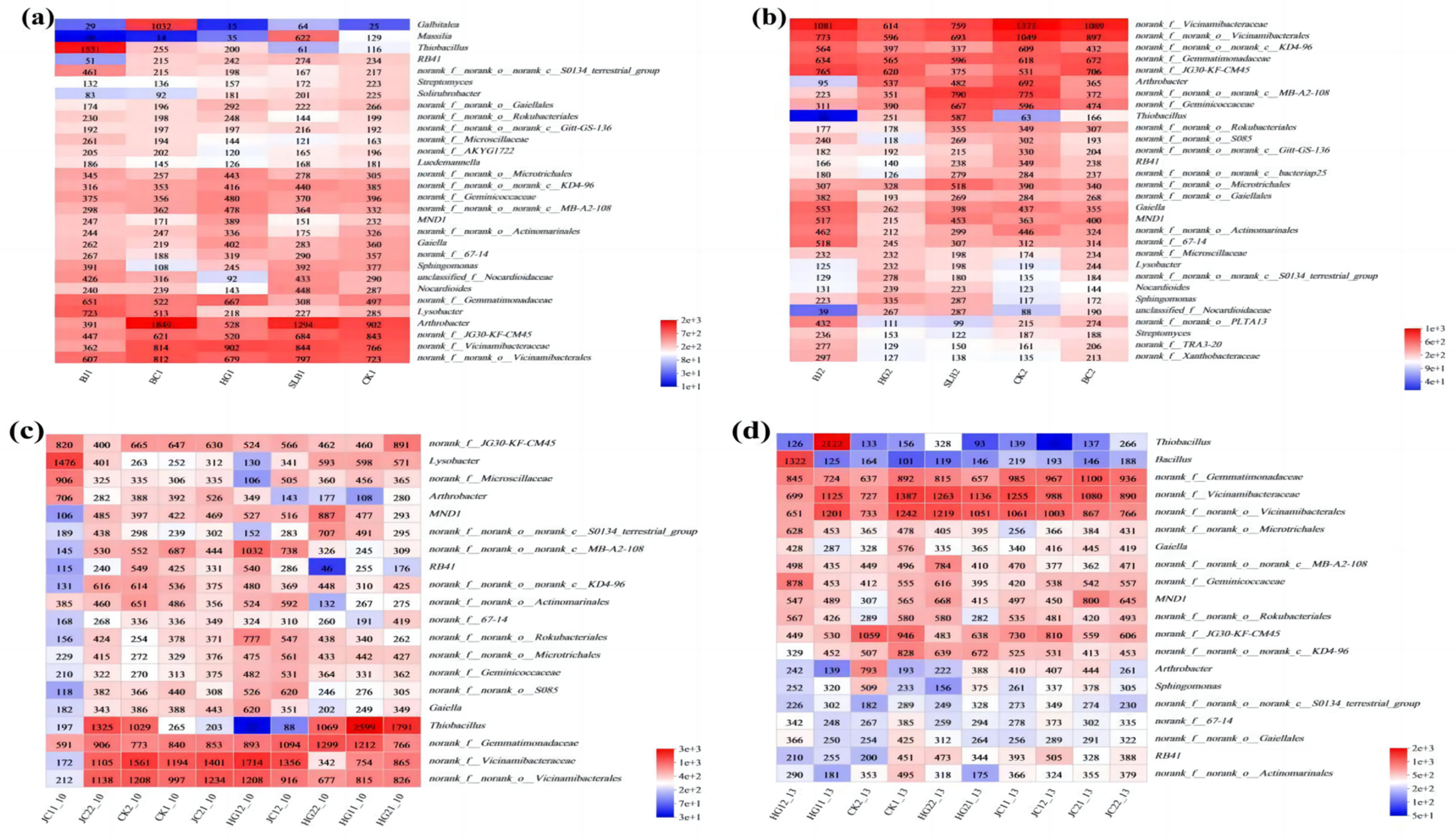
3.2.2. Soil Microbial Community
3.2.3. Soil Hygienic and Sanitary Properties
3.3. Effects of SWIS Treatment of Rural Black-Grey Water on Vegetable Harvest
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Yang, B.; Zhang, C.; Wei, X.; Cao, H.; Zheng, X. Human Waste Substitute Strategies Enhanced Crop Yield, Crop Quality, and Soil Fertility in Vegetable Cultivation Soils in North China. Agronomy 2021, 11, 2232. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Z.; Uddin, S.M.N.; Mang, H.P.; Zhou, X.; Zhang, J.; Zheng, L.; Zhang, L. Toilet revolution in China. J. Environ. Manag. 2018, 216, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Zheng, T.; Yan, Y.; Li, P.; Odey, E.A.; Mang, H.P.; Uddin, S.M.N. Review of global sanitation development. Environ. Int. 2018, 120, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Y.; Chai, B.; Wu, H. Evaluating the effects of intermittent aeration and biochar addition on enhancing removal performance of subsurface wastewater infiltration systems with loess soil. Bioresour. Technol. Rep. 2019, 5, 12–19. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Dong, L.; Zhao, X.; Wu, H. Accumulation and characteristics of fluorescent dissolved organic matter in loess soil-based subsurface wastewater infiltration system with aeration and biochar addition. Environ. Pollut. 2021, 269, 116100. [Google Scholar] [CrossRef]
- Jung, Y.T.; Narayanan, N.C.; Cheng, Y.L. Cost comparison of centralized and decentralized wastewater management systems using optimization model. J. Environ. Manag. 2018, 213, 90–97. [Google Scholar] [CrossRef]
- Yang, S.L.; Zheng, Y.F.; Mao, Y.X.; Xu, L.; Jin, Z.; Zhao, M.; Kong, H.N.; Huang, X.F.; Zheng, X.Y. Domestic wastewater treatment for single household via novel subsurface wastewater infiltration systems (SWISs) with NiiMi process: Performance and microbial community. J. Clean. Prod. 2021, 279, 123434. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Chi, L.; Wang, J. The design and operation of subsurface wastewater infiltration systems for domestic wastewater. Water Environ. Res. 2019, 91, 843–854. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, H.B.; Yang, L.; Wang, S.; Su, F.; Xu, X.Y. How does high C load affect nitrous oxide emission of subsurface wastewater infiltration system? Sci. Total Environ. 2020, 698, 134174. [Google Scholar] [CrossRef]
- Paranychianakis, N.V.; Angelakis, A.N.; Leverenz, H.; Tchobanoglous, G. Treatment of Wastewater With Slow Rate Systems: A Review of Treatment Processes and Plant Functions. Crit. Rev. Environ. Sci. Technol. 2006, 36, 187–259. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, J.; Zheng, P.; Yuan, K.; Chu, S.; Mu, K.; Hu, L. Plant uptake in subsurface wastewater infiltration systems plays an important role in removing nitrogen from sewage. Ecol. Eng. 2018, 123, 193–201. [Google Scholar] [CrossRef]
- Yang, P.; Hou, R.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Effect of intermittent operation and shunt wastewater on pollutant removal and microbial community changes in subsurface wastewater infiltration system. Process Saf. Environ. Prot. 2022, 165, 255–265. [Google Scholar] [CrossRef]
- Poustie, A.; Yang, Y.; Verburg, P.; Pagilla, K.; Hanigan, D. Reclaimed wastewater as a viable water source for agricultural irrigation: A review of food crop growth inhibition and promotion in the context of environmental change. Sci. Total Environ. 2020, 739, 139756. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Li, Y.; Zheng, X.; Zhang, C.; Gao, Y.; Chen, P.; Li, Q.; Tan, L. Systematically assess the advancing and limiting factors of using the multi-soil-layering system for treating rural sewage in China: From the economic, social, and environmental perspectives. J. Environ. Manag. 2022, 312, 114912. [Google Scholar] [CrossRef]
- Xie, Y.D.; Zhang, Q.H.; Dzakpasu, M.; Zheng, Y.C.; Tian, Y.; Jin, P.K.; Yang, S.J.; Wang, X.C. Towards the formulation of rural sewage discharge standards in China. Sci. Total Environ. 2021, 759, 143533. [Google Scholar] [CrossRef]
- Burt, T.P.; Howden, N.J.K.; Worrall, F.; Whelan, M.J.; Bieroza, M. Nitrate in United Kingdom Rivers: Policy and Its Outcomes Since 1970. Environ. Sci. Technol. 2011, 45, 175–181. [Google Scholar] [CrossRef]
- Li, Y.; Su, F.; Li, M.; Wang, Y.; Qian, J. Effects of freeze-thaw intensities on N2O release from subsurface wastewater infiltration system. J. Environ. Chem. Eng. 2023, 11, 110134. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, X.; Fang, L.; Huang, X. Nitrogen removal in improved subsurface wastewater infiltration system: Mechanism, microbial indicators and the limitation of phosphorus. J. Environ. Manag. 2023, 335, 117456. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Xia, X. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- Ren, J.; Liu, X.; Yang, W.; Yang, X.; Li, W.; Xia, Q.; Li, J.; Gao, Z.; Yang, Z. Rhizosphere soil properties; microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Li, G.W.; Li, X.G.; Wang, H.Q.; Wu, H.B.; Li, J.X.; Li, C.L.; Li, W.; Zhang, L.Y.; Xi, B.D. The cotreatment of old landfill leachate and domestic sewage in rural areas by deep subsurface wastewater infiltration system (SWIS): Performance and bacterial community. Environ. Pollut. 2021, 274, 115800. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; van Geest, J.; Kleerebezem, R.; van Loosdrecht, M.C.M. Short- and long-term temperature effects on aerobic polyhydroxybutyrate producing mixed cultures. Water Res. 2010, 44, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Huang, G.; An, C.; Xin, X.; Zhang, P.; Chen, X.; Ren, S.; Xu, Z.; Yang, X. Exploring the decentralized treatment of sulfamethoxazole-contained poultry wastewater through vertical-flow multi-soil-layering systems in rural communities. Water Res. 2021, 188, 116480. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.S. Influent Constituent Characteristics of the Modern Waste Stream from Single Sources; IWA Publishing: London, UK, 2010; Volume 9. [Google Scholar] [CrossRef]
- Wang, C.; Feng, B.; Wang, P.; Guo, W.; Li, X.; Gao, H.; Zhang, B.; Chen, J. Revealing factors influencing spatial variation in the quantity and quality of rural domestic sewage discharge across China. Process Saf. Environ. Prot. 2022, 162, 200–210. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, X.; Wu, S.; Kang, M.; Guo, J.; Chen, F. Effect of hydraulic loading rate on pollutant removal efficiency in subsurface infiltration system under intermittent operation and micro-power aeration. Bioresour. Technol. 2016, 205, 174–182. [Google Scholar] [CrossRef]
- Dong, H.; Yuan, X.; Wang, W.; Qiang, Z. Occurrence and removal of antibiotics in ecological and conventional wastewater treatment processes: A field study. J. Environ. Manag. 2016, 178, 11–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Dong, L.; Han, C.; Li, M.; Wu, H. Effects of biochar dosage on treatment performance, enzyme activity and microbial community in aerated constructed wetlands for treating low C/N domestic sewage. Environ. Technol. Innov. 2021, 24, 101919. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Peng, Y.; Mironov, V.; Chen, J.; Jin, H.; Zhang, S. Feasibility of sewage sludge and food waste aerobic co-composting: Physicochemical properties, microbial community structures, and contradiction between microbial metabolic activity and safety risks. Sci. Total Environ. 2022, 825, 154047. [Google Scholar] [CrossRef]
- Fu, J.; Yan, B.; Gui, S.; Fu, Y.; Xia, S. Anaerobic co-digestion of thermo-alkaline pretreated microalgae and sewage sludge: Methane potential and microbial community. J. Environ. Sci. 2023, 127, 133–142. [Google Scholar] [CrossRef]
- Sheng, Q.; Lu, Y.; Yuan, S.; Li, X.; Dai, X.; Guo, Y.; Dong, B. Effect of nitrite on hydrolysis-acidification, biogas production and microbial community in semi-continuous two-phase anaerobic digestion of sewage sludge. J. Environ. Sci. 2023, 126, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Liao, R.; Zhang, X.X.; Wang, Y.; Wang, Z.; Shi, P.; Liu, B.; Li, A. Metagenomic insights into Cr(VI) effect on microbial communities and functional genes of an expanded granular sludge bed reactor treating high-nitrate wastewater. Water Res. 2015, 76, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vilela, P.B.; Starling, M.C.V.M.; Neto, R.P.M.; de Souza, F.A.R.; Pires, G.F.F.; Amorim, C.C. Solar photo-Fenton mediated by alternative oxidants for MWWTP effluent quality improvement: Impact on microbial community, priority pathogens and removal of antibiotic-resistant genes. Chem. Eng. J. 2022, 441, 136060. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Q.; Yan, G.; Zhu, H.; Xu, X.Y.; Zhu, L. Seasonal bacterial community succession in four typical wastewater treatment plants: Correlations between core microbes and process performance. Sci. Rep. 2018, 8, 4566. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Zheng, M.; Chen, Q.; Ni, J. Performance Assessment of Full-Scale Wastewater Treatment Plants Based on Seasonal Variability of Microbial Communities via High-Throughput Sequencing. PLoS ONE 2016, 11, e0152998. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, Y.; Feng, K.; Li, S.; Wang, S.; Jin, D.; Zhang, Y.; Chen, H.; Yin, H.; Xu, M.; et al. The divergence between fungal and bacterial communities in seasonal and spatial variations of wastewater treatment plants. Sci. Total Environ. 2018, 628–629, 969–978. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Z.; Fang, W.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Dudits, D.; Kakuk, B.; Bagi, Z.; Shetty, P.; Kovacs, K.L.; Maroti, G. Genome-centric investigation of anaerobic digestion using sustainable second and third generation substrates. J. Biotechnol. 2021, 339, 53–64. [Google Scholar] [CrossRef]
- Peng, X.; Yang, W.; Jin, Q.; Su, S.; Guo, P.; Li, M.; Liu, H.; Li, W. Biofilter-constructed wetland-trophic pond system: A new strategy for effective sewage treatment and agricultural irrigation in rural area. J. Environ. Manag. 2023, 332, 117436. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, S.; Li, Z.; Song, H.; Guo, M.; Li, Z.; Mang, H.P.; Xu, Y.; Chen, C.; Basandorj, D.; et al. Using system dynamics to assess the complexity of rural toilet retrofitting: Case study in eastern China. J. Environ. Manag. 2021, 280, 111655. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical fertilizer reduction with organic fertilizer effectively improve soil fertility and microbial community from newly cultivated land in the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Qin, S.; Huang, F.; Jiang, B.; Jia, L. Evaluation of the removal performance in long-term operation of bioaugmented subsurface wastewater infiltration systems under a high hydraulic loading rate. Environ. Technol. Innov. 2021, 24, 101918. [Google Scholar] [CrossRef]
- Lehmann, J.; Lan, Z.; Hyland, C.; Sato, S.; Solomon, D.; Ketterings, Q.M. Long-term dynamics of phosphorus forms and retention in manure-amended soils. Environ. Sci. Technol. 2005, 39, 6672. [Google Scholar] [CrossRef]
- He, J.; He, Y.; Gao, W.; Chen, Y.; Ma, G.; Ji, R.; Liu, X. Soil depth and agricultural irrigation activities drive variation in microbial abundance and nitrogen cycling. Catena 2022, 219, 106596. [Google Scholar] [CrossRef]
- Haoyu, C.; Bo, Y.; Tao, Z.; Bo, L.; Chunxue, Z.; Xiaocheng, W. Rural mixed pond water irrigation affected microbial community and eco-enzymatic stoichiometry of soil profiles. Appl. Soil Ecol. 2023, 188, 104863. [Google Scholar] [CrossRef]
- Castro, H.; Fortunel, C.; Freitas, H. Effects of land abandonment on plant litter decomposition in a Montado system: Relation to litter chemistry and community functional parameters. Plant Soil 2010, 333, 181–190. [Google Scholar] [CrossRef]
- Guo, W.; Andersen, M.N.; Qi, X.-B.; Li, P.; Li, Z.-Y.; Fan, X.-Y.; Zhou, Y. Effects of reclaimed water irrigation and nitrogen fertilization on the chemical properties and microbial community of soil. J. Integr. Agric. 2017, 16, 679–690. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Nakajima, A. Electron spin resonance study of copper biosorption by bacteria. Water Res. 2002, 36, 2091–2097. [Google Scholar] [CrossRef]
- Hayward, A.C.; Fegan, N.; Fegan, M.; Stirling, G.R. Stenotrophomonas and Lysobacter: Ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J. Appl. Microbiol. 2010, 108, 756–770. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge by indigenous iron-oxidizing microorganisms using ammonium ferrous sulfate and ferrous sulfate as energy sources: A comparative study. J. Hazard. Mater. 2009, 171, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Han, S.; Hong, H.J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.K.; Crusemann, M.; Lee, Y.B.; Kim, J.F.; et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Du, T.-Y.; He, H.-Y.; Zhang, Q.; Lu, L.; Mao, W.-J.; Zhai, M.-Z. Positive effects of organic fertilizers and biofertilizers on soil microbial community composition and walnut yield. Appl. Soil Ecol. 2022, 175, 104457. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Xue, J.; Shi, K.; Chen, C.; Bai, Y.; Cui, Q.; Li, N.; Fu, X.; Qiao, Y. Evaluation of response of dynamics change in bioaugmentation process in diesel-polluted seawater via high-throughput sequencing: Degradation characteristic, community structure, functional genes. J. Hazard. Mater. 2021, 403, 123569. [Google Scholar] [CrossRef]
- Zhanhui, Z.; Congzhi, Z.; Jiabao, Z.; Changhua, L.; Qicong, W. Fertilizer impacts on soil aggregation and aggregate-associated organic components. Plant Soil Environ. 2018, 64, 338–343. [Google Scholar]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Tang, Q.; Xia, Y.; Ti, C.; Shan, J.; Zhou, W.; Li, C.; Yan, X.; Yan, X. Partial Organic Fertilizer Substitution Promotes Soil Multifunctionality by Increasing Microbial Community Diversity and Complexity. Pedosphere 2022, 33, 407–420. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, Y.; Xiong, W.; Ran, W.; Shen, B.; Shen, Q.; Zhang, R. Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS ONE 2014, 9, e85301. [Google Scholar] [CrossRef]
- Bei, S.; Zhang, Y.; Li, T.; Christie, P.; Li, X.; Zhang, J. Response of the soil microbial community to different fertilizer inputs in a wheat-maize rotation on a calcareous soil. Agric. Ecosyst. Environ. 2018, 260, 58–69. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Liang, P. Influence of drip irrigation by reclaimed water on the dynamic change of the nitrogen element in soil and tomato yield and quality. J. Clean. Prod. 2016, 139, 561–566. [Google Scholar] [CrossRef]
- Lyu, S.; Wu, L.; Wen, X.; Wang, J.; Chen, W. Effects of reclaimed wastewater irrigation on soil-crop systems in China: A review. Sci. Total Environ. 2022, 813, 152531. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hu, Y.; Guan, X.; Xu, L. Advances in research of reclaimed water irrigation in China. Irrig. Drain. 2020, 69, 119–126. [Google Scholar] [CrossRef]
- Li, J.; Wen, J. Effects of water managements on transport of E. coli in soil-plant system for drip irrigation applying secondary sewage effluent. Agric. Water Manag. 2016, 178, 12–20. [Google Scholar] [CrossRef]
- Farhadkhani, M.; Nikaeen, M.; Yadegarfar, G.; Hatamzadeh, M.; Pourmohammadbagher, H.; Sahbaei, Z.; Rahmani, H.R. Effects of irrigation with secondary treated wastewater on physicochemical and microbial properties of soil and produce safety in a semi-arid area. Water Res. 2018, 144, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Orlofsky, E.; Bernstein, N.; Sacks, M.; Vonshak, A.; Benami, M.; Kundu, A.; Maki, M.; Smith, W.; Wuertz, S.; Shapiro, K.; et al. Comparable levels of microbial contamination in soil and on tomato crops after drip irrigation with treated wastewater or potable water. Agric. Ecosyst. Environ. 2016, 215, 140–150. [Google Scholar] [CrossRef]

| Sample | Shannon | Ace | Coverage |
|---|---|---|---|
| H_0 | 1.300 ± 0.045 b | 30.152 ± 3.055 a | 0.999 ± 0.000 a |
| H_1 | 0.971 ± 0.057 c | 38.944 ± 15.587 a | 0.999 ± 0.000 a |
| H_13 | 1.600 ± 0.017 a | 36.557 ± 4.608 a | 0.999 ± 0.000 a |
| Indexes | Shannon | Ace | Coverage | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Top Soil | Deep Soil | Top Soil | Deep Soil | Top Soil | Deep Soil | |
| BJ | 6.184 ± 0.13899 b | 6.2434 ± 0.0657 b | 3758.57 ± 428.34 a | 3051.90 ± 114.92 c | 0.956 ± 0.002 a | 0.964 ± 0.000 a | |
| CK | 6.607 ± 0.05961 a | 6.5576 ± 0.0395 a | 4017.65 ± 298.15 a | 4126.78 ± 314.93 a | 0.952 ± 0.001 a | 0.952 ± 0.001 b | |
| BC | 5.945 ± 0.21989 c | 6.6366 ± 0.0132 a | 3658.23 ± 151.71 a | 4555.95 ± 112.86 a | 0.956 ± 0.001 a | 0.949 ± 0.001 b | |
| HG | 6.713 ± 0.09131 a | 6.5482 ± 0.1132 a | 3945.15 ± 324.38 a | 3781.61 ± 224.30 b | 0.948 ± 0.001 b | 0.953 ± 0.002 b | |
| SLB | 6.493 ± 0.09850 a | 6.5414 ± 0.0661 a | 3944.56 ± 216.07 a | 3702.72 ± 181.45 b | 0.952 ± 0.001 a | 0.951 ± 0.002 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, L.; Zhang, C.; Wei, X.; Zheng, X. Effect of Rural Black-Gray Water Treatment by Subsurface Wastewater Infiltration System on Soil Environment of Vegetable Crop Field. Agronomy 2023, 13, 2206. https://doi.org/10.3390/agronomy13092206
Wang S, Liu L, Zhang C, Wei X, Zheng X. Effect of Rural Black-Gray Water Treatment by Subsurface Wastewater Infiltration System on Soil Environment of Vegetable Crop Field. Agronomy. 2023; 13(9):2206. https://doi.org/10.3390/agronomy13092206
Chicago/Turabian StyleWang, Songmin, Liyuan Liu, Chunxue Zhang, Xiaocheng Wei, and Xiangqun Zheng. 2023. "Effect of Rural Black-Gray Water Treatment by Subsurface Wastewater Infiltration System on Soil Environment of Vegetable Crop Field" Agronomy 13, no. 9: 2206. https://doi.org/10.3390/agronomy13092206




