Influence of White, Red, Blue, and Combination of LED Lights on In Vitro Multiplication of Shoots, Rooting, and Acclimatization of Gerbera jamesonii cv. ‘Shy Pink’ Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
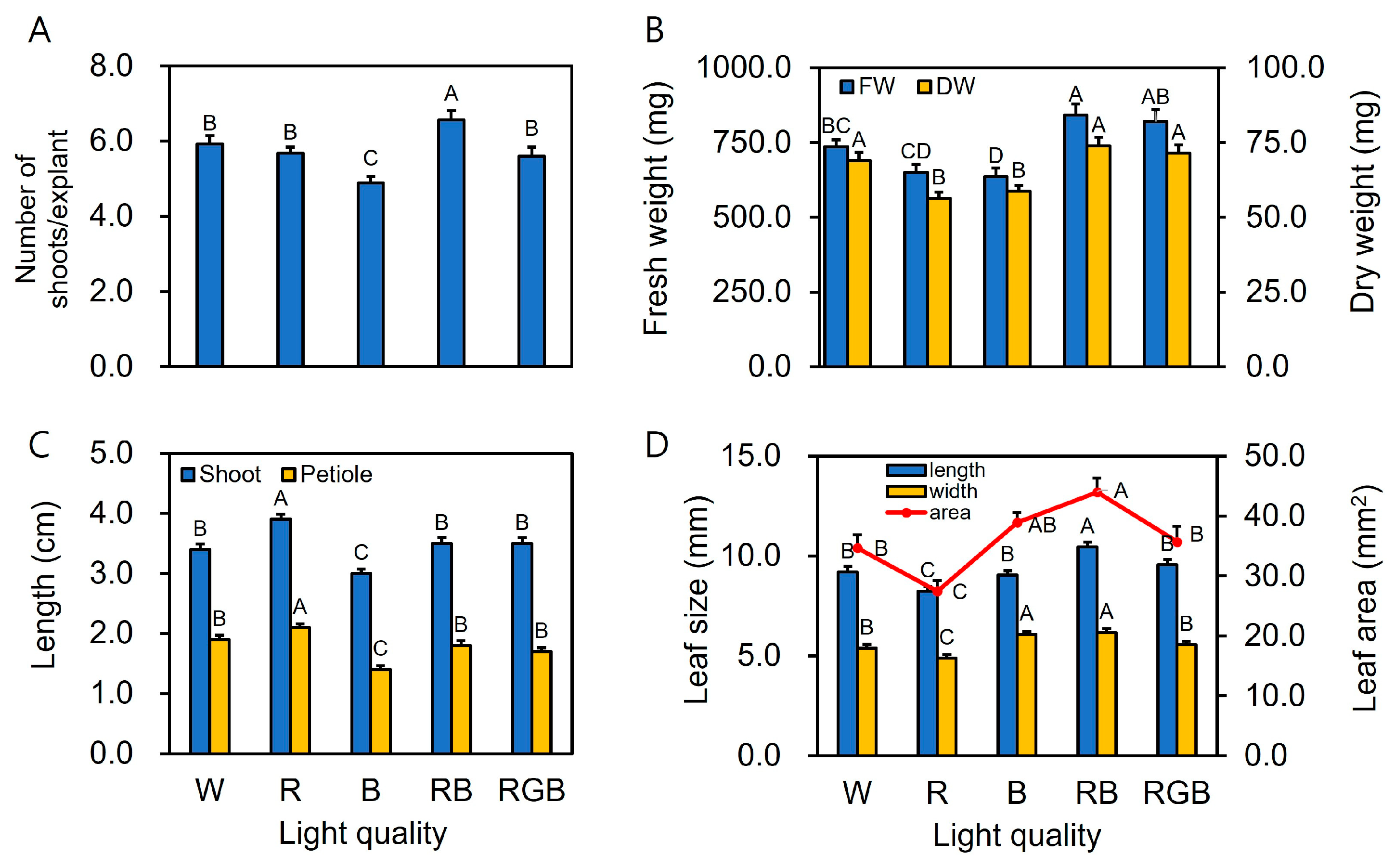
2.2. Data Collection
2.3. Acclimatization
2.4. Estimation of Chlorophyll and Carotenoid Content
2.5. Measurement of Photosynthetic Characteristics and Chlorophyll Fluorescence
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of artificial light on plant physiology and secondary metabolism: A review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Higuchi, Y.; Hisamatsu, T. Light acts as a signal for regulation of growth and development. In LED Lighting for Urban Agriculture; Kozai, T., Fujiwara, K., Runkle, E., Eds.; Springer: Singapore, 2016; pp. 57–73. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Batista, D.S.; Felipe, S.H.S.; Silva, T.D.; de Castro, K.M.; Mamedes-Rodriques, T.; Miranda, N.A.; Rios-Rois, A.M.; Faria, D.V.; Fortini, E.A.; Chagas, K.; et al. Light quality in plant tissue culture: Does it matter? Vitr. Cell. Dev. Biol.–Plant 2018, 54, 195–2015. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D. Impact of light-emitting diodes (LEDs) and its potential on growth and development in controlled environment plant production system. Curr. Biotechnol. 2016, 5, 28–43. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, M.; Abbas, F.; Sun, Y.; Gao, T.; Yan, F.; Li, X.; Yu, Y.; Yue, Y.; Yu, R.; et al. Classification and association analysis of gerbera (Gerbera hybrida) flower color traits. Font. Plant Sci. 2022, 12, 779288. [Google Scholar] [CrossRef]
- Song, H.Y.; Park, J.T.; Jeong, J.A.; Lim, S.H.; Kwon, O.K. A high-yielding standard gerbera cultivar ‘Shy Pink’ with light pink colored petals and semi-double type for cut flower. Flower Res. J. 2022, 30, 227–231. [Google Scholar]
- Cardoso, J.C.; Teixeira da Silva, J.A. Gerbera micropropagation. Biotechnol. Adv. 2013, 31, 1344–1357. [Google Scholar] [CrossRef]
- Hahn, E.J.; Kozai, T.; Paek, K.Y. Blue and red light-emitting diodes with or without sucrose and ventilation affect in vitro growth of Rehmannia glutinosa plantlets. J. Plant Biol. 2000, 43, 247–250. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Kim, Y.W.; Moon, H.K. Enhancement of somatic embryogenesis and plant regeneration in Japanese red pine (Pinus densiflora). Plant Biotechnol. Rep. 2014, 8, 259–266. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effect of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Lian, M.L.; Murthy, H.N.; Paek, K.Y. Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’. Sci. Hortic. 2002, 94, 365–370. [Google Scholar] [CrossRef]
- Park, Y.G.; Oh, H.J.; Jeong, B.R. Growth and anthocyanin concentration of Perilla frutescence var. acuta Kudo as affected by light source and DIF under controlled environment. Hortic. Environ. Biotechnol. 2013, 54, 103–108. [Google Scholar] [CrossRef]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Pawlowska, B.; Zupnik, M.; Szewczyk-Taranek, B.; Cioc, M. Impact of LED light sources on morphogenesis and levels of photosynthetic pigments in Gerbera jamesonii grown in vitro. Horticul. Environ. Biotechnol. 2018, 59, 115–123. [Google Scholar] [CrossRef]
- Cioc, M.; Kalisz, A.; Zupnik, M.; Pawlowska, B. Different LED light intensities and 6-benzyladenine concentration in relation to shoot development, leaf architecture, and photosynthetic pigments of Gerbera jamesonii Bolus in vitro. Agronomy 2019, 9, 358. [Google Scholar] [CrossRef]
- Cioc, M.; Pawlowska, B. Leaf response to differential light spectrum compositions during micropropagation of Gerbera axillary shoots. Agronomy 2020, 10, 1832. [Google Scholar] [CrossRef]
- Cioc, M.; Dziurka, M.; Pawlowska, B. Changes in endogenous phytohormones of Gerbera jamesonii axillary shoots multiplied under different light emitting diodes light quality. Molecules 2022, 27, 1804. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Z.; He, S.; Shi, L.; Song, Y.; Lou, X.; He, D. LED-supplied red and blue light alters the growth, antioxidant status, and photochemical potential of in vitro-grown Gerbera jamesonii plantlets. Horticul. Sci. Technol. 2019, 37, 473–489. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatohu, B. Fundamentals and applications of light emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectrophotometry. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; Wiley: New York, NY, USA, 2001; pp. F4.3-1–F4.3.8. [Google Scholar] [CrossRef]
- Faukda, N. Advanced light control technologies in protected horticulture: A review on morphological and physiological responses in plant to light quality and its applications. J. Dev. Sustain. Agric. 2013, 8, 32–40. [Google Scholar] [CrossRef]
- Paradiso, R.; Meinen, E.; Snel, J.F.; De Visser, P.; Van Ieperen, W.; Hogewoning, S.W.; Marcelis, L.F.M. Spectral dependence of photosynthesis and light absorptance in single leaves and canopy in rose. Sci. Horitc. 2011, 127, 548–554. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse role in plant processes. J. Exp. Bot. 2017, 68, 2099–2119. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of tsuru-rindo (Tripoterospermum japanicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Nhut, D.T.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of strawberry plantlets cultured in vitro under super bright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult. 2003, 73, 43–52. [Google Scholar] [CrossRef]
- Cashmore, A.R.; Jarillo, J.A.; Wu, Y.J.; Liu, D.M. Cryptochromes: Blue receptors for plants and animals. Science 1999, 284, 760–765. [Google Scholar] [CrossRef]
- Quail, P.H. Phytochrome photosensory signaling networks. Nat. Rev. Mol. Cell. Biol. 2002, 3, 85. [Google Scholar] [CrossRef]
- Sellaro, R.; Hoecker, U.; Yanovsky, M.; Chory, J.; Casal, J.J. Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr. Biol. 2009, 19, 1216–1220. [Google Scholar] [CrossRef]
- Poudel, P.R.; Kataoka, I.; Mochioka, R. Effect of red and blue light-emitting diodes on growth and morphogenesis of grapes. Plant Cell Tissue Organ Cult. 2008, 92, 147–153. [Google Scholar] [CrossRef]
- Fukuda, N.; Ajima, C.; Yukawa, T.; Olsen, J.E. Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ. Exp. Bot. 2016, 121, 102–111. [Google Scholar] [CrossRef]
- Macedo, A.F.; Leal-Costa, M.V.; Tavares, E.S.; Lage, C.L.S.; Esquibel, M.A. The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Tang, C. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. 2010, 103, 155–163. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 414–415. [Google Scholar] [CrossRef]
- Lin, Y.; Li, J.; Li, B.; He, T.; Chun, Z. Effects of light quality on growth and development of protocorm-like bodies of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Matuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supple mental blue light. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.X.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomata devel opment of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Fornt. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Daud, N.; Faizal, A.; Grelen, D. Adventitious rooting of Jatropha curcus L. is stimulated by phloroglucinol and by red LED light. Vitr. Cell Dev. Biol.–Plant 2013, 31, 1344–1357. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, Y.; Yang, M. Effect of composite LED light on root growth and antioxidant capacity of Cunnighamia lanceolata tissue culture seedlings. Sci. Rep. 2019, 9, 9766. [Google Scholar] [CrossRef] [PubMed]
- Stuefer, J.F.; Huber, H. Differential effect of light quantity and spectral light quality on growth, morphology and development of two stoloniferous Potentilla species. Oecologia 1998, 117, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dougher, T.A.; Bugbee, B.G. Is blue light good or bad for plants? Life Support Biosph. Sci. 1998, 5, 129–136. [Google Scholar]
- Tripathy, B.C.; Brwon, C.S. Root-shoot interaction in greening of wheat seedlings grown under red light. Plant Physiol. 1995, 107, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LED on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Mengxi, L.; Zhigang, X.; Yang, Y.; Yijie, F. Effect of different spectral lights on Oncidium PLBs induction, proliferation and plant regeneration. Plant Cell Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.M.K.; Liu, G.H.; Liu, H. Growth, photosynthetic characteristics, antioxidant capacity and biomass yield and quality of wheat (Triticum aestivum L.) exposed to LED light sources with different spectra combination. J. Agron. Crop Sci. 2014, 200, 219–230. [Google Scholar] [CrossRef]
- Lee, S.H.; Tewari, R.K.; Hanh, E.J.; Paek, K.Y. Photon flux and light quality induce changes in growth, stomatal development, photosynthesis and transpiration of Withania somnifera (L.) Dunal. Plantlets. Plant Cell Tissue Organ Cult. 2007, 90, 141–151. [Google Scholar] [CrossRef]
- Lin, K.H.; Hunag, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Yang, L.Y.; Wang, L.T.; Ma, E.D.; Li, J.Y.; Gong, M. Effects of light quality on growth and development, photosynthetic character istics and content of carbohydrates in tobacco (Nicotiana tabacum L.) plants. Photosynthetica 2017, 55, 467–477. [Google Scholar] [CrossRef]
- Shang, W.; Song, Y.; Zhang, C.; Shi, L.; Shen, Y.; Li, X.; Wang, Z.; He, S. Effects of light quality on growth, photosynthetic charac teristics, and endogenous hormones in in vitro-cultured Lilium plantlets. Hortic. Environ. Biotechnol. 2023, 64, 65–81. [Google Scholar] [CrossRef]
- Inoue, S.; Takemiya, A.; Shimazaki, K. Phototropin signaling and stomatal opening as a model case. Curr. Opin. Plant Biol. 2010, 13, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Babani, F. Light adaption and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters, and photosynthetic activity. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 713–736. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2015, 53, 213–222. [Google Scholar] [CrossRef]




| Light Quality | Total Fresh Weight (mg) | Total Dry Weight (mg) | Plantlet Height (cm) | Length (cm) | Number (per Plant) | ||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Roots | Leaves | ||||
| W z | 506.37 b y | 47.89 c | 6.27 c | 5.01 c | 1.83 b | 6.60 bc | 7.26 a |
| R | 581.26 a | 52.66 b | 8.35 a | 7.01 a | 2.10 a | 6.11 c | 6.77 b |
| B | 542.77 b | 53.09 b | 6.05 c | 4.79 c | 1.77 b | 7.00 b | 7.26 a |
| RB | 607.89 a | 56.29 a | 6.68 b | 5.41 b | 1.80 b | 7.77 a | 7.29 a |
| RGB | 515.40 b | 47.83 c | 6.23 c | 4.83 c | 1.83 b | 7.00 b | 7.40 a |
| Light Quality | Petiole Length (cm) | Leaf Length (mm) | Leaf Width (mm) | Leaf Index (length/width) | Leaf Area (mm2) |
|---|---|---|---|---|---|
| W z | 2.54 b y | 14.60 b | 9.47 b | 1.55 b | 94.35 b |
| R | 4.24 a | 13.79 c | 7.51 c | 1.83 a | 67.40 c |
| B | 2.15 c | 16.52 a | 11.32 a | 1.46 d | 127.41 a |
| RB | 2.61 b | 16.44 a | 11.05 a | 1.49 c | 124.11 a |
| RGB | 2.47 b | 14.65 b | 9.91 b | 1.48 cd | 99.39 b |
| Light Quality | Total Fresh Weight (g) | Total Dry Weight (mg) | Plantlet Height (cm) | Length (cm) | Number (per Plant) | ||
|---|---|---|---|---|---|---|---|
| Shoot | Root | Roots | Leaves | ||||
| W z | 1.36 ab y | 144.13 a | 12.37 ab | 8.95 a | 5.27 a | 9.88 a | 4.88 b |
| R | 1.14 b | 115.25 b | 12.47 ab | 9.11 a | 4.36 b | 7.00 b | 5.13 b |
| B | 1.39 a | 149.25 a | 13.46 a | 9.08 a | 5.48 a | 10.13 a | 5.13 b |
| RB | 1.39 a | 144.13 a | 12.95 ab | 9.56 a | 4.47 b | 9.63 a | 6.63 a |
| RGB | 1.25 ab | 128.25 ab | 12.25 b | 8.72 a | 4.95 ab | 8.38 ab | 5.00 b |
| Light Quality | Petiole Length (cm) | Leaf Length (cm) | Leaf Width (cm) | Leaf Index (Length/Width) | Leaf Area (cm2) |
|---|---|---|---|---|---|
| W z | 5.78 a y | 3.60 ab | 2.91 a | 1.25 b | 7.22 ab |
| R | 6.14 a | 2.87 c | 2.17 b | 1.35 ab | 4.67 c |
| B | 5.98 a | 3.72 a | 3.07 a | 1.23 b | 8.57 a |
| RB | 5.48 ab | 3.54 ab | 2.87 a | 1.25 b | 7.54 a |
| RGB | 5.04 b | 3.19 bc | 2.29 b | 1.41 a | 5.23 bc |
| Light Quality | Total Chlorophyll | Chlorophyll a | Chlorophyll b | Carotenoids |
|---|---|---|---|---|
| W z | 1.77 b y | 1.18 b | 0.60 ab | 0.29 b |
| R | 1.75 b | 1.18 b | 0.57 b | 0.26 b |
| B | 2.05 a | 1.33 a | 0.73 a | 0.35 a |
| RB | 1.80 ab | 1.21 ab | 0.60 ab | 0.28 b |
| RGB | 1.73 b | 1.16 b | 0.57 b | 0.29 b |
| Light Quality | Transpiration Rate (Tr) (mol·H2O·m−2·s−1) | Photosynthetic Rate (Pn) (µmol·CO2·m−2·s−1) | Internal CO2 Concentration (Ci) (µmol·CO2·mol−1) | Stomatal Conductance (Gs) (mol·H2O·m−2·s−1) |
|---|---|---|---|---|
| W z | 0.0005 b y | 3.86 b | 276.74 c | 0.037 b |
| R | 0.0005 b | 3.40 c | 297.71 b | 0.040 b |
| B | 0.0010 a | 5.31 a | 318.12 a | 0.077 a |
| RB | 0.0010 a | 5.44 a | 325.78 a | 0.080 a |
| RGB | 0.0009 a | 5.40 a | 313.98 a | 0.072 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, M.-J.; Murthy, H.N.; Song, H.-Y.; Lee, S.-Y.; Park, S.-Y. Influence of White, Red, Blue, and Combination of LED Lights on In Vitro Multiplication of Shoots, Rooting, and Acclimatization of Gerbera jamesonii cv. ‘Shy Pink’ Plants. Agronomy 2023, 13, 2216. https://doi.org/10.3390/agronomy13092216
Lim M-J, Murthy HN, Song H-Y, Lee S-Y, Park S-Y. Influence of White, Red, Blue, and Combination of LED Lights on In Vitro Multiplication of Shoots, Rooting, and Acclimatization of Gerbera jamesonii cv. ‘Shy Pink’ Plants. Agronomy. 2023; 13(9):2216. https://doi.org/10.3390/agronomy13092216
Chicago/Turabian StyleLim, Myeong-Jin, Hosakatte Niranjana Murthy, Hyun-Young Song, Su-Young Lee, and So-Young Park. 2023. "Influence of White, Red, Blue, and Combination of LED Lights on In Vitro Multiplication of Shoots, Rooting, and Acclimatization of Gerbera jamesonii cv. ‘Shy Pink’ Plants" Agronomy 13, no. 9: 2216. https://doi.org/10.3390/agronomy13092216
APA StyleLim, M.-J., Murthy, H. N., Song, H.-Y., Lee, S.-Y., & Park, S.-Y. (2023). Influence of White, Red, Blue, and Combination of LED Lights on In Vitro Multiplication of Shoots, Rooting, and Acclimatization of Gerbera jamesonii cv. ‘Shy Pink’ Plants. Agronomy, 13(9), 2216. https://doi.org/10.3390/agronomy13092216





