Effects of Saline-Alkali Stress on Sugar Metabolism of Jujube Fruit: A Metabolomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of the Microstructure of Jujube Fruit
2.3. Determination of Sugar Composition
2.4. Metabolomics Assay
2.5. Quantitative Real-Time PCR (qRT-PCR)
2.6. Data Analysis
3. Results
3.1. Phenotypic Changes in Jujube Fruit under Different Salinity-Alkali Treatments
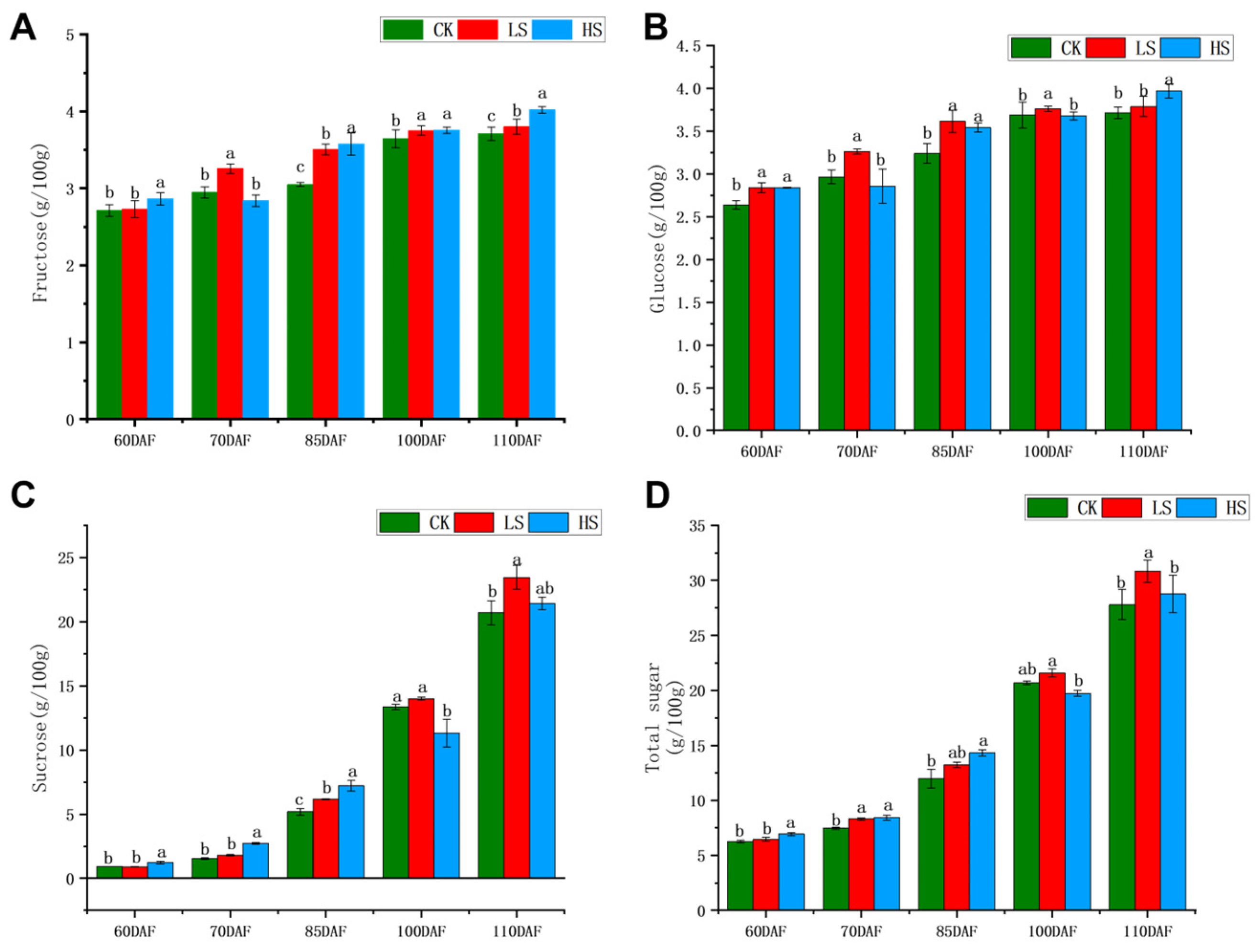
3.2. Change in Sugar Component Content
3.3. Metabolomics Analysis
3.4. Screening of Differentially-Accumulated Metabolites
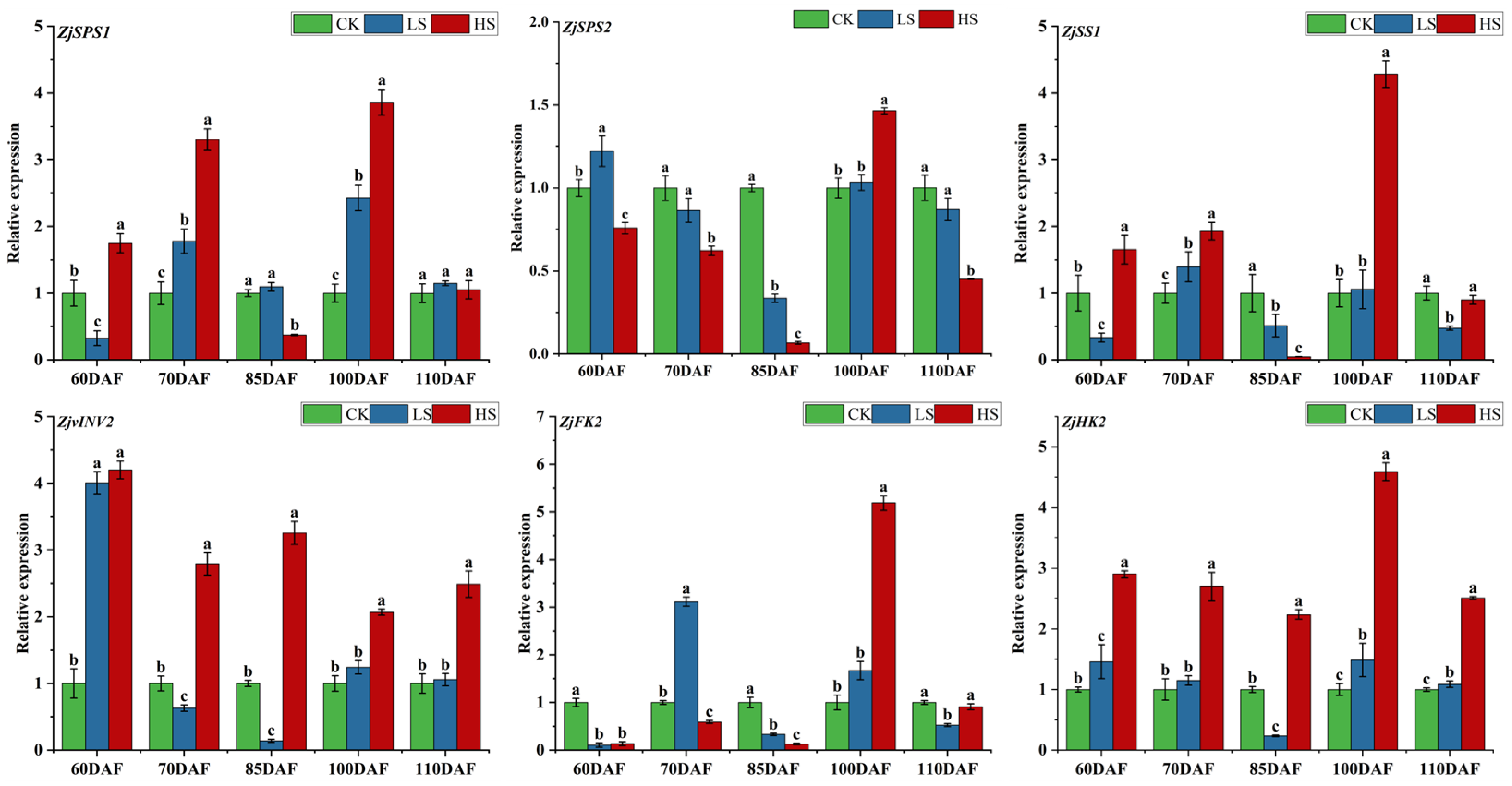
3.5. Expression Analysis of Genes Related to Carbohydrate Metabolism Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Feng, Y.; Yan, M.; Yu, J.; Zhou, X.; Bao, J.; Zhang, Q.; Wu, C. Effect of Saline-Alkali Stress on Sugar Metabolism of Jujube Fruit. Horticulturae 2022, 8, 474. [Google Scholar] [CrossRef]
- Wang, X.; Geng, S.; Ri, Y.-J.; Cao, D.; Liu, J.; Shi, D.; Yang, C. Physiological responses and adaptive strategies of tomato plants to salt and alkali stresses. Sci. Hortic. 2011, 130, 248–255. [Google Scholar] [CrossRef]
- Abdel-Hameed, A.A. Effect of Salt and Water Stresses on Jujube Trees under Ras Sudr Conditions. IOSR J. Agric. Vet. Sci. 2015, 8, 92–107. [Google Scholar]
- Afifia, A.A.; Refat, A.Y.; Hussein, M.M. Fourier Transform Infrared Spectometry Study on Early Stage of Salt Stress in Jojoba Plant. Life Sci. J. 2013, 10, 1973–1981. [Google Scholar]
- Li, M.; Guo, Y.; Liu, S.; Zhao, Y.; Pang, X.; Li, Y. Autotetraploidization in Ziziphus jujuba Mill. var. spinosa enhances salt tolerance conferred by active, diverse stress responses. Environ. Exp. Bot. 2019, 165, 92–107. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, L.; Wang, Y.; Tao, H.X.; Jun, L.; Zhao, Z.Y.; Guo, Y.P. Effects of soil water stress on fruit yield, quality and their relationship with sugar metabolism in ‘Gala’ apple. Sci. Hortic. 2019, 258, 108753. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Fan, X.-W.; Sun, J.-L.; Cai, Z.; Zhang, F.; Li, Y.-Z.; Palta, J.A. MeSWEET15a/b genes play a role in the resistance of cassava (Manihot esculenta Crantz) to water and salt stress by modulating sugar distribution. Plant Physiol. Biochem. 2023, 194, 394–405. [Google Scholar] [CrossRef]
- Liu, L.; Gai, Z.; Qiu, X.; Liu, T.; Li, S.; Ye, F.; Jian, S.; Shen, Y.; Li, X. Salt stress improves the low-temperature tolerance in sugar beet in which carbohydrate metabolism and signal transduction are involved. Environ. Exp. Bot. 2023, 208, 105239. [Google Scholar] [CrossRef]
- Sher, A.; Hassan, M.U.; Sattar, A.; Ul-Allah, S.; Ijaz, M.; Hayyat, Z.; Bibi, Y.; Hussain, M.; Qayyum, A. Exogenous application of melatonin alleviates the drought stress by regulating the antioxidant systems and sugar contents in sorghum seedlings. Biochem. Syst. Ecol. 2023, 107, 104620. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soy bean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, Y.; Zhang, X.; Tian, P.; Li, J.; Tian, Y. Comprehensive comparison of different saline water irrigation strategies for tomato production: Soil properties, plant growth, fruit yield and fruit quality. Agric. Water Manag. 2019, 213, 521–533. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Tang, T.; Liu, Y.; Tahir, M.M.; Wang, C.; Meng, Z.; Niu, J.; Yang, W.; Ma, J.; et al. Comparison of morphological, physiological, and related-gene expression responses to Saline-Alkali stress in eight apple rootstock genotypes. Sci. Hortic. 2022, 306, 11145. [Google Scholar] [CrossRef]
- Jia, X.; Zhu, Y.; Zhang, R.; Zhu, Z.; Zhao, T.; Cheng, L.; Gao, L.; Liu, B.; Zhang, X.; Wang, Y. Ionomic and metabolomic analyses reveal the resistance response mechanism to Saline-Alkali stress in Malus halliana seedlings. Plant Physiol. Biochem. 2019, 147, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-W.; Li, T.-L.; Jiang, J. Tomato Key Sucrose Metabolizing Enzyme Activities and Gene Expression Under NaCl and PEG Iso-Osmotic Stresses. Agric. Sci. China 2009, 8, 1046–1052. [Google Scholar] [CrossRef]
- Morabito, C.; Secchi, F.; Schubert, A. Grapevine TPS (trehalose-6-phosphate synthase) family genes are differentially regulated during development, upon sugar treatment and drought stress. Plant Physiol. Biochem. 2021, 164, 54–62. [Google Scholar] [CrossRef]
- Li, H.; Tiwari, M.; Tang, Y.; Wang, L.; Yang, S.; Long, H.; Guo, J.; Wang, Y.; Wang, H.; Yang, Q.; et al. Metabolomic and transcriptomic analyses reveal that sucrose synthase regulates maize pollen viability under heat and drought stress. Ecotoxicol. Environ. Saf. 2022, 246, 114191. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Z.; Yan, G.; Wang, F.; Zhao, L.; Liu, N.; Abudurezike, A.; Li, Y.; Wang, W.; Shi, S. Salt-responsive transcriptome analysis of triticale reveals candidate genes involved in the key metabolic pathway in response to salt stress. Sci. Rep. 2020, 10, 20669. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.; Wang, Y.; Wu, C.; Huang, J. Genome-Wide Identification of WRKY Transcription Factors in Chinese jujube (Ziziphus jujuba Mill.) and Their Involvement in Fruit Developing, Ripening, and Abiotic Stress. Genes 2019, 10, 360. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Wang, Y.; Yang, D.; Tan, H.; Zhan, Y.; Yang, Y.; Luo, Y.; Chen, G. Optimization extraction, structural features and antitumor activity of polysaccharides from Z. jujuba cv. Ruoqiangzao seeds. Int. J. Biol. Macromol. 2019, 135, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bian, Y.; Hou, S.; Li, X. Sugar transport played a more important role than sugar biosynthesis in fruit sugar accumulation during Chinese jujube domestication. Planta 2018, 248, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, Z.; Shi, Q.; Duan, X.; Du, J.; Wu, C.; Li, X. Methyl Jasmonate- and Salicylic Acid-Induced Transcription Factor ZjWRKY18 Regulates Triterpenoid Accumulation and Salt Stress Tolerance in Jujube. Int. J. Mol. Sci. 2023, 24, 3899. [Google Scholar] [CrossRef]
- Chang, X.; Sun, J.; Liu, L.; He, W.; Zhao, B. Transcriptome Analysis of Differentially Expressed Genes in Wild Jujube Seedlings under Salt Stress. J. Am. Soc. Hortic. Sci. 2020, 145, 174–185. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, L.; Wang, L.; Li, X.; Ban, Z.; Chen, C.; Zhu, Y. Partial compression increases acidity, but decreases phenolics in jujube fruit: Evidence from targeted metabolomics. Food Res. Int. 2023, 164, 112388. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Xu, M.; Deng, B.; Liu, H.; Zhao, X. Phytochemical analysis of Ziziphus jujube leaf at different foliar ages based on widely targeted metabolomics. Open Chem. 2022, 20, 1485–1493. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, A.; Wang, Y.; Ren, H.; Li, Y.; Li, D.; Du, J. Metabolomics-based analysis of flavonoid metabolites in Chinese jujube and sour jujube fruits from different harvest periods. J. Food Sci. 2022, 87, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef]
- Zou, J.; Yu, H.; Yu, Q.; Jin, X.; Cao, L.; Wang, M.; Wang, M.; Ren, C.; Zhang, Y. Physiological and UPLC-MS/MS widely targeted metabolites mechanisms of alleviation of drought stress-induced soybean growth inhibition by melatonin. Ind. Crop. Prod. 2021, 163, 113323. [Google Scholar] [CrossRef]
- Zheng, Q.; Yin, X.; Yang, A.; Yu, N.; Xing, R.; Chen, Y.; Deng, R.; Cao, J. Precise Authenticity of Quinoa, Coix Seed, Wild Rice and Chickpea Components Using Optimized TaqMan Real-Time PCR. Foods 2023, 12, 852. [Google Scholar] [CrossRef]
- Zozio, S.; Servent, A.; Hubert, O.; Hiol, A.; Pallet, D.; Mbéguié, D. Physicochemical and biochemical characterization of ripening in jujube (Ziziphus mauritiana Lamk) fruits from two accessions grown in Guadeloupe. Sci. Hortic. 2014, 175, 290–297. [Google Scholar] [CrossRef]
- Tian, S.-L.; Lu, B.-Y.; Gong, Z.-H.; Shah, S.N.M. Effects of drought stress on capsanthin during fruit development and ripening in pepper (Capsicum annuum L.). Agric. Water Manag. 2014, 137, 46–51. [Google Scholar] [CrossRef]
- Kesari, R.; Trivedi, P.K.; Nath, P. Ethylene-induced ripening in banana evokes expression of defense and stress related genes in fruit tissue. Postharvest Biol. Technol. 2007, 46, 136–143. [Google Scholar] [CrossRef]
- Steelheart, C.; Alegre, M.L.; Baldet, P.; Rothan, C.; Bres, C.; Just, D.; Okabe, Y.; Ezura, H.; Ganganelli, I.M.; Grozeff, G.E.G.; et al. High light stress induces H2O2 production and accelerates fruit ripening in tomato. Plant Sci. 2022, 322, 111348. [Google Scholar] [CrossRef]
- Galindo, A.; Calín-Sánchez, Á.; Griñán, I.; Rodríguez, P.; Cruz, Z.; Girón, I.; Corell, M.; Martínez-Font, R.; Moriana, A.; Carbonell-Barrachina, A.; et al. Water stress at the end of the pomegranate fruit ripening stage produces earlier harvest and improves fruit quality. Sci. Hortic. 2017, 226, 68–74. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, M.; Sun, P.; Gao, H.; Yang, H.; Jing, Y.; Hussain, M.A.; Saxena, R.K.; Carther, F.I.; Wang, Q.; et al. Tonoplast inositol transporters: Roles in plant abiotic stress response and crosstalk with other signals. J. Plant Physiol. 2022, 271, 153660. [Google Scholar] [CrossRef]
- Ju, F.; Pang, J.; Sun, L.; Gu, J.; Wang, Z.; Wu, X.; Ali, S.; Wang, Y.; Zhao, W.; Wang, S.; et al. Integrative transcriptomic, metabolomic and physiological analyses revealed the physiological and molecular mechanisms by which potassium regulates the salt tolerance of cotton (Gossypium hirsutum L.) roots. Ind. Crop. Prod. 2023, 193, 116177. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Liu, Y.; Yang, S.; Zhang, J.; Gong, X.; Ma, F. Overexpression of MdMIPS1 enhances salt tolerance by improving osmosis, ion balance, and antioxidant activity in transgenic apple. Plant Sci. 2020, 301, 110654. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-R.; Li, X.-Y.; Yao, Y.-X.; Hao, Y.-J. Modifications of Kyoho grape berry quality under long-term NaCl treatment. Food Chem. 2013, 139, 931–937. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, X.; Li, Y.; Sun, Q.; Dai, L.; Li, J.; Guo, Z.; Zhang, L.; Ci, L. Functional carbon nanodots improve soil quality and tomato tolerance in Saline-Alkali soils. Sci. Total. Environ. 2022, 830, 154817. [Google Scholar] [CrossRef]
- Sagar, M.; Chervin, C.; Mila, I.; Hao, Y.; Roustan, J.-P.; Benichou, M.; Gibon, Y.; Biais, B.; Maury, P.; Latché, A.; et al. SlARF4, an Auxin Response Factor Involved in the Control of Sugar Metabolism during Tomato Fruit Development. Plant Physiol. 2013, 161, 1362–1374. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, C.; Zhao, X.; Fei, Z.; Wan, K.; Zhang, Z.; Pang, X.; Yin, X.; Bai, Y.; Sun, X.; et al. The Jujube Genome Provides Insights into Genome Evolution and the Domestication of Sweetness/Acidity Taste in Fruit Trees. PLoS Genet. 2016, 12, e1006433. [Google Scholar] [CrossRef]
- Pi, E.; Zhu, C.; Fan, W.; Huang, Y.; Qu, L.; Li, Y.; Zhao, Q.; Ding, F.; Qiu, L.; Wang, H.; et al. Quantitative Phosphoproteomic and Metabolomic Analyses Reveal GmMYB173 Optimizes Flavonoid Metabolism in Soybean under Salt Stress. Mol. Cell. Proteom. 2018, 17, 1209–1224. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Huang, S.; Wang, Z.; Wang, W.; Zou, J.; Wang, F.; Luo, M.; Zhang, J. Integrated transcriptomics and metabolomics analyses provide insights into salt-stress response in germination and seedling stage of wheat (Triticum aestivum L.). Curr. Plant Biol. 2023, 33, 100274. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Gong, B.; Yan, Y.; Shi, Q. Proteomics and metabolomics analysis of tomato fruit at different maturity stages and under salt treatment. Food Chem. 2019, 311, 126009. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Wang, L.; Li, X.; Liu, Y.; Wang, X.; Gao, T.; Ma, Y. Integrated transcriptomic and metabolomics analysis reveals abscisic acid signal transduction and sugar metabolism pathways as defense responses to cold stress in Argyranthemum frutescens. Environ. Exp. Bot. 2023, 205, 105115. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289. [Google Scholar] [CrossRef]
- Prathap, V.; Aruna, T. Correlation between expression and activity of ADP glucose pyrophosphorylase and starch synthase and their role in starch accumulation during grain filling under drought stress in rice. Plant Physiol. Biochem. 2020, 157, 239–243. [Google Scholar]
- Liao, C.T.; Lin, C.H. Photosynthetic responses of grafted bitter melon seedlings to flood stress. Environ. Exp. Bot. 1996, 36, 167–172. [Google Scholar] [CrossRef]
- Shekhawat, S.; Mathur, G.S. Influence of NaCl Salt on Stevioside Production and Antioxidant Enzymes in Stevia rebaudiana Suspension culture: An Anti-Diabetic Sweetener Plant. Int. J. Adv. Sci. Eng. Technol. 2018, 4, 2321–8991. [Google Scholar]
- Sun, N.; Ma, Y.; Wang, X.; Ying, Q.; Huang, Y.; Zhang, L.; Zhu, Z.; Wang, Y.; He, Y. Grafting onto pumpkin alters the evolution of fruit sugar profile and increases fruit weight through invertase and sugar transporters in watermelon. Sci. Hortic. 2023, 314, 111936. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W. Regulation of accumulation and metabolism circadian rhythms of starch and sucrose in two leaf-color lettuces by red:blue ratios of LED continuous light. Environ. Exp. Bot. 2022, 196, 104811. [Google Scholar] [CrossRef]
- Solis, G.; Arguello, M.G.; Lopez, B.G.; Ruiz, H.J.; Lopez, L.F.; Sanchez, J.E.; Carreon, L. Arabidopsis thaliana sucrose phosphate synthase (sps) genes are expressed differentially in organs and tissues, and their transcription is regulated by osmotic stress. Gene Exp. Patterns 2017, 25, 92–101. [Google Scholar] [CrossRef]
- Maloney, V.J.; Park, J.Y.; Unda, F.; Mansfield, S.D. Sucrose phosphate synthase and sucrose phosphate phosphatase interact in planta and promote plant growth and biomass accumulation. J. Exp. Bot. 2015, 66, 4383–4394. [Google Scholar] [CrossRef] [PubMed]



| Sugar Constituent | Regression Equation | Correlation Coefficient |
|---|---|---|
| Fructose | y = 749.23X1.6239 | R2 = 0.9993 |
| Glucose | y = 1046.9X1.5509 | R2 = 0.9991 |
| Sucrose | y = 1055X1.6852 | R2 = 0.9989 |
| Gene Symbol | Primer Sequence (5′-3′) | Primer Sequence (3′-5′) |
|---|---|---|
| ZjSPS1 | AGTCCCACTCGCTACTTCGT | TCCAAATCCTCCAGCACATA |
| ZjSPS2 | TCCCAAGCCCTCAGGTATTT | GTAGTTTCTGTTTGCGTGTAG |
| ZjSS1 | AAGTCATAAGATCCGCACAG | AACACGAACATATTCCCAAA |
| ZjvINV2 | ACCCGATAACCCGAAGGAAG | GTCTGTACGGACGCCCAACC |
| ZjFK2 | CCCTCCTTTCCTACGACCCG | ATCGCTAACCTTGATCTCCTCTGC |
| ZjHK2 | TGATAGCCCTAATCCAAACT | TATCTGCCTCTTCTGACATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, Y.; Yan, M.; Zhou, X.; Yuan, Z.; Zhang, Q.; Yan, H.; Wu, C. Effects of Saline-Alkali Stress on Sugar Metabolism of Jujube Fruit: A Metabolomic Analysis. Agronomy 2023, 13, 2239. https://doi.org/10.3390/agronomy13092239
Wang Y, Feng Y, Yan M, Zhou X, Yuan Z, Zhang Q, Yan H, Wu C. Effects of Saline-Alkali Stress on Sugar Metabolism of Jujube Fruit: A Metabolomic Analysis. Agronomy. 2023; 13(9):2239. https://doi.org/10.3390/agronomy13092239
Chicago/Turabian StyleWang, Yan, Yifeng Feng, Min Yan, Xiaofeng Zhou, Ze Yuan, Qiaoqiao Zhang, Haoyu Yan, and Cuiyun Wu. 2023. "Effects of Saline-Alkali Stress on Sugar Metabolism of Jujube Fruit: A Metabolomic Analysis" Agronomy 13, no. 9: 2239. https://doi.org/10.3390/agronomy13092239
APA StyleWang, Y., Feng, Y., Yan, M., Zhou, X., Yuan, Z., Zhang, Q., Yan, H., & Wu, C. (2023). Effects of Saline-Alkali Stress on Sugar Metabolism of Jujube Fruit: A Metabolomic Analysis. Agronomy, 13(9), 2239. https://doi.org/10.3390/agronomy13092239





