Towards Improved Grain Yield and Soil Microbial Communities of Super Hybrid Rice through Sustainable Management
Abstract
:1. Introduction
2. Methods and Materials
2.1. Experimental Site Description
2.2. Experimental Design
2.3. Sample Collection and Analyses
2.3.1. Soil Sample Collection
2.3.2. Determination of Soil Physicochemical Indexes
2.3.3. Grain Yield
2.3.4. Soil DNA Extraction and High-Throughput Sequencing Analysis
2.4. Sequence Processing
3. Results
3.1. Soil Properties
3.2. Alpha Diversity of Bacterial and Fungal Communities
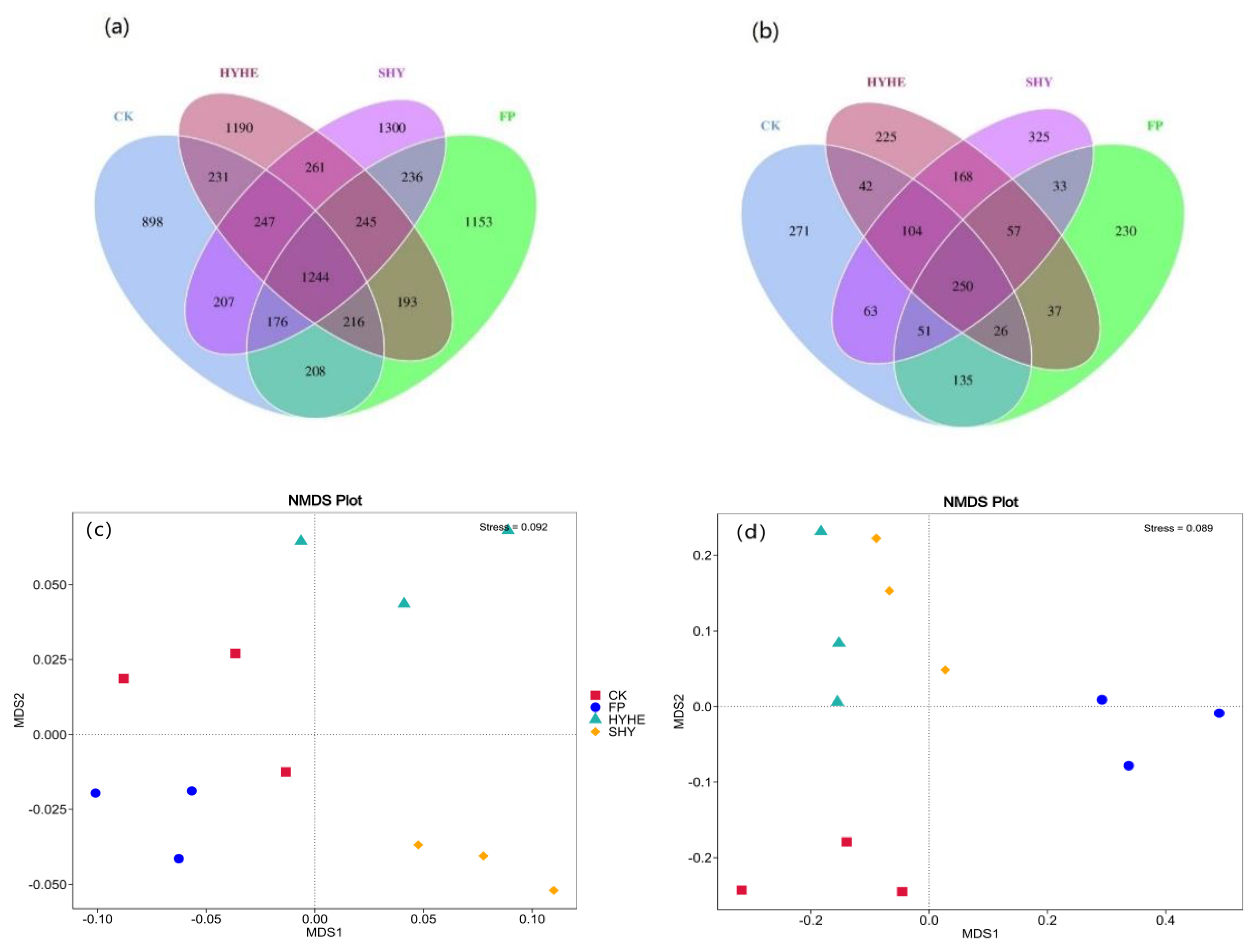
3.3. Beta Diversity of Bacterial and Fungal Communities
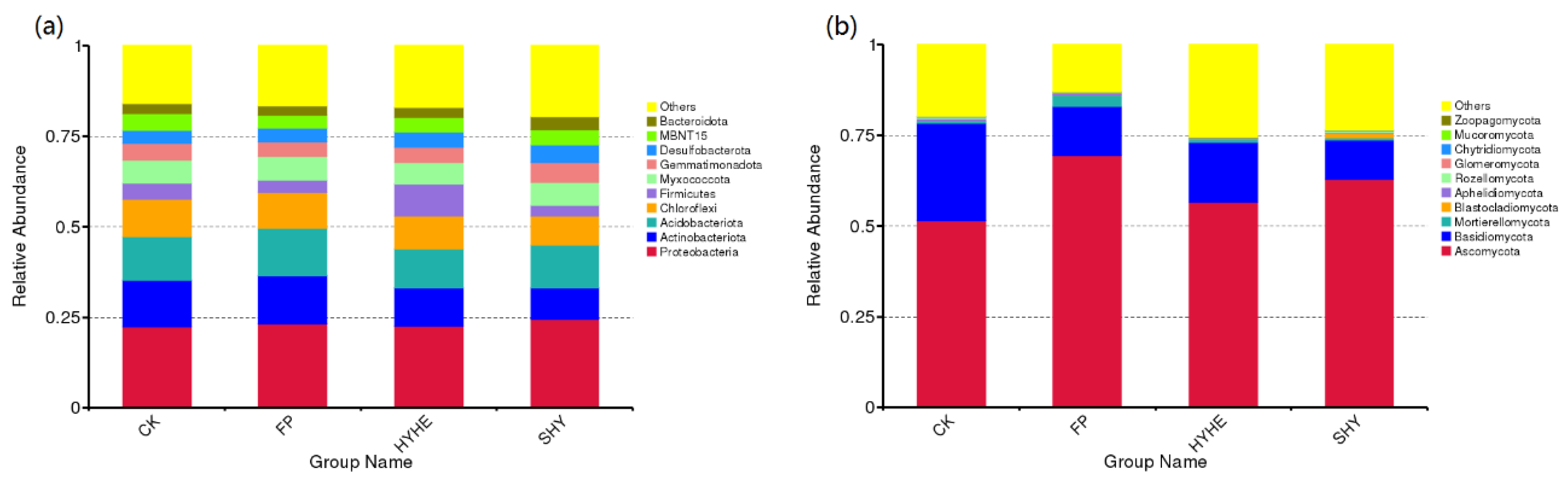
3.4. Composition of Bacterial and Fungal Communities
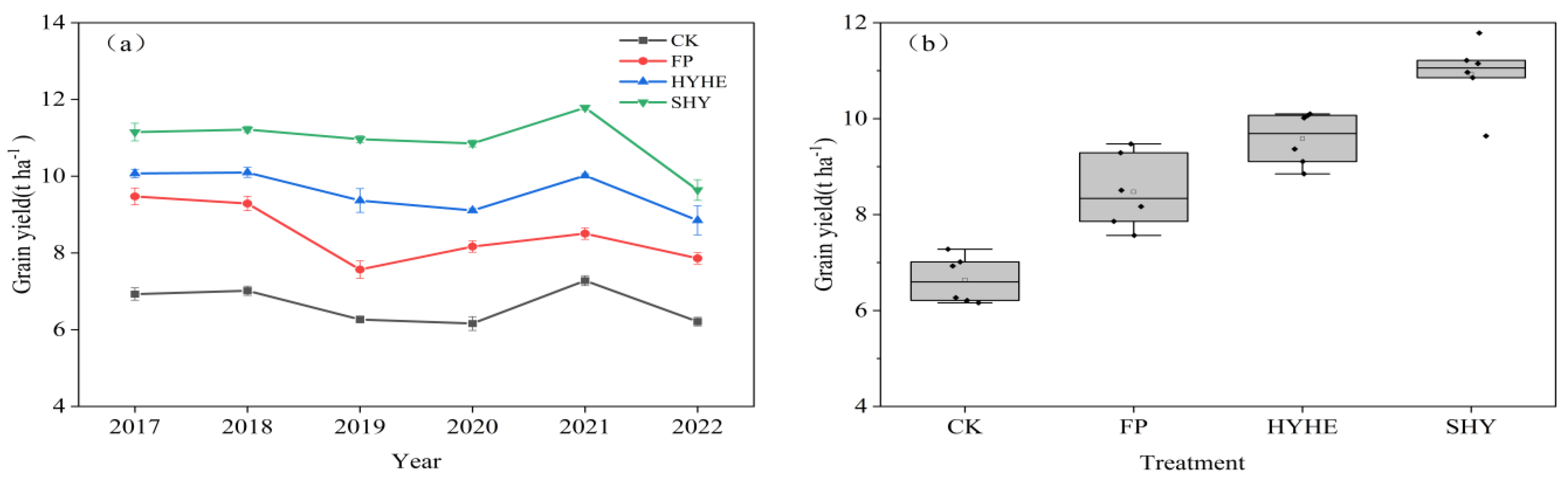
3.5. Grain Yield
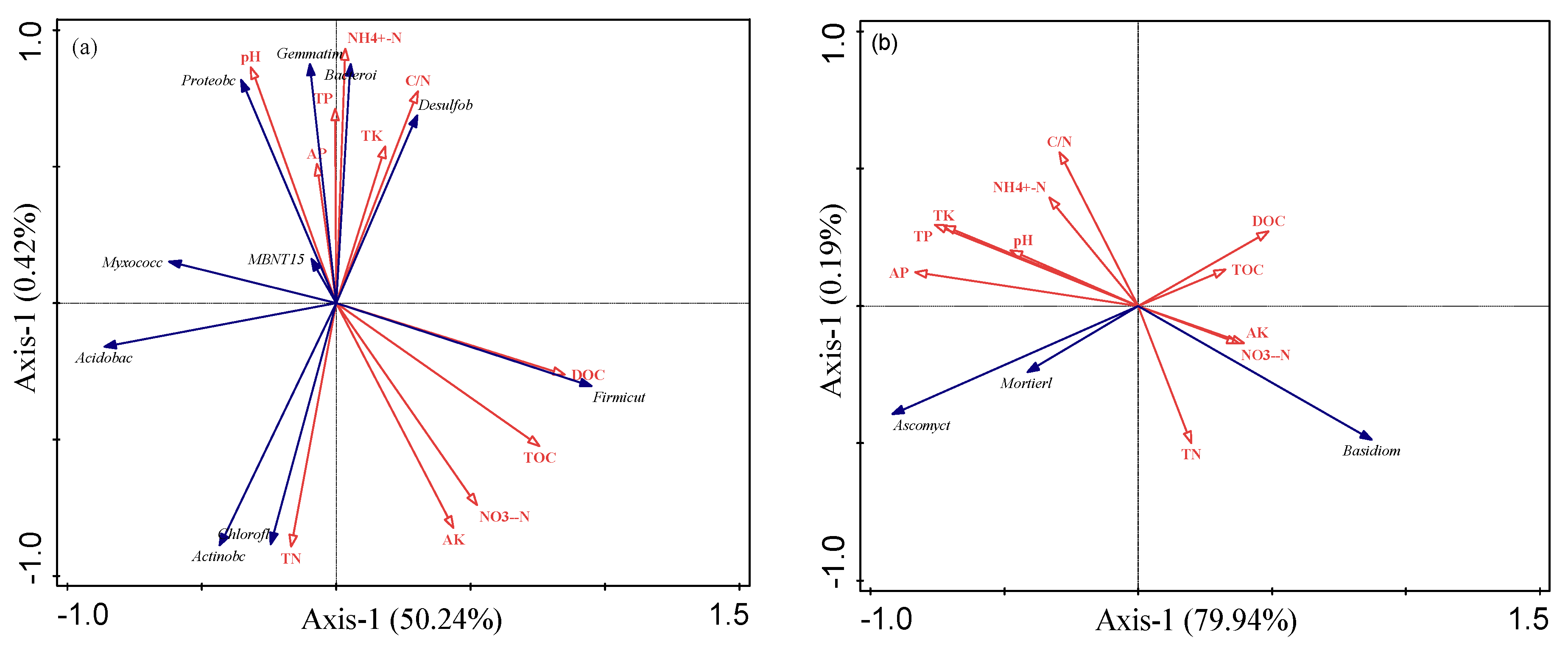
3.6. Correlation of Dominant Microbial Communities with Soil Properties
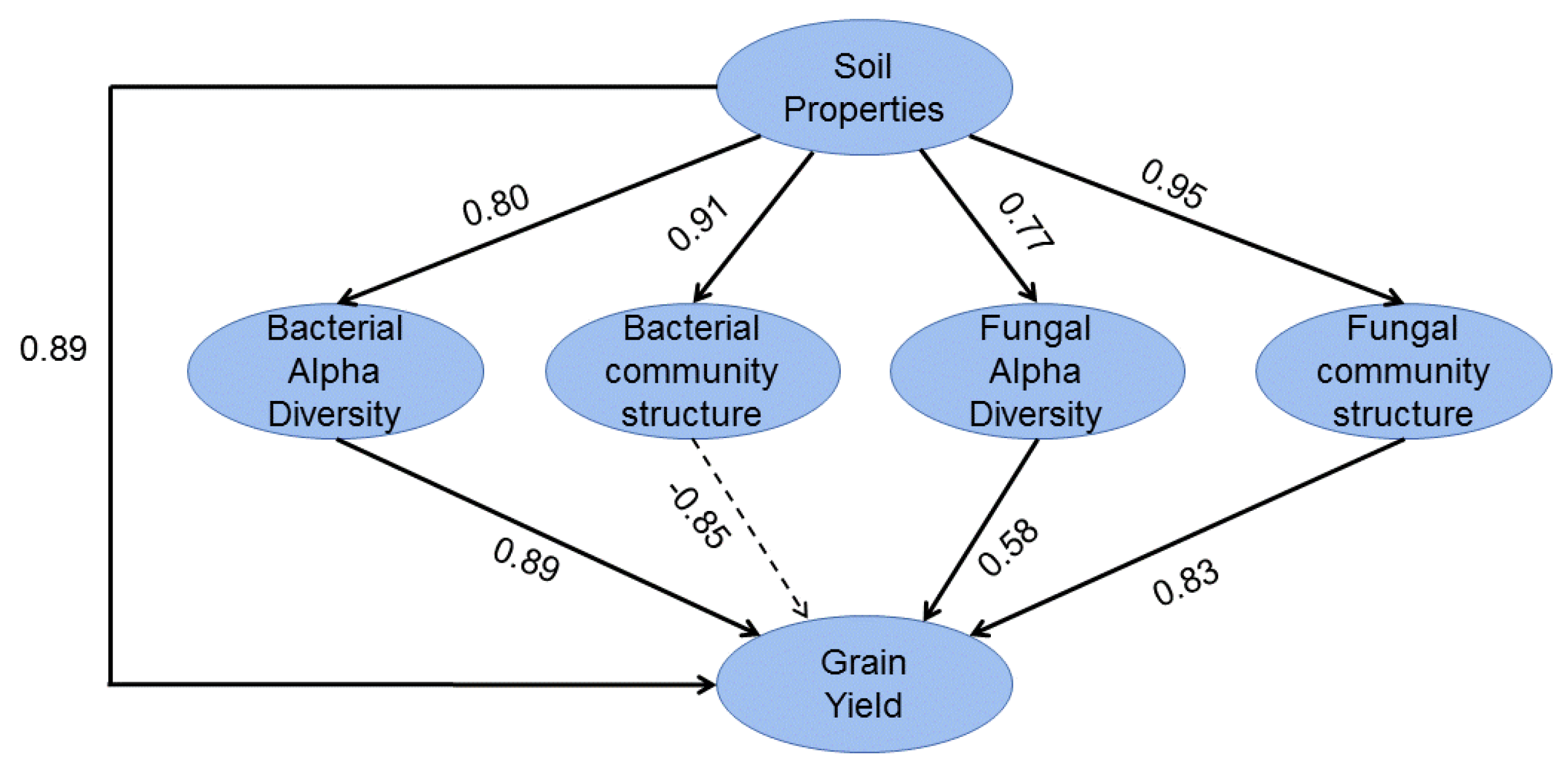
3.7. Effects of Environmental Factors on Soil Microorganisms and Rice Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, S.; Hu, P. Development strategy of rice science and technology in China. Chin. J. Rice Sci. 2008, 22, 223–226. [Google Scholar] [CrossRef]
- Yuan, L.P. Progress in Breeding of Super Hybrid Rice. J. Agric. 2018, 1, 71–73. [Google Scholar] [CrossRef]
- Deng, J.; Harrison, M.T.; Liu, K.; Ye, J.Y.; Xiong, X.; Fahad, S.; Huang, L.Y.; Tian, X.H.; Zhang, Y.B. Integrated crop management practices improve grain yield and resource use efficiency of super hybrid rice. Front. Plant Sci. 2022, 13, 851562. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.B.; Buresh, J.R.; Huang, J.L.; Yang, J.C.; Zou, Y.B.; Zhong, X.H.; Wang, G.H.; Zhang, F.S. Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop. Res. 2006, 96, 37–47. [Google Scholar] [CrossRef]
- Liu, K.; Deng, J.; Lu, J.; Wang, X.Y.; Lu, B.L.; Tian, X.H.; Zhang, Y.B. High nitrogen levels alleviate yield loss of super hybrid rice caused by high temperatures during the flowering stage. Front. Plant Sci. 2019, 10, 357. [Google Scholar] [CrossRef]
- Strickland, M.S.; Lauber, C.; Fierer, N.; Bradford, M. Testing the functional significance of microbial community composition. Ecology 2009, 90, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tian, J.; Fang, H.J.; Gao, Y.; Xu, M.G.; Lou, Y.L.; Zhou, B.K.; Kuzyakov, Y. Functional soil organic matter fractions, microbial community, and enzyme activities in a mollisol under 35 years manure and mineral fertilization. J. Soil Sci. Plant Nutr. 2019, 19, 430–439. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Patra, A.; Sharma, V.K.; Nath, D.J.; Ghosh, A.; Purakayastha, T.J.; Barman, M.; Kumar, S.; Chobhe, K.A.; Anil, S.; Rekwar, R.K. Impact of soil acidity influenced by long-term integrated use of enriched compost, biofertilizers, and fertilizer on soil microbial activity and biomass in rice under acidic soil. J. Soil Sci. Plant Nutr. 2021, 21, 756–767. [Google Scholar] [CrossRef]
- Zhao, J.; Ni, T.; Li, J.; Lu, Q.; Fang, Z.Y.; Huang, Q.W.; Zhang, R.F.; Li, R.; Shen, B.; Shen, Q. Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Agriculture 2016, 99, 1–12. [Google Scholar] [CrossRef]
- Cassman, N.A.; Leite, M.F.A.; Pan, Y.; De, H.M.; Van, V.J.A.; Kuramae, E.E. Plant and soil fungal but not soil bacterial communities are linked in long-term fertilized grassland. Sci. Rep. 2016, 6, 23680. [Google Scholar] [CrossRef] [PubMed]
- César, F.P.V.; Karl, K.; Márcia, R.; Teotonio, S.C.; Fatima, M.S.M. Correction to: Ora-pro-nobis (pereskia aculeata mill.) nutrition as related to soil chemical and physical attributes and plant growth-promoting microorganisms. J. Soil Sci. Plant Nutr. 2020, 20, 1637–1654. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, J.L.; Wang, C.; Wang, Q. The 19-years inorganic fertilization increased bacterial diversity and altered bacterial community composition and potential functions in a paddy soil. Agriculture 2019, 144, 60–67. [Google Scholar] [CrossRef]
- Simon, J.; Dannenmann, M.; Pena, R.; Gessler, A.; Rennenberg, H. Nitrogen nutrition of beech forests in a changing climate: Importance of plant-soil-microbe water, carbon, and nitrogen interactions. Plant Soil 2017, 418, 89–114. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.L.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Zhou, M.X.; Hoogenboom, G.; Wang, B.; Peng, B.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Andersen, M.N.; Qi, X.B.; Li, P.; Li, Z.Y.; Fan, X.Y.; Zhou, Y. Effects of reclaimed water irrigation and nitrogen fertilization on the chemical properties and microbial community of soil. J. Integr. Agric. 2016, 16, 679–690. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Ali, S. Multifunctional Pseudomonas putida strain FBKV2 from arid rhizosphere soil and its growth promotional effects on maize under drought stress. Rhizosphere 2016, 1, 4–13. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.C.; Liu, B.R.; Zhang, J.B.; Hua, J.N.; Liu, D.; Bhople, P.; Zhang, Y.R.; Zhang, H.G.; Zhang, C.H.; et al. Exploration of Soil Microbial Diversity and Community Structure along Mid-Subtropical Elevation Gradients in Southeast China. Forests 2023, 14, 769. [Google Scholar] [CrossRef]
- Jia, D.; Lu, J.J.; Sun, Y.J.; Song, S.; Du, H.; Han, L. Effect of Different Nitrogen Fertilizer Application Strategies on Rice Growth and Yield. Asian J. Agric. Res. 2016, 1, 33–39. [Google Scholar]
- Purwanto; Widiatmoko, T.; Wijonarko, B.R. Net assimilation rate, growth and yield of rice (Oryza sativa L. cv Inpago Unsoed 1) with the application of PGPR in different rate of nitrogen. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012064. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, B.; Guo, Z.; Wang, D.; Li, D.; Xu, J.; Li, Z.; Zhang, J. Divergent responses of bacterial activity, structure, and co-occurrence patterns to long-term unbalanced fertilization without nitrogen, phosphorus, or potassium in a cultivated vertisol. Environ. Sci. Pollut. Res. 2019, 26, 12741–12754. [Google Scholar] [CrossRef]
- Liu, G.; Feng, Y.; Yin, Z.; Yan, G.; Wang, Q.; Xing, Y. Exogenous organic C inputs profit soil C sequestration under different long-term N addition levels in a boreal forest. J. Soil Sci. Plant Nutr. 2023, 23, 1740–1750. [Google Scholar] [CrossRef]
- Chatterjee, D.; Nayak, A.K.; Mishra, A.; Swain, C.K.; Kumar, U.; Bhaduri, D.; Panneerselvam, P.; Lal, B.; Gautam, P.; Pathak, H. Effect of Long-Term Organic Fertilization in Flooded Rice Soil on Phosphorus Transformation and Phosphate Solubilizing Microorganisms. J. Soil Sci. Plant Nutr. 2021, 21, 1368–1381. [Google Scholar] [CrossRef]
- Li, J.F.; Gan, G.Y.; Chen, X.; Zou, J.L. Effects of Long-Term Straw Management and Potassium Fertilization on Crop Yield, Soil Properties, and Microbial Community in a Rice–Oilseed Rape Rotation. Agriculture 2021, 12, 1233. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Archontoulis, S.V.; Huth, N.; Yang, R.; Liu, D.L.; Yan, H.L.; Meinke, H.; Huber, I.; Feng, P.Y.; et al. Climate change shifts forward flowering and reduces crop waterlogging stress. Environ. Res. Lett. 2021, 16, 094017. [Google Scholar] [CrossRef]
- Zhao, M.L.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Hale, Q.W.; Vivanco, J.M.; Zhou, J.Z.; Kowalchuk, G.A.; Shen, Q.R. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2022, 44, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ye, J.Y.; Liu, K.; Harrison, M.T.; Zhong, X.F.; Wang, C.H.; Tian, X.H.; Huang, L.Y.; Zhang, Y.B. Optimizing agronomy improves super hybrid rice yield and nitrogen use efficiency through enhanced post-heading carbon and nitrogen metabolism. Agronomy 2023, 13, 13. [Google Scholar] [CrossRef]
- Han, Y.L.; Ma, W.; Zhou, B.Y.; Salah, A.; Geng, M.; Cao, C.G.; Zhan, M.; Zhao, M. Straw return increases crop grain yields and K-use efficiency under a maize-rice cropping system. Crop J. 2021, 9, 168–180. [Google Scholar] [CrossRef]
- Wu, J.; Brookes, P.C. The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol. Biochem. 2005, 37, 507–515. [Google Scholar] [CrossRef]
- Lisek, J.; Sas, P.L.; Mika, A.; Lisek, A. The Response of Weeds and Apple Trees to Beneficial Soil Microorganisms and Mineral Fertilizers Applied in Orchards. Agronomy 2022, 12, 2882. [Google Scholar] [CrossRef]
- Xu, Y.L.; Tang, H.M.; Xiao, X.P.; Guo, L.J.; Li, E.Y.; Li, J.M. Effects of different long-term fertilization regimes on the soil microbiological properties of a paddy field. Acta Ecol. Sin. 2016, 36, 5847–5855. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, X.; Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Du, B.H.; Li, J. Characteristics and driving factors of soil bacterial and archaeal communities under long-term fertilization regimes in black soil. J. Plant Nutr. 2015, 21, 1590–1598. [Google Scholar] [CrossRef]
- Nacke, H.; Thurmer, A.; Wollherr, A.; Will, C.; Hodac, L. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tremblay, J.; Bainard, L.D.; Cade-Menun, B.; Hamel, C. Long-term effects of nitrogen and phosphorus fertilization on soil microbial community structure and function under continuous wheat production. Environ. Microbiol. 2020, 22, 1066–1088. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.C.; Guan, D.W.; Ma, M.C.; Zhang, W.; Li, J.; Shen, D.L. Effects of fertilization on bacterial community under the condition of continuous soybean monoculture in black soil in northeast China. Sci. Agric. Sin. 2017, 50, 1271–1281. [Google Scholar] [CrossRef]
- Thuy, D.; Corinne, B.; Yvan, B.; Bouvier, T.; Henry-des-Tureaux, T.; Janeau, J.L.; Jouquet, P. Influence of buffalo manure, compost, vermicompost and biochar amendments on bacterial and viral communities in soil and adjacent aquatic systems. Agriculture 2014, 73, 78–86. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.L.; Chen, J.; Xie, J.; Jiang, Y.; Deng, G.Q.; Li, D.M.; Guan, X.J.; Liang, X.H.; Chen, X.M.; et al. New Insights from Soil Microorganisms for Sustainable Double Rice-Cropping System with 37-Year Manure Fertilization. Agronomy 2023, 13, 261. [Google Scholar] [CrossRef]
- Fan, K.K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H.Y. Wheat rhizosphere harbors a less complex and more stable microbial cooccurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 251–260. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Wang, Y.X.; Wang, P.Z.; Wang, C.N.; Ma, J.H.; Yang, X.F.; Ma, D.; Li, M.H. Study on the diversity of fungal and bacterial communities in continuous cropping fields of Chinese chives. BioMed Res. Int. 2020, 5, 3589758. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Song, Y.; Ma, T.F.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q.R. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Agriculture 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Tan, G.; Liu, Y.J.; Peng, S.G.; Yin, H.Q.; Meng, D.L.; Tao, J.M.; Gu, Y.B.; Li, J.; Yang, S.; Xiao, N.W.; et al. Soil potentials to resist continuous cropping obstacle: Three field cases. Environ. Res. 2021, 200, 111319. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Fertilizer | Spacing | Water Management |
|---|---|---|---|
| N-P-K-Zn-Si | (cm) | ||
| CK | 0-0-0-0-0 | 30 × 18 | Continuous flooding; field drained after flowering |
| FP | 210-100-220-0-0 | 30 × 18 | Continuous flooding; field drained after flowering |
| HYHE | 195-100-180-5-0 | 30 × 16 | SWD; Dehydrate 1 week before harvest |
| SHY | 270-120-240-5-150 | 30 × 16 | SWD; Dehydrate 1 week before harvest |
| Soil Properties | Treatment | |||
|---|---|---|---|---|
| CK | FP | HYHE | SHY | |
| pH | 7.25 ± 0.15 a | 7.37 ± 0.05 a | 7.39 ± 0.09 a | 7.35 ± 0.16 a |
| TN (g·kg−1) | 3.75 ± 0.06 a | 3.69 ± 0.22 a | 3.62 ± 0.06 a | 3.19 ± 0.09 b |
| NH4+-N (mg·kg−1) | 3.08 ± 0.12 c | 3.05 ± 0.04 c | 3.31 ± 0.09 b | 4.38 ± 0.16 a |
| NO3−-N (mg·kg−1) | 3.95 ± 0.12 b | 3.96 ± 0.14 b | 4.48 ± 0.14 a | 3.15 ± 0.10 c |
| TK (g·kg−1) | 185.36 ± 5.67 b | 204.9 ± 1.40 a | 207.47 ± 4.25 a | 212.47 ± 4.81 a |
| AK (mg·kg−1) | 82.19 ± 0.65 b | 81.33 ± 1.80 b | 87.44 ± 1.29 a | 78.62 ± 0.28 c |
| TP (g·kg−1) | 3.28 ± 0.13 c | 5.81 ± 0.14 b | 5.73 ± 0.22 b | 7.4 ± 0.030 a |
| AP (mg·kg−1) | 20.76 ± 0.10 c | 23.69 ± 0.11 a | 23.16 ± 0.14 b | 23.85 ± 0.16 a |
| SOC (g·kg−1) | 15.73 ± 0.19 b | 15.21 ± 0.52 b | 16.52 ± 0.17 a | 15.59 ± 0.40 b |
| C/N | 4.19 ± 0.02 c | 4.16 ± 0.19 c | 4.56 ± 0.11 b | 4.88 ± 0.04 a |
| Microbe Type | Treatment | Richness Index | Diversity Index | ||
|---|---|---|---|---|---|
| ACE | Chao1 | Shannon | Simposn | ||
| Bacteria | CK | 1827 ± 46 b | 1827 ± 45 b | 10.19 ± 0.08 b | 0.999 ± 0.001 a |
| FP | 1934 ± 9 ab | 1977 ± 93 ab | 10.29 ± 0.08 ab | 0.999 ± 0 a | |
| HYHE | 2064 ± 24 ab | 2034 ± 51 a | 10.28 ± 0.10 ab | 0.999 ± 0.001 a | |
| SHY | 2188 ± 81 a | 2121 ± 115 a | 10.41 ± 0.09 a | 0.999 ± 0 a | |
| Fungi | CK | 495 ± 4 b | 495 ± 15 bc | 6.16 ± 0.34 a | 0.958 ± 0.015 a |
| FP | 464 ± 6 c | 464 ± 20 c | 5.68 ± 0.18 b | 0.917 ± 0.049 a | |
| HYHE | 511 ± 8 b | 511 ± 27 b | 6.34 ± 0.10 a | 0.969 ± 0.007 a | |
| SHY | 556 ± 10 a | 556 ± 27 a | 6.52 ± 0.17 a | 0.969 ± 0.021 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Zhong, X.; Harrison, M.T.; Kang, K.; Sheng, T.; Shang, C.; Wang, C.; Deng, J.; Huang, L.; Tian, X.; et al. Towards Improved Grain Yield and Soil Microbial Communities of Super Hybrid Rice through Sustainable Management. Agronomy 2023, 13, 2259. https://doi.org/10.3390/agronomy13092259
Ye J, Zhong X, Harrison MT, Kang K, Sheng T, Shang C, Wang C, Deng J, Huang L, Tian X, et al. Towards Improved Grain Yield and Soil Microbial Communities of Super Hybrid Rice through Sustainable Management. Agronomy. 2023; 13(9):2259. https://doi.org/10.3390/agronomy13092259
Chicago/Turabian StyleYe, Jiayu, Xuefen Zhong, Matthew Tom Harrison, Kai Kang, Tian Sheng, Cheng Shang, Chunhu Wang, Jun Deng, Liying Huang, Xiaohai Tian, and et al. 2023. "Towards Improved Grain Yield and Soil Microbial Communities of Super Hybrid Rice through Sustainable Management" Agronomy 13, no. 9: 2259. https://doi.org/10.3390/agronomy13092259






