Characterization of the TcCYPE2 Gene and Its Role in Regulating Trehalose Metabolism in Response to High CO2 Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Feeding Method
2.2. Bioinformatics Analysis
2.3. Collection of Tissue and Developmental Expression Samples
2.4. Total RNA Extraction and dsRNA Synthesis
2.5. Microinjection of dsRNA
2.6. Determination of Trehalase Activity, Carbohydrate Content, and ATP Content
2.7. qRT-PCR
2.8. Data Analysis
3. Results and Analysis
3.1. Sequence Analysis of TcCYP9E2 Gene of T. castaneum
3.2. Temporal and Spatial Expression Pattern of T. castaneum TcCYP9E2
3.3. Detection of Silencing Efficiency of T. castaneum TcCYP9E2 and Survival Rate after CO2 Stress
3.4. Determination of Carbohydrate Content and Trehalase Activity in T. castaneum under Conditions of dsCYP9E2 Combined with 75% CO2
3.5. Change in T. Castaneum ATP Content under Conditions of dsCYP9E2 Combined with 75% CO2
3.6. The Expression Changes in Trehalose Metabolism Pathway Genes under Conditions of dsCYP9E2 Combined with 75% CO2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Shi, Y.; Wang, L.; Liu, S.; Wu, S.; Yang, Y.; Feyereisen, R.; Wu, Y. CYP6AE gene cluster knockout in Helicoverpa armigera reveals role in detoxification of phytochemicals and insecticides. Nat. Commun. 2018, 9, 4820. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Kar, A.; Giri, S.K. Insect Pest Management in Stored Pulses: An Overview. Food Bioprocess Technol. 2015, 8, 239–265. [Google Scholar] [CrossRef]
- Wong-Corra, F.J.; Castane, C.; Riudavets, J. Lethal effects of CO2-modified atmospheres for the control of three Bruchidae species. J. Stored Prod. Res. 2013, 55, 62–67. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2021, 102, 213–229. [Google Scholar] [CrossRef]
- Nieri, R.; Anfora, G.; Mazzoni, V.; Stacconi, R. Semiochemicals, semiophysicals and their integration for the development of innovative multi-modal systems for agricultural pests’ monitoring and control. Entomol. Gen. 2022, 42, 167–183. [Google Scholar] [CrossRef]
- Morrison, W.R., 3rd; Scully, E.D.; Campbell, J.F. Towards developing areawide semiochemical-mediated, behaviorally-based integrated pest management programs for stored product insects. Pest Manag. Sci. 2021, 77, 2667–2682. [Google Scholar] [CrossRef]
- Navarro, S. Modified atmospheres for the control of stored-product insects and mites. Insect Manag. Food Storage Process. 2006, 55, 3564–3574. [Google Scholar] [CrossRef]
- Helenius, I.T.; Krupinski, T.; Turnbull, D.W.; Gruenbaum, Y.; Silverman, N.; Johnson, E.A.; Sporn, P.H.; Sznajder, J.I.; Beitel, G.J. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc. Natl. Acad. Sci. USA 2009, 106, 18710–18715. [Google Scholar] [CrossRef]
- Yang, W.Q.; Lei, G.M.; Liu, X.; LI, X.H. Effects of controlled atmospheres with gigh CO2 concentrations on eating quality of rice. Storage Process 2012, 12, 20–22 + 31. [Google Scholar] [CrossRef]
- Kharel, K.; Mason, L.J.; Murdock, L.L.; Baributsa, D. Efficacy of hypoxia against Tribolium castaneum (Coleoptera: Tenebrionidae) Tthroughout ontogeny. J. Econ. Entomol. 2019, 112, 1463–1468. [Google Scholar] [CrossRef]
- Beall, C.M. Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc. Natl. Acad. Sci. USA 2007, 104, 8655–8660. [Google Scholar] [CrossRef]
- Nika, E.P.; Kavallieratos, N.G.; Papanikolaou, N.E.; Malesios, C. Interactions of Oryzaephilus surinamensis (L.) (Coleoptera: Silvanidae) with two key stored-product pests under variable abiotic conditions. Entomol. Gen. 2022, 42, 471–478. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Guan, L.W.; Pan, B.Y.; Xu, H.T.; Luo, Y.J.; Zhou, M.; Zhang, J.Y.; Wang, S.G.; Li, C.; Tang, B. Mechanism of HIF1-α-mediated regulation of Tribolium castaneum metabolism under high CO2 concentration elucidated. J. Stored Prod. Res. 2022, 99, 102030. [Google Scholar] [CrossRef]
- Wong, D.M.; Shen, Z.; Owyang, K.E.; Martinez-Agosto, J.A. Insulin- and warts-dependent regulation of tracheal plasticity modulates systemic larval growth during hypoxia in Drosophila melanogaster. PLoS ONE 2014, 26, e115297. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Ji, R.; Lei, C.; Zhu-Salzman, K. Parental hypoxic exposure influences performance of offspring in Callosobruchus maculatus. Pest Manag. Sci. 2019, 75, 2810–2819. [Google Scholar] [CrossRef]
- Nandety, R.S.; Kuo, Y.W.; Nouri, S.; Falk, B.W. Emerging strategies for RNA interference (RNAi) applications in insects. Bioengineered 2015, 6, 8–19. [Google Scholar] [CrossRef]
- Yan, S.; Yin, M.Z.; Shen, J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: Mechanisms, current status and challenges. Entomol. Gen. 2022, 43, 21–30. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalaka, C.T.; Skourti, A.; Nika, E.P.; Maggi, F.; Spinozzi, E.; Mazzara, E.; Petrelli, R.; Lupidi, G.; et al. Efficacy of 12 commercial essential oils as wheat protectants against stored-product beetles, and their acetylcholinesterase inhibitory activity. Entomol. Gen. 2021, 41, 385–414. [Google Scholar] [CrossRef]
- Shah, S.; Elgizawy, K.K.; Shi, C.M.; Yao, H.; Yan, W.H.; Li, Y.; Wang, X.P.; Wu, G.; Yang, F.L. Diallyl trisulfide, a biologically active component of garlic essential oil, decreasesmale fertility in Sitotroga cerealella by impairing dimorphic spermatogenesis, sperm motility and lipid homeostasis. Cells 2023, 12, 669. [Google Scholar] [CrossRef]
- Shah, S.; Zhang, S.S.; Elgizawy, K.K.; Yan, W.H.; Tang, N.; Wu, G.; Yang, F.L. Diallyl trisulfide reduced the reproductive capacity of male Sitotroga cerealella via the regulation of juvenile and ecdysone hormones. Ecotoxicol. Environ. Saf. 2022, 248, 114304. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, K.; Zhu, X.; Bai, Y.; Yang, W.; Li, C. Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol. 2019, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Z.J.; Chen, Z.S.; Wang, X.X.; Cong, H.S.; Fan, Y.L.; Liu, T.X. Connection between cuticular hydrocarbons and melanization in Harmonia axyridis revealed by RNAi-mediated silencing of the CYP4G79. Entomol. Gen. 2021, 41, 83–96. [Google Scholar] [CrossRef]
- Katsavou, E.; Riga, M.; Ioannidis, P.; King, R.; Zimmer, C.T.; Vontas, J. Functionally characterized arthropod pest and pollinator cytochrome P450s associated with xenobiotic metabolism. Pestic. Biochem. Physiol. 2022, 181, 105005. [Google Scholar] [CrossRef]
- López, M.F.; Cano-Ramírez, C.; Cesar-Ayala, A.K.; Ruiz, E.A.; Zúñiga, G. Diversity and expression of P450 genes from Dendroctonus valens LeConte (Curculionidae: Scolytinae) in response to different kairomones. Insect Biochem. Mol. Biol. 2013, 43, 417–432. [Google Scholar] [CrossRef]
- Rasool, A.; Joußen, N.; Lorenz, S.; Ellinger, R.; Schneider, B.; Khan, S.A.; Ashfaq, M.; Heckel, D.G. An independent occurrence of the chimeric P450 enzyme CYP337B3 of Helicoverpa armigera confers cypermethrin resistance in Pakistan. Insect Biochem. Mol. Biol. 2014, 53, 54–65. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Liu, Y. Spatial shifts in grain production increases in China and implications for food security. Land Use Policy 2018, 74, 204–213. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, C.; Gao, X.; Luo, J.; Zhu, X.; Wan, S. Characterization of P450 monooxygenase gene family in the cotton aphid, Aphis gossypii Glover. J. Asia-Pac. Entomol. 2022, 25, 101861. [Google Scholar] [CrossRef]
- Gilbert, L.I.; Rybczynski, R.; Warren, J.T. Control and biochemical nature of the ecdysteroidogenic pathway. Annu. Rev. Entomol. 2002, 47, 883–916. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Shah, K.; Palli, S.R. Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genom. 2013, 14, 174. [Google Scholar] [CrossRef]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef]
- Bergé, J.B.; Feyereisen, R.; Amichot, M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 29, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yang, Y.; Hu, J.; Wang, Y.; Zhao, Z.; Ma, R.; Gao, L.; Guo, Y. Identification and characterization of CYP6 family genes from the Oriental Fruit Moth (Grapholita molesta) and Their Responses to Insecticides. Insects 2022, 17, 300. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Li, F.F.; He, C.; Cui, J.; Song, W.; Li, M.L. Molecular cloning and sequence analysis of novel cytochrome P450 cDNA fragments from Dastarcus helophoroides. J. Insect Sci. 2014, 26, 28. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Bao, H.; Liu, Z. Sequence analysis of insecticide action and detoxification-related genes in the insect pest natural enemy pardosa pseudoannulata. PLoS ONE 2015, 29, e0125242. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Rothe, M.; Zheng, S.; Bhatla, N.; Pender, C.L.; Menzel, R.; Horvitz, H.R. Cytochrome P450 drives a HIF-regulated behavioral response to reoxygenation by C. elegans. Science 2013, 2, 554–558. [Google Scholar] [CrossRef]
- Pender, C.L.; Horvitz, H.R. Hypoxia-inducible factor cell non-autonomously regulates C. elegans stress responses and behavior via a nuclear receptor. eLife 2018, 16, e36828. [Google Scholar] [CrossRef]
- White, G.G. Field estimates of population growth rates of Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) (Coleoptera: Tenebrionidae and Bostrychidae) in bulk wheat. J. Stored Prod. Res. 1988, 24, 13–22. [Google Scholar] [CrossRef]
- Campbell, J.F.; Runnion, C. Patch exploitation by female red flour beetles, Tribolium castaneum. J. Insect Sci. 2003, 3, 20. [Google Scholar] [CrossRef][Green Version]
- Tatun, N.; Singtripop, T.; Tungjitwitayakul, J.; Sakurai, S. Regulation of soluble and membrane-bound trehalase activity and expression of the enzyme in the larval midgut of the bamboo borer Omphisa fuscidentalis. Insect Biochem. Mol. Biol. 2008, 38, 788–795. [Google Scholar] [CrossRef]
- Tatun, N.; Singtripop, T.; Sakurai, S. Dual control of midgut trehalase activity by 20-hydroxyecdysone and an inhibitory factor in the bamboo borer Omhisa fuscidentalis Hampson. J. Insect Physiol. 2008, 54, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Jiang, W.; Dou, P.; Tang, P.A.; An, F.M. Identification, Characterization, and expression of P450 gene encoding CYP6BQ13v2 from the Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Agric. Sci. China 2009, 8, 1210–1218. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, H.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Gao, X.; Luo, J.; et al. Knockdown of cytochrome P450 gene CYP6AB12 based on nanomaterial technology reduces the detoxification ability of Spodoptera litura to gossypol. Pestic. Biochem. Physiol. 2022, 188, 105284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xue, H.; Zhu, X.; Wang, L.; Zhang, K.; Li, D.; Ji, J.; Niu, L.; Gao, X.; Luo, J.; et al. Silencing of cytochrome P450 gene CYP321A1 effects tannin detoxification and metabolism in Spodoptera litura. Int. J. Biol. Macromol. 2022, 1, 895–902. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, X.; Kong, Y.; Zhao, Z.; Mahmoud, A.; Wu, L.; Moussian, B.; Zhang, J. CYP311A1 in the anterior midgut is involved in lipid distribution and microvillus integrity in Drosophila melanogaster. Cell Mol. Life Sci. 2022, 28, 261. [Google Scholar] [CrossRef]
- Senger, K.; Harris, K.; Levine, M. GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc. Natl. Acad. Sci. USA 2006, 24, 15957–15962. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Skaer, H.; Dow, J.A. The developmental, molecular, and transport biology of Malpighian tubules. Annu. Rev. Entomol. 2010, 55, 351–374. [Google Scholar] [CrossRef]
- Hou, Y.; Zou, Y.; Wang, F.; Gong, J.; Zhong, X.; Xia, Q.; Zhao, P. Comparative analysis of proteome maps of silkworm hemolymph during different developmental stages. Proteome Sci. 2010, 8, 45. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, Q.; Chen, H.; Li, Z.; Yin, F.; Feng, X. Identification of a novel cytochrome P450 gene, CYP321E1 from the diamondback moth, Plutella xylostella (L.) and RNA interference to evaluate its role in chlorantraniliprole resistance. Bull. Entomol. Res. 2014, 104, 716–723. [Google Scholar] [CrossRef]
- Wang, K.X.; Liu, M.W.; Wang, Y.Z.; Song, W.; Tang, P.A. Identification and functional analysis of cytochrome P450 CYP346 family genes associated with phosphine resistance in Tribolium castaneum. Pestic. Biochem. Physiol. 2020, 168, 104622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Gao, S.S.; Xue, S.; An, S.H.; Zhang, K.P. Disruption of the cytochrome P450 CYP6BQ7 gene reduces tolerance to plant toxicants in the red flour beetle, Tribolium castaneum. Int. J. Biol. Macromol. 2021, 1, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, J.; Wu, H.; Zhang, H.; Zhang, J.; Ma, E. Knockdown of cytochrome P450 CYP6 family genes increases susceptibility to carbamates and pyrethroids in the migratory locust, Locusta migratoria. Chemosphere 2019, 223, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mitsumasu, K.; Azuma, M.; Niimi, T.; Yamashita, O.; Yaginuma, T. Membrane-penetrating trehalase from silkworm Bombyx mori. Molecular cloning and localization in larval midgut. Insect Mol. Biol. 2005, 14, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.A.; Forbes, J.M. Glucose and glycogen in the diabetic kidney: Heroes or villains? eBioMedicine 2019, 47, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Radziuk, J.; Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab. Res. Rev. 2001, 17, 250–272. [Google Scholar] [CrossRef]
- Tabata, E.; Kashimura, A.; Kikuchi, A.; Masuda, H.; Miyahara, R.; Hiruma, Y.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; et al. Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci. Rep. 2018, 23, 1461. [Google Scholar] [CrossRef]
- Tetreau, G.; Cao, X.; Chen, Y.R.; Muthukrishnan, S.; Jiang, H.; Blissard, G.W.; Kanost, M.R.; Wang, P. Overview of chitin metabolism enzymes in Manduca sexta: Identification, domain organization, phylogenetic analysis and gene expression. Insect Biochem. Mol. Biol. 2015, 62, 114–126. [Google Scholar] [CrossRef]
- Cao, Y.C. Toxicity of CO2 to Oryzaephilus surinamensis and content and utilization of its energy substances. J. Northwest A F Univ. 2015, 43, 123–128. [Google Scholar] [CrossRef]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef]
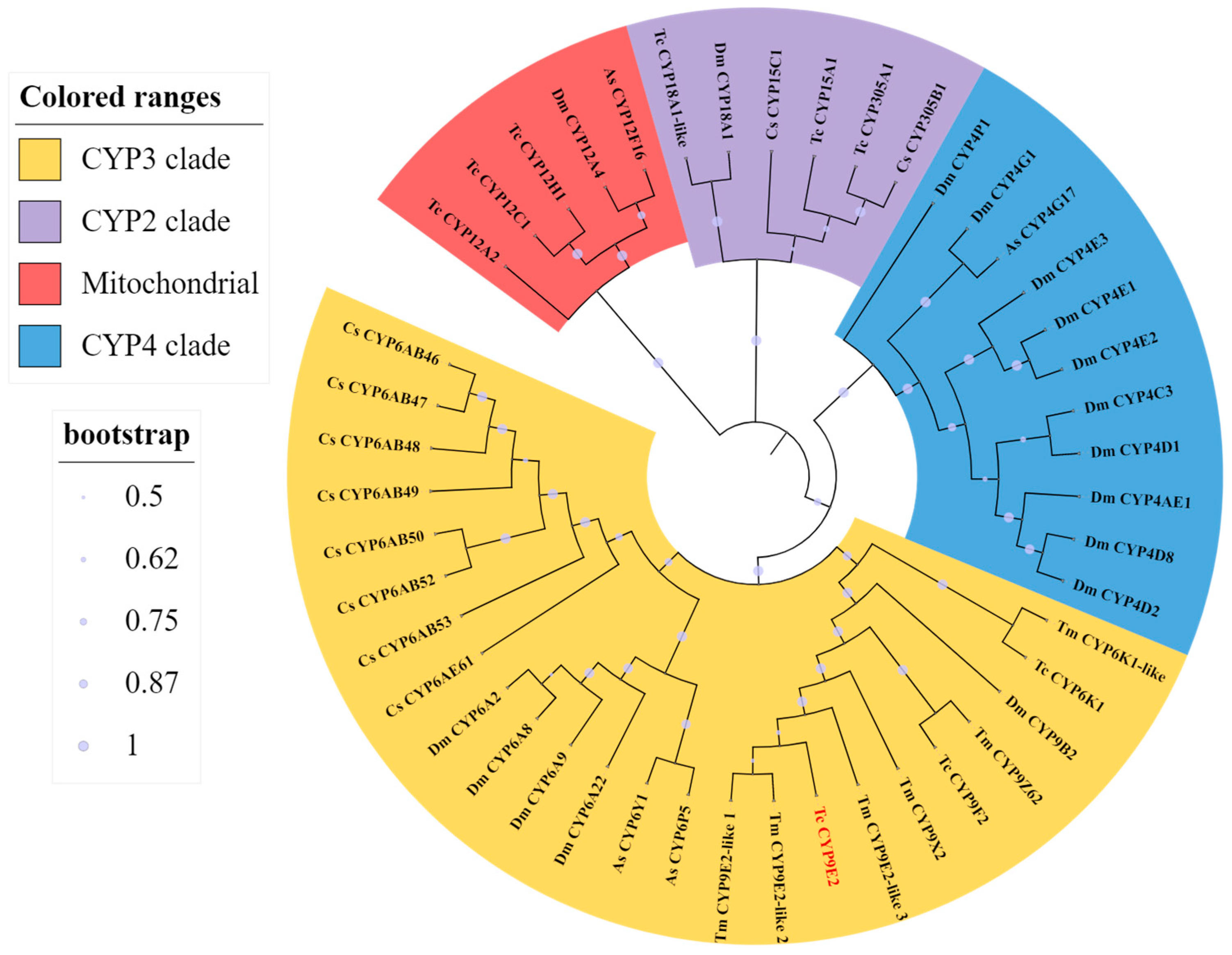

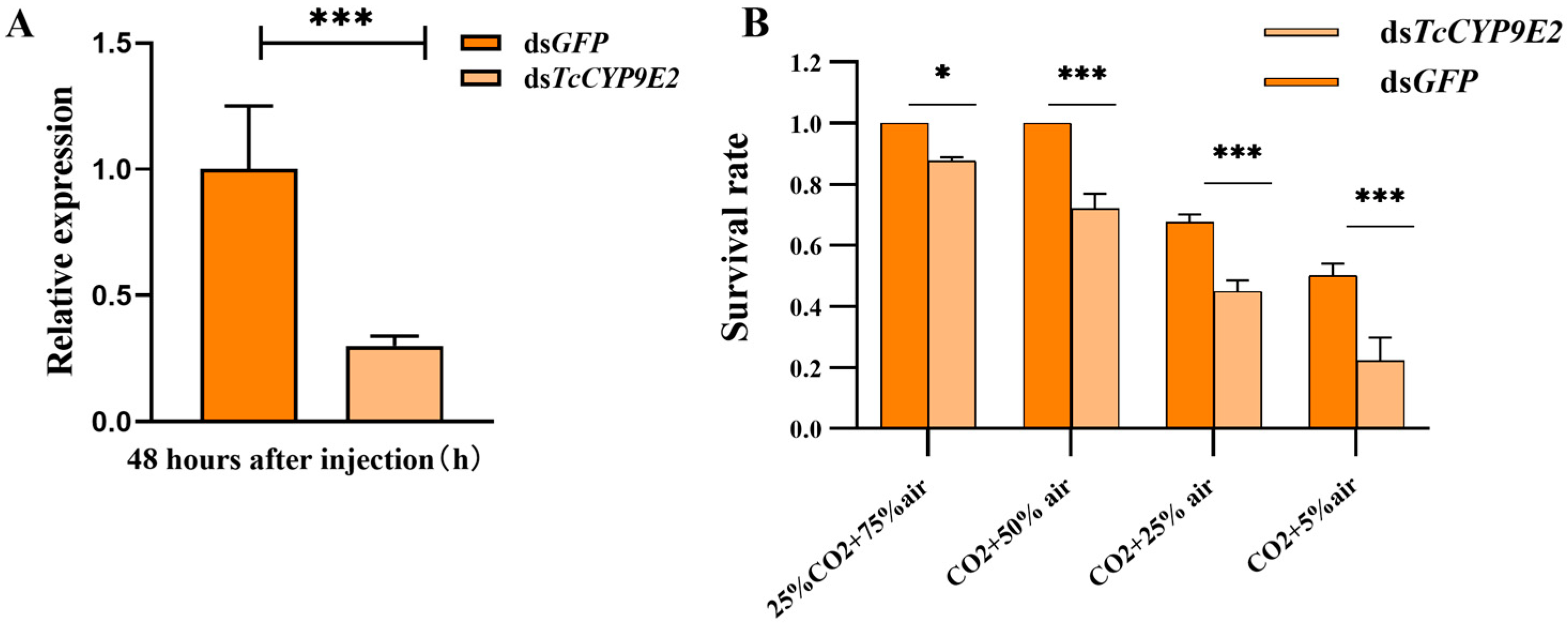
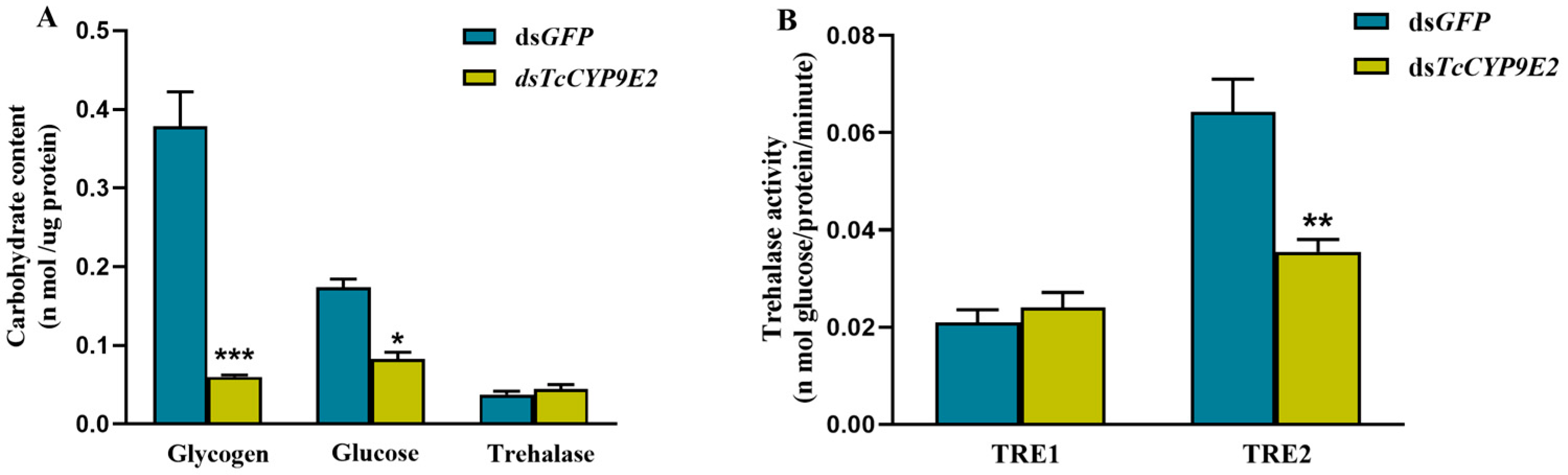

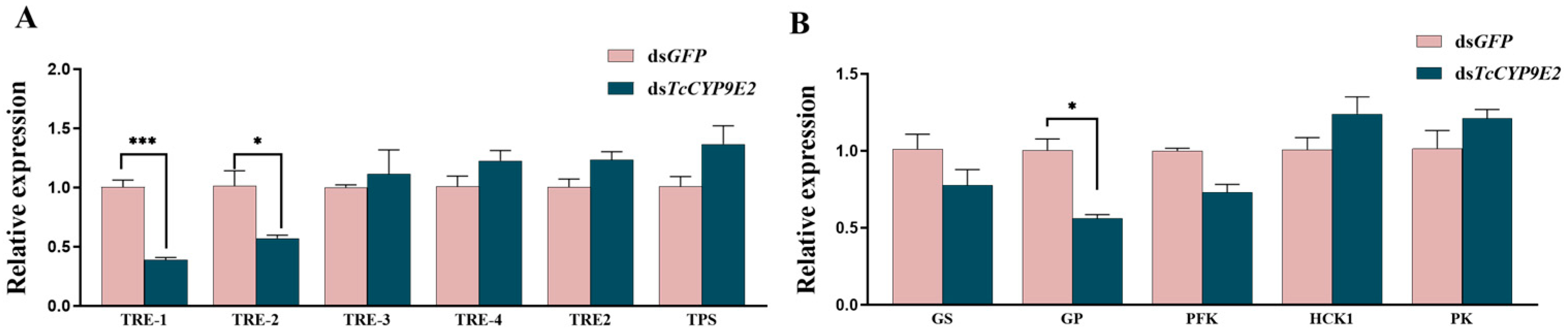
| Application Type | Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| dsRNA synthesis | CYP9E2 | TAAGTGTGGTTTTGGGTGCG | CTGGGCTTGAATGTTAGATG |
| T7-CYP9E2 | T7-TAAGTGTGGTTTTGGGTGCG | T7-CTGGGCTTGAATGTTAGATG | |
| dsCYP9E2 | ACGACCATCTTGCCATAA | AAACCATCACCACCTTCAT | |
| dsGFP | AAGGGCGAGGAGCTGTTCACCG | GCAGGACCATGTGATCGCGC | |
| qRT-PCR | TcRPL13a | ACCATATGACCGCAGGAAAC | GGTGAATGGAGCCACTTGTT |
| TcCYP9E2 | ACCGGTTTCAGTTTCATGGC | ACAAAGTCGGTGTTGCAGAA | |
| TcTRE-1 | AACCAAACACTCACTCATTCC | AATCCAATAAGTGTCCCAGTAG | |
| TcTRE-2 | GAAGTATCGGTTGGCTCG | GAGTGGGGTTGATTGTGC | |
| TcTRE-3 | CTTGAACGCCTTCCTCTG | CCATCCTCGTGGTCATAAA | |
| TcTRE-4 | CTACCTAAACCGCTCCCA | TGTCCAGCCAGTACCTCAG | |
| TcTRE2 | TGTTGTGCGTTTGTGCTC | GGACGGCTTATTGTTGTTTA | |
| TcTPS | GATTCGCTACATTTACGGG | GAACGGAGACACTATGAGGAC | |
| TcGS | ATTGGAGGAGTCTAGGAGTGTAC | CCGAATCGCTTTCATCAT | |
| TcGP | CCGATGGCTCCTTATGTG | GTATGCGTTTGACGTGGAT | |
| TcPFK | CTACGAAAATGTCCGAAGG | GTTGCGGTCAAAAGGTGT | |
| TcHCK1 | GAGGTATGTCTGCGAATGC | TGGAAATGTGGGTGGAAC | |
| TcPK | CAACCGACGAAAAGTATGC | TTCACCCCTTTACTACTCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-F.; Zhou, M.; Wang, Y.-Y.; Jiang, X.-Y.; Zhang, P.; Xu, K.-K.; Tang, B.; Li, C. Characterization of the TcCYPE2 Gene and Its Role in Regulating Trehalose Metabolism in Response to High CO2 Stress. Agronomy 2023, 13, 2263. https://doi.org/10.3390/agronomy13092263
Zhou Y-F, Zhou M, Wang Y-Y, Jiang X-Y, Zhang P, Xu K-K, Tang B, Li C. Characterization of the TcCYPE2 Gene and Its Role in Regulating Trehalose Metabolism in Response to High CO2 Stress. Agronomy. 2023; 13(9):2263. https://doi.org/10.3390/agronomy13092263
Chicago/Turabian StyleZhou, Yan-Fei, Min Zhou, Yuan-Yuan Wang, Xin-Yi Jiang, Pei Zhang, Kang-Kang Xu, Bin Tang, and Can Li. 2023. "Characterization of the TcCYPE2 Gene and Its Role in Regulating Trehalose Metabolism in Response to High CO2 Stress" Agronomy 13, no. 9: 2263. https://doi.org/10.3390/agronomy13092263
APA StyleZhou, Y.-F., Zhou, M., Wang, Y.-Y., Jiang, X.-Y., Zhang, P., Xu, K.-K., Tang, B., & Li, C. (2023). Characterization of the TcCYPE2 Gene and Its Role in Regulating Trehalose Metabolism in Response to High CO2 Stress. Agronomy, 13(9), 2263. https://doi.org/10.3390/agronomy13092263





