Evaluation of the Relationship between Cultivar, Endophyte and Environment on the Expression of Persistence in Perennial Ryegrass Populations Using High-Throughput Phenotyping
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials for the Field Experiment
2.2. Endophyte Status of Plant Materials
2.3. Experimental Design
2.4. Manual Pasture Measurements
2.5. Sensor-Based Pasture Height
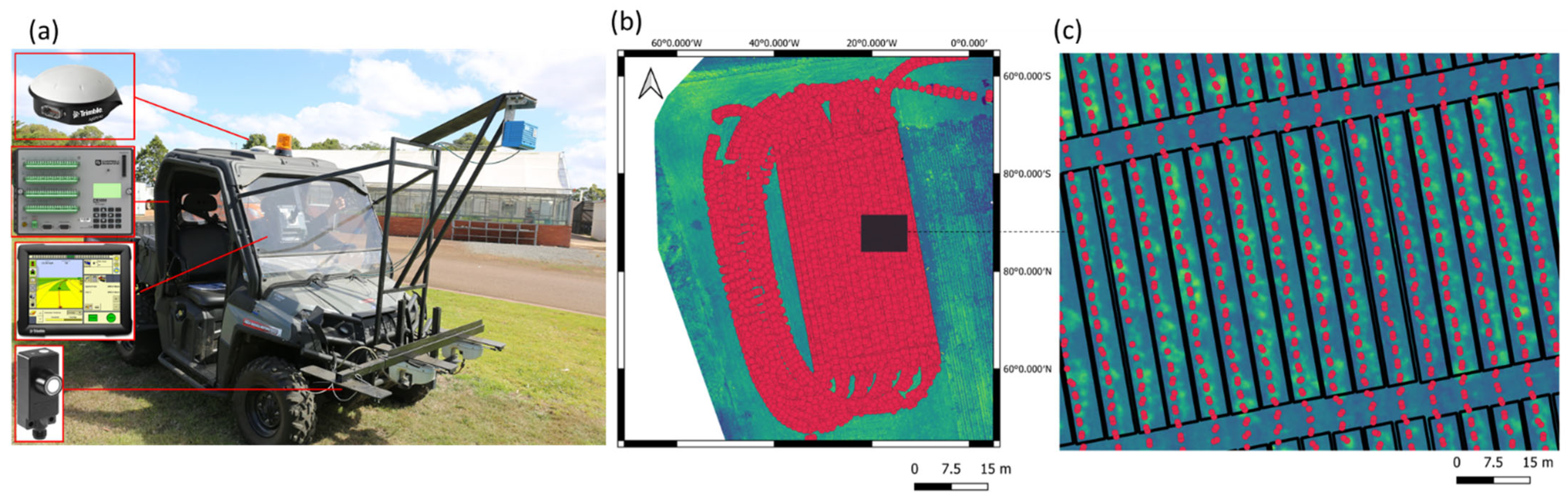
2.6. Airborne Phenomic Data Acquisition
2.7. Vegetation Indices Extraction
2.8. Ground Cover Extraction from Multispectral Images
2.9. Statistical Analysis
3. Results
3.1. Meteorological Data
3.2. Endophyte Frequency
3.3. Manual Pasture Measurement
3.4. Sensor-Based Pasture Measurements
3.5. Interaction of Endophyte, Cultivar, and Environment on Pasture Persistence
4. Discussion
4.1. Pasture Traits for Pasture Persistence Estimation
4.2. Effect of the Environment on the Expression of Pasture Persistence
4.3. Interaction of Cultivar, Endophyte, and Environment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waller, R.A.; Sale, P.W.G. Persistence and productivity of perennial ryegrass in sheep pastures in south-western Victoria: A review. Aust. J. Exp. Agric. 2001, 41, 117–144. [Google Scholar] [CrossRef]
- Kemp, D.R.; Dowling, P.M. Towards sustainable temperate perennial pastures. Aust. J. Exp. Agric. 2000, 40, 125–132. [Google Scholar] [CrossRef]
- Cunningham, P.J.; Blumenthal, M.J.; Anderson, M.W.; Prakash, K.S.; Leonforte, A. Perennial ryegrass improvement in Australia. N. Z. J. Agric. Res. 1994, 37, 295–310. [Google Scholar] [CrossRef]
- Cunningham, P.; Graves, W.; Chakroun, M.; Mezni, M.; Saidi, S.; Ounejmate, M.; Porqueddu, C.; Reed, K. Novel perennial forage germplasm from North Africa and Sardinia. Aust. Plant Introd. Rev. 1997, 27, 13–46. [Google Scholar]
- Fulkerson, W.; Sinclair, K.; Moore, K.; Rolfe, C. Management of Lolium perenne/Trifolium repens pastures in the subtropics. I. Effect of defoliation interval, seeding rate and application of N and lime. Aust. J. Agric. Res. 1993, 44, 1947–1958. [Google Scholar] [CrossRef]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic Stress Responses in Plants: Present and Future. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar] [CrossRef]
- Anjum, S.; Xie, X.-Y.; Wang, L.-C.; Saleem, M.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Malcolm, B.; Smith, K.F.; Jacobs, J.L. Perennial pasture persistence: The economic perspective. Crop Pasture Sci. 2014, 65, 713–720. [Google Scholar] [CrossRef]
- Bolaric, S.; Barth, S.; Melchinger, A.E.; Posselt, U.K. Molecular characterization of genetic diversity in European germplasm of perennial ryegrass. Euphytica 2005, 146, 39–44. [Google Scholar] [CrossRef]
- Guthridge, K.M.; Dupal, M.P.; Kölliker, R.; Jones, E.S.; Smith, K.F.; Forster, J.W. AFLP analysis of genetic diversity within and between populations of perennial ryegrass (Lolium perenne L.). Euphytica 2001, 122, 191–201. [Google Scholar] [CrossRef]
- Skøt, L.; Sanderson, R.; Thomas, A.; Skøt, K.; Thorogood, D.; Latypova, G.; Asp, T.; Armstead, I. Allelic Variation in the Perennial Ryegrass flowering locus T Gene Is Associated with Changes in Flowering Time across a Range of Populations. Plant Physiol. 2011, 155, 1013–1022. [Google Scholar] [CrossRef]
- Thom, E.R.; Popay, A.J.; Hume, D.E.; Fletcher, L.R. Evaluating the performance of endophytes in farm systems to improve farmer outcomes—A review. Crop Pasture Sci. 2012, 63, 927–943. [Google Scholar] [CrossRef]
- Hume, D.E.; Sewell, J.C. Agronomic advantages conferred by endophyte infection of perennial ryegrass (Lolium perenne L.) and tall fescue (Festuca arundinacea Schreb.) in Australia. Crop Pasture Sci. 2014, 65, 747–757. [Google Scholar] [CrossRef]
- Popay, A.J.; Hume, D.E.; Davis, K.L.; Tapper, B.A. Interactions between endophyte (Neotyphodium spp.) and ploidy in hybrid and perennial ryegrass cultivars and their effects on Argentine stem weevil (Listronotus bonariensis). N. Z. J. Agric. Res. 2003, 46, 311–319. [Google Scholar] [CrossRef]
- Latch, G.C.M. Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes. Agric. Ecosyst. Environ. 1993, 44, 143. [Google Scholar] [CrossRef]
- Culvenor, R.A.; Simpson, R.J. Persistence traits in perennial pasture grasses: The case of phalaris. Crop Pasture Sci. 2014, 65, 1165–1176. [Google Scholar] [CrossRef]
- Finch, H.J.S.; Samuel, A.M.; Lane, G.P.F. 21—Grazing management. In Lockhart & Wiseman’s Crop Husbandry Including Grassland, 9th ed.; Finch, H.J.S., Samuel, A.M., Lane, G.P.F., Eds.; Woodhead Publishing: London, UK, 2014; pp. 499–512. [Google Scholar] [CrossRef]
- Boschma, S.P.; Hill, M.J.; Scott, J.M.; Lutton, J.J. Carbohydrate reserves of perennial ryegrasses: Effect drought and defoliation intensity. In Proceedings of the Grasslands 2000—Proceedings of the XVIII International Grassland Congress, Winnipeg, MB, Saskatoon, SK, Canada, 8–19 June 1997; pp. 22–42. [Google Scholar]
- Frank, D.A.; McNaughton, S.J.; Tracy, B.F. The Ecology of the Earth’s Grazing Ecosystems: Profound functional similarities exist between the Serengeti and Yellowstone. BioScience 1998, 48, 513–521. [Google Scholar] [CrossRef]
- Kemp, D.R.; Culvenor, R.A. Improving the grazing and drought tolerance of temperate perennial grasses. N. Z. J. Agric. Res. 1994, 37, 365–378. [Google Scholar] [CrossRef]
- Sanford, P.; Cullen, B.R.; Dowling, P.M.; Chapman, D.F.; Garden, D.L.; Lodge, G.M.; Andrew, M.H.; Quigley, P.E.; Murphy, S.R.; King, W.M.; et al. SGS Pasture Theme: Effect of climate, soil factors and management on pasture production and stability across the high rainfall zone of southern Australia. Aust. J. Exp. Agric. 2003, 43, 945–959. [Google Scholar] [CrossRef]
- Tozer, K.; Bourdôt, G.W.; Edwards, G.R. What factors lead to poor pasture persistence and weed ingress? NZG Res. Pract. Ser. 2011, 15, 129–137. [Google Scholar] [CrossRef]
- Brougham, R.W. Some factors affecting the persistency of short-rotation ryegrass. N. Z. J. Agric. Res. 1961, 4, 516–522. [Google Scholar] [CrossRef][Green Version]
- Jayasinghe, C.; Badenhorst, P.; Jacobs, J.; Spangenberg, G.; Smith, K. Image-based high-throughput phenotyping for the estimation of persistence of perennial ryegrass (Lolium perenne L.)—A review. Grass Forage Sci. 2021, 76, 321–339. [Google Scholar] [CrossRef]
- Pratap, A.; Tomar, R.; Kumar, J.; Pandey, V.R.; Mehandi, S.; Katiyar, P.K. High-Throughput Plant Phenotyping Platforms. In Phenomics in Crop Plants: Trends, Options and Limitations; Kumar, J., Pratap, A., Kumar, S., Eds.; Springer: New Delhi, India, 2015; pp. 285–296. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Qiu, Q.; Sun, N.; Bai, H.; Wang, N.; Fan, Z.; Wang, Y.; Meng, Z.; Li, B.; Cong, Y. Field-Based High-Throughput Phenotyping for Maize Plant Using 3D LiDAR Point Cloud Generated With a “Phenomobile”. Front. Plant Sci. 2019, 10, 554. [Google Scholar] [CrossRef]
- Gebremedhin, A.; Badenhorst, P.; Wang, J.; Giri, K.; Spangenberg, G.; Smith, K. Development and Validation of a Model to Combine NDVI and Plant Height for High-Throughput Phenotyping of Herbage Yield in a Perennial Ryegrass Breeding Program. Remote Sens. 2019, 11, 2494. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Badenhorst, P.; Wang, J.; Jacobs, J.; Spangenberg, G.; Smith, K. An Object-Based Image Analysis Approach to Assess Persistence of Perennial Ryegrass (Lolium perenne L.) in Pasture Breeding. Agronomy 2019, 9, 501. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Badenhorst, P.; Jacobs, J.; Spangenberg, G.; Smith, K. High-Throughput Ground Cover Classification of Perennial Ryegrass (Lolium perenne L.) for the Estimation of Persistence in Pasture Breeding. Agronomy 2020, 10, 1206. [Google Scholar] [CrossRef]
- Siebring, J.; Valente, J.; Domingues Franceschini, M.H.; Kamp, J.; Kooistra, L. Object-Based Image Analysis Applied to Low Altitude Aerial Imagery for Potato Plant Trait Retrieval and Pathogen Detection. Sensors 2019, 19, 5477. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, D.; Fulkerson, B. Principles for Developing an Effective Grazing Management System for Ryegrass-Based Pastures. 1999. Available online: https://www.dairyaustralia.com.au/resource-repository/2020/07/08/principles-for-developing-an-effective-grazing-management-system-for-ryegrass-based-pastures (accessed on 24 July 2019).
- Alem, G.; Badenhorst, P.E.; Wang, J.; Spangenberg, G.C.; Smith, K.F. Prospects for Measurement of Dry Matter Yield in Forage Breeding Programs Using Sensor Technologies. Agronomy 2019, 9, 65. [Google Scholar]
- Hunt, R.; Hively, W.; McCarty, G.; Daughtry, C.; Forrestal, P.; Kratochvil, R.; Carr, J.; Allen, N.; Fox-Rabinovitz, J.; Miller, C. NIR-Green-Blue High-Resolution Digital Images for Assessment of Winter Cover Crop Biomass. GIScience Remote Sens. 2011, 48, 86–98. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, G.; Wang, J.; Feng, H. A comparative analysis of spectral vegetation indices to estimate crop leaf area index. Intell. Autom. Soft Comput. 2013, 19, 315–326. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Prabhakara, K.; Hively, W.D.; McCarty, G.W. Evaluating the relationship between biomass, percent groundcover and remote sensing indices across six winter cover crop fields in Maryland, United States. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 88–102. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of Leaf-Area Index from Quality of Light on the Forest Floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 688. [Google Scholar] [CrossRef]
- Sripada, R.; Heiniger, R.; White, J.; Meijer, A. Aerial Color Infrared Photography for Determining Early In-Season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 200. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially Located Platform and Aerial Photography for Documentation of Grazing Impacts on Wheat. Geocarto Int. 2001, 16, 65–70. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E. Comparing narrow and broad-band vegetation indices to estimate leaf chlorophyll content in planophile crop canopies. Precis. Agric. 2011, 12, 334–344. [Google Scholar] [CrossRef]
- Woebbecke, D.M.; Meyer, G.E.; Von Bargen, K.; Mortensen, D.A. Color Indices for Weed Identification Under Various Soil, Residue, and Lighting Conditions. Trans. ASAE 1995, 38, 259–269. [Google Scholar] [CrossRef]
- Payero, J.O.; Christopher, N.; Wright, J.L. Comparison of eleven vegetation indices for estimating plant height of alfalfa and grass. Appl. Eng. Agric. 2004, 20, 57. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Sripada, R. Determining In-Season Nitrogen Requirements for Corn Using Aerial Color-Infrared Photography. 2005. Available online: https://repository.lib.ncsu.edu/handle/1840.16/4200 (accessed on 24 July 2019).
- Jago, R.A.; Cutler, M.E.J.; Curran, P.J. Estimating Canopy Chlorophyll Concentration from Field and Airborne Spectra. Remote Sens. Environ. 1999, 68, 217–224. [Google Scholar] [CrossRef]
- Chen, P.-F.; Nicolas, T.; Wang, J.-H.; Philippe, V.; Huang, W.-J.; Li, B.-G. New Index for Crop Canopy Fresh Biomass Estimation. Spectrosc. Spectr. Anal. 2010, 30, 512–517. [Google Scholar] [CrossRef]
- Wilkins, P.W. Breeding perennial ryegrass for agriculture. Euphytica 1991, 52, 201–214. [Google Scholar] [CrossRef]
- Cullen, B.R.; Rawnsley, R.P.; Eckard, R.J.; Christie, K.M.; Bell, M.J. Use of modelling to identify perennial ryegrass plant traits for future warmer and drier climates. Crop Pasture Sci. 2014, 65, 758–766. [Google Scholar] [CrossRef]
- Hume, D.E.; Barker, D.J. Growth and Management of Endophytic Grasses in Pastoral Agriculture. In Neotyphodium in Cool-Season Grasses; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 201–226. [Google Scholar] [CrossRef]
- Lowe, K.F.; Bowdler, T.M.; Hume, D.E.; Casey, N.D.; Tapper, B.A. The effect of endophyte on the performance of irrigated perennial ryegrasses in subtropical Australia. Aust. J. Agric. Res. 2008, 59, 567–577. [Google Scholar] [CrossRef]
- Sutherland, B.L.; Hoglund, J.H. Effcet of ryegrass containing the endophyte (Acremonium lolii), on the performance of associated white clover and subsequent crops. Proc. N. Z. Grassl. Assoc. 1989, 50, 265–269. [Google Scholar]
- Cashman, P.; Gilliland, T.; McEvoy, M.; Watson, S.; O’Donovan, M. Changes in plant morphological expression in 12 perennial ryegrass cultivars following frequent and infrequent cutting management. J. Agric. Sci. 2015, 154, 456–471. [Google Scholar] [CrossRef]
- Kaloki, P.; Trethowan, R.; Tan, D.K.Y. Effect of genotype × environment × management interactions on chickpea phenotypic stability. Crop Pasture Sci. 2019, 70, 453–462. [Google Scholar] [CrossRef]
- Biddiscombe, E.; Rogers, A.; Maller, R. Summer dormancy, regeneration and persistence of perennial grasses in south-western Australia. Aust. J. Exp. Agric. 1977, 17, 795–801. [Google Scholar] [CrossRef]
- Nie, Z.; Norton, M.R. Stress Tolerance and Persistence of Perennial Grasses: The Role of the Summer Dormancy Trait in Temperate Australia. Crop Sci. 2009, 49, 2405–2411. [Google Scholar] [CrossRef]
- Tian, P.; Le, T.-N.; Ludlow, E.J.; Smith, K.F.; Forster, J.W.; Guthridge, K.M.; Spangenberg, G.C. Characterisation of novel perennial ryegrass host-Neotyphodium endophyte associations. Crop Pasture Sci. 2013, 64, 716–725. [Google Scholar] [CrossRef]
- Faville, M.J.; Crush, J.R.; Hong, W.; Phillips, H.; Lee, J.M.; Chapman, D. Effects of pasture age on the genotype and phenotype of perennial ryegrass. Grass Forage Sci. 2020, 75, 135–144. [Google Scholar] [CrossRef]









| Image Acquisition Date | Image Overlap Forward/Side | Flight Speed (m/s) | Flight Height (m) | Mean RMS Error (m) | GSD (cm/pixels) |
|---|---|---|---|---|---|
| 2019 Autumn | 80%/75% | 6 | 30 | 0.019 | 2.26 |
| 2020 Autumn | 80%/75% | 6 | 30 | 0.010 | 2.16 |
| Vegetation Index | Abbreviation | Equation |
|---|---|---|
| Normalised Difference Vegetation Index | NDVI | (Rn − Rr)/(Rn + Rr) [33] |
| Green Normalised Difference Vegetation Index | GNDVI | (Rn − Rg)/(Rn + Rg) [34] |
| Red Edge Normalised Difference Vegetation Index | ReNDVI | (Rn − Rre)/(Rn + Rre) |
| Renormalised Difference Vegetation Index | RDVI | (Rn − Rr)/(Rn + Rr)1/2 [35] |
| Soil Adjusted Vegetation Index | SAVI | (Rn − Rr)/(Rn + Rr + 0.5) × (1 + 0.5) [36] |
| Normalised green-red Difference Index | NGRDI | (Rg − Rr)/(Rg + Rr) [37] |
| Simple Ratio Index | SRI | Rn/Rr [38] |
| Red Edge Simple Ratio Index | ReSRI | Rn/Rre [39] |
| Green Simple Ratio Index | GSRI | Rn/Rg [40] |
| Green Leaf Index | GLI | (2 × Rg − Rr- Rb)/(2 × Rg + Rr + Rb) [41] |
| Chlorophyll Vegetation Index | CVI | Rn × Rr/Rg [42] |
| Normalised Green Intensity | NGI | Rg/(Rr + Rg + Rb) [43] |
| Infrared Percentage Vegetation Index | IPVI | Rn/(Rn + Rr) [44] |
| Visible Atmospherically Resistant Index | VARI | (Rn − Rr)/(Rr + Rg + Rb) [45] |
| Red Difference Index | RDI | Rn − Rr [46] |
| Green Difference Index | GDI | Rn − Rg [47] |
| Canopy Chlorophyll Concentration Index | CCCI | ((Rn − Rre)/(Rn + Rre))/NDVI [48] |
| Core Red Edge Triangular Vegetation Index | CReTVI | 100(Rn − Rre) − 10(Rn − Rg) [49] |
| Variable | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HY | PSP | GC | PH | NDVI | GNDVI | RDVI | SAVI | SRI | IPVI | RDI | GLI | |
| Host grass | <0.001 | 0.006 | 0.003 | 0.186 | 0.055 | 0.0943 | 0.057 | 0.049 | <0.001 | 0.058 | <0.001 | 0.027 |
| Endophyte | <0.001 | <0.001 | <0.001 | 0.07 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Population | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Season | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Host grass × Endophyte | <0.001 | <0.0039 | <0.001 | 0.336 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Host grass × Population | <0.001 | <0.001 | 0.012 | 0.069 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Endophyte × Population | <0.001 | <0.001 | <0.001 | 0.047 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Host grass × Season | 0.28 | 0.001 | 0.001 | 0.749 | 0.055 | 0.154 | 0.055 | 0.059 | 0.063 | 0.055 | 0.262 | 0.474 |
| Endophyte × Season | 0.356 | <0.001 | <0.001 | 0.53 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 |
| Population × Season | <0.001 | <0.001 | <0.001 | 0.028 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Host grass × Endophyte × Population | 0.003 | 0.291 | 0.896 | 0.346 | 0.081 | 0.087 | 0.071 | 0.074 | <0.001 | 0.071 | 0.262 | 0.027 |
| Host grass × Endophyte × Season | 0.831 | 0.212 | <0.001 | 0.635 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.273 |
| Host grass × Population × Season | 0.395 | 0.006 | 0.001 | 0.562 | 0.079 | 0.08 | 0.079 | 0.08 | 0.009 | 0.089 | 0.201 | 0.038 |
| Endophyte × Population × Season | 0.192 | <0.001 | <0.001 | 0.335 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, C.; Jacobs, J.; Thomson, A.; Smith, K. Evaluation of the Relationship between Cultivar, Endophyte and Environment on the Expression of Persistence in Perennial Ryegrass Populations Using High-Throughput Phenotyping. Agronomy 2023, 13, 2292. https://doi.org/10.3390/agronomy13092292
Jayasinghe C, Jacobs J, Thomson A, Smith K. Evaluation of the Relationship between Cultivar, Endophyte and Environment on the Expression of Persistence in Perennial Ryegrass Populations Using High-Throughput Phenotyping. Agronomy. 2023; 13(9):2292. https://doi.org/10.3390/agronomy13092292
Chicago/Turabian StyleJayasinghe, Chinthaka, Joe Jacobs, Anna Thomson, and Kevin Smith. 2023. "Evaluation of the Relationship between Cultivar, Endophyte and Environment on the Expression of Persistence in Perennial Ryegrass Populations Using High-Throughput Phenotyping" Agronomy 13, no. 9: 2292. https://doi.org/10.3390/agronomy13092292
APA StyleJayasinghe, C., Jacobs, J., Thomson, A., & Smith, K. (2023). Evaluation of the Relationship between Cultivar, Endophyte and Environment on the Expression of Persistence in Perennial Ryegrass Populations Using High-Throughput Phenotyping. Agronomy, 13(9), 2292. https://doi.org/10.3390/agronomy13092292







