Plant Allelopathy in Response to Biotic and Abiotic Factors
Abstract
1. Introduction
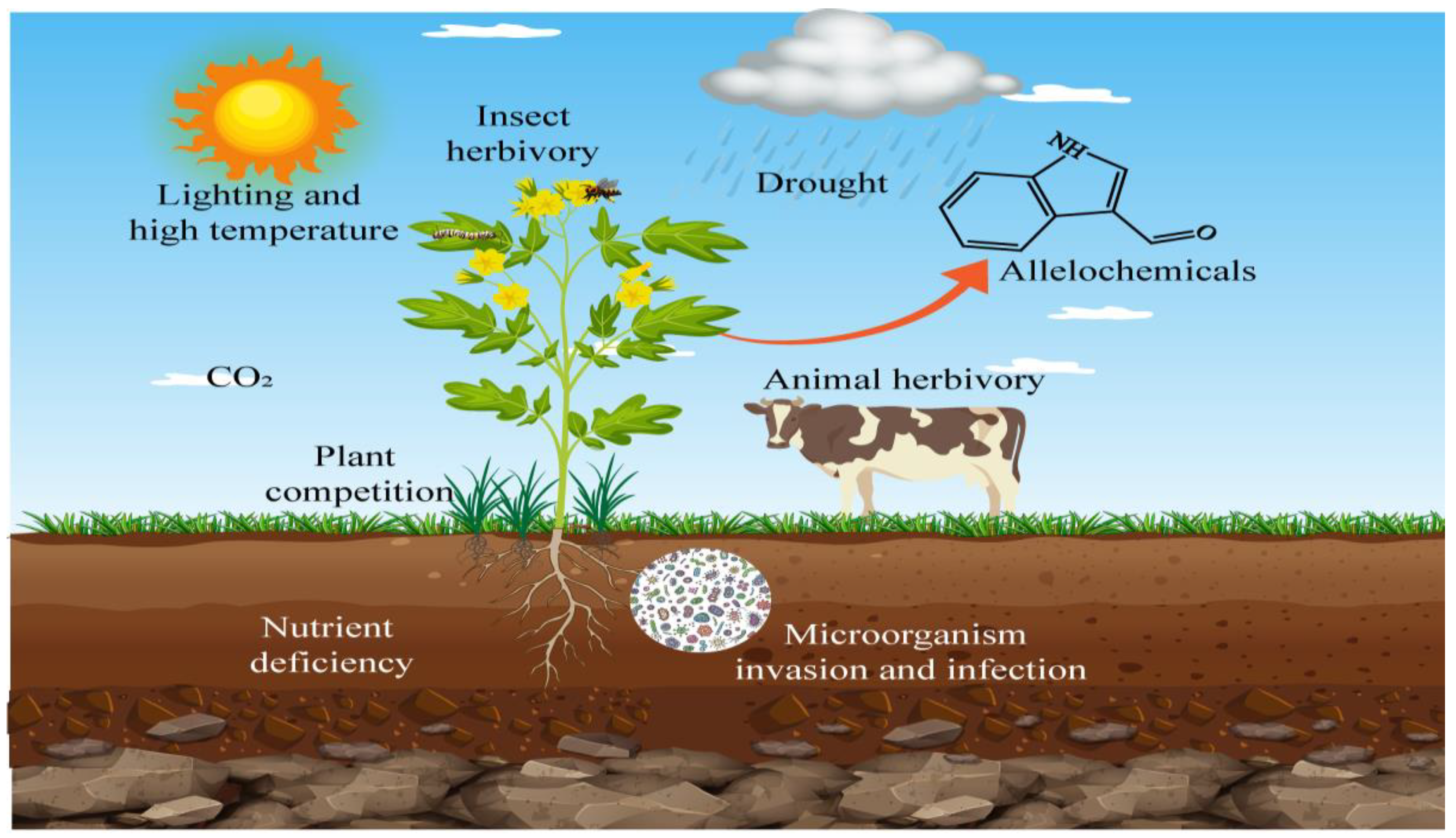
2. Biological Factors
2.1. Plant Competition
2.2. Animals
2.3. Insects
2.4. Microorganisms
3. Abiotic Factors
3.1. Light
3.2. Temperature
3.3. Drought
3.4. Carbon Dioxide
4. Nutrient Deficiency
5. Other Environmental Factors
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willis, R.J. The History of Allelopathy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Mallik, A.U. Allelopathy: Advances, challenges and opportunities. In Allelopathy in Sustainable Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 2008; pp. 25–38. [Google Scholar]
- Kong, C.-H.; Xuan, T.D.; Khanh, T.D.; Tran, H.-D.; Trung, N.T. Allelochemicals and signaling chemicals in plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Gries, G.A. Juglone, the active agent in walnut toxicity. North. Nut. Grow. Assoc. Annu. Rep. 1943, 32, 52–55. [Google Scholar]
- Shang, L.; Xu, Y.; Leaw, C.P.; Lim, P.T.; Wang, J.; Chen, J.; Deng, Y.; Hu, Z.; Tang, Y.Z. Potent allelopathy and non-PSTs, non-spirolides toxicity of the dinoflagellate Alexandrium leei to phytoplankton, finfish and zooplankton observed from laboratory bioassays. Sci. Total Environ. 2021, 780, 146484. [Google Scholar]
- Jilani, G.; Mahmood, S.; Chaudhry, A.N.; Hassan, I.; Akram, M. Allelochemicals: Sources, toxicity and microbial transformation in soil—A review. Ann. Microbiol. 2008, 58, 351–357. [Google Scholar] [CrossRef]
- Zuo, Z. Why algae release volatile organic compounds—The emission and roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Bókony, V.; Üveges, B.; Móricz, Á.M.; Hettyey, A. Competition induces increased toxin production in toad larvae without allelopathic effects on heterospecific tadpoles. Funct. Ecol. 2018, 32, 667–675. [Google Scholar] [CrossRef]
- Hadacek, F. Secondary metabolites as plant traits: Current assessment and future perspectives. Crit. Rev. Plant Sci. 2002, 21, 273–322. [Google Scholar] [CrossRef]
- Arafat, Y.; Ud Din, I.; Tayyab, M.; Jiang, Y.; Chen, T.; Cai, Z.; Zhao, H.; Lin, X.; Lin, W.; Lin, S. Soil sickness in aged tea plantation is associated with a shift in microbial communities as a result of plant polyphenol accumulation in the tea gardens. Front. Plant Sci. 2020, 11, 601. [Google Scholar] [CrossRef]
- Arafat, Y.; Wei, X.; Jiang, Y.; Chen, T.; Saqib, H.S.A.; Lin, S.; Lin, W. Spatial distribution patterns of root-associated bacterial communities mediated by root exudates in different aged ratooning tea monoculture systems. Int. J. Mol. Sci. 2017, 18, 1727. [Google Scholar] [CrossRef]
- Niemeyer, H.M.; Perez, F.J. Potential of Hydroxamic Acids in the Control of Cereal Pests, Diseases, and Weeds; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Wang, C.; Liu, Z.; Wang, Z.; Pang, W.; Zhang, L.; Wen, Z.; Zhao, Y.; Sun, J.; Wang, Z.-Y.; Yang, C. Effects of autotoxicity and allelopathy on seed germination and seedling growth in Medicago truncatula. Front. Plant Sci. 2022, 13, 908426. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.D. Field testing for pollen allelopathy: A review. J. Chem. Ecol. 2000, 26, 2155–2172. [Google Scholar] [CrossRef]
- Wink, M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008, 9, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; Cabi: Boston, MA, USA, 2018. [Google Scholar]
- Wink, M. Ecological Roles of Alkaloids; Wiley Online Library: Weinheim, Germany, 2008; pp. 3–52. [Google Scholar]
- Zwenger, S.; Basu, C. Plant terpenoids: Applications and future potentials. Biotechnol. Mol. Biol. Rev. 2008, 3, 1. [Google Scholar]
- Fattorusso, E.; Taglialatela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis, and Biology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Schenk, S.U.; Werner, D. β-(3-isoxazolin-5-on-2-yl)-alanine from Pisum: Allelopathic properties and antimycotic bioassay. Phytochemistry 1991, 30, 467–470. [Google Scholar] [CrossRef]
- Morris, P.F.; Ward, E. Chemoattraction of zoospores of the soybean pathogen, Phytophthora sojae, by isoflavones. Physiol. Mol. Plant Pathol. 1992, 40, 17–22. [Google Scholar] [CrossRef]
- Gfeller, A.; Glauser, G.; Etter, C.; Signarbieux, C.; Wirth, J. Fagopyrum esculentum alters its root exudation after Amaranthus retroflexus recognition and suppresses weed growth. Front. Plant Sci. 2018, 9, 50. [Google Scholar] [CrossRef]
- Einhellig, F.A. Interactions involving allelopathy in cropping systems. Agron. J. 1996, 88, 886–893. [Google Scholar] [CrossRef]
- Berenbaum, M. Brementown revisited: Interactions among allelochemicals in plants. In Chemically Mediated Interactions between Plants and Other Organisms; Springer: Berlin/Heidelberg, Germany, 1985; pp. 139–169. [Google Scholar]
- Romeo, J.T. Functional multiplicity among nonprotein amino acids in Mimosoid legumes: A case against redundancy. Ecoscience 1998, 5, 287–294. [Google Scholar] [CrossRef]
- Saiki, H.; Yoneda, K. Possible dual roles of an allelopathic compound, cis-dehydromatricaria ester. J. Chem. Ecol. 1982, 8, 185–193. [Google Scholar] [CrossRef]
- Seigler, D.; Price, P.W. Secondary compounds in plants: Primary functions. Am. Nat. 1976, 110, 101–105. [Google Scholar] [CrossRef]
- Bi, H.H.; Zeng, R.S.; Su, L.M.; An, M.; Luo, S.M. Rice allelopathy induced by methyl jasmonate and methyl salicylate. J. Chem. Ecol. 2007, 33, 1089–1103. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. Allelopathy and exotic plant invasion. Plant Soil 2003, 256, 29–39. [Google Scholar] [CrossRef]
- Putnam, A.R.; Duke, W.B. Biological suppression of weeds: Evidence for allelopathy in accessions of cucumber. Science 1974, 185, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Scavo, A.; Pandino, G.; Restuccia, A.; Caruso, P.; Lombardo, S.; Mauromicale, G. Allelopathy in durum wheat landraces as affected by genotype and plant part. Plants 2022, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- Xian, Q.; Chen, H.; Zou, H.; Yin, D. Allelopathic activity and nutrients competition between Ceratophyllum demersum and Microcystis aeruginosa. In Proceedings of the 4th World Congress on Allelopathy, Establishing the Scientific Base, Wagga Wagga, New South Wales, Australia, 21–26 August 2005; pp. 403–406. [Google Scholar]
- Keating, K.I. Allelochemistry in plankton communities. In Principles and Practices in Plant Ecology; CRC Press: Boca Raton, FL, USA, 1999; pp. 165–178. [Google Scholar]
- Kong, C. Allelopathy: Chemistry and Mode of Action of Allelochemicals. J. Environ. Qual. 2005, 34, 1453. [Google Scholar] [CrossRef]
- Li, L.-L.; Zhao, H.-H.; Kong, C.-H. (–)-Loliolide, the most ubiquitous lactone, is involved in barnyardgrass-induced rice allelopathy. J. Exp. Bot. 2020, 71, 1540–1550. [Google Scholar] [CrossRef]
- Kost, C.; Heil, M. The defensive role of volatile emission and extrafloral nectar secretion for lima bean in nature. J. Chem. Ecol. 2008, 34, 2–13. [Google Scholar] [CrossRef] [PubMed]
- De Lacy Costello, B.; Evans, P.; Ewen, R.; Gunson, H.; Jones, P.R.; Ratcliffe, N.M.; Spencer-Phillips, P.T. Gas chromatography–mass spectrometry analyses of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 2001, 50, 489–496. [Google Scholar] [CrossRef]
- Bonsang, B.; Gros, V.; Peeken, I.; Yassaa, N.; Bluhm, K.; Zöllner, E.; Sarda-Esteve, R.; Williams, J. Isoprene emission from phytoplankton monocultures: The relationship with chlorophyll-a, cell volume and carbon content. Environ. Chem. 2010, 7, 554–563. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Molinillo, J.M.; Varela, R.M.; Mauromicale, G.; Macías, F.A. Effect of shading on the sesquiterpene lactone content and phytotoxicity of cultivated cardoon leaf extracts. J. Agric. Food Chem. 2020, 68, 11946–11953. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Zhu, Y.; Bai, Y.; Wang, Y. Acetic acid-induced programmed cell death and release of volatile organic compounds in Chlamydomonas reinhardtii. Plant Physiol. Biochem. 2012, 51, 175–184. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Loreto, F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia 1993, 95, 328–333. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, L.; Yang, W.; Bai, Y.; Hou, P.; Zhao, J.; Zhou, L.; Zuo, Z. Volatile organic compounds released from Microcystis flos-aquae under nitrogen sources and their toxic effects on Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2017, 135, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Teuber, M.; Zimmer, I.; Kreuzwieser, J.; Ache, P.; Polle, A.; Rennenberg, H.; Schnitzler, J.P. VOC emissions of Grey poplar leaves as affected by salt stress and different N sources. Plant Biol. 2008, 10, 86–96. [Google Scholar] [CrossRef]
- Uddin, M.N.; Asaeda, T.; Shampa, S.H.; Robinson, R.W. Allelopathy and its coevolutionary implications between native and non-native neighbors of invasive Cynara cardunculus L. Ecol. Evol. 2020, 10, 7463–7475. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, B.; Wang, P. Barnyardgrass root recognition behaviour for rice allelopathy. Agronomy 2018, 8, 39. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Knorr, D.; Smetanska, I. Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 2011, 59, 1400–1405. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Van Wees, S.C. Shifting from priming of salicylic acid-to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Berg, M.P.; Staudt, M.; Holmstrup, M.; Glasius, M.; Ellers, J.; Tomiolo, S.; Madsen, R.B.; Slotsbo, S.; Penuelas, J. Plant secondary compounds in soil and their role in belowground species interactions. Trends Ecol. Evol. 2020, 35, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; Von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions:” talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Xu, X.; Zhou, B.; Hu, F.; Zhang, C.; Zhang, M. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 2004, 65, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Rivoal, A.; Fernandez, C.; Greff, S.; Montes, N.; Vila, B. Does competition stress decrease allelopathic potential? Biochem. Syst. Ecol. 2011, 39, 401–407. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, Y.; Zhu, W.; Tang, J.; Hu, S.; Zhou, T.; Chen, X. Baicalin released from Scutellaria baicalensis induces autotoxicity and promotes soilborn pathogens. J. Chem. Ecol. 2010, 36, 329–338. [Google Scholar] [CrossRef]
- Singh, H.; Batish, D.R.; Kohli, R. Autotoxicity: Concept, organisms, and ecological significance. Crit. Rev. Plant Sci. 1999, 18, 757–772. [Google Scholar] [CrossRef]
- Skoneczny, D.; Zhu, X.; Weston, P.A.; Gurr, G.M.; Callaway, R.M.; Weston, L.A. Production of pyrrolizidine alkaloids and shikonins in Echium plantagineum L. in response to various plant stressors. Pest Manag. Sci. 2019, 75, 2530–2541. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H.; Yang, X.; Li, Y.F. Interference of allelopathic rice with penoxsulam-resistant barnyardgrass. Pest Manag. Sci. 2017, 73, 2310–2317. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. The chemical-mediated allelopathic interaction between rice and barnyard grass. Plant Soil 2013, 370, 267–275. [Google Scholar] [CrossRef]
- Dayan, F.E. Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor. Planta 2006, 224, 339–346. [Google Scholar] [CrossRef]
- Ling, S.; Rizvi, S.A.H.; Xiong, T.; Liu, J.; Gu, Y.; Wang, S.; Zeng, X. Volatile signals from guava plants prime defense signaling and increase jasmonate-dependent herbivore resistance in neighboring citrus plants. Front. Plant Sci. 2022, 13, 833562. [Google Scholar] [CrossRef] [PubMed]
- Rakwal, R.; Komatsu, S. Role of jasmonate in the rice (Oryza sativa L.) self-defense mechanism using proteome analysis. ELECTROPHORESIS: Int. J. 2000, 21, 2492–2500. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassão, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [PubMed]
- Gu, Y.; Li, H.; Kong, C. Allelopathic potential of barnyard grass on rice and soil microbes in paddy. Allelopath. J. 2008, 21, 389. [Google Scholar]
- You, L.; Wang, P. Rice-barnyard grass allelopathic interaction: A role of jasmonic acid and salicylic acid. Adv. Mater. Res. 2010, 113, 1782–1786. [Google Scholar] [CrossRef]
- Zhou, B.; Kong, C.H.; Wang, P.; Li, Y.H. Chemical constituents of the essential oils of wild oat and crabgrass and their effects on the growth and allelochemical production of wheat. Weed Biol. Manag. 2013, 13, 62–69. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [Google Scholar] [CrossRef]
- Fang, C.-X.; Xiong, J.; Qiu, L.; Wang, H.-B.; Song, B.-Q.; He, H.-B.; Lin, R.-Y.; Lin, W.-X. Analysis of gene expressions associated with increased allelopathy in rice (Oryza sativa L.) induced by exogenous salicylic acid. Plant Growth Regul. 2009, 57, 163–172. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The chemical cross talk between rice and barnyardgrass. Plant Signal. Behav. 2011, 6, 1207–1209. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Zheng, X.-Y.; Lin, S.-X.; Gu, C.-Z.; Li, L.; Li, J.-Y.; Fang, C.-X.; He, H.-B. Transcriptome analysis reveals that barnyard grass exudates increase the allelopathic potential of allelopathic and non-allelopathic rice (Oryza sativa) accessions. Rice 2019, 12, 30. [Google Scholar]
- Zhang, S.Z.; Li, Y.H.; Kong, C.H.; Xu, X.H. Interference of allelopathic wheat with different weeds. Pest Manag. Sci. 2016, 72, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.A.; Laue, G.; Baldwin, I.T. Methyl jasmonate is blowing in the wind, but can it act as a plant–plant airborne signal? Biochem. Syst. Ecol. 2001, 29, 1007–1023. [Google Scholar] [CrossRef]
- Van Donk, E.; Ianora, A.; Vos, M. Induced defences in marine and freshwater phytoplankton: A review. Hydrobiologia 2011, 668, 3–19. [Google Scholar]
- Ma, Z.; Fang, T.; Thring, R.W.; Li, Y.; Yu, H.; Zhou, Q.; Zhao, M. Toxic and non-toxic strains of Microcystis aeruginosa induce temperature dependent allelopathy toward growth and photosynthesis of Chlorella vulgaris. Harmful Algae 2015, 48, 21–29. [Google Scholar] [PubMed]
- Van Donk, E.; van de Bund, W.J. Impact of submerged macrophytes including charophytes on phyto-and zooplankton communities: Allelopathy versus other mechanisms. Aquat. Bot. 2002, 72, 261–274. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Renne, I.J.; Sinn, B.T.; Shook, G.W.; Sedlacko, D.M.; Dull, J.R.; Villarreal, D.; Hierro, J.L. Eavesdropping in plants: Delayed germination via biochemical recognition. J. Ecol. 2014, 102, 86–94. [Google Scholar] [CrossRef]
- Tollrian, R.; Harvell, C.D. The Ecology and Evolution of Inducible Defenses; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Toth, G.B.; Pavia, H. Induced herbivore resistance in seaweeds: A meta-analysis. J. Ecol. 2007, 95, 425–434. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Turlings, T.C.; Ton, J. Exploiting scents of distress: The prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr. Opin. Plant Biol. 2006, 9, 421–427. [Google Scholar] [CrossRef]
- Vivanco, J.M.; Cosio, E.; Loyola-Vargas, V.M.; Flores, H.E. Mecanismos químicos de defensa en las plantas. Investig. Y Cienc. 2005, 341, 68–75. [Google Scholar]
- Benlarbi, K.H.; Elmtili, N.; Macías, F.A.; Galindo, J.C.G. Influence of in vitro growth conditions in the production of defence compounds in Mentha pulegium L. Phytochem. Lett. 2014, 8, 233–244. [Google Scholar] [CrossRef]
- Provenza, F.D. Postingestive feedback as an elementary determinant of food preference and intake in ruminants. Rangel. Ecol. Manag. J. Range Manag. Arch. 1995, 48, 2–17. [Google Scholar] [CrossRef]
- Arimura, G.-i.; Shiojiri, K.; Karban, R. Acquired immunity to herbivory and allelopathy caused by airborne plant emissions. Phytochemistry 2010, 71, 1642–1649. [Google Scholar] [CrossRef]
- Rice, E.L. Pest Control with Nature’s Chemicals. Allelochemics and Pheromones in Gardening and Agriculture; University of Oklahoma Press: Norman, OK, USA, 1983. [Google Scholar]
- Mahmood, K.; Khan, M.B.; Ijaz, M.; Zeng, R.S.; Luo, S.M. Molecular, biochemical and bioassay based evidence of lower allelopathic potential in genetically modified rice. Plant Growth Regul. 2014, 74, 73–82. [Google Scholar] [CrossRef]
- Farmer, E.E. Surface-to-air signals. Nature 2001, 411, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Bodnaryk, R.P. Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry 1992, 31, 2671–2677. [Google Scholar] [CrossRef]
- Arimura, G.-i.; Köpke, S.; Kunert, M.; Volpe, V.; David, A.; Brand, P.; Dabrowska, P.; Maffei, M.E.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 2008, 146, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Kruidhof, H.M.; van Dam, N.M.; Ritz, C.; Lotz, L.A.; Kropff, M.J.; Bastiaans, L. Mechanical wounding under field conditions: A potential tool to increase the allelopathic inhibitory effect of cover crops on weeds? Eur. J. Agron. 2014, 52, 229–236. [Google Scholar] [CrossRef]
- Myers, T.L.; Prince, E.K.; Naar, J.; Kubanek, J. Loss of waterborne brevetoxins from exposure to phytoplankton competitors. Harmful Algae 2008, 7, 762–771. [Google Scholar] [CrossRef]
- Paul, C.; Barofsky, A.; Vidoudez, C.; Pohnert, G. Diatom exudates influence metabolism and cell growth of co-cultured diatom species. Mar. Ecol. Prog. Ser. 2009, 389, 61–70. [Google Scholar] [CrossRef]
- Poulson-Ellestad, K.L.; Jones, C.M.; Roy, J.; Viant, M.R.; Fernández, F.M.; Kubanek, J.; Nunn, B.L. Metabolomics and proteomics reveal impacts of chemically mediated competition on marine plankton. Proc. Natl. Acad. Sci. USA 2014, 111, 9009–9014. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.K.; Myers, T.L.; Naar, J.; Kubanek, J. Competing phytoplankton undermines allelopathy of a bloom-forming dinoflagellate. Proc. R. Soc. B Biol. Sci. 2008, 275, 2733–2741. [Google Scholar] [CrossRef]
- Zuo, S.; Fang, Z.; Zhou, S.; Ye, L. Benthic fauna promote algicidal effect of allelopathic macrophytes on Microcystis aeruginosa. J. Plant Growth Regul. 2016, 35, 646–654. [Google Scholar] [CrossRef]
- Jang, M.-H.; Jung, J.-M.; Takamura, N. Changes in microcystin production in cyanobacteria exposed to zooplankton at different population densities and infochemical concentrations. Limnol. Oceanogr. 2007, 52, 1454–1466. [Google Scholar] [CrossRef]
- Jang, M.H.; Ha, K.; Joo, G.J.; Takamura, N. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshw. Biol. 2003, 48, 1540–1550. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Chuihua, K. Chemical interactions between plant and other organisms: A potential strategy for pest management. Sci. Agric. Sin. 2007, 40, 712–720. [Google Scholar]
- Pickett, J.; Rasmussen, H.; Woodcock, C.; Matthes, M.; Napier, J. Plant stress signalling: Understanding and exploiting plant–plant interactions. Biochem. Soc. Trans. 2003, 31, 123–127. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids—Biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Ye, M.; Li, C.Y.; Wang, R.L.; Wei, X.C.; Luo, S.M.; Zeng, R.S. Priming of anti-herbivore defense in tomato by arbuscular mycorrhizal fungus and involvement of the jasmonate pathway. J. Chem. Ecol. 2013, 39, 1036–1044. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Garriga, A.; Sardans, J.; Pérez-Trujillo, M.; Rivas-Ubach, A.; Oravec, M.; Vecerova, K.; Urban, O.; Jentsch, A.; Kreyling, J.; Beierkuhnlein, C. Opposite metabolic responses of shoots and roots to drought. Sci. Rep. 2014, 4, 6829. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.; Turlings, T.; Jones, T.; Stenhagen, G.; Loughrin, J.; Tumlinson, J. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Pickett, J.A.; Birkett, M.; Moraes, B.; Bruce, T.; Chamberlain, K.; Gordon-Weeks, R.; Matthes, M.; Napier, J.; Smart, L.; Wadhams, L. cis-Jasmone as allelopathic agent in inducing plant defence. Allelopath. J. 2007, 19, 109–118. [Google Scholar]
- Mishra, S.; Upadhyay, R.S.; Nautiyal, C.S. Unravelling the beneficial role of microbial contributors in reducing the allelopathic effects of weeds. Appl. Microbiol. Biotechnol. 2013, 97, 5659–5668. [Google Scholar] [CrossRef]
- Rashidi, S.; Yousefi, A.R.; Pouryousef, M.; Goicoechea, N. Effect of arbuscular mycorrhizal fungi on the accumulation of secondary metabolites in roots and reproductive organs of Solanum nigrum, Digitaria sanguinalis and Ipomoea purpurea. Chem. Biol. Technol. Agric. 2022, 9, 23. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Holighaus, G.; Rohlfs, M. Fungal allelochemicals in insect pest management. Appl. Microbiol. Biotechnol. 2016, 100, 5681–5689. [Google Scholar] [CrossRef]
- Pedrol, N.; González, L.; Reigosa, M.J. Allelopathy and abiotic stress. In Allelopathy: A Physiological Process with Ecological Implications; Springer: Berlin/Heidelberg, Germany, 2006; pp. 171–209. [Google Scholar]
- Inderjit; Weston, L.A. Are laboratory bioassays for allelopathy suitable for prediction of field responses? J. Chem. Ecol. 2000, 26, 2111–2118. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Alías, J.C.; Chaves Lobón, N. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Hartikainen, K.; Riikonen, J.; Nerg, A.-M.; Kivimäenpää, M.; Ahonen, V.; Tervahauta, A.; Kärenlampi, S.; Mäenpää, M.; Rousi, M.; Kontunen-Soppela, S. Impact of elevated temperature and ozone on the emission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth). Environ. Exp. Bot. 2012, 84, 33–43. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Riikonen, J.; Ahonen, V.; Tervahauta, A.; Holopainen, T. Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. Environ. Exp. Bot. 2013, 90, 32–42. [Google Scholar] [CrossRef]
- Lankau, R. A chemical trait creates a genetic trade-off between intra-and interspecific competitive ability. Ecology 2008, 89, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Ladwig, L.M.; Meiners, S.J.; Pisula, N.L.; Lang, K.A. Conditional allelopathic potential of temperate lianas. Plant Ecol. 2012, 213, 1927–1935. [Google Scholar] [CrossRef]
- Dudt, J.F.; Shure, D.J. The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 1994, 75, 86–98. [Google Scholar] [CrossRef]
- Johnson, J.D.; Tognetti, R.; Michelozzi, M.; Pinzauti, S.; Minotta, G.; Borghetti, M. Ecophysiological responses of Fagus sylvatica seedlings to changing light conditions. II. The interaction of light environment and soil fertility on seedling physiology. Physiol. Plant. 1997, 101, 124–134. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kujime, H.; Ino, T. UV-induced momilactone B accumulation in rice rhizosphere. J. Plant Physiol. 2007, 164, 1548–1551. [Google Scholar] [CrossRef]
- Voirin, B.; Brun, N.; Bayet, C. Effects of daylength on the monoterpene composition of leaves of Mentha x piperita. Phytochemistry 1990, 29, 749–755. [Google Scholar] [CrossRef]
- Khanh, T.D.; Anh, L.H.; Nghia, L.T.; Huu Trung, K.; Bich Hien, P.; Minh Trung, D.; Dang Xuan, T. Allelopathic responses of rice seedlings under some different stresses. Plants 2018, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Blum, U.; Fites, R.C. Stress modification of allelopathy of Helianthus annuus L. debris on seed germination. Am. J. Bot. 1982, 69, 776–783. [Google Scholar] [CrossRef]
- He, Y.; Ma, D.; Li, Q.; Wang, Y.; Tian, J.; Cheng, W. Temperature influences on the allelopathy effect of aqueous extracts from Trifolium repens L. Bull. Bot. Res. 2010, 30, 243–247. [Google Scholar]
- Gao, Y.; Yuan, X.; Lin, X.; Sun, B.; Zhao, Z. Low-molecular-weight organic acids enhance the release of bound PAH residues in soils. Soil Tillage Res. 2015, 145, 103–110. [Google Scholar] [CrossRef]
- San Emeterio, L.; Arroyo, A.; Canals, R. Allelopathic potential of Lolium rigidum Gaud. on the early growth of three associated pasture species. Grass Forage Sci. 2004, 59, 107–112. [Google Scholar] [CrossRef]
- Tang, C.-S.; Cai, W.-F.; Kohl, K.; Nishimoto, R.K. Plant Stress and Allelopathy; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Selmar, D.; Kleinwächter, M. Stress enhances the synthesis of secondary plant products: The impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Constable, J.V.H.; Guenther, A.B.; Schimel, D.S.; Monson, R.K. Modelling changes in VOC emission in response to climate change in the continental United States. Glob. Change Biol. 1999, 5, 791–806. [Google Scholar] [CrossRef]
- Himanen, S.J.; Nerg, A.M.; Nissinen, A.; Pinto, D.M.; Stewart Jr, C.N.; Poppy, G.M.; Holopainen, J.K. Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytol. 2009, 181, 174–186. [Google Scholar] [CrossRef]
- Possell, M.; Nicholas Hewitt, C.; Beerling, D.J. The effects of glacial atmospheric CO2 concentrations and climate on isoprene emissions by vascular plants. Glob. Change Biol. 2005, 11, 60–69. [Google Scholar] [CrossRef]
- Rapparini, F.; Baraldi, R.; Miglietta, F.; Loreto, F. Isoprenoid emission in trees of Quercus pubescens and Quercus ilex with lifetime exposure to naturally high CO2 environment. Plant Cell Environ. 2004, 27, 381–391. [Google Scholar] [CrossRef]
- Tiiva, P.; Faubert, P.; Michelsen, A.; Holopainen, T.; Holopainen, J.K.; Rinnan, R. Climatic warming increases isoprene emission from a subarctic heath. New Phytol. 2008, 180, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-L.; Staehelin, C.; Peng, S.-L.; Wang, W.-T.; Xie, X.-M.; Lu, H.-N. Responses of Mikania micrantha, an invasive weed to elevated CO 2: Induction of β-caryophyllene synthase, changes in emission capability and allelopathic potential of β-Caryophyllene. J. Chem. Ecol. 2010, 36, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H. Roles of allelopathy in plant biodiversity and sustainable agriculture. Crit. Rev. Plant Sci. 1999, 18, 609–636. [Google Scholar] [CrossRef]
- Shen, L.; Lin, W. Effects of phosphorus levels on allelopathic potential of rice co-cultured with barnyardgrass. Allelopath. J. 2007, 19, 393. [Google Scholar]
- Wang, H.; He, H.; Ye, C.; Lu, J.; Chen, R.; Liu, C.; Guo, X.; Lin, W. Molecular physiological mechanism of increased weed suppression ability of allelopathic rice mediated by low phosphorus stress. Allelopath. J. 2010, 25, 239–248. [Google Scholar]
- Morcuende, R.; Bari, R.; Gibon, Y.; Zheng, W.; Pant, B.D.; Bläsing, O.; Usadel, B.; Czechowski, T.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of metabolism and regulatory networks of Arabidopsis in response to phosphorus. Plant Cell Environ. 2007, 30, 85–112. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, J.; Xu, Q.; Zhou, L.; Gan, L.; Zuo, Z. Phosphorus deficiency inducing volatile organic compounds from Microcystis aeruginosa and their effects on Chlamydomonas reinhardtii. J. Lake Sci. 2018, 30, 449–457. [Google Scholar]
- Hasegawa, M.; Nishizawa, A.; Tsuji, K.; Kimura, S.; Harada, K.-i. Volatile organic compounds derived from 2-keto-acid decarboxylase in Microcystis aeruginosa. Microbes Environ. 2012, 27, 525–528. [Google Scholar] [CrossRef]
- Jiesheng, L.; Jin, X.; Weidong, Y.; Lixuan, L. Allelopathic effect of alexandrium tamarense on prorocentrum donghaiense under limited nutrient conditions. J. Trop. Subtrop. Bot. 2006, 14, 207–212. [Google Scholar]
- Ye, C.; Yang, Y.; Xu, Q.; Ying, B.; Zhang, M.; Gao, B.; Ni, B.; Yakefu, Z.; Bai, Y.; Zuo, Z. Volatile organic compound emissions from Microcystis aeruginosa under different phosphorus sources and concentrations. Phycol. Res. 2018, 66, 15–22. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Stress-induced allelopathic activity and momilactone B in rice. Plant Growth Regul. 2009, 59, 153–158. [Google Scholar] [CrossRef]
- Laturnus, F.; Giese, B.; Wiencke, C.; Adams, F.C. Low-molecular-weight organoiodine and organobromine compounds released by polar macroalgae–The influence of abiotic factors. Fresenius J. Anal. Chem. 2000, 368, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, A.; Esmaeili, M.; Heidarzade, A. Salicylic acid and abiotic stress influence allelochemicals and inhibitory potential of root exudates of two rice (Oryza sativa) cultivars against barnyardgrass (Echinochloa crus-galli L.). Int. J. Farm. Allied Sci. 2013, 2, 779–784. [Google Scholar]
- Batish, D.R.; Singh, H.P.; Pandher, J.K.; Arora, V.; Kohli, R.K. Phytotoxic effect of Parthenium residues on the selected soil properties and growth of chickpea and radish. Weed Biol. Manag. 2002, 2, 73–78. [Google Scholar] [CrossRef]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress; Springer: Berlin/Heidelberg, Germany, 1984; pp. 273–320. [Google Scholar]
- Yamasaki, Y.; Nagasoe, S.; Matsubara, T.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Allelopathic interactions between the bacillariophyte Skeletonema costatum and the raphidophyte Heterosigma akashiwo. Mar. Ecol. Prog. Ser. 2007, 339, 83–92. [Google Scholar] [CrossRef]
| S.No | Factor | Target Plant | Description | Reference |
|---|---|---|---|---|
| 1 | Plant competition | Rice (O. sativa) | Barnyard grass may emit signaling chemicals such as (−)-loliolide from their roots to stimulate the generation of protective metabolites such as momilactone B and tricinin in rice against competitors. | [38] |
| Moreover, barnyard grass–rice system experiments showed that the proximity of competitive weeds and their root exudates can increase rice allelochemicals such as momilactones and flavone, resulting in an improvement in rice allelopathy by the chemical components in barnyard grass root exudates. | [48] | |||
| B. rapa ssp. | Elicitor application of salicylic acid (SA) and methyl jasmonate (MJ) induces a targeted rhizosecretion of high levels of anticarcinogenic glucosinolates in the turnip organs, as well as in turnip root exudates. | [49] | ||
| E. plantagineum | E. plantagineum is a noxious invasive weed in Australia forming monocultural stands in pastures and rangelands. It produces a naphthoquinones (NQs) which suppress competition from weeds, insects, and pathogens, and influence invasion success. | [57] | ||
| pigweed (A. retroflexus L.) | Buckwheat (Fagopyrum esculentum Moench) root exudates repressed pigweed growth when both plant species were growing next to each other, and pigweed recognition by buckwheat induced changes in the buckwheat root exudation profile. | [24] | ||
| Sorghum (S. bicolor) | The root exudate of sorghum includes sorgoleone, which was discharged in crucial amounts in the presence of a crude extract of velvet leaf root, indicating that seedlings could have increased phytotoxicity and improved allelopathic potential in the presence of other plants. | [60] | ||
| Wheat (T. aestivum) | Many studies have shown that some wheat varieties can produce benzoxazinoids such as DIMBOA against different pests, most notably in allelopathy, influencing the germination and development of weeds in a wheat crop. | [71,72] | ||
| 2 | Animals | Nicotiana attenuate, Arabidopsis, maize, Chinese cabbage | As a reaction to herbivory, plants can alter their phenotype to increase certain compounds or synthesize new chemicals. | [81,82,83] |
| Algae | Herbivores trigger induced protection (the generation of allelochemicals such as phlorotannin) by damaging macroalgae. | [73] | ||
| Higher plants (Mulberry) | When plants are attacked by herbivores, lignin and tannin are released to affect herbivores. Lignin affects the digestion and absorption of livestock, and tannin reduces the feeding rate of animals. | [85] | ||
| Rice | Herbivore feeding enhances the chemosensory effect of rice. | [88] | ||
| 3 | Insects | Cabbage leaves, maize seedlings, N. attenuata system, tomato | When plants are damaged by phytophagous insects, they can release some volatile secondary metabolites have an important role to play in regulating the interrelationship between plants, phytophagous insects, and their natural enemies. | [100] |
| Arabidopsis thaliana L., Brassica vegetables (Brassica napus L., B. rapa, and B. juncea) | Glucosinolates are unique to cruciferous plants, and their chemical combination and discharge can be induced by insect feeding. | [103] | ||
| A. conyzoides | The main ecological function of volatiles from A. conyzoides affected by E. cichoracearum DC. or exposed to aphid feeding may not be to affect neighboring plant development. Infection and aphid feeding are, in fact, more destructive to A. conyzoides than competition with neighboring plants | [75] | ||
| Maize | When the beet moth feeds on corn, the corn releases volatile terpene molecules into the air, triggering a chemical defense mechanism that attracts the moth’s natural enemies, parasitic wasps. | [108] | ||
| A. thaliana | Studies of plant–insect interactions, especially in multitrophic systems, have the potential to identify insect signaling chemicals that may elicit plant defense responses. | [109] | ||
| 4 | Microorganisms | tomato (S. lycopersicum) | Jasmonic acid (JA)- and salicylic acid (SA)-regulated defensive pathways in tomato beneficial root endophytes (Trichoderma spp.) induced resistance to the root knot nematode Meloidogyne incognita. | [50] |
| E. plantagineum | When sensing elicitors of pathogen origin, many plants can accumulate allelochemicals around infection sites of pathogens. | [59] | ||
| Solanum nigrum, Digitaria sanguinalis, Ipomoea purpurea | Arbuscular mycorrhizal fungi (AMFs) could induce the accumulation and synthesis of allelochemicals in the tissues of host plants, impacting their allelopathic potential. AMFs have an important role in enhancing different plants’ allelochemicals. | [111] |
| S.No | Factor | Target Plant | Description | Reference |
|---|---|---|---|---|
| 1. | Light | Cynara cardunculus L. | A previous study suggested that 60% plant shading in cultivated cardoons increased the sesquiterpene lactone content and phytotoxicity of its leaf extracts. | [42] |
| Toxicodendron radicans L., Parthenocissus quinquefolia L., Celastrus orbiculatus Thunb, Lonicera japonica Thunb, and Vitis vulpina L. | Photoinduced allelochemicals can increase the success rate of liana substitution by making liana redistribute resources according to light differences during early forest regeneration. Understanding how allelopathic potential changes with light accessibility may help with clarifying the dynamic role of allelochemicals in plant communities. | [120] | ||
| Fagus sylvatica L. | Changes in light quality can also affect the production of allelochemicals in plants. | [122] | ||
| O. sativa | After UV irradiation, plants release mycorolactone B. UV radiation increases the concentration of momilactone B in the shoots and roots of rice seedlings. | [123] | ||
| Mentha × piperita L. | In the case of mint, abiotic stressors such as UV radiation induce the production of mint essential oils, the main ingredients of which are monoterpenes. | [124] | ||
| 2. | temperature | Synechococcus spp. | High temperature could induce the generation of enormous amounts of ROS in algae, which benefits the oxidation of halide ions, carotenoids, and fatty acids, driving the formation of halogenated hydrocarbons, GLVs, and carotenoid degradants | [41] |
| E. plantagineum | E. plantagineum roots exposed to the highest temperature regime appeared to show improved accumulation of naphthoquinones over time in contrast to roots created for the lowest temperature treatment. Allelochemicals such as deoxyshikonin, dimethylshikonin, and shikonin showed significantly higher concentrations over time in roots exposed to a high-temperature regime. | [57] | ||
| M. aeruginosa | Climatic warming and eutrophication may lead to a shift in Microcystis populations toward blooms that contain a more prominent rate of toxic Microcystis cells and, consequently, more noteworthy concentrations of microcystin. When the temperature is over 25.8 °C, with a further increase in temperature, some toxic or nontoxic M. aeruginosa strains will release more allelochemicals to inhibit the growth of the green alga Chlorella vulgaris Beyerinck. | [74] | ||
| O. sativa | The rice cultivar Koshihikari may produce more syringic, p-hydroxybenzoic, vanillic, sinapic, and benzoic acids, which conceivably suppress the plants’ growth. Extracts and root exudates had the highest amount of total phenolic and flavonoid substances when rice seedlings were treated at 37 °C under abiotic stress. | [125] | ||
| R. officinalis | Some researchers found that the discharge of monoterpenes from rosemary (R. officinalis) was significantly higher in the high-temperature season than in other seasons, and its release was significantly influenced by the environment. | [126] | ||
| V. faba | Allelopathy of the extract increased V. faba root tip cells’ toxicity, with more prominent inhibition of cell mitosis, and induced a higher frequency of chromosomal aberrations and micronucleus. At high temperatures, the impact of allelopathy was more prominent than at low temperatures. | [127] | ||
| H. lanatus and A. pratensis | It has been observed that simultaneous exposure to high temperature and dry stress brought about an accumulation of allelochemicals in the roots and stimulation of secondary metabolites in the foliage of H. lanatus and A. pratensis. | [128] | ||
| 3. | Drought | E. plantagineum | E. plantagineum, under stress, including drought and elevated temperature, showed improved generation of shikonins, including those related to improved allelopathic or weed-suppressive activity and those acting as potent antimicrobials. | [57] |
| B. pilosa | B. pilosa appeared to enhance phytotoxicity in periods of drought. | [112] | ||
| T. erecta | Under water deficiency, the level of phenolic substances in T. erecta was much higher than that in normal water conditions. | [130] | ||
| P. sativum | Drought stress triggers accumulation of ROS and bioaccumulation of bioactive chemicals such as terpenes, phenols, and alkaloids to facilitate defense against pathogens, insects, and weeds. Water stress (45% of field water capacity) and treatment with prohydrojasmon before sowing seem to improve the chemosensory resistance to grass induction in a few tested wheat varieties. | [131] | ||
| A. thaliana | The biosynthesis of anthocyanins by A. thaliana improved under drought conditions, and generation was related to protection against drought stress | [132] | ||
| 4. | Carbon dioxide | R. officinalis | The monoterpene of R. officinalis was significantly enhanced with an increase in CO2 concentration, particularly in the high-temperature season compared to other seasons | [126] |
| B. napus | Elevated carbon dioxide on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (B. napus). | [134] | ||
| Mucuna pruriens L. and Arundo donax L. | We found a significant enhancement of isoprene emissions perunit leaf area in M. pruriens under subambient CO2 concentrations relative to ambient controls but not for A. donax. | [135] | ||
| M. micrantha | Increasing air CO2 levels may improve the biosynthesis and phytotoxicity of allelochemicals in M. micrantha, one of the most obtrusive weeds in the world, which in turn might improve its potential allelopathic effect on neighboring local plants if discharged in bioactive concentrations. | [138] | ||
| 5. | Nutrient deficiency | O. sativa | The main nutrient deficiencies are of nitrogen and phosphate. In rice, allelopathic activity may be increased under nutrient starvation conditions. Rice has shown strong allelopathic activity under phosphorus and nitrogen deficiency, which indicates the influence of nutrient starvation. | [69] |
| O. sativa | Rice produces more allelochemicals in nitrogen- and phosphate-limited conditions. | [124] | ||
| O. sativa | The inducible phenomenon in rice was observed when the P content dropped below the optimum level in hydroponic culture. | [140] | ||
| O. sativa | In lower P conditions, the defensive enzyme activities and other physiological and biochemical indices of barnyard grass were restrained. Further analysis revealed that the activity of phenylalanine ammonia-lyase and the total phenol content in root and leaf tissues increased remarkably compared with that in non-allelogenic rice. | [141] | ||
| Microcystis spp. | In Microcystis, the discharge of VOCs was dramatically increased in a low-nitrogen medium. | [45] | ||
| A. thaliana | In A. thaliana, P deficiency induces the expression of more than 1000 traits, of which a large number of genes are related to terpene VOCs and phenylalanine metabolism. | [142] | ||
| M. aeruginosa | M. aeruginosa increased the release of VOCs and β-cyclocitral when N availability was insufficient. | [144] | ||
| A. tamarense | It has been reported that the production of allelochemicals was stimulated in A. tamarense after exposure to N-limited and P-limited conditions | [145] | ||
| 6. | heavy metals | O. sativa | These heavy metals increase the generation and emission of momilactone B in rice. The rice allelopathic activity might be enhanced due to heavy metals, with a rise in the emission of momilactone B, which has formidable phytotoxin and allelopathic efficacy. | [147] |
| 7. | high salinity | Solieria chordalis J. Agardh and Gymnogongrus antarcticus Skottsberg | During high salinity, S. chordalis and G. antarcticus increase the production of allelochemicals. | [148] |
| O. sativa | Similarly, when rice cultivars are influenced by high salt, the production of salicylic acid is significantly elevated. Allelochemical production by rice is an effective defense for rice seedlings against barnyard grass. | [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, Z.; Zhou, S.; Shah, A.; Arafat, Y.; Arif Hussain Rizvi, S.; Shao, H. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy 2023, 13, 2358. https://doi.org/10.3390/agronomy13092358
Shan Z, Zhou S, Shah A, Arafat Y, Arif Hussain Rizvi S, Shao H. Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy. 2023; 13(9):2358. https://doi.org/10.3390/agronomy13092358
Chicago/Turabian StyleShan, Zixiang, Shixing Zhou, Asma Shah, Yasir Arafat, Syed Arif Hussain Rizvi, and Hua Shao. 2023. "Plant Allelopathy in Response to Biotic and Abiotic Factors" Agronomy 13, no. 9: 2358. https://doi.org/10.3390/agronomy13092358
APA StyleShan, Z., Zhou, S., Shah, A., Arafat, Y., Arif Hussain Rizvi, S., & Shao, H. (2023). Plant Allelopathy in Response to Biotic and Abiotic Factors. Agronomy, 13(9), 2358. https://doi.org/10.3390/agronomy13092358





