Responses of Two-Row and Six-Row Barley Genotypes to Elevated Carbon Dioxide Concentration and Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Site and Design
- Control irrigation condition and Ambient CO2 concentration.
- Control irrigation condition and Elevated CO2 concentration.
- Water stress condition and Ambient CO2 concentration.
- Water stress condition and Elevated CO2 concentration.
2.2. Water Stress Treatment
2.3. Measurement of the Physiological Traits
2.3.1. Plant Leaf Area (LA)
2.3.2. Relative Water Content (RWC)
2.3.3. Chlorophyll a (Chla), Chlorophyll b (Chlb) and carotenoids (Car)
2.3.4. Maximum Quantum Efficiency of PSII (Fv/Fm)
2.3.5. Proline Content
2.3.6. MDA Concentration
2.3.7. Antioxidant Enzyme Activities
- Catalase activity (CAT)
- b.
- Ascorbate peroxidase activity (APX)
- c.
- Peroxidase activity (POX)
2.3.8. DPPH Radical Scavenging Assay
2.3.9. Dry Weight of Shoots and Roots
2.3.10. Seed Weight per Plant
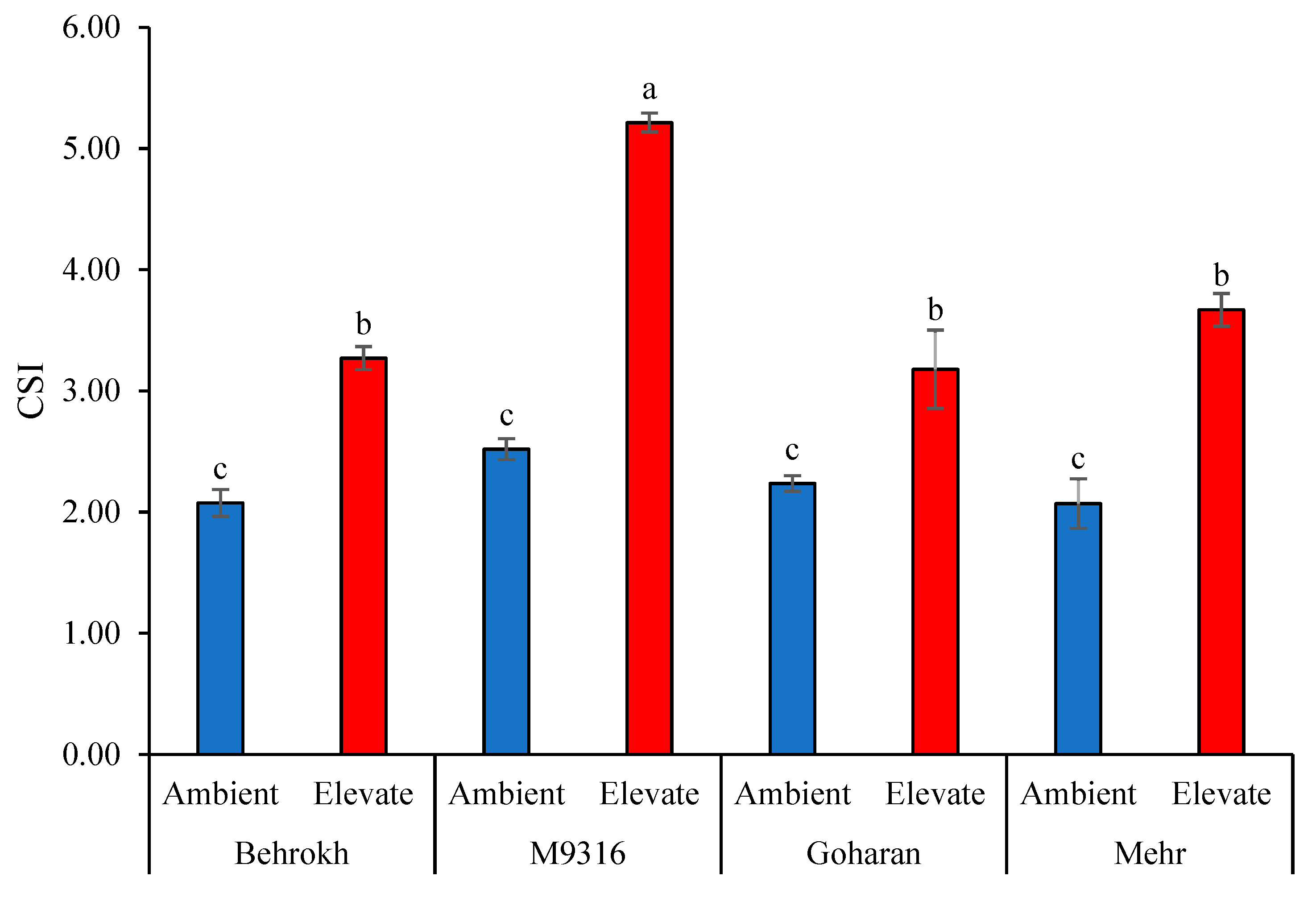
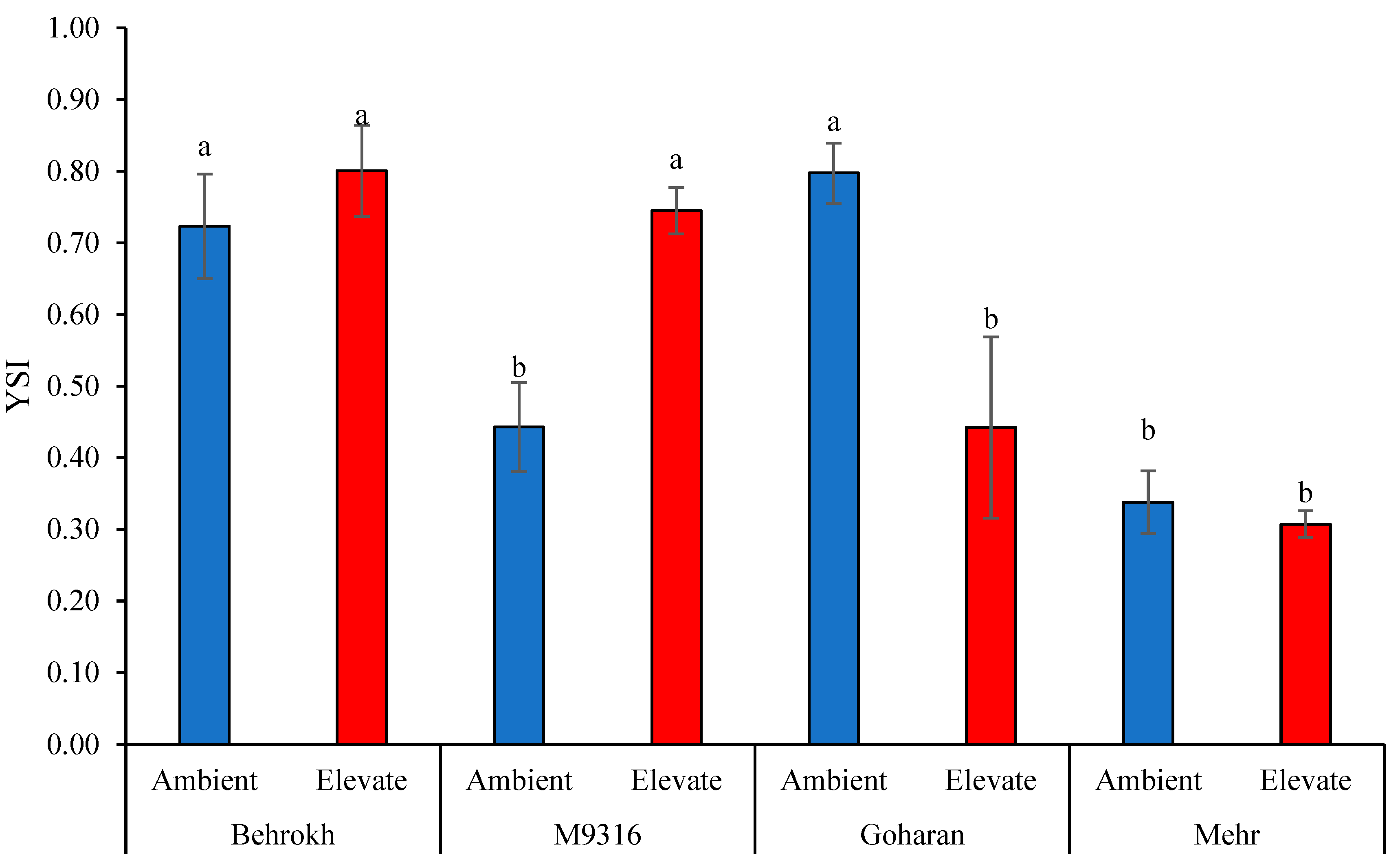
2.3.11. Combination of Significant Indices (CSI) and Yield Stability Index (YSI)
2.3.12. Statistical Analysis
3. Results
3.1. Univariate Data Analysis
3.1.1. Interaction of CO2 and Water Stress
3.1.2. Interaction of CO2 and Different Genotypes
3.1.3. Interaction Effects of Water Stress and Different Genotypes
3.1.4. Correlation between Ambient CO2 and Two Moisture Conditions (Normal, Water Stress)
3.1.5. Correlation between Elevated CO2 and Two Moisture Conditions (Normal, Water Stress)
3.2. Multivariate Data Analysis
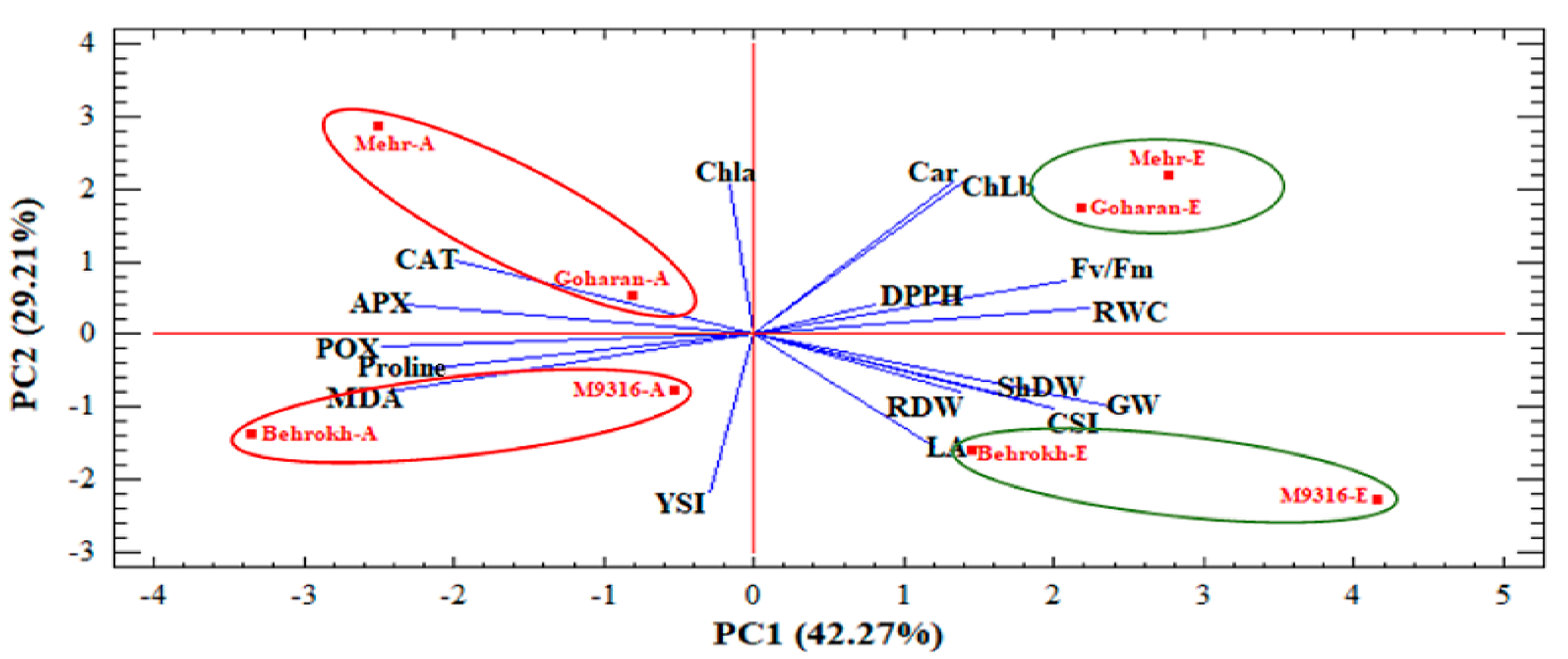
3.2.1. Principal Component Analysis
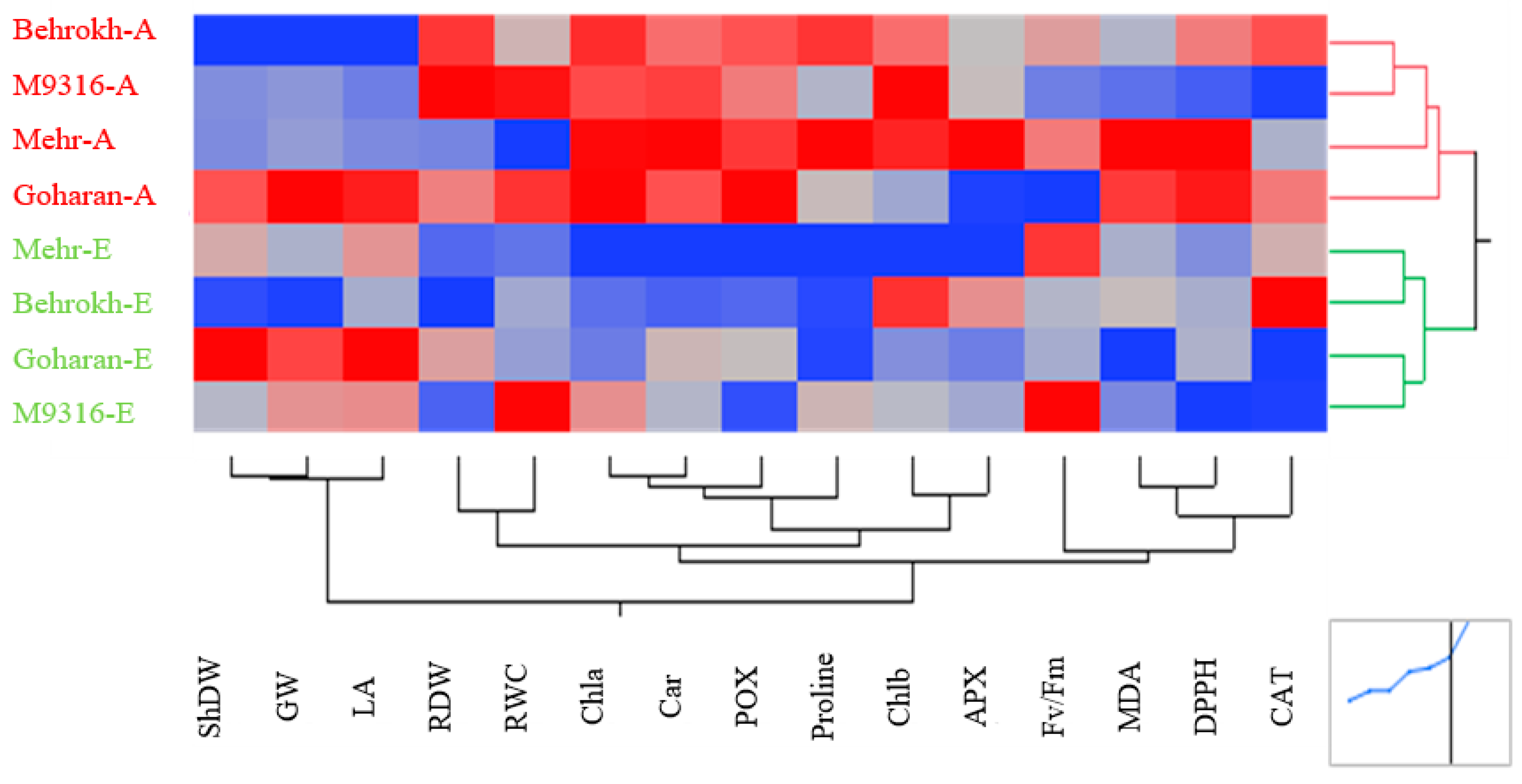
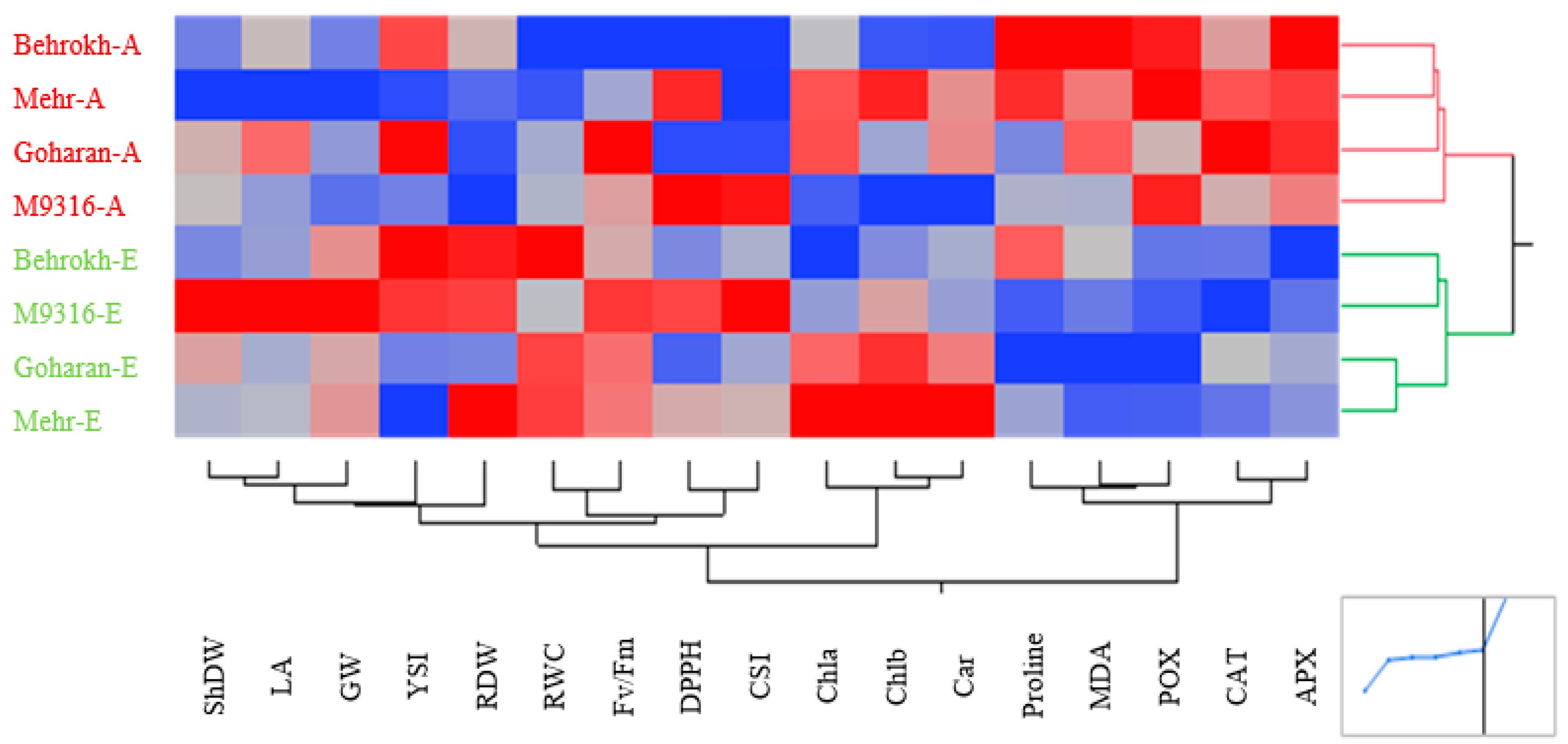
3.2.2. Heatmapping Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Karimifard, S.; Moghadasi, R.; Yazdani, S.; Mohammadinejad, A. The Economic Impact of Climate Change on Agricultural Crops Yield in Khuzestan (Case study: Wheat, Barley, and Rice). Eur. Online J. Nat. Soc. Sci. Proc. 2016, 4, 2254. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Chandio, A.A.; Jiang, Y.; Amin, A.; Akram, W.; Ozturk, I.; Sinha, A.; Ahmad, F. Modeling the impact of climatic and non-climatic factors on cereal production: Evidence from Indian agricultural sector. Environ. Sci. Pollut. Res. 2022, 29, 14634–14653. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Dhileepan, K.; Zalucki, M.P.; Adkins, S.W. Biological control under a changing climate: The efficacy of the parthenium weed stem-galling moth under an atmosphere enriched with CO2. Biol. Control 2019, 139, 104077. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Kundu, S.; Shanker, A.K.; Naik, R.P.; Vanaja, M.; Venkanna, K.; Sankar, M.; Naresh, K. Continuous cropping under elevated CO2: Differential effects on C4 and C3 crops, soil properties and carbon dynamics in semi-arid alfisols. Agric. Ecosyst. Environ. 2016, 218, 73–86. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate Change Regulated Abiotic Stress Mechanisms in Plants: A Comprehensive Review; Springer: Berlin/Heidelberg, Germany, 2022; Volume 41, ISBN 0029902102759. [Google Scholar]
- Chen, Y.; Wei, Z.; Wan, H.; Zhang, J.; Liu, J.; Liu, F. CO2 Elevation and Nitrogen Supply Alter the Growth and Physiological Responses of Tomato and Barley Plants to Drought Stress. Agronomy 2022, 12, 1821. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The Impact of Drought Stress on Soil Microbial Community, Enzyme Activities and Plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of Plant Responses to Water Stress and Related Genes: A Review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Amare, K.; Mashilla, D.; Alex Albert, V.; Firew, M. Saved barley (Hordeum vulgare) seed quality in mid-altitudes and high-lands of Southern Ethiopia. Afr. J. Agric. Res. 2014, 9, 448–454. [Google Scholar] [CrossRef]
- Kreszies, T.; Shellakkutti, N.; Osthoff, A.; Yu, P.; Baldauf, J.A.; Zeisler-Diehl, V.V.; Ranathunge, K.; Hochholdinger, F.; Schreiber, L. Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: Analysis of chemical, transcriptomic and physiological responses. New Phytol. 2019, 221, 180–194. [Google Scholar] [CrossRef]
- Feiziasl, V.; Jafarzadeh, J.; Sadeghzadeh, B.; Mousavi Shalmani, M.A. Water deficit index to evaluate water stress status and drought tolerance of rainfed barley genotypes in cold semi-arid area of Iran. Agric. Water Manag. 2022, 262, 107395. [Google Scholar] [CrossRef]
- Miralles, D.J.; Abeledo, L.G.; Prado, S.A.; Chenu, K.; Serrago, R.A.; Savin, R. Barley. In Crop Physiology Case Histories for Major Crops; Elsevier: Amsterdam, The Netherlands, 2021; pp. 164–195. [Google Scholar]
- Munaweera, T.I.K.; Jayawardana, N.U.; Rajaratnam, R.; Dissanayake, N. Modern plant biotechnology as a strategy in addressing climate change and attaining food security. Agric. Food Secur. 2022, 11, 26. [Google Scholar] [CrossRef]
- Bardehji, S.; Eshghizadeh, H.R.; Zahedi, M.; Sabzalian, M.R.; Gheisari, M. The combined effect of nitrogen fertilizer and sowing season on response to water-limited stress in barley (Hordeum vulgare L.). J. Agric. Sci. 2021, 159, 31–49. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Techniques for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembrane. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified Thiobarbituric Acid Assay for Measuring Lipid Oxidation in Sugar-Rich Plant Tissue Extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Nematpour, A.; Eshghizadeh, H.R.; Abraheh, M. Interactive effects of CO2 and nitrogen supply on growth and physiological traits of millet cultivars under drought stress. Arch. Agron. Soil Sci. 2019, 12, 1476–3567. [Google Scholar] [CrossRef]
- Aebi, H.I. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1983; Volume 105, pp. 673–686. [Google Scholar]
- Nakano, Y.; Asada, K. APX Nakano & Asada 1981.pdf. Plant Cell Physiol. 2018, 22, 867–880. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Sabouri, A.; Dadras, A.R.; Azari, M.; Saberi Kouchesfahani, A.; Taslimi, M.; Jalalifar, R. Screening of rice drought-tolerant lines by introducing a new composite selection index and competitive with multivariate methods. Sci. Rep. 2022, 12, 2163. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Jogaiah, S.; Burritt, D.J.; Tran, L.S.P. Legume genetic resources and transcriptome dynamics under abiotic stress conditions. Plant Cell Environ. 2018, 41, 1972–1983. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. Clim. Chang. Plant Abiotic Stress Toler. 2013, 9, 209–250. [Google Scholar]
- Leakey, A.D.B.; Uribelarreà, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Sharkey, T.D.; Loreto, F. Formation of Reactive Oxygen Species; Landes Bioscience: Austin, TX, USA, 2012; pp. 1–3. [Google Scholar]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 479. [Google Scholar] [CrossRef]
- Valero-Galván, J.; González-Fernández, R.; Navarro-Cerrillo, R.M.; Gil-Pelegrín, E.; Jorrín-Novo, J.V. Physiological and proteomic analyses of drought stress response in Holm oak provenances. J. Proteome Res. 2013, 12, 5110–5123. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Rahman, M.S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Zare, S.; Mirlohi, A.; Sabzalian, M.R.; Saeidi, G.; Koçak, M.Z.; Hano, C. Water Stress and Seed Color Interacting to Impact Seed and Oil Yield, Protein, Mucilage, and Secoisolariciresinol Diglucoside Content in Cultivated Flax (Linum usitatissimum L.). Plants 2023, 12, 1632. [Google Scholar] [CrossRef]
- Vasilaki, C.; Katsileros, A.; Doulfi, D.; Karamanos, A.; Economou, G. Evaluation of seven barley genotypes under water stress conditions. Agron. Res. 2022, 20, 1–14. [Google Scholar]

| Country | Pedigree | Number of Rows | Genotype |
|---|---|---|---|
| Iran | Rhn-03//L.527/NK1272 | 6 | Goharan |
| Iran | Roho/Mazorka/Trompilo | 6 | Mehr |
| Iran | Novosadski-444 | 2 | Behrokh |
| Iran | SLB44-56/Lignee131 | 2 | M9316 |
| S.O.V | Mean Square | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DF | ShDW | RDW | LA | GW | RWC | Chla | Chlb | Car | FV/Fm | Proline | MDA | CAT | APX | POX | DPPH | CSI | YSI | |
| CO2 | 1 | 2.49 ** | 0.492 ** | 5.76 ** | 3.46 ** | 1829 ** | 0.008 ns | 0.021 * | 0.017 * | 0.001 ns | 115 ** | 78.4 ** | 0.273 ** | 0.001 ** | 1.28 ** | 0.002 ns | 15.5 ** | 3.011 ** |
| Rep*CO2 | 4 | 0.032 | 0.005 | 0.81 | 0.042 | 31.07 | 0.013 | 0.016 | 0.001 | 0.001 | 2.89 | 0.192 | 0.006 | 0 | 0.044 | 0.002 | 0.066 | 0.021 |
| Irrigation (I) | 1 | 7.35 ** | 0.127 ** | 47.9 ** | 4.61 ** | 1806 ** | 2.37 ** | 0.021 ** | 0.212 ** | 0.000 ns | 8413 ** | 718 ** | 0.563 ** | 0.003 ** | 0.053 ns | 0.144 ** | - | - |
| Genotype (G) | 3 | 1.49 ** | 0.250 ** | 6.95 ** | 0.387 ** | 136 ns | 0.414 ** | 0.033 ** | 0.061 ** | 0.004 ** | 601 ** | 19.2 ** | 0.026 ** | 0.001 ** | 0.830 ** | 0.142 ** | 1.91 ** | 0.202 ** |
| I*G | 3 | 0.776 ** | 0.086 ** | 4.79 ** | 0.806 ** | 78.7 ns | 0.190 ** | 0.025 ** | 0.149 ** | 0.001 ns | 528 ** | 28.1 ** | 0.003 ns | 0.000 ns | 0.446 ** | 0.054 ** | - | - |
| I*CO2 | 1 | 1.14 ** | 0.047 ** | 11.9 ** | 0.106 * | 3.42 ns | 0.042 * | 0.026 ** | 0.001 ns | 0.000 ns | 135 ** | 17.3 ** | 0.004 ns | 0.000 ns | 2.53 ** | 0.007 ns | - | - |
| G*CO2 | 3 | 0.183 ** | 0.040 ** | 0.434 ns | 0.085 ** | 156 ns | 0.092 ** | 0.011 * | 0.017 * | 0.003 * | 14.4 * | 2.40 * | 0.021 ** | 0.000 * | 0.091 ** | 0.008 ns | 0.897 ** | 0.112 ** |
| I*G*CO2 | 3 | 0.067 ns | 0.004 ns | 1.92 ns | 0.301 ** | 26.6 ns | 0.049 ** | 0.003 | 0.011 * | 0.002 ns | 45.9 ** | 10.6 ** | 0.030 ** | 0.002 ** | 0.299 ** | 0.027 ** | - | - |
| Error | 28 | 0.04 | 0.006 | 0.683 | 0.017 | 55.07 | 0.008 | 0.004 | 0.003 | 0.001 | 4.79 | 0.797 | 0.002 | 0 | 0.012 | 0.002 | 0.086 #12 | 0.012 #12 |
| CV | 12.2 | 16.4 | 12.2 | 11.5 | 9.38 | 11.8 | 9.3 | 14.6 | 4.1 | 15 | 7.7 | 13.1 | 11.1 | 10.2 | 12.2 | 9.6 | 19.7 | |
| MC | Control | Stress | LSD (0.05) | |||
|---|---|---|---|---|---|---|
| CO2 | Ambient | Elevated | Ambient | Elevated | ||
| T | ||||||
| ShDW | 1.56 b | 2.32 a | 1.08 d | 1.23 c | 0.14 | |
| RDW | 0.51 b | 0.64 a | 0.28 c | 0.55 b | 0.03 | |
| LA | 6.10 b | 7.78 a | 4.79 c | 5.09 c | 0.22 | |
| GW | 1.10 b | 1.73 a | 0.57 c | 1.02 b | 0.23 | |
| Chla | 0.55 c | 0.63 b | 1.06 a | 1.02 a | 0.05 | |
| Chlb | 0.25 c | 0.25 c | 0.34 b | 0.43 a | 0.31 | |
| Proline | 1.47 c | 1.21 c | 31.01 a | 24.61 b | 3.17 | |
| MDA | 8.28 c | 6.93 d | 17.02 a | 13.50 b | 1.11 | |
| POX | 1.05 c | 1.19 b | 1.45 a | 0.66 d | 0.12 | |
| CO2 | Ambient | LSD (0.05) | Elevated | LSD (0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G | Behrokh | M9316 | Goharan | Mehr | Behrokh | M9316 | Goharan | Mehr | |||
| T | |||||||||||
| ShDW | 1.11 c | 1.65 a | 1.28 b | 1.23 bc | 0.14 | 1.34 c | 2.44 a | 1.59 b | 1.72 b | 0.25 | |
| RDW | 0.61 a | 0.31 bc | 0.29 c | 0.37 b | 0.12 | 0.74 a | 0.61 b | 0.36 c | 0.67 ab | 0.10 | |
| GW | 0.76 c | 0.98 a | 0.77 c | 0.81 b | 0.03 | 1.14 c | 1.71 a | 1.20 c | 1.44 b | 0.22 | |
| Chla | 0.88 b | 0.50 d | 0.82 c | 0.99 a | 0.05 | 0.65 c | 0.67 c | 0.92 b | 1.07 a | 0.09 | |
| Chlb | 0.26 c | 0.20 d | 0.32 b | 0.39 a | 0.04 | 0.31 b | 0.31 b | 0.37 a | 0.40 a | 0.05 | |
| Car | 0.32 c | 0.23 d | 0.36 b | 0.43 a | 0.03 | 0.38 c | 0.35 c | 0.40 b | 0.49 a | 0.06 | |
| Fv/Fm | 0.71 b | 0.74 a | 0.74 a | 0.73 a | 0.02 | 0.72 ab | 0.73 ab | 0.75 a | 0.67 c | 0.06 | |
| Proline | 22.10 a | 12.11 c | 9.91 d | 20.42 b | 1.21 | 21.01 a | 6.93 c | 5.14 d | 18.91 b | 1.02 | |
| MDA | 13.91 a | 10.90 c | 12.72 b | 13.90 a | 0.65 | 11.60 a | 10.67 a | 8.95 b | 9.12 b | 0.98 | |
| CAT | 0.43 b | 0.38 c | 0.53 a | 0.40 bc | 0.04 | 0.26 b | 0.36 a | 0.29 b | 0.23 c | 0.04 | |
| APX | 0.04 b | 0.03 c | 0.04 b | 0.06 a | 0.01 | 0.02 b | 0.03 a | 0.03 a | 0.02 b | 0.009 | |
| POX | 1.58 a | 1.03 b | 0.88 c | 1.51 a | 0.11 | 1.01 a | 0.97 a | 0.71 c | 0.84 b | 0.12 | |
| MC | Control | LSD (0.05) | Stress | LSD (0.05) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G | Behrokh | M9316 | Goharan | Mehr | Behrokh | M9316 | Goharan | Mehr | |||
| T | |||||||||||
| ShDW | 1.43 d | 1.59 c | 2.60 a | 2.14 b | 0.139 | 1.23 b | 1.50 a | 1.28 b | 0.81 d | 0.177 | |
| RDW | 0.82 a | 0.53 b | 0.39 c | 0.58 b | 0.125 | 0.53 a | 0.39 c | 0.26 d | 0.46 b | 0.210 | |
| LA | 5.07 c | 7.08 b | 8.25 a | 7.35 b | 0.741 | 4.84 b | 5.33 a | 5.11 a | 4.47 b | 0.674 | |
| GW | 0.96 c | 1.24 b | 1.68 a | 1.77 a | 0.461 | 0.84 b | 1.02 a | 0.72 b | 0.48 c | 0.166 | |
| Chla | 0.72 a | 0.37 c | 0.52 b | 0.76 a | 0.140 | 0.82 b | 0.81 b | 1.22 a | 1.30 a | 0.246 | |
| Chlb | 0.28 a | 0.20 c | 0.26 b | 0.26 b | 0.011 | 0.29 b | 0.32 b | 0.42 a | 0.49 a | 0.094 | |
| Car | 0.30 a | 0.21 b | 0.21 b | 0.32 a | 0.072 | 0.39 b | 0.57 a | 0.34 b | 0.60 a | 0.194 | |
| Proline | 1.67 a | 1.155 b | 0.710 c | 1.83 a | 0.337 | 33.40 b | 41.30 a | 13.91 d | 30.51 c | 3.058 | |
| MDA | 6.72 c | 6.19 c | 7.10 b | 10.4 a | 1.25 | 18.42 a | 15.80 b | 12.50 d | 14.60 c | 1.832 | |
| POX | 1.12 a | 0.82 b | 0.65 c | 1.11 a | 0.110 | 1.40 b | 1.61 a | 0.94 d | 1.21 c | 0.170 | |
| DPPH | 0.27 b | 0.26 c | 0.21 d | 0.30 a | 0.003 | 0.50 b | 0.57 a | 0.28 d | 0.35 c | 0.012 | |
| ShDW | RDW | GW | LA | RWC | Chla | Chlb | Car | Fv/Fm | Proline | MDA | CAT | APX | POX | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ShDW | −0.34 | 0.65 ** | 0.55 | 0.61 * | −0.33 | −0.60 * | −0.21 | 0.42 | −0.79 ** | −0.23 | −0.03 | −0.11 | −0.58 * | −0.19 | |
| RDW | −0.49 | 0.06 | 0.28 | −0.65 * | 0.05 | −0.31 | −0.45 | −0.61 * | 0.67 * | 0.60 * | −0.42 | 0.12 | 0.30 | −0.54 | |
| GW | 0.80 ** | −0.31 | 0.56 | 0.00 | 0.07 | −0.40 | −0.04 | 0.03 | −0.41 | 0.32 | 0.15 | 0.32 | −0.58 * | −0.66 * | |
| LA | 0.45 | −0.71 ** | 0.42 | 0.33 | −0.11 | −0.61 * | −0.24 | −0.01 | −0.39 | 0.12 | 0.09 | −0.05 | −0.54 | −0.69 * | |
| RWC | −0.46 | 0.45 | −0.41 | −0.27 | −0.38 | −0.28 | 0.03 | 0.52 | −0.73 ** | −0.57 * | 0.20 | −0.43 | −0.43 | 0.22 | |
| Chla | −0.54 | 0.62 * | −0.25 | −0.67 * | −0.06 | 0.74 ** | 0.81 ** | 0.02 | 0.20 | 0.56 | 0.54 | 0.50 | −0.32 | −0.38 | |
| Chlb | −0.58 * | 0.19 | −0.42 | −0.35 | 0.01 | 0.64 * | 0.85 ** | 0.18 | 0.21 | 0.14 | 0.57 * | 0.25 | −0.02 | 0.22 | |
| Car | −0.41 | 0.52 | −0.08 | −0.52 | −0.09 | 0.93 ** | 0.71 ** | 0.34 | −0.12 | 0.20 | 0.78 ** | 0.34 | −0.45 | −0.06 | |
| Fv/Fm | 0.36 | −0.30 | 0.27 | 0.39 | −0.18 | −0.11 | −0.12 | −0.03 | −0.73 ** | −0.51 | 0.28 | −0.18 | −0.66 * | 0.19 | |
| Proline | −0.33 | 0.61 * | −0.07 | −0.62 * | −0.11 | 0.86 ** | 0.55 | 0.92 ** | −0.26 | 0.53 | −0.37 | 0.31 | 0.67 * | −0.04 | |
| MDA | 0.05 | −0.18 | 0.29 | −0.02 | −0.63 * | 0.41 | 0.10 | 0.46 | −0.01 | 0.49 | 0.17 | 0.47 | −0.07 | −0.75 ** | |
| CAT | −0.64 ** | 0.02 | −0.76 ** | −0.02 | 0.62 * | −0.14 | 0.36 | −0.11 | −0.12 | −0.19 | −0.46 | −0.02 | −0.53 | −0.25 | |
| APX | −0.29 | −0.07 | −0.01 | −0.32 | −0.36 | 0.60 * | 0.38 | 0.54 | −0.21 | 0.50 | 0.81 ** | −0.25 | −0.22 | −0.35 | |
| POX | −0.38 | 0.57 * | −0.16 | −0.76 ** | −0.10 | 0.89 ** | 0.36 | 0.80 ** | −0.07 | 0.82 ** | 0.56 | −0.26 | 0.67 * | 0.54 | |
| DPPH | −0.19 | 0.22 | 0.03 | −0.46 | −0.28 | 0.79 ** | 0.45 | 0.82 ** | 0.16 | 0.75 ** | 0.73 ** | −0.30 | 0.74 ** | 0.88 ** |
| ShDW | RDW | GW | LA | RWC | Chla | Chlb | Car | Fv/Fm | Proline | MDA | CAT | APX | POX | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ShDW | −0.25 | 0.80 ** | 0.59 * | −0.35 | −0.28 | −0.18 | −0.55 | 0.64 * | −0.66 * | −0.15 | −0.04 | −0.48 | −0.03 | 0.31 | |
| RDW | −0.08 | 0.28 | 0.20 | 0.05 | −0.28 | −0.38 | 0.05 | −0.36 | 0.72 ** | 0.47 | −0.46 | 0.16 | −0.68 ** | 0.48 | |
| GW | 0.79 ** | −0.23 | 0.55 | −0.40 | −0.58 * | −0.49 | −0.66 * | 0.50 | −0.23 | 0.27 | −0.25 | −0.53 | −0.35 | 0.52 | |
| LA | 0.77 ** | −0.38 | 0.82 ** | 0.06 | −0.19 | −0.03 | −0.18 | 0.30 | −0.30 | −0.07 | −0.22 | −0.07 | −0.19 | 0.48 | |
| RWC | −0.54 | 0.03 | −0.43 | −0.52 | −0.07 | 0.00 | 0.20 | 0.12 | 0.31 | 0.02 | −0.14 | −0.01 | 0.27 | −0.21 | |
| Chla | −0.55 | 0.24 | −0.09 | −0.35 | 0.53 | 0.81 ** | 0.80 ** | −0.57 * | −0.26 | −0.82 ** | 0.16 | 0.84 ** | 0.00 | −0.01 | |
| Chlb | −0.41 | 0.29 | −0.20 | −0.06 | −0.09 | 0.23 | 0.58 * | −0.43 | −0.45 | −0.85 ** | 0.09 | 0.67 * | 0.30 | 0.05 | |
| Car | −0.10 | 0.69 * | −0.02 | −0.21 | 0.38 | 0.43 | 0.27 | −0.67 * | 0.19 | −0.50 | 0.11 | 0.89 ** | 0.10 | −0.26 | |
| Fv/Fm | −0.24 | −0.19 | −0.26 | −0.20 | 0.30 | −0.24 | 0.23 | −0.10 | −0.40 | 0.14 | −0.02 | −0.75 ** | 0.29 | −0.05 | |
| Proline | −0.61 * | −0.04 | −0.08 | −0.20 | 0.43 | 0.84 ** | 0.38 | 0.32 | 0.07 | 0.59 * | −0.04 | 0.16 | −0.33 | −0.08 | |
| MDA | 0.05 | 0.00 | 0.50 | 0.15 | 0.29 | 0.75 ** | 0.00 | 0.27 | −0.33 | 0.53 | −0.12 | −0.59 * | −0.19 | −0.15 | |
| CAT | 0.33 | 0.14 | 0.67 * | 0.38 | 0.12 | 0.59 * | −0.12 | 0.40 | −0.43 | 0.40 | 0.90 ** | 0.16 | 0.35 | −0.34 | |
| APX | −0.73 ** | 0.07 | −0.92 ** | −0.64 * | 0.18 | −0.09 | 0.24 | −0.14 | 0.26 | 0.03 | −0.69 * | −0.82 ** | −0.16 | 0.12 | |
| POX | 0.24 | 0.66 * | 0.33 | −0.04 | −0.01 | 0.41 | −0.07 | 0.61 * | −0.57 * | 0.02 | 0.54 | 0.65 * | −0.50 | −0.74 ** | |
| DPPH | 0.63* | 0.24 | 0.80 ** | 0.53 | −0.15 | 0.23 | −0.19 | 0.29 | −0.42 | −0.02 | 0.69 * | 0.88 ** | −0.89 ** | 0.73 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardehji, S.; Soltan, S.; Eshghizadeh, H.R.; Zahedi, M.; Zare, S.; Koçak, M.Z.; Nouraein, M.; Vita, F.; Vergine, M. Responses of Two-Row and Six-Row Barley Genotypes to Elevated Carbon Dioxide Concentration and Water Stress. Agronomy 2023, 13, 2373. https://doi.org/10.3390/agronomy13092373
Bardehji S, Soltan S, Eshghizadeh HR, Zahedi M, Zare S, Koçak MZ, Nouraein M, Vita F, Vergine M. Responses of Two-Row and Six-Row Barley Genotypes to Elevated Carbon Dioxide Concentration and Water Stress. Agronomy. 2023; 13(9):2373. https://doi.org/10.3390/agronomy13092373
Chicago/Turabian StyleBardehji, Siavash, Saba Soltan, Hamid Reza Eshghizadeh, Morteza Zahedi, Sara Zare, Mehmet Zeki Koçak, Mojtaba Nouraein, Federico Vita, and Marzia Vergine. 2023. "Responses of Two-Row and Six-Row Barley Genotypes to Elevated Carbon Dioxide Concentration and Water Stress" Agronomy 13, no. 9: 2373. https://doi.org/10.3390/agronomy13092373
APA StyleBardehji, S., Soltan, S., Eshghizadeh, H. R., Zahedi, M., Zare, S., Koçak, M. Z., Nouraein, M., Vita, F., & Vergine, M. (2023). Responses of Two-Row and Six-Row Barley Genotypes to Elevated Carbon Dioxide Concentration and Water Stress. Agronomy, 13(9), 2373. https://doi.org/10.3390/agronomy13092373









