Abstract
Throughout the world, salinity is a major environmental issue that limits agricultural productivity, particularly in arid and semi-arid regions. In addition, climate change is the most important reason for the salinization of agricultural soils in the world, so it is now essential to find solutions to increase salinity tolerance in plants. This study investigated the potential of arbuscular mycorrhizal fungi (AMF) inoculation to enhance the growth and yield performances of flax under different salinity levels by conducting a pot experiment. The experiment was laid out in a two-factor completely randomized design including AMF inoculation (AMF+: with inoculation; AMF−: without inoculation) and irrigation water salinity (0, 50, 100, and 150 mM NaCl). According to the results, it is evident that salt stress caused negative physiological effects, including limited growth, reduced photosynthesis, and decreased nitrogen (N) and phosphorus (P) content in the shoots and roots of flax plants. Moreover, mycorrhizal association improved the salt tolerance of the plants by increasing chlorophyll content, and enhancing N and P shoot and root contents and consequently yield parameters, such as seed and stem fiber yield, particularly at moderate salt concentrations (50 and 100 mM NaCl). In particular, under 100 mM, AMF increased the total chlorophyll content, N shoot and root content, P shoot and root content, and seed and stem fiber yield by 30.4%, 36.1%, 31.0%, 38.9%, 45.4%, 35.2%, and 26.9%, respectively. As a result of using AMF, flax plants grown under salt stress exhibited tolerance, suggesting that AMF could be applied in saline environments to maintain ecological stability.
1. Introduction
Flax (Linum usitatissimum L.) is one of about 230 species in the Linaceae family, and it has considerable potential due to its versatile uses and adaptability [1,2]. In spite of its origins in the Mediterranean region and southwestern Asia, its ability to adapt to differing climatic conditions has enabled its cultivation throughout the Middle East, India, Canada, and several European countries [2,3]. As a multipurpose crop, flax is cultivated either for its fiber or for its seeds and the oil produced from them [2]. Fibers from flax have been considerably used for a variety of industrial applications, ranging from dressing fabrics and bedsheets to twine and ropes [4]. A variety of high-quality textiles (also known as linen fabrics), such as damasks and lace, are made from best grades of flax fibers [4,5]. Additionally, these fibers are used in the paper industry and, interestingly, in the production of banknotes [6]. It is notable that flax seeds contain a variety of bioactive compounds, including alpha-linolenic acid (ALA), proteins, and lignans, which make them a valuable source of nutrition for both humans and animals [7,8]. Flaxseeds can be consumed as whole grains or as ground powders [9] and are high in oil (40%) [5,10]. The oil is edible and highly nutritious, as well as having a pleasant taste and aroma [11,12]. It comprises a high percentage of linolenic acid (from 48.5% to 68.5%), a low percentage of saturated fatty acids, and a high percentage of omega-3 and omega-6 fatty acids [5,12].
Throughout the world, salinity is a major environmental issue that limits agricultural productivity, particularly in arid and semi-arid regions [13]. There are two main reasons for the detrimental effects of sodium chloride (NaCl) on plants: the reduced availability of water, caused by sodium accumulation in the soil, reducing soil water potential and the toxic effects of sodium and chlorine ions on plant. In response to reduced nutrient and water uptake, osmotic stress, ion toxicity, and nutritional imbalances result in significantly reduced plant growth and crop production [14,15,16]. High-salinity conditions adversely affect plant growth and photosynthesis as a result of elevated ethylene levels in roots, ionic imbalance, and hyperosmotic conditions [16,17]. Salt accumulation in the soil decreases the osmotic potential, thereby interfering with the absorption of water by roots and affecting cell growth and metabolism [16]. Moreover, high salinity levels decrease the growth of plants by causing oxidative damage to them as a result of the formation of reactive oxygen species (ROS) [14].
Aside from employing some intrinsic mechanisms of adaptation, plants exposed to soil salinity can enhance their tolerance by developing mutualistic relationships with the microorganisms living in the rhizosphere [18,19]. The arbuscular mycorrhizal fungi (AMF) are among the soil microorganisms that have symbiotic relationships with the majority of terrestrial plants [20]. Specifically, AMF have been proven to enhance salt tolerance in host plants by improving nutrient uptake, and increasing chlorophyll synthesis as well as enhancing the enzyme activity of protection systems [21,22]. It is estimated that 70–90% of terrestrial plant species are associated with these beneficial microorganisms in soil [23]. These organisms play an important role in ecosystems by providing water and cycling nutrients [23,24], both of which have a significant impact on carbon, nitrogen, and phosphorus biogeochemical cycles [25]. It is well known that AMF are able to take up nutrients from the soil and then transfer them to the host plants as compensation for photosynthetically fixed carbon [26,27]. Furthermore, 5 to 10% of the photosynthetically active compounds of host plants are assigned to the AMF partner [28], and when soil phosphorus levels are low, roots and AMF transfer more carbon and phosphorus to each other [23,29]. Under drought conditions, AM fungi can absorb and supply more water and nitrogen to their host plants via their hyphae [27]. The presence of AMF increases the tolerance of their host plants to biotic and abiotic stresses, such as drought, heavy metals and salinity [30,31]. AMF can help plants tolerate salt stress by causing increased plant biomass, which consequently leads to a dilution of Na+ and Cl− compared with non-mycorrhizal plants [16]. Additionally, the AMF colonization of roots assists plants in absorbing more water and nutrients under saline conditions [31]. In general, the plant–mycorrhizal fungi symbiotic interaction has been demonstrated to increase plant yield and help plants resist several biotic and abiotic stresses, including salinity in agriculture [32], making the symbiotic relationship among plants and mycorrhizal fungi agriculturally and ecologically important [33,34].
Climate change is the most important reason for the salinization of agricultural soils in the world, so it is now essential to find solutions to increase salinity tolerance in plants. Despite the significant industrial and nutritional importance of flax, limited research has been conducted on the effect of salinity induced by different salts on plant growth and performance in this plant species, especially on its physiological and biochemical characteristics. As AMF have been shown to ameliorate tolerance to salt stress in several plant species, the present work aimed to determine the potential of AMF inoculation to improve the growth parameters (plant height, leaf area per plant, and root and shoot biomass allocation), AMF root colonization, physiological and biochemical parameters (leaf relative water content, total chlorophyll content, soil plant analysis development (SPAD) and normalized difference vegetation index (NDVI) readings, total proline content), and yield parameters (seed, biological and stem fiber yield) of flax under different salinity levels (0, 50, 100 and 150 mM NaCl) by conducting a pot experiment.
2. Materials and Methods
2.1. Experimental Setup, Plant Materials, and Growth Conditions
A greenhouse pot experiment was conducted in 2023 (from January to May) at the Agricultural University of Athens (latitude 37°59′ N, longitude 23°42′ E, 30 m from the sea surface), Greece, using a fiber flax cultivar (Linum usitatissimum L. cv. Eden) in order to determine the effect of inoculation with an introduced AM fungal inoculum under the influence of irrigation water salinity. The average lowest and highest recorded temperatures during the experiment were 13.1 °C and 35.9 °C, respectively (average 15.6 °C), whereas the relative humidity (RH) ranged from 42% to 84% (average 72%). In addition, the plants were subjected to a natural daylight cycle ranging between 10 and 14 h during the current study.
The experiment was conducted in a two-factor completely randomized design including AMF inoculation and irrigation water salinity. Mycorrhizal inoculation included two levels: control treatment without inoculation (AMF−) and inoculation with the AM inoculum (AMF+). The inoculum used was the commercial microgranulated-based root inoculant product Micoseeds Plus (Microspore Hellas–Sacom Hellas, Athens, Greece), which is based on microorganisms that predominantly contain arbuscular mycorrhizal fungi (AMF) spores of Claroideoglomus etunicatum, Funneliformis mosseae, Glomus aggregatum and Rhizophagus intraradices. In addition to the aforementioned AMF spores, there are fungi and bacterial species belonging to the genera Trichoderma, Streptomyces, Bacillus and Pseudomonas. The inoculation dosage was 2 g of inoculum per plant (~200 applied fungal spores) according to the recommendations of the manufacturer of the inoculant product. For the inoculation group of pots, the inoculum was put 3 cm below the surface of the soil substrate before sowing. Four levels of water salinity were applied (according to the screening results of the preliminary experiment): 0, 50, 100, and 150 mM NaCl. In general, a total of eight treatments were formed, with thirty pots planted in each treatment.
The seeds were sown directly 2 cm deep in 7 L plastic pots, having a 33 cm inner diameter and 21 cm depth, and containing 3 kg of homogenous mixture (2:1:1) of clay loam soil (non-sterilized), peat moss (sterilized) and perlite (sterilized). The soil was collected from the experimental field next to the greenhouse. Some physicochemical properties of the substate used are presented in Table 1. Five seeds were sown in each pot on 17 January 2023, and seedlings were thinned to two per pot after emergence, which was on 26 January 2023. Water salinity treatments were started at 20 days after sowing (DAS). For the salinity groups, in order to avoid osmotic shock, each group was treated with different concentrations of NaCl solution (10 mM, 20 mM, or 30 mM) every 2 days until reaching the final concentration, with 5 replicates. As for the control (0 mM NaCl) group, it was treated with sterilized water. According to the pre-experiment, treatments of 50 and 100 mM NaCl water-salinity levels were set as moderate concentrations in the present research study.

Table 1.
Physicochemical characteristics of the soil substrate.
The traits that were taken into account in order to assess the flax plants were the plant growth parameters and biomass allocation, AMF root colonization, leaf relative water content, total chlorophyll content, SPAD and NDVI readings, total proline content, nitrogen (N) and phosphorus (P) absorption and distribution, seed yield, biological yield, harvest index and stem fiber yield.
2.2. Growth Parameters and AMF Root Colonization
The plant growth parameters and biomass allocation were measured using twenty plant samples from each treatment at 90 DAS. Specifically, the following plant growth parameters were evaluated: the plant height, leaf area per plant, and the fresh and dry weight of the plant aboveground parts (shoot) and roots. For the determination of their dry weight, the samples were oven-dried for 72 h at 65 °C. The leaf area per plant was measured by placing the collected samples on an Epson Perfection V330 Photo Scanner (Seiko Epson Inc., Nagano-ken, Japan) using Delta–T software (Delta–T Scan ver. 2.04; Delta–T Devices Ltd., Burwell, Cambridge, UK). The biomass allocation of each part was calculated according to the following formula: root to shoot ratio = underground biomass/aboveground biomass. Moreover, the arbuscular mycorrhizal fungi (AMF) colonization rate in roots for each plant sample was also determined microscopically using the gridline-intersection method at 30–40× magnification [35], a technique that involves cleaning roots in 10% (w/v) KOH for 10 min at 90 °C and staining them with lactophenol containing 0.05% (w/v) trypan blue [36].
2.3. Physiological and Biochemical Parameters
The leaf relative water content was determined at 90 DAS utilizing the method of Yamasaki and Dillenburg [37]. Specifically, for each treatment, a total of 100 mg of fresh leaf samples were placed in Petri dishes containing distilled water for four hours at room temperature (23 °C). After the samples were taken out and dried off, the turgor weight (TW) was also recorded, after which the samples were dried in an oven at 70 °C overnight. The dry weight (DW) was then measured. The relative water content was calculated using the following equation:
where FW is the fresh weight of the leaf tissue.
RWC (%) = [(FW − DW)/(TW − DW)] × 100
For the estimation of total chlorophyll content, fresh leaf samples collected at 90 DAS from the youngest fully expanded leaves were carefully divided into tiny pieces (about 0.1 g), ground to a powder in 10 mL of 80% acetone and also centrifuged for five minutes at 10,000 rpm. The process was reiterated after collecting the supernatant until the remainder had no color. The absorbance was read at 645 and 663 nm using a near-infra-red spectrometer (Rapid Analyzer XDS; Foss Tecator AB, Höganas, Sweden) with a spectral range from 400 to 2498 nm. The blank solution contained 80% acetone. The total chlorophyll content was determined according to the following formula [38]:
where A is the absorbance at a specific wavelength; V is the final volume of the chlorophyll content in 80% acetone; and W is the fresh weight of the leaf tissue extract.
Soil plant analysis development (SPAD) values of the three fully expanded uppermost leaves were determined at 90 DAS (anthesis stage) using SPAD 502 plus (Konica Minolta Optics Inc., Tokyo, Japan). Based on the manufacturer’s instructions, measurements were taken from the center of each leaf, avoiding the main vein. The mean value of six flax plants was considered as one replication. The normalized difference vegetation index (NDVI) of the total plant biomass in the pot was also measured at 90 DAS, by means of a Trimble GreenSeeker portable handheld spectroradiometer (Trimble Agriculture Division, Westminster, CO, USA). At each pot, the sensor head was placed 100 cm above the surface of the pot, covering the entire area of the plant. About 100 NDVI measurements were taken at each pot. In order to soil-adjust the measurements of NDVI, measurements made in pots containing only soil substrate were subtracted from measurements made in pots containing plants. The proline content was determined spectrophotometrically according to the method of Bates et al. [39]. Specifically, 500 mg fresh leaf material at 90 DAS was taken and homogenized in 10 mL of sulfosalicylic acid. To the extract, 2 mL each of glacial acetic acid and ninhydrin acid were added, and the samples were heated at 100 °C. The mixture was extracted with 4 mL toluene and the free toluene was spectrophotometrically quantified at 520 nm using L-proline as a standard.
2.4. Nitrogen and Phosphorus Distribution
To determine the total nitrogen (N) content of aerial and root biomass, dried samples collected for plant growth parameter measurements at 90 DAS were ground to a fine powder and applied to the Kjeldahl procedure using a Kjeltec 8400 autoanalyzer (Foss Tecator AB, Höganas, Sweden). The phosphorus (P) content was determined by selecting ten plant samples per treatment at 90 DAS, dissolving them in a HNO3H2O2 solution, and heating them under pressure in a CEM MDS 2000 microwave (CEM Ltd., Buckingham, UK). In order to analyze the extract, an iCAP 6500 DUO ICP-emission spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used.
2.5. Yield Parameters
Finally, the plants were harvested at full seed maturity (seed moisture 12%) on 22nd May 2023 (125 DAS). The seed and biological yields were determined using ten randomly selected plants from each treatment. The harvest index (HI) was calculated as the ratio between the seed yield and above-ground biomass (biological) yield (whole weight of plants derived for seed yield). In addition, the fiber yield per plant was determined using the method described by Khan et al. [40]. In particular, fibers were extracted using a water-retting process. For each treatment, twelve plants were taken and tied to make a bundle. Bundles were dipped into fresh water tank for 15 days until complete degradation of the cell wall material due to microbial action. After complete retting, the fibers were separated from woody material by means of an operation known as scutching.
2.6. Statistical Analysis
Data were subjected to a two-way analysis of variance (ANOVA) using SigmaPlot v.12.0 (Systat Software Inc., San Jose, CA, USA). The differences between means were separated using Tukey’s honestly significant difference (HSD) test. Correlation analyses were used to describe the relationships among all the studied measurements using Pearson’s correlation. All comparisons were made at the 5% level of significance (p ≤ 0.05).
3. Results
3.1. Growth Parameters and AMF Root Colonization
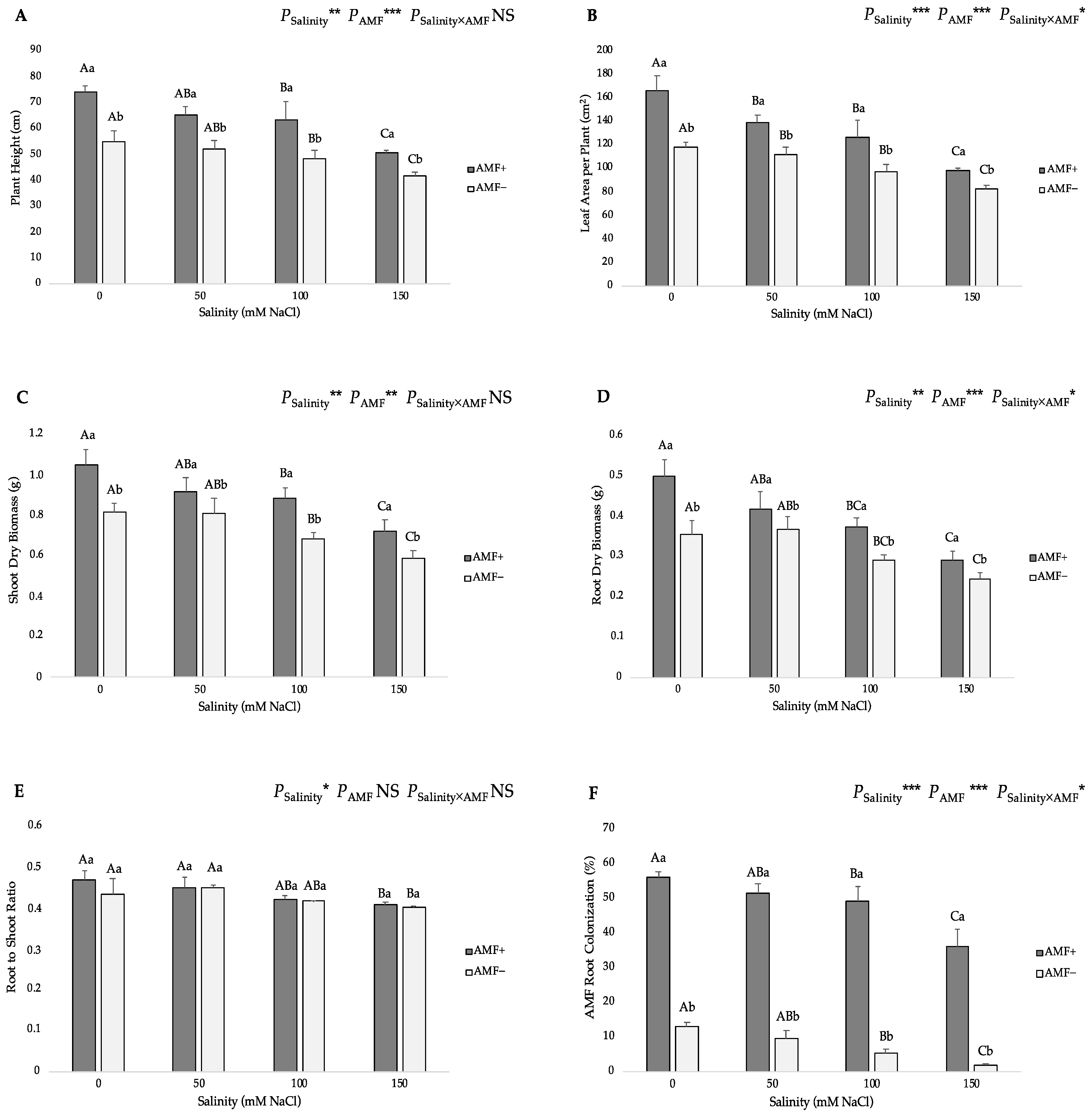
Based on the two-way ANOVA, the effects of salinity and AMF inoculation were significant on the plant height, leaf area per plant, shoot dry biomass, root dry biomass and root-to-shoot ratio, but there was no significant interactive effect on the plant height, shoot dry biomass and root-to-shoot ratio (Figure 1). Inoculation with AMF increased all the above-mentioned growth parameters under all salinity levels versus the non-inoculated plants. Although salinity reduced the values of these studied parameters in mycorrhizal plants, they were higher than those of non-inoculated plants. The highest plant height was related to the AMF-inoculated (AMF+) plants at 0 mM NaCl salinity (74.2 cm), and the lowest one (41.7 cm) was recorded at the 150 mM NaCl level in non-inoculated (AMF−) plants (Figure 1A). The leaf area per plant was higher in the AMF+ plants at the 0 mM salt level (166.25 cm2) and exhibited the lowest (82.58 cm2) value in AMF− plants under 150 mM NaCl (Figure 1B). The highest shoot and root dry biomass values were found in the AMF+ plants at the 0 mM salt level (1.04 and 0.49 g, respectively), whereas the lowest values of these traits were obtained at 150 mM NaCl in the AMF− plants (0.59 and 0.24 g for shoot and root dry biomass, respectively) (Figure 1C,D and Figure 2). As for the root-to-shoot ratio, the highest value was observed in the AMF-inoculated plants at the 0 mM salt level (0.470), whereas the lowest one (0.403) was recorded in non-inoculated plants at the 150 mM salt level; however, the differences among AMF treatments for the same salinity level were not statistically significant (Figure 1E). Consequently, the findings in the growth parameters of the present investigation support the hypothesis that AMF inoculation stimulates plant growth and development under salt stress.
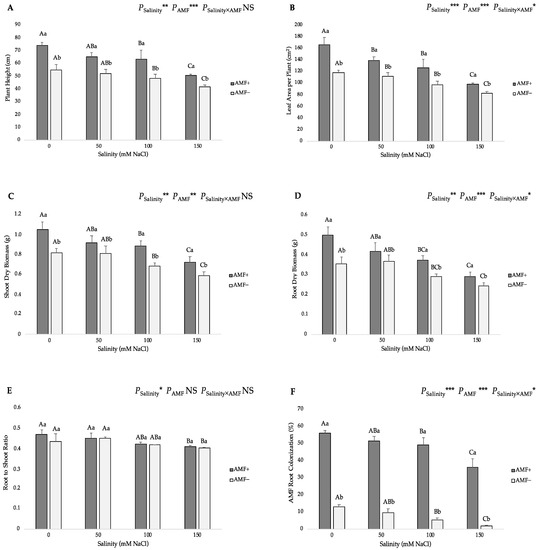
Figure 1.
Effects of arbuscular mycorrhizal fungi (AMF) inoculation on (A) plant height, (B) leaf area per plant, (C) shoot dry biomass, (D) root dry biomass, (E) root-to-shoot ratio, and (F) AMF root colonization of flax at different salinity (0, 50, 100, and 150 mM NaCl) levels. AMF+: with AM inoculum; AMF−: without inoculation. NS: No significant effect; *, ** and ***: Significant at 5%, 1% and 0.1% probability levels, respectively. Values are means ± standard error. The different capital letters indicate significant differences among different salinity levels, and the different lowercase letters for the same salinity level indicate significant differences among different AMF inoculation treatments according to the Tukey’s HSD test (p ≤ 0.05).
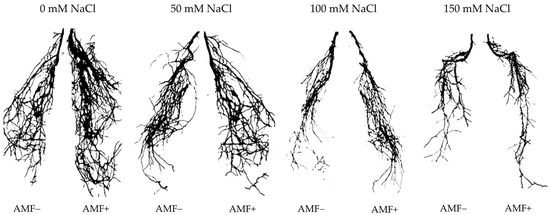
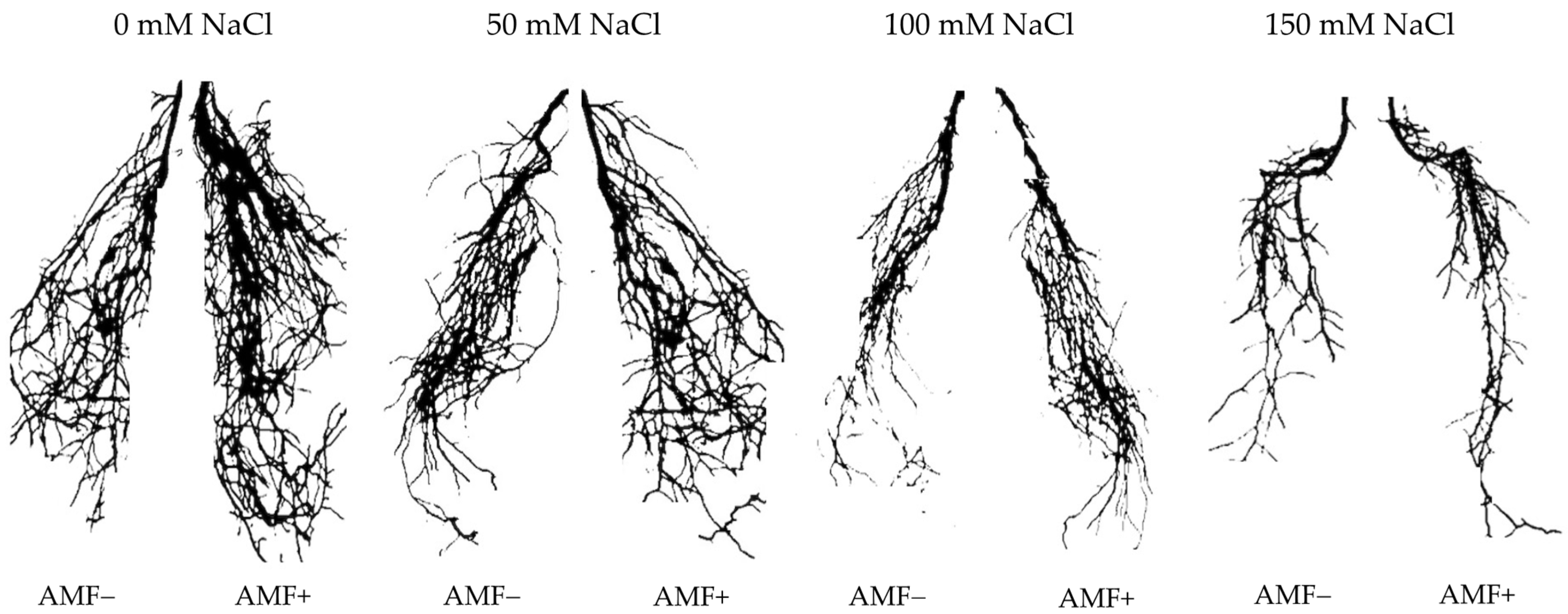
Figure 2.
Effects of arbuscular mycorrhizal fungi (AMF) inoculation on root morphology of flax at different salinity (0, 50, 100, and 150 mM NaCl) levels at 90 days after sowing (DAS). AMF+: with AM inoculum; AMF−: without inoculation. The images were taken by placing the collected root samples on an Epson Perfection V330 Photo Scanner (Seiko Epson Inc., Nagano-ken, Japan).
Salt stress caused significant declines in the AMF root colonization rate in flax plants under NaCl stress from 50 to 150 mM (Figure 1F). The percentage values of AMF root colonization in AMF-inoculated plants decreased from 56.17% (in the 0 mM NaCl treatment) to 35.93% (in the 150 mM NaCl treatment). In addition, the analysis of AMF colonization revealed that the non-inoculated plants presented the lowest mycorrhizal infection. Specifically, the AMF colonization on AMF− plants was kept below 14%, with the highest value recorded at the 0 mM salt level (13.19%), while the lowest one (1.82%) was recorded at the highest (150 mM) salt level.
3.2. Physiological and Biochemical Parameters
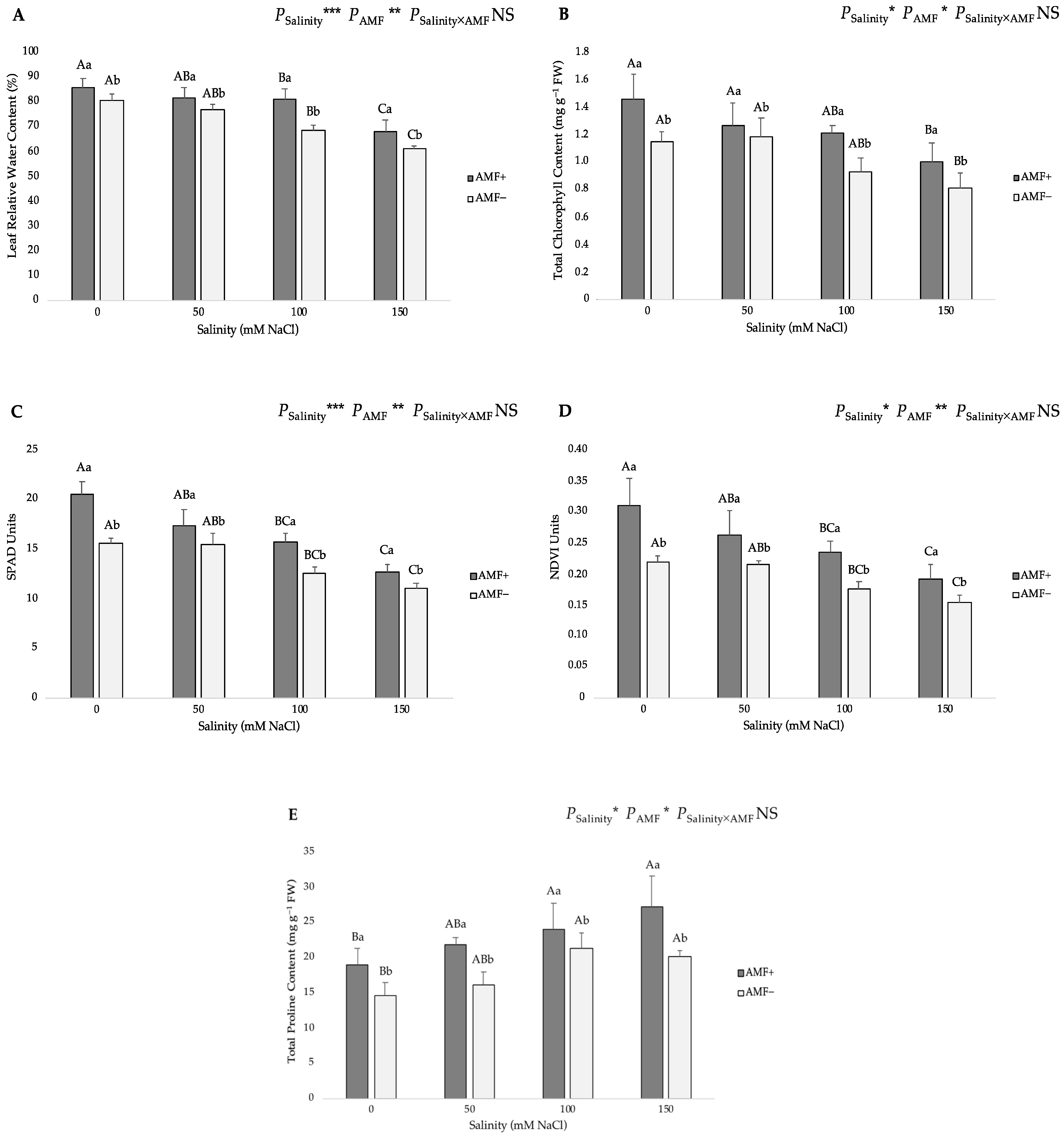
The role of the treatments on the leaf relative water content (RWC), total chlorophyll content and soil plant analysis development (SPAD) index was similar to those found for the shoot dry biomass. Increasing salinity concentrations in the irrigation water from 0 to 150 mM NaCl caused a reduction in the aforementioned traits (Figure 3). AMF inoculation also had a significant effect on these parameters. In particular, the highest RWC was related to the AMF+ plants at 0 mM NaCl salinity (86.04%), while the lowest value (61.16%) was obtained from AMF− plants at the 150 mM NaCl level (Figure 3A). The highest total chlorophyll content was recorded in the AMF+ plants at the 0 mM salt level (1.45 mg g−1 FW), and the lowest one (0.81 mg g−1 FW) was obtained from AMF− plants at 150 mM NaCl level (Figure 3B). Concerning the SPAD index, a non-destructive measurement of the chlorophyll content, the highest value was observed in the AMF+ plants at the 0 mM salt level (20.48 SPAD Units), whereas the lowest one (11.03 SPAD Units) was found in the non-AMF-inoculated plants at the 150 mM salt level (Figure 3C).
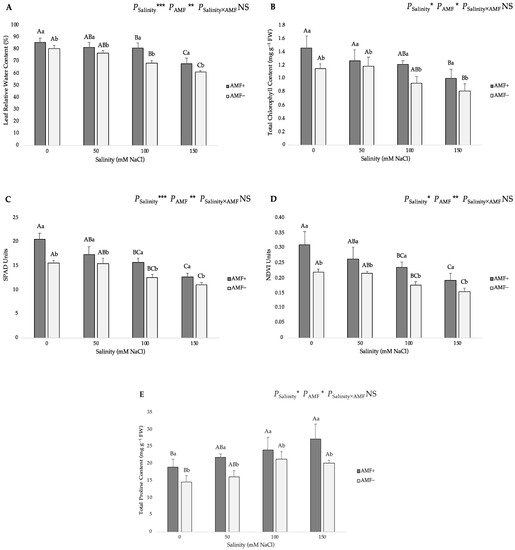
Figure 3.
Effects of arbuscular mycorrhizal fungi (AMF) inoculation on (A) leaf relative water content, (B) total chlorophyll content, (C) soil plant analysis development (SPAD), (D) natural difference vegetation index (NDVI), and (E) total proline content in leaves of flax at different salinity (0, 50, 100, and 150 mM NaCl) levels. AMF+: with AM inoculum; AMF−: without inoculation. NS: No significant effect; *, ** and ***: Significant at 5%, 1% and 0.1% probability levels, respectively. Values are means ± standard error. The different capital letters indicate significant differences among different salinity levels, and the different lowercase letters for the same salinity level indicate significant differences among different AMF inoculation treatments according to Tukey’s HSD test (p ≤ 0.05).
The natural difference vegetation index (NDVI) is a remote sensing method that uses the reflectance of light in red and near-infrared light and can be utilized to determine chlorophyll content by measuring the transmission of these light wavelengths through leaves. As expected, the NDVI index presented a similar trend to the total chlorophyll content. The highest NDVI value was observed in the AMF+ plants at the 0 mM NaCl level (0.31 NDVI Units), whereas the lowest value (0.15 NDVI Units) was obtained from AMF− plants at the 150 mM NaCl level (Figure 3D).
The pattern of total proline content in leaves showed that salinity caused a significant increase in this trait (Figure 3E). AMF inoculation significantly increased the total proline content in flax plants (19.04, 21.77, 24.03 and 27.29 mg g−1 FW at 0, 50, 100 and 150 mM NaCl, respectively). The lowest and highest total proline content (14.52 and 27.29 mg g−1 FW) occurred in non-inoculated plants at 0 mM NaCl and AMF-inoculated plants at 150 mM NaCl, respectively.
3.3. Nitrogen and Phosphorus Distribution
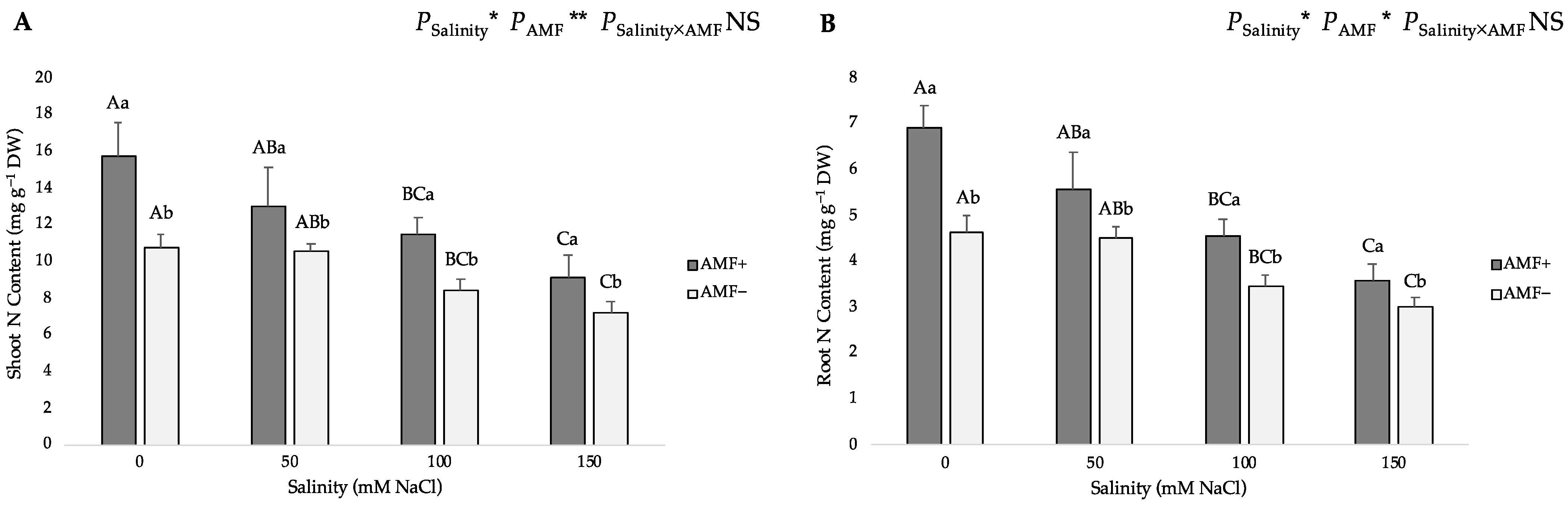
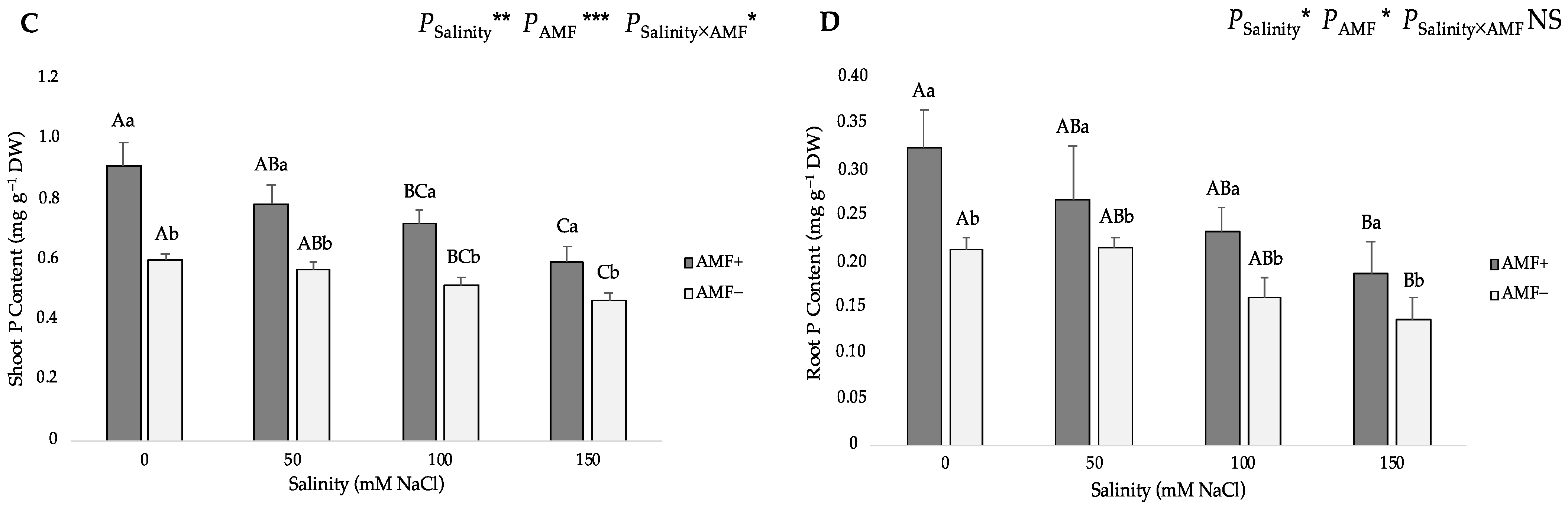
The two-way ANOVA suggested that salinity and AMF inoculation had a significant influence on the nitrogen (N) and phosphorus (P) contents in shoots and roots, and the interaction of the two factors significantly affected shoot P content. Gradually increasing salinity levels from 0 to 150 mM NaCl led to a gradual decrease in the N and P contents of both AMF-inoculated and non-inoculated flax plants (Figure 4). Compared with the roots, the shoots evidently accumulated more N and P as well. In addition, the N and P content of the shoots and roots of AMF-inoculated plants were significantly higher than those of non-inoculated plants at all studied NaCl levels. Under salt stress, AMF inoculation significantly increased the N content by 46.19%, 23.81%, 35.98% and 26.32% at 0, 50, 100 and 150 mM NaCl in shoots (Figure 4A), whereas it increased by 48.82%, 23.94%, 31.03% and 19.33% at 0, 50, 100 and 150 mM NaCl in roots, respectively (Figure 4B). Moreover, compared with non-inoculated plants, AMF-inoculated plants had a significantly higher shoot P content (52.94%, 37.43%, 39.22% and 28.35% at 0, 50, 100 and 150 mM NaCl, respectively) (Figure 4C), and higher root P content (52.60%, 24.77%, 45.38% and 35.76% at 0, 50, 100 and 150 mM NaCl, respectively) (Figure 4D).
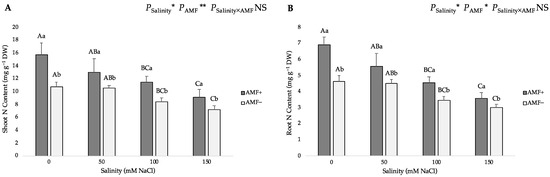
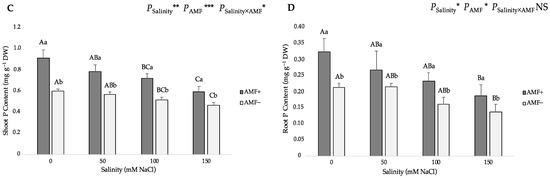
Figure 4.
Effects of arbuscular mycorrhizal fungi (AMF) inoculation on (A) shoot nitrogen (N) content, (B) root N content, (C) shoot phosphorus (P) content, and (D) root P content of flax at different salinity (0, 50, 100, and 150 mM NaCl) levels. AMF+: with AM inoculum; AMF−: without inoculation. NS: No significant effect; *, ** and ***: Significant at 5%, 1% and 0.1% probability levels, respectively. Values are means ± standard error. The different capital letters indicate significant differences among different salinity levels, and the different lowercase letters for the same salinity level indicate significant differences among different AMF inoculation treatments according to the Tukey’s HSD test (p ≤ 0.05).
3.4. Yield Parameters
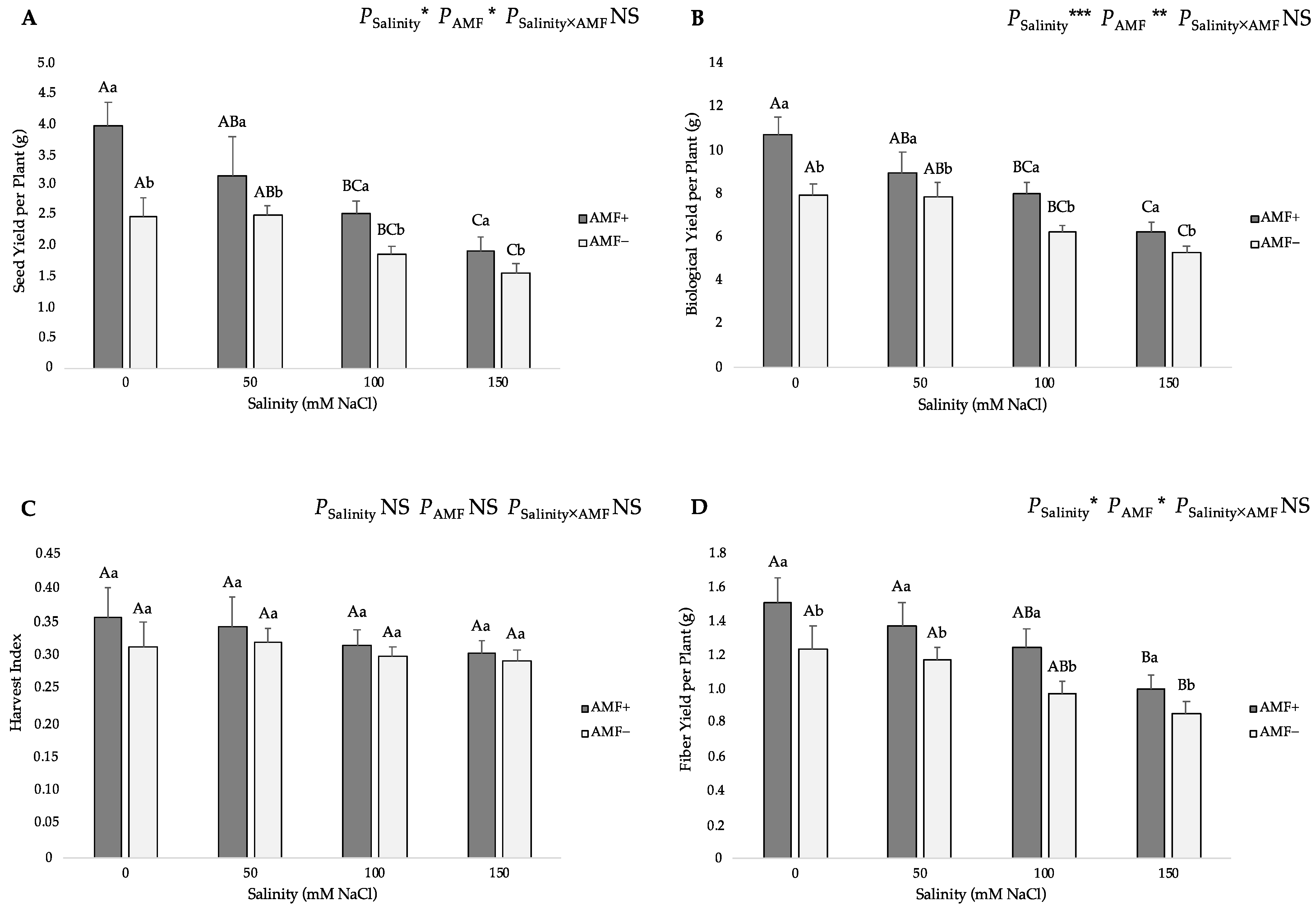
Seed and biological yields per plant were significantly affected by the salinity regimes and AMF colonization. Increasing salt levels in the irrigation water from 0 to 150 mM NaCl caused significant declines in these yield parameters (Figure 5). AMF inoculation also had a significantly positive impact on these traits. Specifically, the highest seed yield per plant was related to the AMF+ plants at 0 mM NaCl salinity (3.98 g), while the lowest value (1.57 g) was recorded in the AMF− plants at the 150 mM NaCl level (Figure 5A). The highest biological yield per plant was recorded in the AMF+ plants at the 0 mM NaCl level (10.79 g), whereas the lowest one (5.35 g) was found in the AMF− plants at 150 mM NaCl level (Figure 5B). In regard to the harvest index (HI), there were no significant differences among the studied salinity regimes and AMF inoculation treatments (Figure 5C); however, under salt stress, the values of AMF-inoculated plants were slightly higher (0.358, 0.344, 0.317 and 0.304 at 0, 50, 100 and 150 mM NaCl, respectively) than those of non-inoculated plants (0.313, 0.321, 0.299 and 0.292 at 0, 50, 100 and 150 mM NaCl, respectively). As for the stem fiber yield per plant, this trait presented similar trends as those described on seed and biological yields per plant values. Salt stress had a negative effect on the stem fiber yield per plant, reflected by an obvious reduction at 100 and 150 mM of AMF-inoculated and non-inoculated flax plants, and this reduction increased as the NaCl levels increased gradually. In addition, AMF inoculation positively affected this trait under salt stress. The highest stem fiber yield per plant was observed in the AMF+ plants at the 0 mM NaCl level (1.51 g), while the lowest value (0.85 g) was obtained from the AMF− plants at the 150 mM NaCl level (Figure 5D).
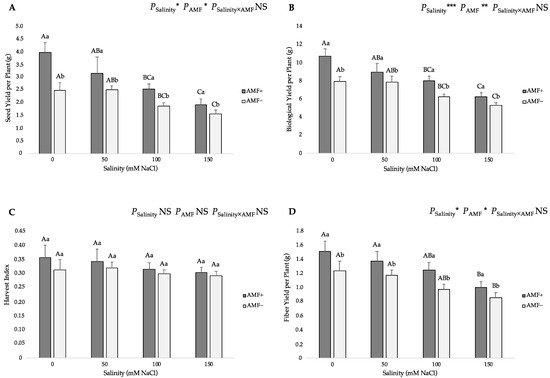
Figure 5.
Effects of arbuscular mycorrhizal fungi (AMF) inoculation on (A) seed yield per plant, (B) biological yield per plant, (C) harvest index, and (D) stem fiber yield per plant of flax at different salinity (0, 50, 100, and 150 mM NaCl) levels. AMF+: with AM inoculum; AMF−: without inoculation. NS: No significant effect; *, ** and ***: Significant at 5%, 1% and 0.1% probability levels, respectively. Values are means ± standard error. The different capital letters indicate significant differences among different salinity levels, and the different lowercase letters for the same salinity level indicate significant differences among different AMF inoculation treatments according to the Tukey’s HSD test (p ≤ 0.05).
4. Discussion
With the advent of natural calamities and the increasing impact of global warming, there is a growing need for food security, plant productivity, and food safety in order to sustain the global population and crop production. A significant environmental stress affecting plant growth is salt stress [41,42]. It is common for plants growing in saline areas to be affected by ion toxicity [42,43]. Salt ions can cause great damage to enzyme structure, disrupt photosynthesis, and result in nutrient deficiencies as a result of their toxic effects [18,21]. Salt stress conditions are a result of global warming, and salt stress management is necessary for better crop production and food security. Consequently, sustainable practices for managing crop production have been emphasized worldwide in recent years. AMF is one of the most widely used symbiotic associations for the promotion of plant growth and the resistance of plants to salt [42,44,45]. To explore the potential function of AMF in flax plants under abiotic stress, the present research examined growth, physiological, biochemical, and yield parameters, as well as nutrient distribution within the plants exposed to salt stress and AMF inoculation.
Salinity decreased the plant height, with a larger effect at higher salinity levels, although AMF-inoculated plants produced higher heights than non-inoculated plants (Figure 1A). These results are consistent with those presented by Mathur et al. [46] on moth bean (Vigna aconitifolia L.), Jamil et al. [47] on radish plant (Raphanus sativus L.), Kapoor and Srivastava [48] on black gram (Vigna mungo L.), Begum et al. [49] on maize (Zea mays L.), Bernardo et al. [50] on wheat (Triticum sp. L.), Hashem et al. [51] on chickpeas (Cicer arietinum L.), and Jerbi et al. [52] on hulless barley (Hordeum vulgare ssp. nudum L.), where they indicated that increasing the concentrations of sodium chloride (NaCl) resulted in the plants decreasing in length. The observed decrease in the plant height of flax plants after the treatment with the NaCl solution could be the result of the negative impact of this salt on the rate of photosynthesis, changes in enzyme activity that directly affect protein synthesis, and a decrease in carbohydrates and growth hormones, both of which can inhibit the plant growth [14,53].
Plants can benefit from the symbiosis between AMFs and host plants in a number of ways, including the promotion of growth and development as well as the enhancement of their resistance to many abiotic stresses. The application of AMF can reduce the inhibition of plant functions caused by salinity stresses, as well as increase plant height and growth generally [53,54,55]. As observed in a previous study [56], salinity-induced detrimental effects on plant height, leaf area, and the number of leaves were mitigated by inoculation with AMF.
The leaf area per plant was significantly influenced by both salinity and AMF inoculation. Regarding the salinity effect, it was observed that with increasing salt levels, the leaf area per plant decreased. In terms of AMF inoculation, this had a positive effect on the leaf area, with the highest values being found in AMF-inoculated plants. The results of Bernardo et al. [50] on wheat and Hashem et al. [51] on chickpeas support our findings that the NaCl treatment reduced the leaf area when compared to the control (0 mM NaCl) plants. As a consequence of the plant’s exposure to higher concentrations of sodium chloride, the present study observed a significant decrease in leaf area, which may be explained by the negative effect salt has on photosynthesis, resulting in a reduction in the leaf growth, chlorophyll content, and plant growth [14].
Salt-induced osmotic stress results in a reduction in leaf area as well as a decrease in stomatal conductance and mesophyll conductance, thus limiting CO2 assimilation and availability [53,57]. A symbiotic relationship with AMF appears to alleviate the negative effects of salinity on the photosynthetic capacity of plants. It has been demonstrated that mycorrhizal symbiosis prevents salt stress from negatively affecting photosynthesis by improving the water status of mycorrhizal plants, which increases leaf area and stomatal conductance, and, thereby, results in better CO2 assimilation [54,55]. Additionally, the increase in leaf area following AMF inoculation can also be attributed to the increased activity of cytokinin, a growth promoter that appears to increase leaf growth through its effect on cell division and expansion [57].
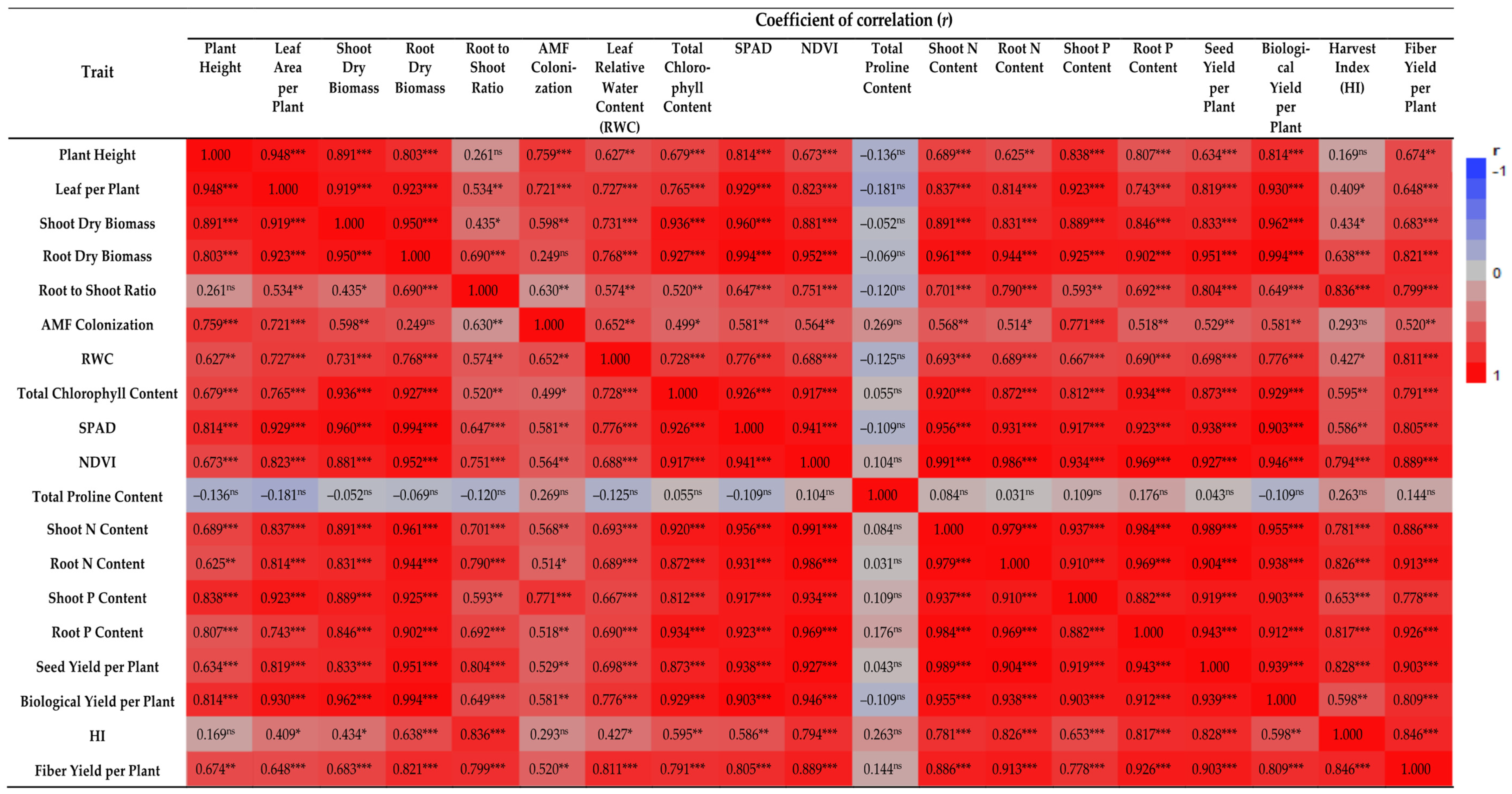
Furthermore, the leaf area per plant was linearly related to the biological and seed yield per plant (r = 0.930, p < 0.001; r = 0.819, p < 0.001, respectively) (Figure 6), suggesting that leaf area is an important yield component that should be considered during the selection of promising accessions. There is evidence that plants with a large number of leaves and a large leaf area are more likely to have a higher ability to perform photosynthetic duties, which contributes to the development and growth of the plant [34,45,46].
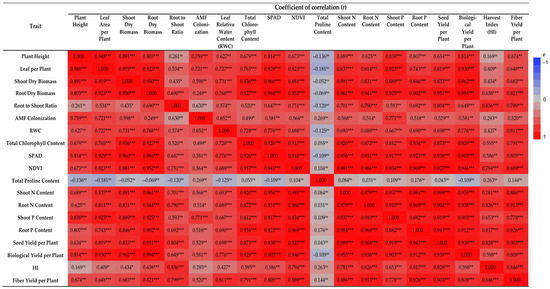
Figure 6.
Correlation coefficient heatmap between evaluated traits. ns: No significant effect; *, ** and ***: Significant at 5%, 1% and 0.1% probability levels, respectively. AMF: Arbuscular mycorrhizal fungi; SPAD: Soil plant analysis development; NDVI: Normalized difference vegetation index; N: Nitrogen; and P: Phosphorus.
In the same manner of the previous growth parameters, the values of the shoot and root dry biomass had a negative response to the increase in salt levels and exhibited a positive performance with AMF inoculation (Figure 1C,D). Various previous studies have demonstrated that AMF can alleviate the negative effects of salinity and improve plant growth by improving the growth of shoots and roots [46,47,49,50,51,52,58,59]. In a study conducted by Jerbi et al. [52], it was found that AMF inoculation significantly increased the plant biomass at various salinity levels. Furthermore, Klinsukon et al. [58] confirmed that AMF colonization affects the root’s dry weight at all salt-stress levels compared to non-inoculated plants. The result is an increase in the number of extraradical hyphae and an increase in the surface area of the mycelia network of the mycorrhiza, thereby contributing to the increase in the biomass of roots [58,59].
The root-to-shoot ratio was not affected by either the different salt levels or the AMF inoculation. At this point, it is worth noting that the plants treated with lower salinity levels, as well as the AMF-inoculated plants, showed slightly higher values (Figure 1E). In particular, the application of AMF increased the root-to-shoot ratio in inoculated plants, revealing that AMF-inoculated plants diverted more biomass to the roots than to the shoots. An increased root-to-shoot ratio is generally thought to enhance the source-to-sink ratio for water and nutrients and could, therefore, prove advantageous under salinity conditions [60,61].
All the above-mentioned findings show a substantial positive relationship between AMF root colonization and plant growth characteristics, including the plant height, leaf area per plant, shoot and root dry biomass, and root-to-shoot ratio (Figure 6). The percentage of AMF root colonization was negatively affected by salinity (Figure 1F). The presence of NaCl in soil generally reduces the colonization of AMF roots by inhibiting their activity [58,62]. In cases in which AMF was inoculated, the colonization rate was higher, resulting in a more intensive symbiotic relationship between AMF and the host plant. AMF colonization was also observed in the non-inoculated plants, suggesting that AMF populations capable of colonizing flax plants were already present in the control soils. As a result of the above-mentioned mechanism, root system reinforcement is promoted and, consequently, plant growth is enhanced [63].
The leaf relative water content (RWC) was also significantly affected by salinity and AMF inoculation. Although salinity decreased, the RWC of mycorrhizal plants increased (Figure 3A). Several factors contribute to the higher RWC in leaves of AMF-inoculated plants: (a) AMF enhance soil–plant hydraulic conductivity at a low water potential, (b) the AMF can reduce the osmotic potential of plants when they are experiencing water deficits by increasing levels of organic osmolytes, (c) AMF-inoculated plants exhibit higher stomatal conductance, and (d) AMF hyphae improve water relation [58,64,65,66].
The chlorophyll content in leaves, a significant physiological parameter of plant photosynthetic capacity, was significantly influenced by both salinity and AMF inoculation. There was a significant reduction in leaf total chlorophyll content due to the increasing salt levels (Figure 3B), possibly as the result of the repression of specific enzymes of the photosynthesis system as well as the reduced uptake of nutrients such as nitrogen (N) and magnesium (Mg) for chlorophyll biosynthesis. A significant increase in leaf total chlorophyll content was observed following AMF inoculation. As a result, the plants were able to take up more nutrients and have a lower Na content, which resulted in a higher capacity for photosynthetic respiration [58,67]. Specifically, AMF enhances photosynthesis by increasing Rubisco activity, electron transport rates, adenosine triphosphate (ATP) synthesis, and even the ratio of ATP to ADP (adenosine diphosphate) in leaves by increasing the mass fractions of N and P in leaves [68,69].
The soil plant analysis development (SPAD) value represents the relative content of chlorophyll in plants. According to the results of the SPAD value, a decrease in chlorophyll content was observed as a result of salt stress. The presence of AMF-compound inoculants increases the SPAD value of flax plants (Figure 3C), thereby promoting their photosynthesis. AMF inoculation has been shown in previous studies to increase the rate of photosynthesis in plants [70,71]. Using AMF-inoculated flax may improve the photosynthetic capacity by increasing the capacity for exchanging gas and absorbing water, thereby reducing salt stress-induced toxicity [70].
Non-destructive optical chlorophyll meters are often utilized to quantify leaf chlorophyll content [72]. The proportion of transmitted red light and near-infrared light released by a red and a near-infrared LED, through a leaf is used to determine the relative chlorophyll content accordingly [73]. Considering that chlorophyll absorbs red light effectively, the amount of red light transmitted through a leaf is proportional to its chlorophyll content [72,74]. As chlorophylls absorb very little near-infrared light, near-infrared light can be used as a measure of the absorption spectrum associated with non-chlorophylls [73,74]. In remote sensing, the natural difference vegetation index (NDVI) sensors equipped with red and near-infrared light detectors are commonly employed to measure vegetation coverage [74]. NDVI sensors are capable of detecting red and near-infrared light from solar radiation, and they can, therefore, be used to determine the chlorophyll content in leaves by measuring the transmitted red and near-infrared light [72]. NDVI values, like the previous measurements assessing chlorophyll content directly (total chlorophyll content) or indirectly (SPAD index), demonstrated a negative reaction to an increase in NaCl levels and a positive response to AMF inoculation (Figure 3D). In addition, all the above-mentioned findings about the total chlorophyll content, SPAD, and NDVI values are also confirmed by the positive and significant correlations of the total chlorophyll content with SPAD (r = 0.926, p < 0.001), total chlorophyll content with NDVI (r = 0.917, p < 0.001) and SPAD with NDVI (r = 0.941, p < 0.001) (Figure 6).
A surplus of salt reduces soil osmotic potential, limiting plants from absorbing soil water and resulting in physiological drought in plants. In plant cells, a substantial number of osmoregulatory metabolites, such as proline, are generated and stored; these metabolites can reduce intracellular osmotic potential and enable appropriate water uptake and use from plant species. This approach is utilized by plants to cope with physiological drought produced by salt stress [75]. This physiological alteration ensures that plants take up and utilize water normally, reducing the physiological drought induced by salt stress. Furthermore, it has been proposed that proline in plants performs a number of crucial roles, including scavenging reactive oxygen species and stabilizing proteins and cell membrane structures as well [76,77]. The present study reported that during salt stress at 50 mM, 100 mM, and 150 mM NaCl, the proline content in flax increased considerably. Inoculation with AMF enhanced the proline content of flax plants even more (Figure 3E). The results demonstrated that inoculating flax with AMF could stimulate proline accumulation under salt stress, result in a lower cellular osmotic potential, and improve flax-plant salt resistance. Nevertheless, the contribution of AMF in the accumulation of proline in plants is not constant: a few investigations found a higher proline content in AMF-inoculated plants under stress, while other research demonstrated a lower proline content [18,51,52,77].
Nitrogen (N) is an essential component of several molecules, including amino acids, amides, polyamines, and proteins, all of which play a role in plant salt tolerance via diverse methods. The appropriate management of N metabolism is critical for plant tolerance to salt. Nevertheless, depending on the level and length of salt stress, the plant species, the stage of plant growth, and the amount, type, and shape of N in the rhizosphere, the interaction between salinity and N metabolism is an extremely intricate network [14]. The mechanism of absorbing N by plants is primarily impeded in a saline environment, likely because of the diametrically opposed impact of salt ions with nitrate (NO3−) and ammonium (NH4+) disruption in the N ions transferring into root xylem, the decreased transpiration rate, reduced water absorption owing to osmotic changes in the root zone, destruction of the root structure of membranes, and less N demand due to the decreased plant growth rate [78]. In the saline environment, chloride (Cl−) constitutes the major anion, and NO3− uptake is known to compete with that of Cl−. At higher chloride concentrations, this interaction results in a lower N uptake and content in plant tissues and decreased plant development [53,78]. Since Cl− competes against NO3− uptake, sodium (Na+) competes against NH4+ uptake at the plasma membrane. Salt-induced membrane protein disruption, which affects plasma membrane integrity, impacts NO3− and NH4+ uptake [78]. Since nitrate reductase constitutes a substrate-inducible enzyme, this competition leads to reduced NO3− flow from soil to roots [79].
AMF colonization in saline-grown host plants has been demonstrated to enhance nutrient absorption and maintain ionic balance. In fact, it has been established that AMF hyphae can deliver up to 25% of the plant’s nitrogen [79]. The higher nitrate reductase activity in mycorrhizal plants is ascribed to AMF-facilitated membrane stability maintenance [79,80,81]. Furthermore, AMF colonization influences the concentration and profile of polyamines in the plant, thereby helping in the maintenance of ion homeostasis in plant cells by increasing nutrient and water uptake [66,82]. In this study, salt stress significantly reduced the N content in the shoots and roots of the flax plant, while inoculation with the AMF inoculant improved flax N-content status (Figure 4A,B). Similar results were recorded in the salt-tolerance tests of AMF-inoculated Euonymus maackii, Lallemantia iberica, and Leymus chinensis plants conducted by Li et al. [42], Heydari and Pirzad [83] and Cao et al. [84], respectively.
In all treatments of the present study, AMF colonization increased the phosphorus (P) contents in the shoots and roots (Figure 4C,D). The current analysis demonstrated that the AMF effect on plant P nutrition was greater in plants inoculated with AMF, likely as a result of a lower level of nutrient mobility under salinity conditions. Under a wide range of salinity conditions, AMFs are able of enhancing the amount of P in plant tissues significantly across all salinity levels. A number of researchers have concluded that the main mechanism of improvement in the plant P concentration in mycorrhizal plants under salt stress is the improvement in plant P concentration [80,82]. Based on a previous study conducted by Frosi et al. [71], P is capable of being transported into the root through protoplasmic circulation by AMF, thereby reducing the transport resistance and increasing its speed [80]. However, other studies have demonstrated that mycorrhizal plants grow better under salt stress than non-AMF-inoculated plants, even if their P levels are similar under unstressed conditions [42,85].
The mycorrhizal contribution to nutrient (N and P) contents in the shoots and roots of flax plants was higher in the AMF-inoculated plants than in non-mycorrhizal plants under salt stress, explaining the better growth performance and biomass accumulation of AMF-inoculated plants under salinity. Thus, this suggests that the increased salinity tolerance of plants can be attributed to the improved uptake and concentration of N and P in plant tissues. It is evident that AMF contributes to the plant’s biomass and nutrient content in a positive manner. This was also confirmed by the positive and significant correlations of shoot dry weight with shoot N content (r = 0.891, p < 0.001), root N content (r = 0.831, p < 0.001), shoot P content (r = 0.889, p < 0.001) and root P content (r = 0.846, p < 0.001) (Figure 6).
Saline solutions have a direct effect on cell proliferation; nevertheless, the specific mechanism through which this arises is unknown [55]. Based on the biophysical model of the elongation of cells, the rate of cell elongation can be modulated or controlled by changes in any of a number of variables (cell wall extensibility, turgor pressure, and yield threshold). The yield threshold is the turgor pressure value below which there is no irreversible expansion of the cell wall. Because an increased salt content reduces the osmotic potential of the soil solution, it is commonly assumed that the root-zone salinity affects growth through lowering cell turgor. Growth inhibition produced by a quick increase in external solute concentrations is definitely mediated by unexpected decreases in turgor pressure [86]. Many yield components reduced as the plant height and leaf area and number decreased, resulting in a reduced seed and biological yield.
In the present experiment, salinity reduced the seed and biological yield, with a greater effect at higher salinity levels, while AMF-inoculated plants resulted in higher values of these yield parameters than non-inoculated plants (Figure 5A,B). A number of investigations on various plants have demonstrated that AMF supports the plant under salt-stress conditions by improving its photosynthetic ability, nutrient absorption, protection against antioxidants, and osmolyte accumulation, leading to enhanced plant growth and tolerance [55,87]. Specifically, mycorrhization increased the biomass and seed yield in maize [49], wheat [50], chickpea [51], hulless barley [52] and Euonymus maackii [42] under salinity stress.
As for the harvest index (HI), it was not affected by AMF inoculation or the various salt-level regimes. The AMF-inoculated plants displayed marginally higher values at all salt levels (Figure 5C). Overall, the range of HI values was between 0.292 and 0.358. Various researchers have reported similar results [40,88,89] when studying the development of flax plants under salinity conditions. Finally, in regard to the fiber yield, this presented a similar trend to the seed and biological yield, with salinity having a negative impact on this trait, especially under high salinity levels. Salinity adversely impacts the stem fiber yield as a result of the reduction in plant height, causing the plants to produce inferior fibers [88]. This is also supported by the considerable positive and significant association between the stem fiber yield and plant height (r = 0.674, p < 0.01) (Figure 6).
5. Conclusions
Based on the findings of the current study and their assessment, it is evident that salt stress caused negative physiological effects, including limited growth, reduced photosynthesis, and decreased nutrient (N and P) contents in the shoots and roots of flax plants. It is clear from our results that AMF symbionts play a beneficial role in reducing salinity-induced adverse effects. Specifically, mycorrhizal association improved the salt tolerance of the plants by increasing their chlorophyll content, enhancing the nutrient contents of their shoots and roots, and consequently their yield parameters, such as their seed, biological and stem fiber yields, particularly at moderate salt concentrations (50 and 100 mM NaCl). As a result of using AMF, flax plants grown under salt stress exhibited tolerance, suggesting that AMF could be applied in saline environments to maintain ecological stability. In addition, it is important to conduct field trials in the future in order to better understand the potential of AMF. The use of native or exotic AMF strains as biofertilizer, in particular, could represent a potential method to ensure the sustainability of agricultural cropping systems in arid and semi-arid regions vulnerable to salinity.
Author Contributions
Conceptualization, I.K., P.S., I.R., A.M. and D.B.; methodology, I.K., P.S., I.R., A.M. and D.B.; validation, I.K., P.S., I.R., A.M. and D.B.; formal analysis, I.K., P.S., I.R., A.M. and D.B.; investigation, I.K., P.S., I.R., A.M. and D.B.; resources, I.K., P.S., I.R., A.M. and D.B.; writing—original draft preparation, I.K., P.S., I.R. and D.B.; writing—review and editing, I.K., I.R. and D.B.; supervision, I.K. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. AJBAS 2010, 4, 4304–4312. [Google Scholar]
- Kiryluk, A.; Kostecka, J. Pro-environmental and health-promoting grounds for restitution of flax (Linum usitatissimum L.) cultivation. J. Ecol. Eng. 2020, 21, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, D.; Roussis, I.; Cheimona, N.; Kakabouki, I.; Travlos, I. Organic agriculture and innovative feed crops. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2018; Volume 23, pp. 55–100. [Google Scholar]
- Debnath, S. Flax fiber extraction to textiles and sustainability: A holistic approach. In Sustainable Fashion and Textiles in Latin America, 1st ed.; Gardetti, M.A., Larios-Francia, R.P., Eds.; Springer: Singapore, 2021; pp. 73–85. [Google Scholar]
- Stavropoulos, P.; Mavroeidis, A.; Papadopoulos, G.; Roussis, I.; Bilalis, D.; Kakabouki, I. On the path towards a “greener” EU: A mini review on flax (Linum usitatissimum L.) as a case study. Plants 2023, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Vaisey-Genser, M.; Morris, D.H. Introduction: History of the cultivation and uses of flaxseed. In Flax, 1st ed.; Muir, A.D., Westcott, N.D., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 13–33. [Google Scholar]
- Cloutier, S. Linseed: Overview. In Encyclopedia of Food Grains, 1st ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 1, pp. 259–264. [Google Scholar]
- Adorian, T.J.; Pianesso, D.; Bender, A.B.B.; Speroni, C.S.; Mombach, P.I.; Kowalski, É.A.; da Silva, L.P. Fractionation of linseed and obtaining ingredients rich in protein and fibers: Alternatives for animal feed. J. Sci. Food Agric. 2021, 102, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.X.; Shi, L.E.; Wang, X.M.; Dai, G.W.; Cheng, L.A.; Wan, Z.X.; He, H.; Wu, Q.; Wang, Y.B.; Jin, X.Y.; et al. Whole flaxseed-based products and their health benefits. Food Sci. Technol. Res. 2020, 26, 561–578. [Google Scholar] [CrossRef]
- Kolodziejczyk, P.; Ozimek, L.; Kozłowska, J. The application of flax and hemp seeds in food, animal feed and cosmetics production. In Handbook of Natural Fibers, 1st ed.; Kozłowski, R.M., Ed.; Woodhead Publishing: Cambridge, UK, 2012; Volume 2, pp. 329–366. [Google Scholar]
- Begum, H.; Alam, A.K.M.M.; Chowdhury, M.J.A.; Hossain, M.I. Genetic divergence in linseed (Linum usitatissimum L.). Int. J. Sustain. Crop. Prod. 2007, 2, 4–6. [Google Scholar]
- Topnikova, E.V.; Pirogova, E.N.; Danilova, E.S. Quality assessment of linseed oil. IOP Conf. Ser. Earth Environ. Sci. 2022, 1, 012096. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant salinity tolerance conferred by arbuscular mycorrhizal fungi and associated mechanisms: A meta-analysis. Front. Plant Sci. 2020, 11, 588550. [Google Scholar] [CrossRef]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995, 109, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Li, Q.S.; Ding, W.Y.; Dong, L.W.; Deng, M.; Chen, J.H.; Tian, X.; Hashem, A.; Al-Arjani, A.F.; Alenazi, M.M.; et al. Arbuscular mycorrhizal fungi inoculation impacts expression of aquaporins and salt overly sensitive genes and enhances tolerance of salt stress in tomato. Chem. Biol. Technol. Agric. 2023, 10, 5. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: New York, NY, USA, 2010. [Google Scholar]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci. Rep. 2016, 6, 37663. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Maucieri, C.; Berruti, A.; Borin, M.; Barbera, A. Responses of different Panicum miliaceum L. genotypes to saline and water stress in a Marginal Mediterranean environment. Agronomy 2018, 8, 8. [Google Scholar] [CrossRef]
- Xiao, X.; Liao, X.; Yan, Q.; Xie, Y.; Chen, J.; Liang, G.; Chen, M.; Xiao, S.; Chen, Y.; Liu, J. Arbuscular mycorrhizal fungi improve the growth, water status, and nutrient uptake of Cinnamomum migao and the soil nutrient stoichiometry under drought stress and recovery. J. Fungi 2023, 9, 321. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, P.; Li, F.; Duan, T. Effects of AM fungi and grass endophytes on perennial ryegrass Bipolaris sorokiniana leaf spot disease under limited soil nutrients. Eur. J. Plant Pathol. 2019, 154, 659–671. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2009, 326, 3–20. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Alguacil, M.; Lozano, Z.; Campoy, M.J.; Roldán, A. Phosphorus fertilization management modifies the biodiversity of am fungi in a tropical savanna forage system. Soil Biol. Biochem. 2010, 42, 1114–1122. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling arbuscular mycorrhiza-induced changes in plant primary and secondary metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Püschel, D.; Bitterlich, M.; Rydlová, J.; Jansa, J. Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol. Biochem. 2021, 157, 108243. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef] [PubMed]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Change Biol. 2017, 24, e171–e182. [Google Scholar] [CrossRef] [PubMed]
- Konvalinková, T.; Püschel, D.; Rezácová, V.; Gryndlerová, H.; Jansa, J. Carbon flow from plant to arbuscular mycorrhizal fungi is reduced under phosphorus fertilization. Plant Soil 2017, 419, 319–333. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Wu, M.; Wu, R.; Zhou, Y.; Gao, Y.; Ren, A. Arbuscular mycorrhizal fungus inoculation reduces the drought-resistance advantage of endophyte-infected versus endophyte-free Leymus chinensis. Mycorrhiza 2017, 27, 791–799. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.R. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Khan, M.M.A.; Naeem, M. Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in linseed genotypes. World J. Agric. Sci. 2007, 3, 685–695. [Google Scholar]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Microenvironment and microbial community in the rhizosphere of dioecious Populus cathayana at Chaka Salt Lake. J. Soils Sediments 2019, 19, 2740–2751. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Meng, S.; Wu, F.; Liu, T. Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii Rupr. at a moderate level of salinity. PLoS ONE 2020, 15, e0231497. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Azcón, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, V.; Shamshiri, M.H.; Alaei, H.; Salehi, H. The role of inoculum identity for growth, photosynthesis, and chlorophyll fluorescence of zinnia plants by arbuscular mycorrhizal fungi under varying water regimes. Photosynthetica 2019, 57, 409–419. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mathur, N.; Singh, J.; Bohra, S.; Bohra, A.; Vyas, A. Biomass production, productivity and physiological changes in moth bean genotypes at different salinity levels. Am. J. Plant Physiol. 2006, 1, 210–213. [Google Scholar]
- Jamil, M.; Rehman, S.U.; Lee, K.J.; Kim, J.M.; Rha, H.K. Salinity reduced growth PS2 photochemistry and chlorophyll content in radish. Sci. Agric. 2007, 64, 111–118. [Google Scholar] [CrossRef]
- Kapoor, K.; Srivastana, A. Assessment of salinity tolerance of Vigna mungo var. Pu-19 using ex vitro and in vitro methods. Asian J. Biotechnol. 2010, 2, 73–85. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, B.F.; Singh, G.; Farooq, M.; Abd-Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Biol. Sci. 2019, 26, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Dalpé, Y.; Lounès-Hadj Sahraoui, A.; Ben Jeddi, F. Contribution of native and exotic arbuscular mycorrhizal fungi in improving the physiological and biochemical response of hulless barley (Hordeum vulgare ssp. nudum L.) to drought. J. Soil Sci. Plant Nutr. 2022, 22, 2187–2204. [Google Scholar] [CrossRef]
- Bilalis, D.J.; Roussis, I.; Kakabouki, I.; Karydogianni, S. Effects of salinity and arbuscular mycorrhizal fungi (AMF) on root growth development and productivity of chia (Salvia hispanica L.), a promising salt-tolerant crop, under Mediterranean conditions. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Wu, Q.S.; Zou, Y.N.; Abd Allah, E.F. Mycorrhizal association and ROS in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Elsevier: New York, NY, USA, 2014; pp. 453–475. [Google Scholar]
- Borde, M.; Dudhane, M.; Kulkarni, M. Role of arbuscular mycorrhizal fungi (AMF) in salinity tolerance and growth response in plants under salt stress conditions. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 71–86. [Google Scholar]
- Bheemareddy, V.S.; Lakshman, H.C. Effect of salt and acid stress on Triticum aestivum inoculated with Glomus fasciculatum. J. Anim. Plant Sci. 2011, 7, 945–956. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Klinsukon, C.; Lumyong, S.; Kuyper, T.W.; Boonlue, S. Colonization by arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci. Rep. 2021, 11, 4362. [Google Scholar] [CrossRef]
- Da Silva, H.F.O.; Tavares, O.C.H.; da Silva, L.S.; Zonta, E.; da Silva, E.M.R.; Júnior, O.J.S.; Nobre, C.P.; Berbara, R.L.L.; García, A.C. Arbuscular mycorrhizal fungi and humic substances increased the salinity tolerance of rice plants. Biocatal. Agric. Biotechnol. 2022, 44, 102472. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martinez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Glaría, A.; Eichler-Löbermann, B.; Loiret, F.G.; Ortega, E.; Kavka, M. Root-system architectures of two Cuban rice cultivars with salt stress at early development stages. Plants 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Juniper, S.; Abbott, L.K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 2006, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Nacoon, S.; Ekprasert, J.; Riddech, N.; Mongkolthanaruk, W.; Jogloy, S.; Vorasoot, N.; Cooper, J.; Boonlue, S. Growth enhancement of sunchoke by arbuscular mycorrhizal fungi under drought condition. Rhizosphere 2021, 17, 100308. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Kapoor, R.; Sharma, D.; Bhatnagar, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 2008, 116, 227–239. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 2003, 38, 170–175. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-dependent regulation of growth and stresses management in plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Chang, W.; Sui, X.; Fan, X.X.; Jia, T.T.; Song, F.Q. Arbuscular mycorrhizal symbiosis modulates antioxidant response and ion distribution in salt-stressed Elaeagnus angustifolia seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Santos, M.; Ramos, D.G.; Maia, L.C.; Santos, M.G.J.T.P. Arbuscular mycorrhizal fungi and foliar phosphorus inorganic supply alleviate salt stress effects in physiological attributes, but only arbuscular mycorrhizal fungi increase biomass in woody species of a semiarid environment. Tree Physiol. 2018, 38, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ferrarezi, R.; Geiger, T.C.; Greenidge, J.; Dennery, S.; Weiss, S.A.; Vieira, G.S. Microirrigation equipment for okra cultivation in the U.S. Virgin Islands. HortScience 2020, 55, 1045–1052. [Google Scholar] [CrossRef]
- Monje, O.A.; Bugbee, B. Inherent limitations of nondestructive chlorophyll meters: A comparison of two types of meters. HortScience 1992, 27, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dunn, B.L.; Arnall, D.B.; Mao, P. Use of an active canopy sensor and SPAD chlorophyll meter to quantify geranium nitrogen status. HortScience 2012, 47, 45–50. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, F.; Zheng, X.; Pan, H.; Wen, Y.; Song, F. Effects of AMF compound inoculants on growth, ion homeostasis, and salt tolerance-related gene expression in Oryza sativa L. under salt treatments. Rice 2023, 16, 18. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants—Focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1108. [Google Scholar] [CrossRef]
- Hoff, T.; Stummann, B.M.; Henningsen, K.W. Structure, function and regulation of nitrate reductase in higher plants. Physiol. Plant. 1992, 84, 616–624. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J. Plant Nutr. Soil Sci. 2011, 174, 283–291. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef]
- Heydari, S.; Pirzad, A. Mycorrhizal fungi and Thiobacillus co-inoculation improve the physiological indices of Lallemantia iberica under salinity stress. Curr. Microbiol. 2020, 77, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wu, X.; Zhukova, A.; Tang, Z.; Weng, Y.; Li, Z.; Yang, Y. Arbuscular mycorrhizal fungi (AMF) species and abundance exhibit different effects on saline-alkaline tolerance in Leymus chinensis. J. Plant Interact. 2020, 15, 266–279. [Google Scholar] [CrossRef]
- Poss, J.A.; Pond, E.; Menge, J. Effect of salinity on mycorrhizal onion and tomato in soil with and without additional phosphate. Plant Soil. 1985, 88, 307–309. [Google Scholar] [CrossRef]
- Volkamar, K.M.; Hu, Y.; Steppuhn, H. Physiological responses of plants to salinity: A review. Can. J. Plant Sci. 1998, 78, 19–27. [Google Scholar] [CrossRef]
- Hameed, A.; Egamberdieva, D.; Abd Allah, E.F.; Hashem, A.; Kumar, A.; Ahmad, P. Salinity stress and arbuscular mycorrhizal symbiosis in plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; Volume 1, pp. 139–159. [Google Scholar]
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. 2020, 48, 954–966. [Google Scholar] [CrossRef]
- Abdullah; Mahmood, A.; Bibi, S.; Naqve, M.; Javaid, M.M.; Zia, M.A.; Jabbar, A.; Ud-Din, W.; Attia, K.A.; Khan, N.; et al. Physiological, biochemical, and yield responses of linseed (Linum usitatissimum L.) in α-tocopherol-mediated alleviation of salinity stress. Front. Plant Sci. 2022, 13, 867172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).