Effect of Some Herbicides on Primary Photosynthesis in Malva moschata as a Prospective Plant for Agricultural Grass Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation and Experimental Conditions
2.2. Herbicide Application
- (1)
- Propaquizafop (Propachizafop), i.e., (2-(propan-2-ylidenamino)oxyethyl (2R)-2-[4-(6-chlorchinoxalin-2-yl)oxyphenoxy]propanoate (a dose of 100 g·L−1, resp. 4.5 mL·L−1), recommended application dose: 1.35 L·ha−1 per 300 l of water.
- (2)
- Clopyralid, i.e., 3,6-dichloropyridine-2-carboxylic acid (a dose of 0.999 mL·L−1), recommended application dose: 0.3 L·ha−1 per 300 L of water.
- (3)
- Metamitron—525 g (4-amino-4,5-dihydro-3-methyl-6-phenyl-1,2,4-triazin-5-one), Quinmerac—40 g (7-chloro-3-methylquinoline-8-carboxylic acid), (a dose of 3.3 mL·L−1) recommended application dose: 1.0 L·ha−1 per 300 L of water.
2.3. Measurements of Chlorophyll Fluorescence Parameters
2.4. Non-Photochemical Quenching Induction
2.5. Fast Chlorophyll Fluorescence Transients
2.6. Statistical Analysis
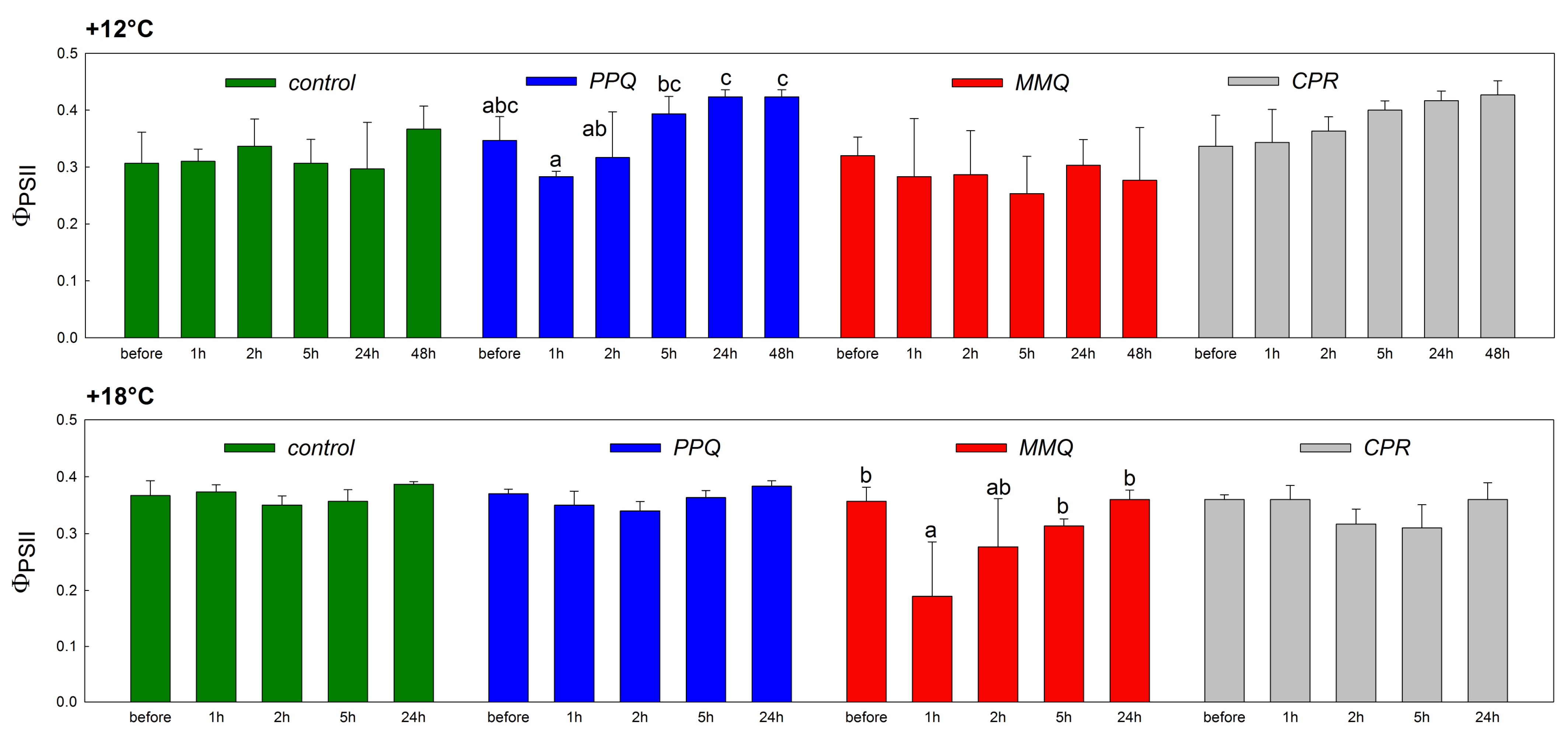
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolova, M. Screening of radical scavenging activity and polyphenol content of Bulgarian plant species. Pharmacogn. Res. 2011, 3, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.E. Malva sp. (Mallow): In vitro culture and the production of secondary metabolites. In Medicinal and Aromatic Plants XII. Biotechnology in Agriculture and Forestry; Nagata, T., Ebizuka, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 51. [Google Scholar]
- Mousavi, S.M.; Hashemi, S.A.; Behbudi, G.; Mazraedoost, S.; Omidifar, N.; Gholami, A.; Chiang, W.H.; Babapoor, A.; Pynadathu Rumjit, N. A review on health benefits of Malva sylvestris L. nutritional compounds for metabolites, antioxidants, and anti-inflammatory, anticancer, and antimicrobial applications. Evid. Based Complement. Altern. Med. eCAM 2021, 2021, 5548404. [Google Scholar] [CrossRef] [PubMed]
- Terninko, I.I.; Onishchenko, U.E.; Frolova, A. Research phenolic compounds Malva sylvestris by high performance liquid chromatography. Pharma Innov. J. 2014, 3, 46–50. [Google Scholar]
- Irfan, A.; Imran, M.; Ullah, M.; Khalid, N.; Assiri, M.; Thomas, R.; Muthu, S.; Raza, A.; Al-Atabi, M.; Al-sehemi, A.; et al. Phenolic and flavonoid contents in Malva sylvestris and exploration of active drugs as antioxidant and anti-COVID19 by quantum chemical and molecular docking studies. J. Saudi Chem. Soc. 2021, 25, 101277. [Google Scholar] [CrossRef]
- Redžić, S.; Hodžić, N.; Tuka, M. Plant pigments (antioxidants) of medicinal plants Malva silvestris L. and Malva moschata L. (Malvaceae). Bosn. J. Basic Med. Sci. 2005, 5, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Schmidgall, J.; Schnetz, E.; Hensel, A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med. 2000, 66, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Altyar, A.E.; Munir, A.; Ishtiaq, S.; Rizwan, M.; Abbas, K.; Kensara, O.; Elhady, S.S.; Rizg, W.Y.; Youssef, F.S.; Ashour, M.L. Malva parviflora leaves and fruits mucilage as natural sources of anti-inflammatory, antitussive and gastro-protective agents: A comparative study using rat models and gas chromatography. Pharmaceuticals 2022, 15, 427. [Google Scholar] [CrossRef]
- Samavati, V.; Manoochehrizade, A. Polysaccharide extraction from Malva sylvestris and its anti-oxidant activity. Int. J. Biol. Macromol. 2013, 60, 427–436. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X.; Chen, T.; Chen, X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci. Technol. 2022, 123, 264–280. [Google Scholar] [CrossRef]
- de Sousa, C.P.; de Farias, M.E.; Schock, A.A.; Bacarin, M.A. Photosynthesis of soybean under the action of a photosystem II-inhibiting herbicide. Acta Physiol. Plant. 2014, 36, 3051–3062. [Google Scholar] [CrossRef]
- Todorova, D.; Aleksandrov, V.; Anev, S.; Sergiev, I. Photosynthesis alterations in wheat plants induced by herbicide, soil drought or flooding. Agronomy 2022, 12, 390. [Google Scholar] [CrossRef]
- Onwuchekwa-Henry, C.B.; Coe, R.; Ogtrop, F.V.; Roche, R.; Tan, D.K.Y. Efficacy of pendimethalin rates on barnyard grass (Echinochloa crus-galli (L.) Beauv) and their effect on photosynthetic performance in rice. Agronomy 2023, 13, 582. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, N.; Yang, M.; Li, X.; Han, J.; Lin, X.; Yang, Q.; Lv, G.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. 2022, 29, 37248–37265. [Google Scholar] [CrossRef]
- Hussain, M.I.; González, L.; Reigosa Roger, M. Phytotoxic effects of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca saliva. Allelopath. J. 2010, 3, 157–174. [Google Scholar]
- Lang, J.; Zikmundová, B.; Hájek, J.; Barták, M.; Váczi, P. The effects of foliar application of phenoxy and imidazoline family herbicides on the limitation of primary photosynthetic processes in Galega orientalis L. Agronomy 2022, 12, 96. [Google Scholar] [CrossRef]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early identification of herbicide modes of action by the use of chlorophyll fluorescence measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef]
- Boisvert, S.; Joly, D.; Carpentier, R. Quantitative analysis of the experimental O–J–I–P chlorophyll fluorescence induction kinetics: Apparent activation energy and origin of each kinetic step. FEBS J. 2006, 273, 4770–4777. [Google Scholar] [CrossRef]
- Neubauer, C.; Schreiber, U. The polyphasic rice of chlorophyll fluorescence upon onset of strong continuous illumination: I. Saturation characteristics and partial control by the photosystem II acceptor side. Z. Naturforsch. 1987, 42, 1246–1254. [Google Scholar] [CrossRef]
- Ilík, P.; Schansker, G.; Kotabová, E.; Váczi, P.; Strasser, R.J.; Barták, M. A dip in the chlorophyll fluorescence induction at 0.2—2 s in Trebouxia-possessing lichens reflects a fast reoxidation of photosystem I. A comparison with higher plants. Biochim. Et Biophys. Acta 2006, 1757, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Begović, L.; Jurišić, N.; Šrajer Gajdošik, M.; Mikuška, A.; Mlinarić, S. Photosynthetic efficiency and antioxidative response of soybean exposed to selective herbicides: A field study. Agriculture 2023, 13, 1385. [Google Scholar] [CrossRef]
- Saura, P.; Quiles, M.J. Assessment of photosynthesis tolerance to herbicides, heat and high illumination by fluorescence imaging. Open Plant Sci. J. 2009, 3, 7–13. [Google Scholar] [CrossRef]
- Pinnamaneni, S.R.; Anapalli, S.S.; Reddy, K.N. Photosynthetic response of soybean and cotton to different irrigation regimes and planting geometries. Front. Plant Sci. 2022, 13, 894706. [Google Scholar] [CrossRef]
- Quan, L.; Chen, K.; Chen, T.; Li, H.; Li, W.; Cheng, T.; Xia, F.; Lou, Z.; Geng, T.; Sun, D.; et al. Monitoring weed mechanical and chemical damage stress based on chlorophyll fluorescence imaging. Front. Plant Sci. 2023, 14, 1188981. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Délye, C. Weed resistance to acetyl coenzyme a carboxylase inhibitors: An update. Weed Sci. 2005, 53, 728–746. Available online: http://www.jstor.org/stable/4047044 (accessed on 9 November 2023). [CrossRef]
- Kaundun, S.S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 2014, 70, 1405–1417. [Google Scholar] [CrossRef]
- Doležal, M.; Kra’ľova, K. Synthesis and evaluation of pyrazine derivatives with herbicidal activity. In Herbicides, Theory and Applications; InTech: Rijeka, Croatia, 2011; pp. 581–610. [Google Scholar] [CrossRef]
- Rusjan, A.; Dębski, H.; Koczkodaj, D.; Mitrus, J.; Horbowicz, M. Effect of propaquizafop treatment on pigments content and growth of sweet maize seedlings. Acta Sci. Polonorum. Agric. 2017, 16, 227–234. [Google Scholar]
- Manová, A.; Hýžová, B.; Darriba Canora, D.; Castrillo Antolin, A.; Dufková, K. Vitality and growth rate of agar plate-cultivated Antarctic microautotrophs: Analysis of PSII functioning by chlorophyll fluorescence parameters. Czech Polar Rep. 2022, 12, 269–279. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Barták, M.; Hájek, J.; Halıcı, M.G.; Bednaříková, M.; Casanova-Katny, A.; Váczi, P.; Puhovkin, A.; Mishra, K.B.; Giordano, D. Resistance of primary photosynthesis to photoinhibition in Antarctic lichen Xanthoria elegans: Photoprotective mechanisms activated during a short period of high light stress. Plants 2023, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Špundová, M.; Strzałka, K.; Nauš, J. Xanthophyll cycle activity in detached barley leaves senescing under dark and light. Photosynthetica 2005, 43, 117–124. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plantarum 2019, 167, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Lazár, D.; Nauš, J.; Matoušková, M.; Flašarová, M. Mathematical modeling of changes in chlorophyll fluorescence induction caused by herbicides. Pesticicides Biochem. Physiol. 1997, 57, 200–210. [Google Scholar] [CrossRef]
- Abbaspoor, M.; Teicher, H.B.; Streibig, J.C. The effect of root-absorbed PSII inhibitors on Kautsky curve parameters in sugar beet. Weed Res. 2006, 46, 226–235. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestič, M.; Puthur, J.T. Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Battaglino, B.; Grinzato, A.; Pagliano, C. Binding properties of photosynthetic herbicides with the QB site of the D1 protein in plant photosystem II: A combined functional and molecular docking study. Plants 2021, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Weber, J.F.; Gerhards, R. Early identification of herbicide stress in soybean (Glycine max (L.) Merr.) using chlorophyll fluorescence imaging technology. Sensors 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Kunz, C.; Peteinatos, G.; Santel, H.; Gerhards, R. Utilization of chlorophyll fluorescence imaging technology to detect plant injury by herbicides in sugar beet and soybean. Weed Technol. 2017, 31, 523–535. [Google Scholar] [CrossRef]
- Tadmor, Y.; Raz, A.; Reikin-Barak, S.; Ambastha, V.; Shemesh, E.; Leshem, Y.; Crane, O.; Stern, R.A.; Goldway, M.; Tchernov, D.; et al. Metamitron, a photosynthetic electron transport chain inhibitor, modulates the photoprotective mechanism of apple trees. Plants 2021, 10, 2803. [Google Scholar] [CrossRef]
- Stern, R.A. The photosynthesis inhibitor metamitron is a highly effective thinner for ‘Golden Delicious’ apple in a warm climate. Fruits 2015, 70, 127–134. [Google Scholar] [CrossRef]
- McArtney, S.J.; Obermiller, J.D.; Arellano, C. Comparison of the effects of metamitron on chlorophyll fluorescence and fruit set in apple and peach. Hort. Sci. 2012, 47, 509–514. [Google Scholar] [CrossRef]
- Christensen, M.G.; Teicher, H.B.; Streibig, J.C. Linking fluorescence induction curve andbiomass in herbicide screening. Pest Manag. Sci. 2003, 59, 1303–1310. [Google Scholar] [CrossRef]
- Lang, J.; Barták, M.; Hájek, J.; Váczi, P.; Zikmundová, B. Chilling effects on primary photosynthetic processes in Medicago sativa: Acclimatory changes after short- and long-term exposure to low temperatures. Biologia 2020, 75, 1105–1114. [Google Scholar] [CrossRef]
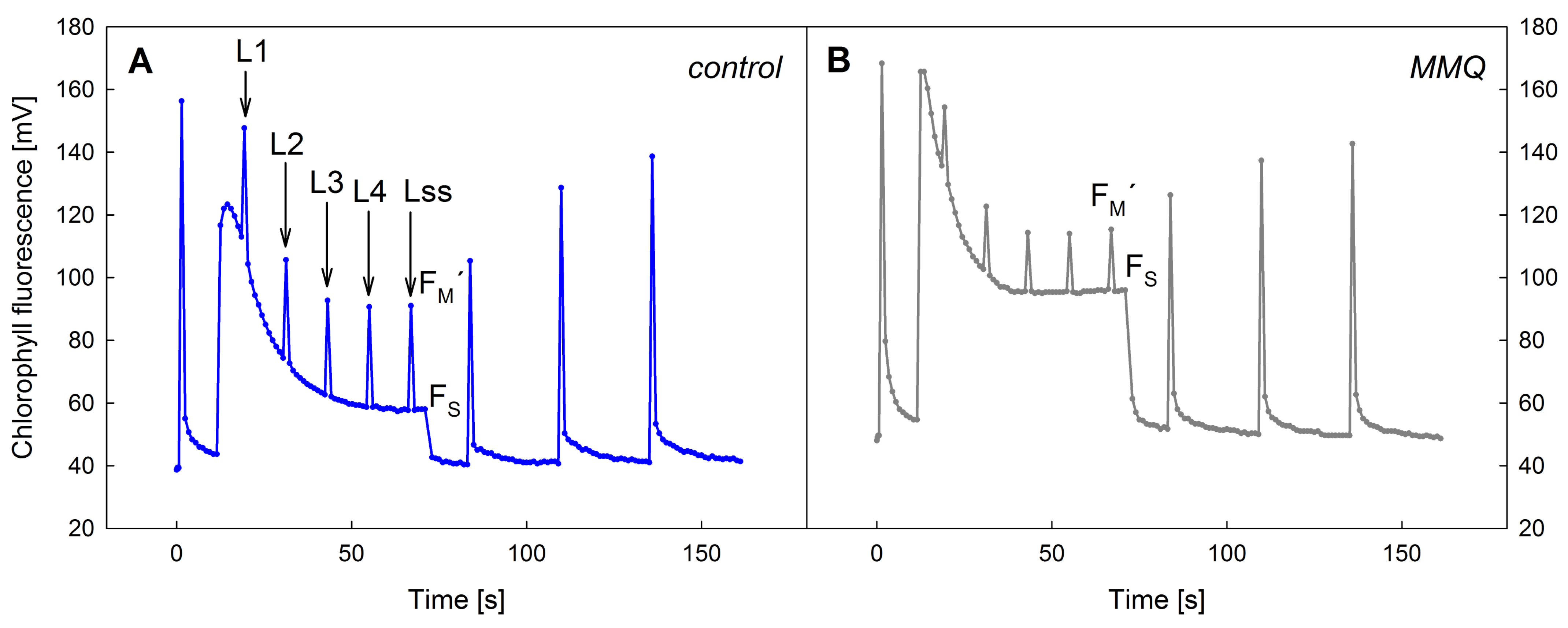

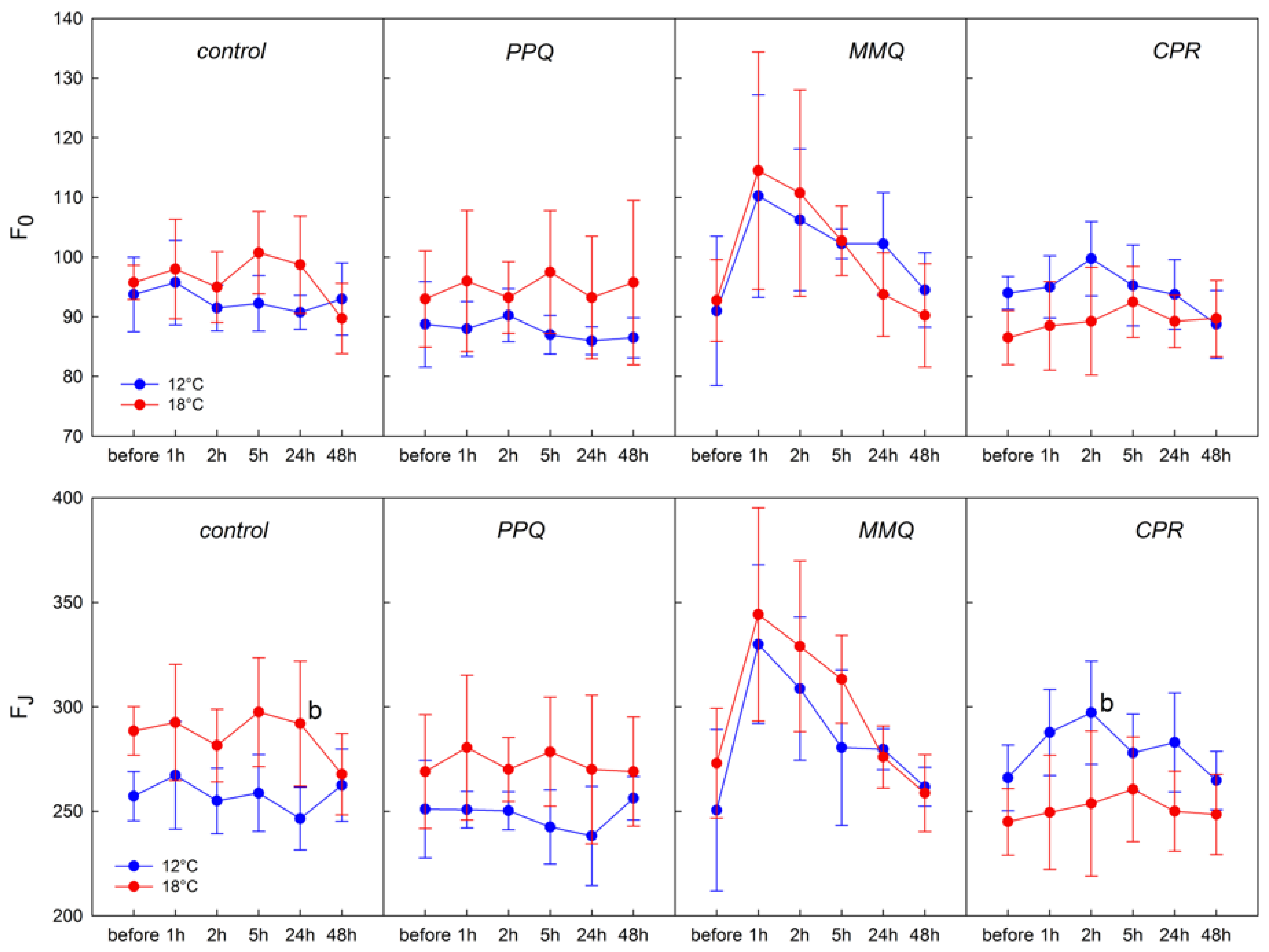
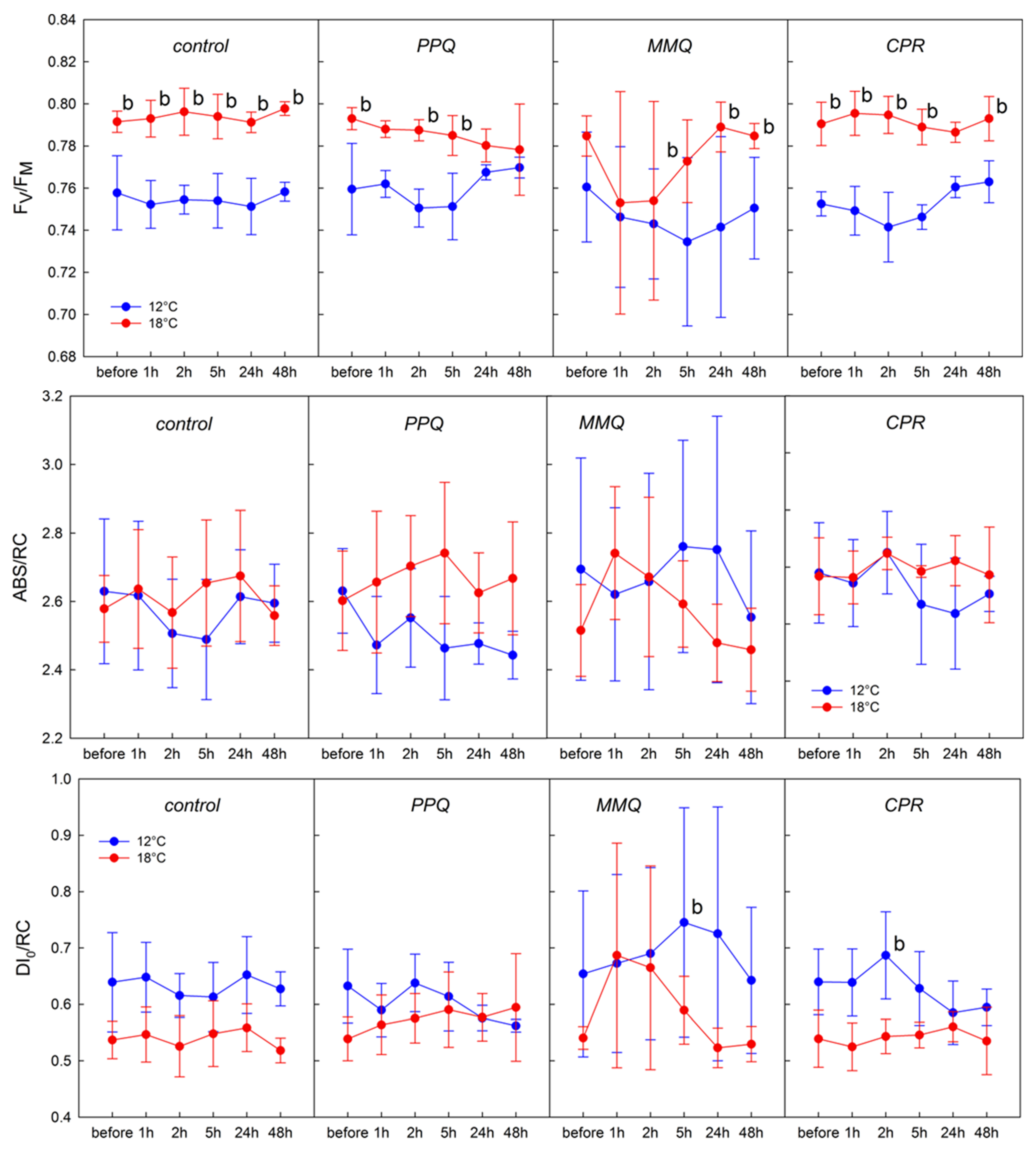
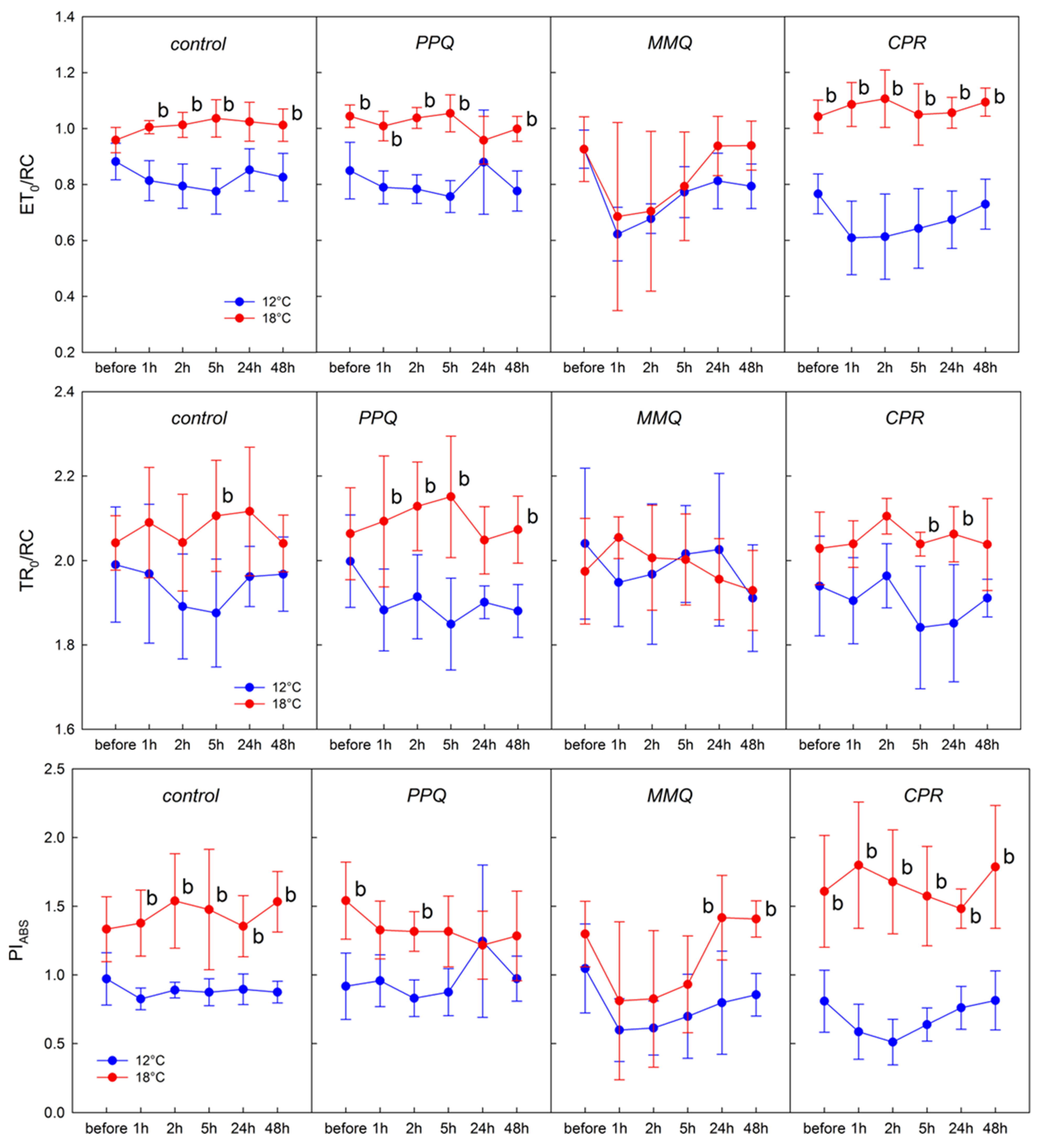
| Abbrev. | Formula/Equations | Explanation |
|---|---|---|
| F0 | Background chlorophyll fluorescence (F0, O point) | |
| FJ | Chlorophyll fluorescence signal at J point | |
| FV/FM | FV/FM = (FM − F0)/FM | Maximal quantum yield of PSII fluorescence |
| NPQ | NPQ = (FM − FM’)/FM’ | Non-photochemical quenching |
| PIABS | PIABS = (RC/ABS) · [φP0/(1 − φP0)] · [ψ0/(1 − ψ0)] | Performance index (potential) for energy conservation from exciton to the reduction in intersystem electron acceptors |
| ABS/RC | ABS/RC = M0(1/VJ)(1/φP0) | Absorption flux (of antenna chlorophylls) per RC |
| TR0/RC | TR0/RC = M0(1/VJ) | Trapped energy flux (leading to QA reduction) per RC |
| ET0/RC | ET0/RC = M0 · (1/VJ) · ψ0 | Electron transport flux (further than QA) per RC |
| DI0/RC | DI0/RC = (ABS/RC) − (TR0/RC) | The flux of dissipated excitation energy at time 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, J.; Barták, M.; Váczi, P.; Hájek, J. Effect of Some Herbicides on Primary Photosynthesis in Malva moschata as a Prospective Plant for Agricultural Grass Mixtures. Agronomy 2024, 14, 10. https://doi.org/10.3390/agronomy14010010
Lang J, Barták M, Váczi P, Hájek J. Effect of Some Herbicides on Primary Photosynthesis in Malva moschata as a Prospective Plant for Agricultural Grass Mixtures. Agronomy. 2024; 14(1):10. https://doi.org/10.3390/agronomy14010010
Chicago/Turabian StyleLang, Jaroslav, Miloš Barták, Peter Váczi, and Josef Hájek. 2024. "Effect of Some Herbicides on Primary Photosynthesis in Malva moschata as a Prospective Plant for Agricultural Grass Mixtures" Agronomy 14, no. 1: 10. https://doi.org/10.3390/agronomy14010010
APA StyleLang, J., Barták, M., Váczi, P., & Hájek, J. (2024). Effect of Some Herbicides on Primary Photosynthesis in Malva moschata as a Prospective Plant for Agricultural Grass Mixtures. Agronomy, 14(1), 10. https://doi.org/10.3390/agronomy14010010






