Comparative Study on the Effects of Different Soil Improvement Methods in Blueberry Soil
Abstract
:1. Introduction
2. Materials and Methods
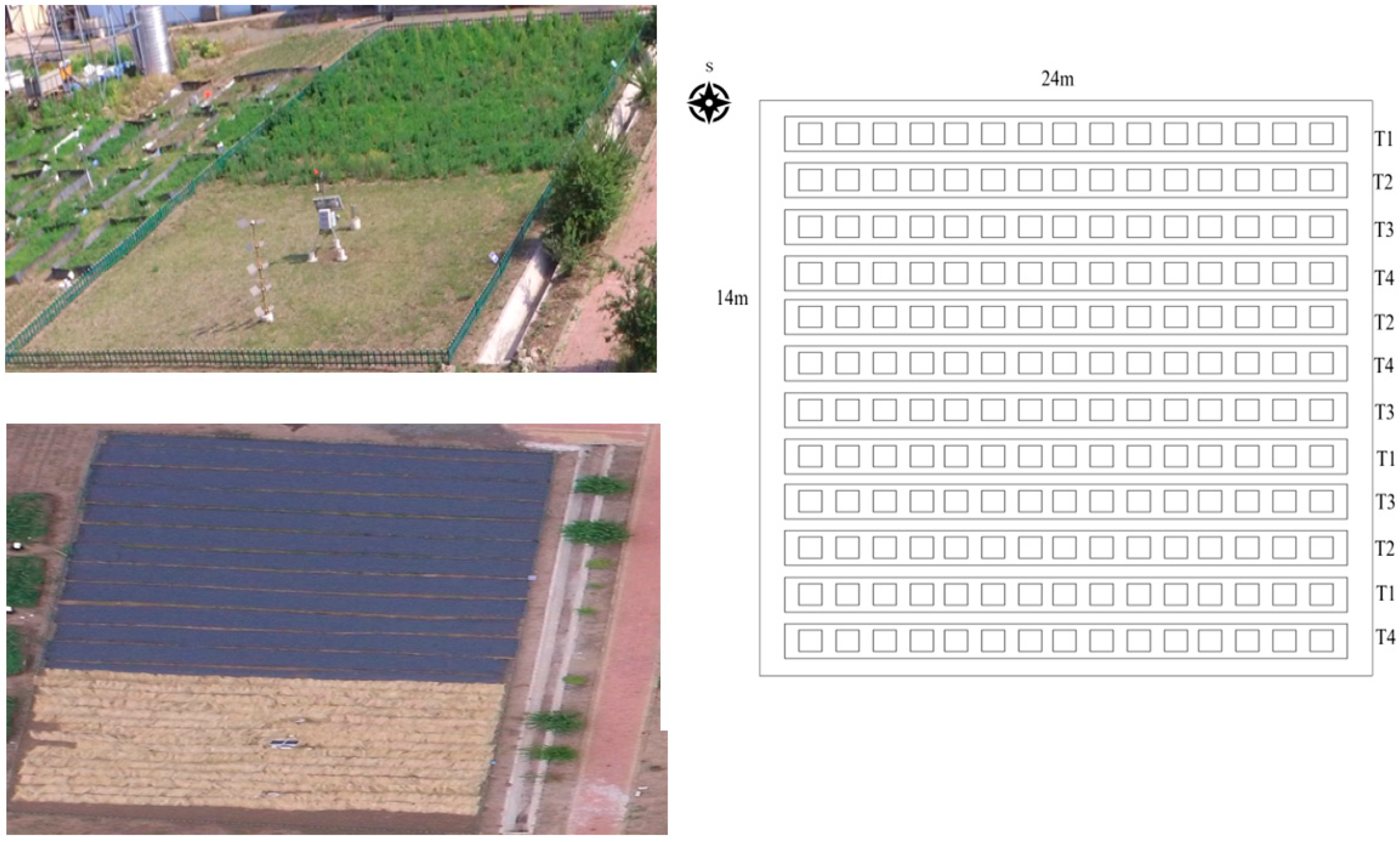
2.1. Experimental Design
2.2. Sequencing
2.3. Data Analysis
3. Results
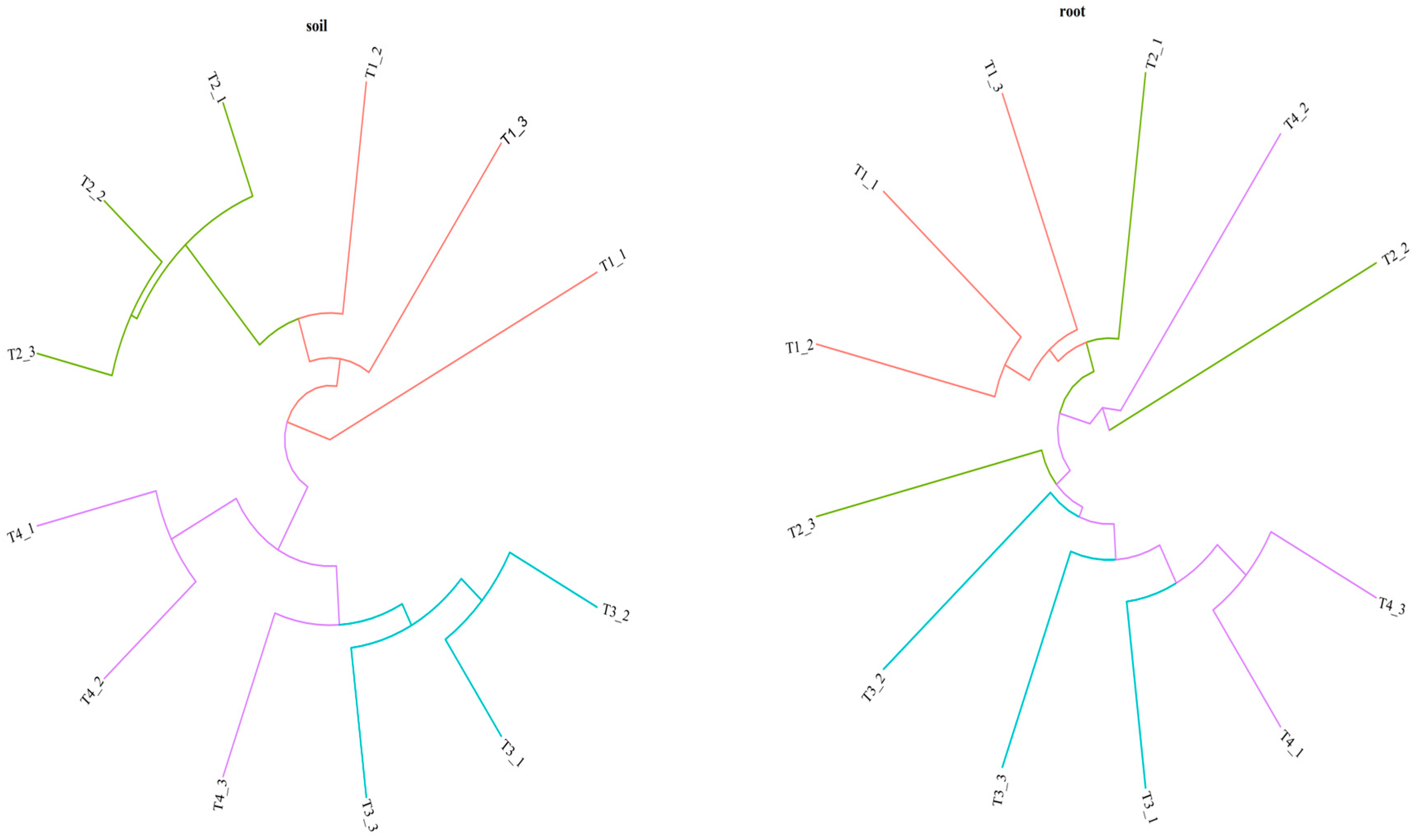
3.1. Bacterial Community Diversity and Composition
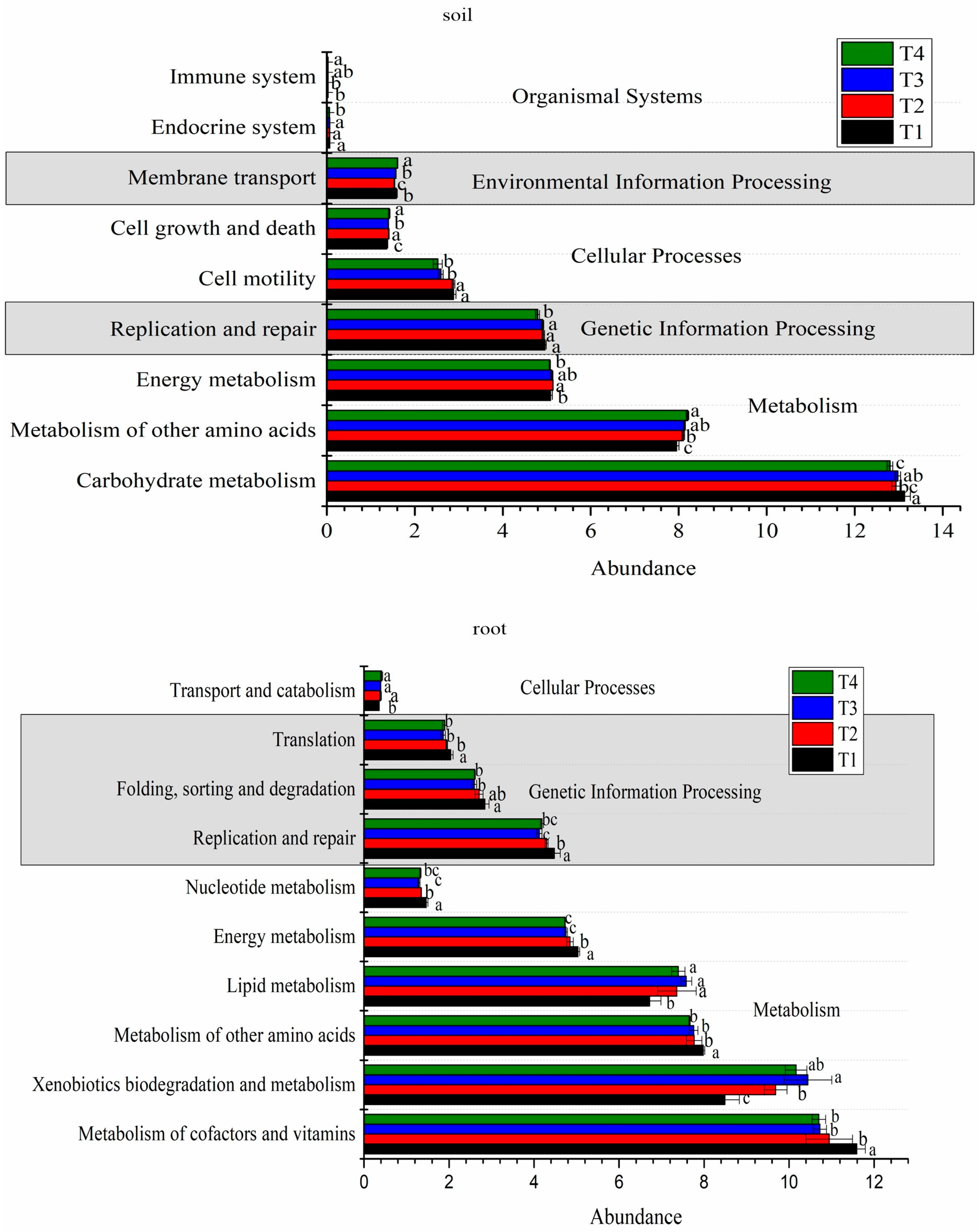
3.2. Impact of Different Soil Improvement Methods on Microbial Function in the Rhizosphere and Roots of Blueberry
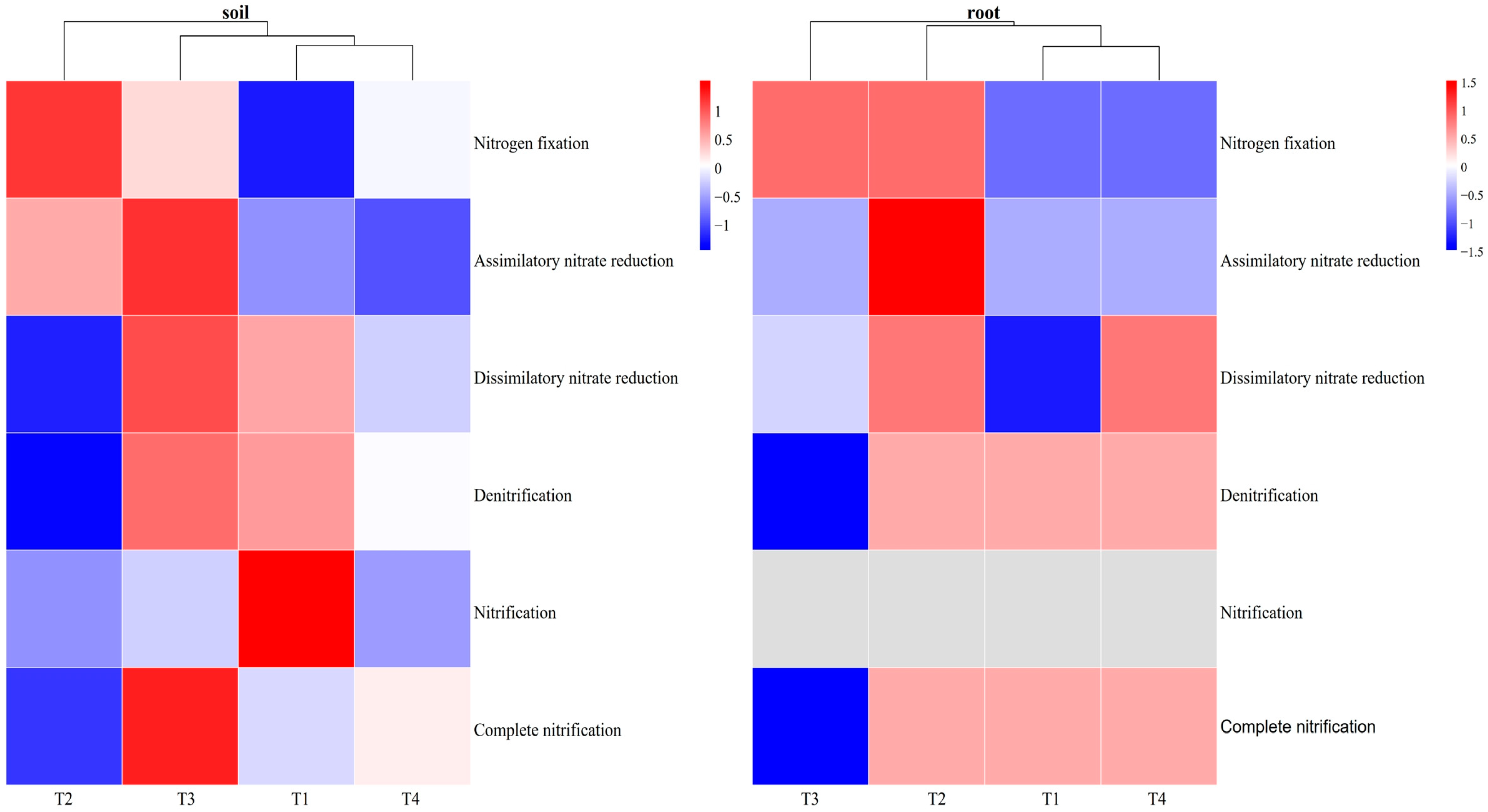
3.3. Functional Bacteria and Modules Involved in the N Cycle
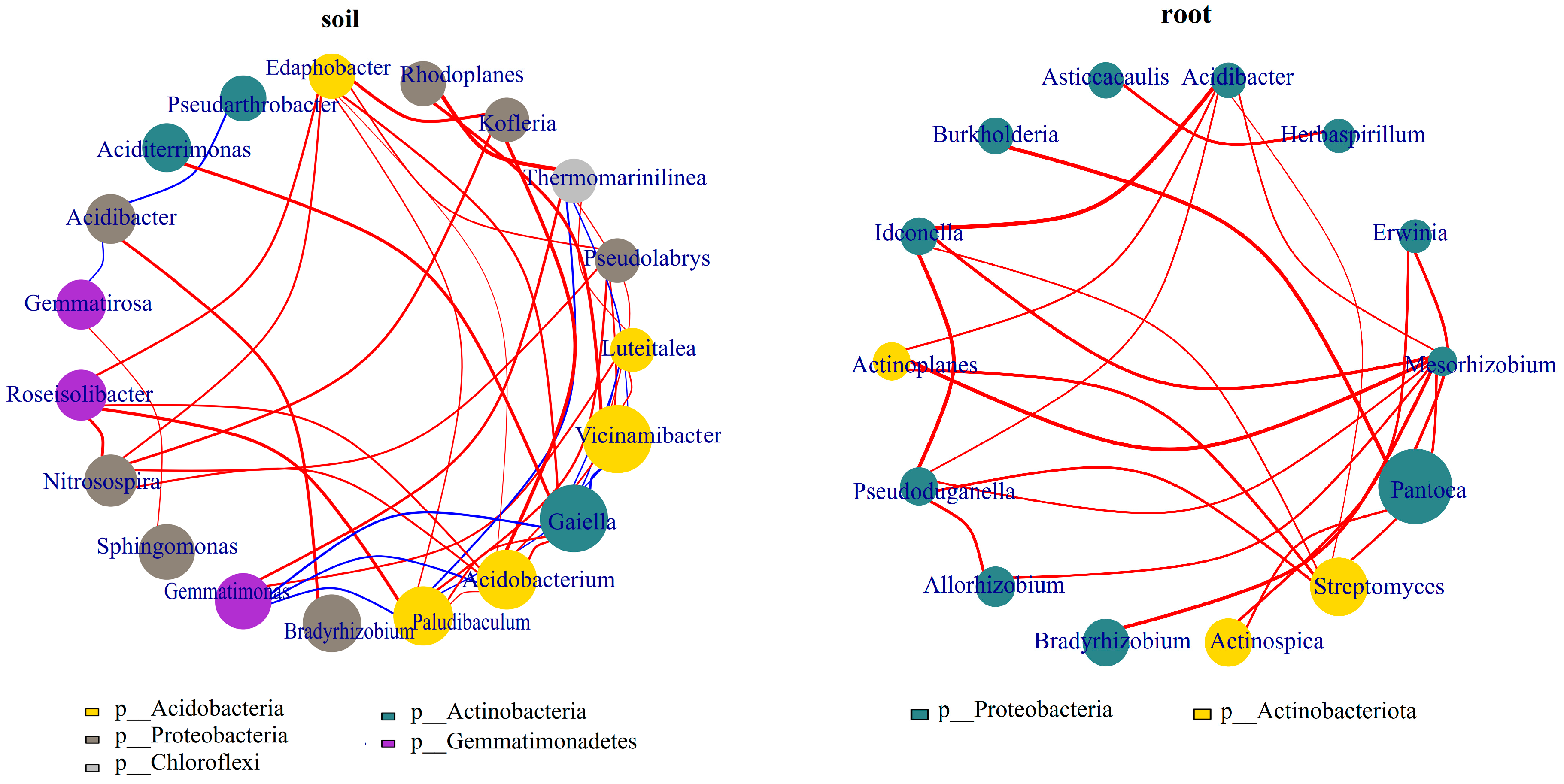
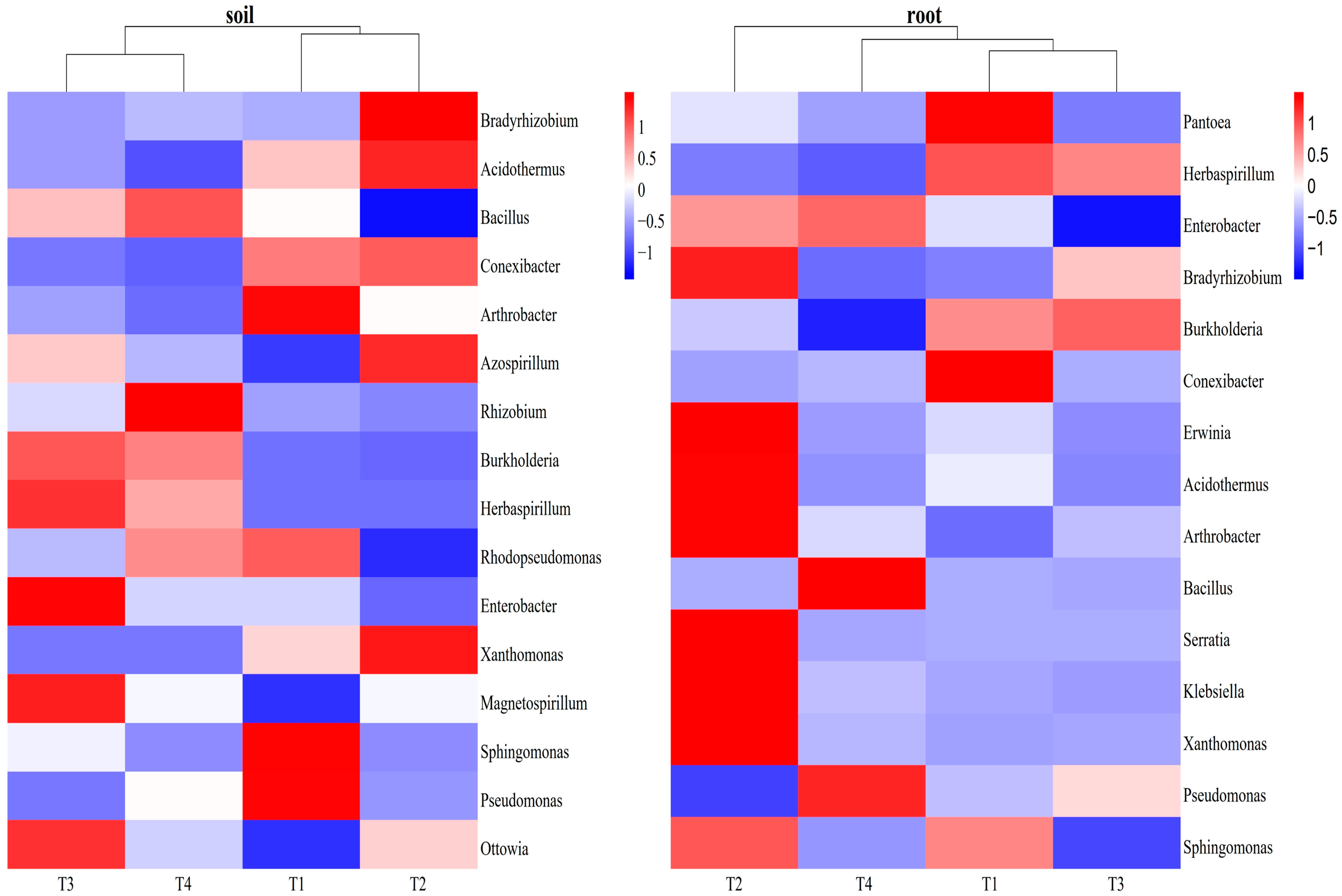
3.3.1. Influences of Soil Improvement on Functional Bacteria in Blueberry Rhizosphere Soil and Roots
3.3.2. The Effect of Soil Improvement on N Cycle at the Module Level in Blueberry Rhizosphere Soil and Roots
4. Discussion
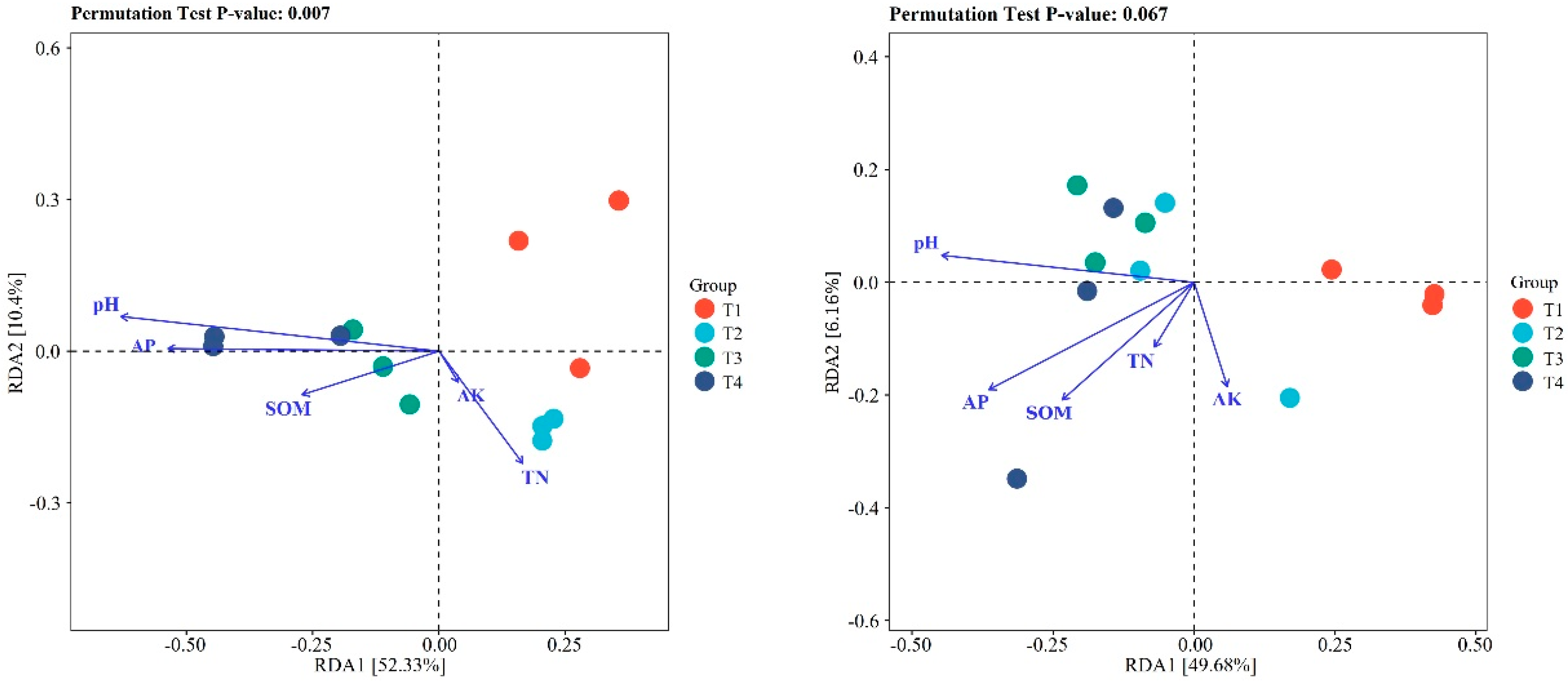
4.1. Differences in Microbial Community Diversity and Structure in Blueberry Rhizosphere and Roots under Different Soil Improvement Methods
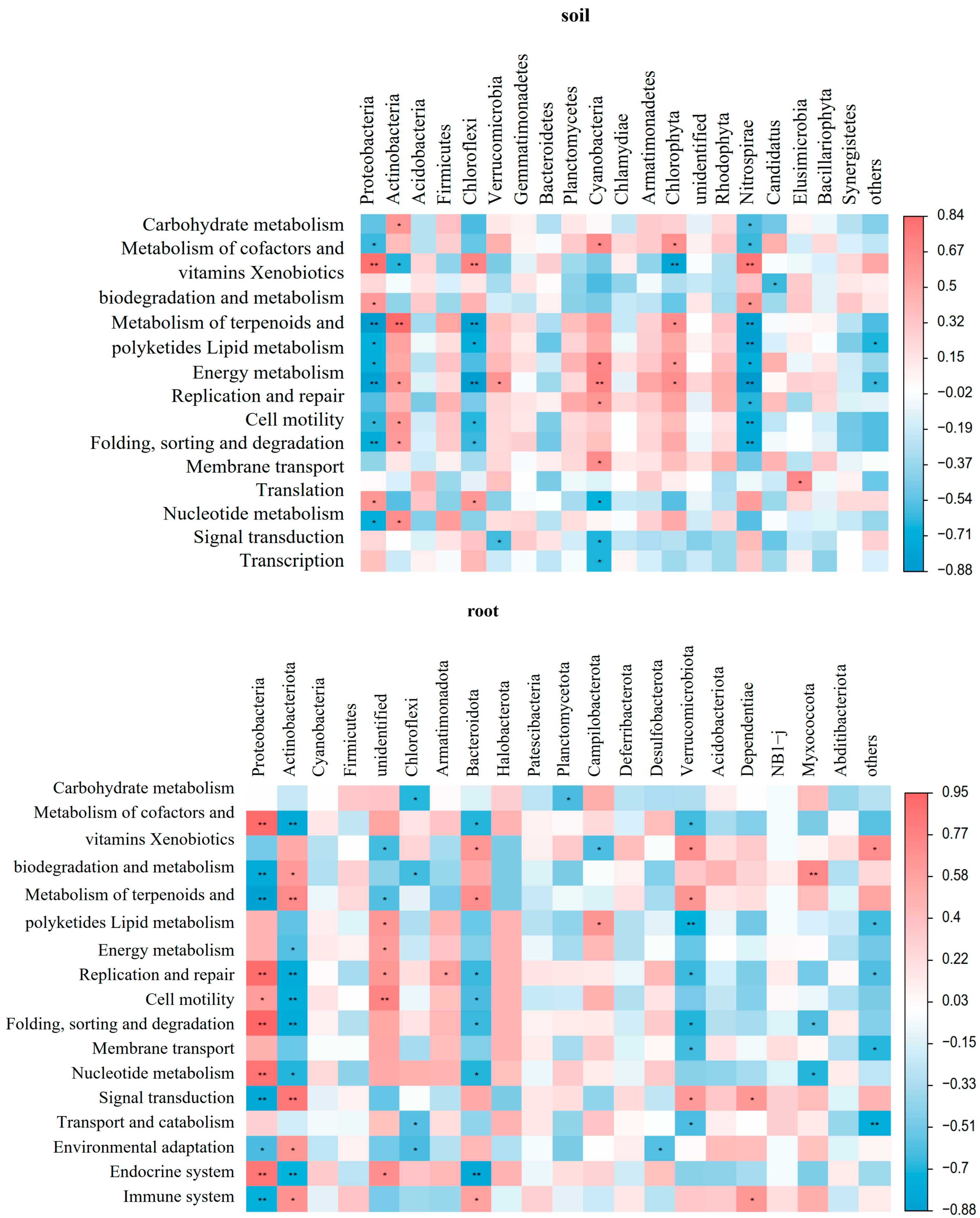
4.2. The Function of Microbial Community in Blueberry Rhizosphere Soil and Roots
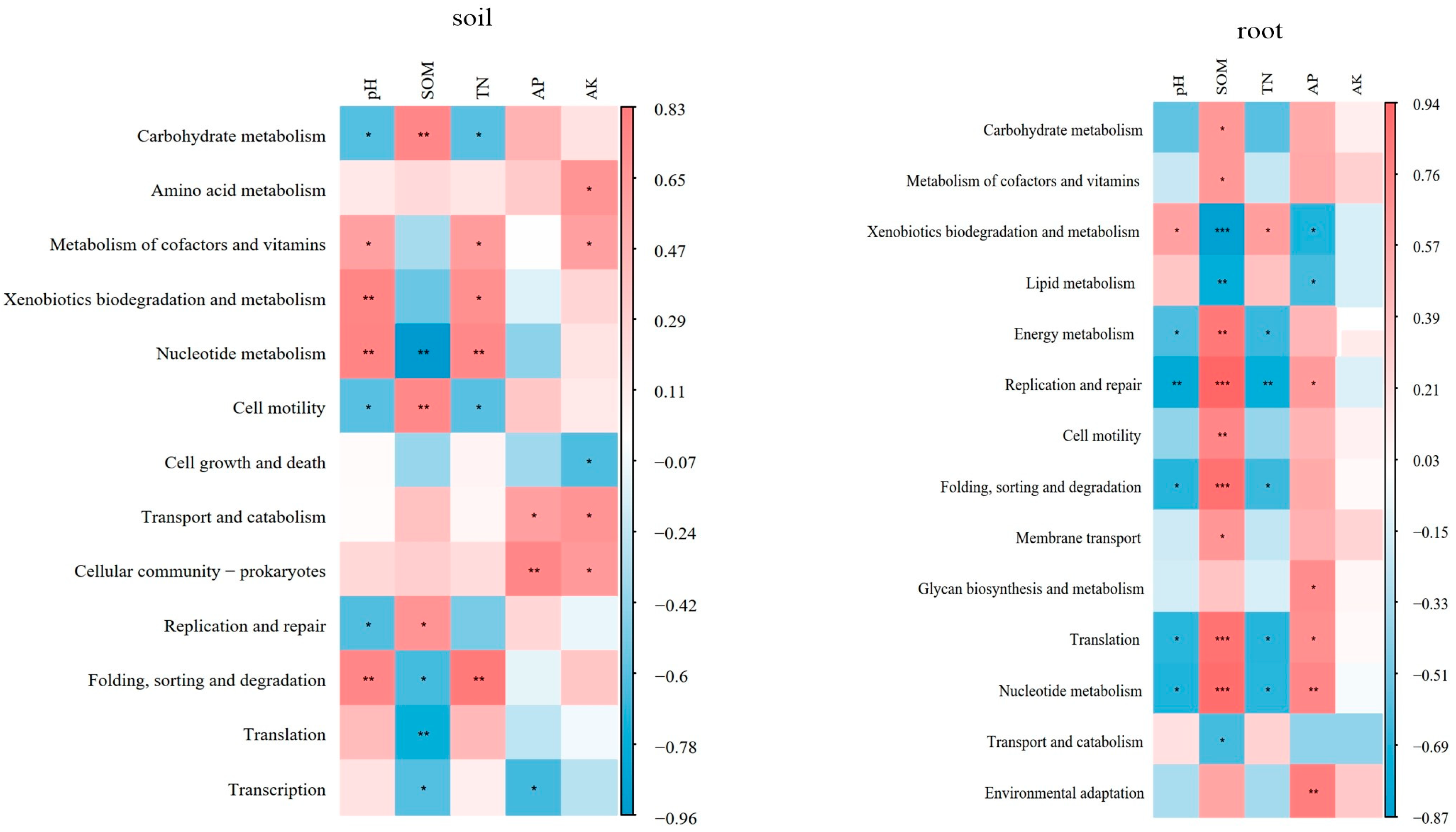
4.3. Effects of Different Soil Improvement Methods on N Cycle
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Peat | Sulfur | Rice Husk | Mushroom Bran | |
|---|---|---|---|---|
| T1 | 1.47 | 0.02 | 0 | 0 |
| T2 | 1.47 | 0 | 0.02 | 0 |
| T3 | 0 | 0 | 0 | 1.53 |
| T4 | 0.74 | 0 | 0 | 0.77 |
| pH | SOM % | TN g/kg | AP g/kg | AK g/kg |
|---|---|---|---|---|
| 5.73 ± 0.01 c | 9.35 ± 0.38 a | 3.99 ± 0.11 bc | 33.60 ± 4.90 a | 152.53 ± 3.00 a |
| 5.53 ± 0.01 d | 8.77 ± 0.13 b | 3.89 ± 0.05 c | 23.33 ± 2.02 b | 118.80 ± 6.45 c |
| 6.15 ± 0.00 a | 7.87 ± 0.06 c | 4.32 ± 0.01 a | 23.07 ± 3.05 b | 154.27 ± 6.72 a |
| 6.13 ± 0.00 b | 8.61 ± 0.01 b | 4.12 ± 0.11 b | 24.20 ± 2.10 b | 139.00 ± 3.00 b |
| Soil | T1 | T2 | T3 | T4 |
|---|---|---|---|---|
| Carbohydrate metabolism | 13.13 ± 0.13 a | 12.93 ± 0.10 bc | 12.98 ± 0.06 ab | 12.80 ± 0.06 c |
| Amino acid metabolism | 13.01 ± 0.14 a | 12.90 ± 0.09 a | 12.96 ± 0.08 a | 12.92 ± 0.02 a |
| Replication and repair | 4.96 ± 0.03 a | 4.92 ± 0.03 a | 4.91 ± 0.03 a | 4.79 ± 0.04 b |
| Cell motility | 2.88 ± 0.06 a | 2.87 ± 0.04 a | 2.59 ± 0.05 b | 2.52 ± 0.10 b |
| Membrane transport | 1.57 ± 0.03 b | 1.54 ± 0.01 c | 1.57 ± 0.00 b | 1.61 ± 0.01 a |
| Environmental adaptation | 0.16 ± 0.00 a | 0.16 ± 0.00 a | 0.16 ± 0.00 a | 0.16 ± 0.00 a |
| root | T1 | T2 | T3 | T4 |
| Carbohydrate metabolism | 13.22 ± 0.36 a | 13.11 ± 0.27 a | 12.71 ± 0.27 a | 12.81 ± 0.24 a |
| Amino acid metabolism | 13.31 ± 0.39 a | 13.53 ± 0.33 a | 13.88 ± 0.33 a | 13.19 ± 0.24 a |
| Replication and repair | 4.48 ± 0.04 a | 4.29 ± 0.06 a | 4.12 ± 0.06 a | 4.46 ± 0.04 a |
| Cell motility | 3.99 ± 0.46 a | 3.59 ± 0.15 a | 3.53 ± 0.15 a | 3.83 ± 0.41 a |
| Membrane transport | 2.76 ± 0.27 a | 2.36 ± 0.11 a | 2.38 ± 0.11 a | 2.50 ± 0.16 a |
| Environmental adaptation | 0.19 ± 0.01 a | 0.16 ± 0.02 a | 0.15 ± 0.02 a | 0.29 ± 0.00 a |
| pH | SOM | TN | AP | AK | |
|---|---|---|---|---|---|
| soil | |||||
| Bradyrhizobium | −0.58 | 0.15 | −0.54 | −0.29 | −0.54 |
| Burkholderia | 0.81 ** | −0.76 ** | 0.82 ** | −0.20 | 0.20 |
| Bacillus | 0.63 * | −0.34 | 0.51 | 0.01 | 0.22 |
| Azospirillum | −0.20 | −0.39 | −0.13 | −0.63 * | −0.54 |
| Rhizobium | 0.77 ** | −0.59 * | 0.68 * | −0.09 | 0.29 |
| Herbaspirillum | 0.88 ** | −0.81 ** | 0.88 ** | −0.31 | 0.34 |
| Rhodopseudomonas | 0.24 | 0.18 | 0.17 | 0.54 | 0.44 |
| Magnetospirillum | 0.48 | −0.72 ** | 0.56 | −0.37 | −0.02 |
| Enterobacter | 0.33 | −0.32 | 0.18 | −0.28 | 0.30 |
| Arthrobacter | −0.50 | 0.62 * | −0.46 | 0.30 | 0.11 |
| Xanthomonas | −0.57 | 0.25 | −0.44 | −0.16 | −0.41 |
| Klebsiella | 0.11 | 0.18 | −0.05 | 0.50 | 0.71 ** |
| Conexibacter | −0.72 ** | 0.57 | −0.63 * | 0.17 | −0.20 |
| Acidothermus | −0.69 * | 0.60 * | −0.54 | 0.34 | −0.24 |
| Sphingomonas | 0.26 | 0.27 | 0.19 | 0.48 | 0.74 ** |
| Pseudomonas | −0.02 | 0.50 | 0.06 | 0.87 ** | 0.55 |
| Ottowia | 0.37 | −0.63 * | 0.50 | −0.63 * | −0.13 |
| root | |||||
| Pantoea | −0.78 ** | 0.97 ** | −0.77 ** | 0.59 * | −0.13 |
| Burkholderia | 0.40 | −0.17 | 0.42 | 0.08 | 0.66 * |
| Bradyrhizobium | −0.39 | −0.01 | −0.32 | −0.46 | −0.30 |
| Herbaspirillum | −0.03 | 0.27 | −0.05 | 0.34 | 0.37 |
| Bacillus | 0.01 | −0.16 | 0.07 | −0.26 | −0.56 |
| Enterobacter | −0.53 | 0.31 | −0.54 | −0.26 | −0.82 ** |
| Erwinia | −0.98 ** | 0.77 ** | −0.95 ** | 0.10 | −0.59 * |
| Serratia | −0.55 | 0.22 | −0.058 * | −0.45 | −0.38 |
| Arthrobacter | −0.25 | −0.34 | −0.21 | −0.55 | −0.59 * |
| Xanthomonas | −0.29 | −0.01 | −0.17 | −0.46 | −0.76 ** |
| Klebsiella | −0.36 | 0.25 | −0.37 | −0.32 | −0.49 |
| Conexibacter | 0.08 | 0.42 | 0.03 | 0.23 | 0.40 |
| Acidothermus | −0.57 | 0.19 | −0.55 | 0.05 | −0.36 |
| Pseudomonas | 0.72 ** | −0.55 | 0.69 * | −0.10 | 0.11 |
| Sphingomonas | −0.87 ** | 0.83 ** | −0.76 ** | 0.29 | −0.51 |
References
- Delpino, F.M.; Figueiredo, L.M.; Gonçalves da Silva, T.; Flores, T.R. Effects of blueberry and cranberry on type 2 diabetes parameters in individuals with or without diabetes: A systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovas. 2022, 32, 1093–1109. [Google Scholar] [CrossRef]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary blueberry and soluble fiber supplementation reduces risk of gestational diabetes in women with obesity in a randomized controlled trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef]
- Stote, K.S.; Wilson, M.M.; Hallenbeck, D.; Thomas, K.; Rourke, J.M.; Sweeney, M.I.; Gottschall-Pass, K.T.; Gosmanov, A.R. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: An 8-week, double-blind, randomized, placebo-controlled trial. Curr. Dev. Nutr. 2020, 4, nzaa030. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. Lebensm.-Wiss. Technol. 2019, 109, 233–240. [Google Scholar] [CrossRef]
- He, L.; Jing, G.; Zhao, N.; Lu, Q.; Zhang, Z.; Chen, Z.; Huang, B.; Ding, X. Soil nutrients and the responses of microbial community structure to pine bark and vinegar residues in blueberry cultivation. Appl. Soil. Ecol. 2023, 189, 104907. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Shao, T.; Long, X.; Gao, X.; Zhou, Z. Relationship between rhizosphere soil properties and disease severity in highbush blueberry (Vaccinium corymbosum). Appl. Soil. Ecol. 2019, 137, 187–194. [Google Scholar] [CrossRef]
- Chen, J.; Ren, R.; Wang, H.; Luo, T.; Yao, X.; Zhang, Z.; Hu, K. Effect of Lou soil pH change on selenium forms and availability. Northwest. Geol. 2020, 53, 254–260. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Y.; Tavakkoli, E.; Pan, X.; Liao, F.; Chen, W.; Guo, W. Preferential ammonium: Nitrate ratio of blueberry is regulated by nitrogen transport and reduction systems. Sci. Hortic. 2021, 288, 110345. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, Y.; Wei, J.; Yu, H. Common problems in the process of blueberry cultivation-soil organic matter improvement is not in place. Fruit Grower’s Friend 2017, 6, 25–26. [Google Scholar]
- Wei, H.; Li, G.; Ding, Q. Common problems and solutions of blueberry planting soil management. Fruit Grower’s Friend 2014, 10, 19. [Google Scholar] [CrossRef]
- Gagnon, B.; Simard, R.R.; Lalande, R.; Lafond, J. Improvement of soil properties and fruit yield of native lowbush blueberry by papermill sludge addition. Can. J. Soil. Sci. 2003, 83, 1–9. [Google Scholar] [CrossRef]
- Naghman, R.; Bhatti, M.T.; Najabat, Z.; Hyder, S.; Rizvi, Z.F.; Gondal, A.S.; Zafar, Z.; Malik, S.; Iqbal, R.; Hafeez, A.; et al. Organic amendments: A natural way to suppress phytopathogens: A sustainable approach to go green. Turk. J. Agric. For. 2023, 47, 602–622. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, C.; Xu, C. Progress in research on function, utilization and organic cultivation of blueberry. J. JLIT 2009, 25, 49–54. [Google Scholar]
- Sun, J.; Dong, L.; Xu, G.; Shao, H. Effects of furfural and it biocha additions on physcial-chemical characteristics of a sline soil. J. Agro-Environ. Sci. 2014, 33, 532–538. [Google Scholar]
- Zhou, X.; Tang, Z.; Bai, H.; Li, J.; Mo, L. Research status and prospect of soil environment on blueberry plant growth and development. J. JLNU 2019, 40, 100–105. [Google Scholar]
- Liu, Y.; Li, H. Research on Blueberry Cultivation for Soil Improvement in Shaanxi. SXFST 2019, 47, 48–51. [Google Scholar]
- Ochmian, I.; Malinowski, R.; Kubus, M.; Malinowska, K.; Sotek, Z.; Racek, M. The feasibility of growing highbush blueberry (V. corymbosum L.) on loamy calcic soil with the use of organic substrates. Sci. Hortic. 2019, 257, 108690. [Google Scholar] [CrossRef]
- Kang, B. Effects of Mixed Application of Natural Soil Improvement Materials and PAM on Soil Physcial and Chemical Properties. Master’s Thesis, Northweat A&F University, Xianyang, China, 2014. [Google Scholar]
- Dymov, A.A.; Gorbach, N.M.; Goncharova, N.N.; Karpenko, L.V.; Gabov, D.N.; Kutyavin, I.N.; Startsev, V.V.; Mazur, A.S.; Grodnitskaya, I.D. Holocene and recent fires influence on soil organic matter, microbiological and physico-chemical properties of peats in the European North-East of Russia. Catena 2022, 217, 106449. [Google Scholar] [CrossRef]
- Tian, X. Effects of Soil Compaction on Sulfur Metabolism of Yong Apple Tree and Improvement of Rice Husk Biochar. Master’s Thesis, Shandong Agriculture University, Tai’an, China, 2023. [Google Scholar]
- Chen, H. Research on the Recycle Unilization of Spent Mushroom Substrates. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2009. [Google Scholar]
- Chen, Y.; Xie, Y.; Zhou, H.; Chen, F.; Liu, Y.; Chen, Q. Effects of different organic materials mulching on jasmine flower and its soil. Mod. Horticult. 2022, 45, 10–11. [Google Scholar]
- Lin, B. Effecting of spent mushroom sunstrates (SMS) and biogas residue on improving navel orange production and its fruit quality. Fujian J. Agric. Sci. 2006, 21, 293–295. [Google Scholar] [CrossRef]
- Yurgel, S.N.; Douglas, G.M.; Dusault, A.; Percival, D.; Langille, M.G.I. Dissecting community structure in wild blueberry root and soil microbiome. Front. Microbiol. 2018, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, J.; Li, Y.; Koziol, L.; Podzikowski, L.; Delgado-Baquerizo, M.; Wang, G.; Zhang, J. Relationships between soil biodiversity and multifunctionality in croplands depend on salinity and organic matter. Geoderma 2023, 429, 116273. [Google Scholar] [CrossRef]
- Guo, X.; Wan, Y.; Shakeel, M.; Wang, D.; Xiao, L. Effect of mycorrhizal fungi inoculation on bacterial diversity, community structure and fruit yield of blueberry. Rhizosphere 2021, 19, 100360. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, R.; Zhu, W.; Shi, Y.; Ni, S.; Wu, C.; Li, T. Impact of dielectric barrier discharge cold plasma on the quality and phenolic metabolism in blueberries based on metabonomic analysis. Postharvest Biol. Technol. 2023, 197, 112208. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Zheng, H. Research progress on plant endophytic nitrogen-fixing bacteria and their nitrogen fixation mechanism. Mod. Horticult. 2022, 12, 17–26. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Santoyo, G. Plant-microbial endophytes interactions: Scrutinizing their beneficial mechanisms from genomic explorations. Curr. Plant Biol. 2021, 25, 100189. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; He, Y.; Yu, X.; Chen, S.; Penttinen, P.; Liu, S.; Yang, Y.; Zhao, K.; Zou, L. Organic fertilizers shape soil microbial communities and increase soil amino acid metabolites content in a blueberry orchard. Microb. Ecol. 2022, 85, 234–246. [Google Scholar] [CrossRef]
- Chamkhi, I.; Sbabou, L.; Aurag, J. Improved growth and quality of saffron (Crocus sativus L.) in the field conditions through inoculation with selected native plant growth-promoting rhizobacteria (PGPR). Ind. Crop Prod. 2023, 197, 116606. [Google Scholar] [CrossRef]
- Khan, A.; Bano, A.; Khan, R.A.; Khan, N. Role of PGPR in suppressing the growth of Macrophomina phaseolina by regulating antioxidant enzymes and secondary metabolites in Vigna radiata (L.) R. Wilczek. S. Afr. J. Bot. 2023, 158, 443–451. [Google Scholar] [CrossRef]
- Castillo, P.; Molina, R.; Andrade, A.; Vigliocco, A.; Alemano, S.; Cassán, F.D. Phytohormones and other plant growth regulators produced by PGPR: The genus Azospirillum. In Handbook for Azospirillum; Springer International Publishing: Cham, Switzerland, 2015; pp. 115–138. [Google Scholar] [CrossRef]
- Khan, A.L.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Gou, Z.; Zheng, H.; He, Z.; Su, Y.; Chen, S.; Chen, H.; Chen, G.; Ma, N.L.; Sun, Y. The combined action of biochar and nitrogen-fixing bacteria on microbial and enzymatic activities of soil N cycling. Environ. Pollut. 2023, 317, 120790. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhao, X.; Zhu, L.; Wu, J.; Mohamed, T.A.; Zhang, X.; Chen, X.; Zhao, Y.; Wei, Z. Elucidating the negative effect of denitrification on aromatic humic substance formation during sludge aerobic fermentation. J. Hazard. Mater. 2020, 388, 122086. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, Y.; Wang, Z.; Wang, W.; Ren, N. Insight into effects of electro-dewatering pretreatment on nitrous oxide emission involved in related functional genes in sewage sludge composting. Bioresour. Technol. 2018, 265, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, M.H.; Mustapha, N.A.; Maeda, T.; Ramli, N.; Sakai, K.; Hassan, M. Biochar enhanced the nitrifying and denitrifying bacterial communities during the composting of poultry manure and rice straw. Waste Manag. 2020, 106, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wu, J.; Zhang, Y.; Li, Q.; Liu, S.; Gao, Y. The effects of localized plant-soil-microbe interactions on soil nitrogen cycle in maize rhizosphere soil under long-term fertilizers. Agronomy 2023, 13, 2114. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, X.; Gao, X.; Shao, T.; Long, X.; Rengel, Z. Effects of Soil Properties and Microbiome on Highbush Blueberry (Vaccinium corymbosum) Growth. Agronomy 2022, 12, 1263. [Google Scholar] [CrossRef]
- Darnell, R.L.; Casamali, B.; Williamson, J.G. Nutrient assimilation in southern highbush blueberry and implications for the field. HortTechnology 2015, 25, 460–463. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Growth and physiological characteristics of four blueberry cultivars under different high soil pH treatments. Environ. Exp. Bot. 2022, 197, 104842. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Q.; Wei, J.; Jiang, J.; Yu, H. Growth, fruit yield, photosynthetic characteristics, and leaf microelement concentration of two blueberry cultivars under different long-term soil pH treatments. Agronomy 2019, 9, 357. [Google Scholar] [CrossRef]
- Yan, J. Effects of Different Fertilizers on Physiological and Fruit Quality of Blueberry. Master’s Thesis, Guizhou University, Guiyang, China, 2018. [Google Scholar]
- Aqeel, M.; Ran, J.; Hu, W.; Irshad, M.K.; Dong, L.; Akram, M.A.; Eldesoky, G.E.; Aljuwayid, A.M.; Chuah, L.F.; Deng, J. Plant-soil-microbe interactions in maintaining ecosystem stability and coordinated turnover under changing environmental conditions. Chemosphere 2023, 318, 137924. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Kang, L.; Liu, C.; Li, M. Study on the effect of planting pattern adjustment on the growth of kiwifruit inter-root microorganisms and fruit quality. Turk. J. Agric. For. 2023, 47, 263–272. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Li, R.; Zhang, J.; Liu, Y.; Lv, L.; Zhu, H.; Wu, W.; Li, W. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil. Biol. Biochem. 2017, 109, 145–155. [Google Scholar] [CrossRef]
- Khan, A.; Ding, Z.; Ishaq, M.; Khan, I.; Ahmed, A.A.; Khan, A.Q.; Guo, X. Applications of beneficial plant growth promoting rhizobacteria and mycorrhizae in rhizosphere and plant growth: A review. Int. J. Agric. Biol. Eng. 2020, 13, 199–208. [Google Scholar] [CrossRef]
- Robinson, R.J.; Fraaije, B.A.; Clark, I.M.; Jackson, R.W.; Hirsch, P.R.; Mauchline, T.H. Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant Soil 2015, 405, 381–396. [Google Scholar] [CrossRef]
- Ma, Y.; Weisenhorn, P.; Guo, X.; Wang, D.; Yang, T.; Shi, Y.; Zhang, H.; Chu, H. Effect of long-term fertilization on bacterial communities in wheat endosphere. Pedosphere 2021, 31, 538–548. [Google Scholar] [CrossRef]
- Chen, J.; Dai, J.; Song, X.; Jiang, Q.; Zhao, C.; Sun, C.; Chen, C.; Chen, N.; Han, B. Endophytic microbiota comparison of Dendrobium huoshanense root and stem in different growth years. Planta Med. 2020, 86, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zheng, S.; Hu, Y.; Li, H.; Chen, Y.; Chun, Z. Endophytic bacterial diversity of the medicinal orchid Dendrobium nobile. S. Afr. J. Bot. 2023, 158, 90–97. [Google Scholar] [CrossRef]
- Ma, S.; Verheyen, K.; Props, R.; Wasof, S.; Vanhellemont, M.; Boeckx, P.; Boon, N.; De Frenne, P. Plant and soil microbe responses to light, warming and nitrogen addition in a temperate forest. Funct. Ecol. 2018, 32, 1293–1303. [Google Scholar] [CrossRef]
- Du, X.; Hu, H.; Wang, T.; Zou, L.; Zhou, W.; Gao, H.; Ren, X.; Wang, J.; Hu, S. Long-term rice cultivation increases contributions of plant and microbial-derived carbon to soil organic carbon in saline-sodic soils. Sci. Total Environ. 2023, 904, 166713. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, T.; Thomas, B.W.; Chen, J.; Xie, J.; Hu, Y.; Kong, F.; Yang, Y.; Chen, X.; Zhang, Y.; et al. Legume cover crops enhance soil organic carbon via microbial necromass in orchard alleyways. Soil Till. Res. 2023, 234, 105858. [Google Scholar] [CrossRef]
- Mir, I.R.; Rather, B.A.; Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide in co-ordination with nitrogen reverses cadmium-inhibited photosynthetic activity by interacting with ethylene synthesis, strengthening the antioxidant system, and nitrogen and sulfur assimilation in mustard (Brassica juncea L.). Sci. Hortic. 2023, 314, 111958. [Google Scholar] [CrossRef]
- Dey, S.; Kundu, R.; Gopal, G.; Mukherjee, A.; Nag, A.; Paul, S. Enhancement of nitrogen assimilation and photosynthetic efficiency by novel iron pulsing technique in Oryza sativa L. var Pankaj. Plant Physiol. Biochem. 2019, 144, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef] [PubMed]
- Cid-Pérez, T.S.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Avila-Sosa, R. The relation between drying conditions and the development of volatile compounds in saffron (Crocus sativus). Molecules 2021, 26, 6954. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.K.; Malik, K.A.; Hameed, S.; Saddique, M.J.; Ayesha; Fatima, K.; Naqqash, T.; Majeed, A.; Iqbal, M.J.; Imran, A. Growth stimulatory effect of AHL producing Serratia spp. from potato on homologous and non-homologous host plants. Microbiol. Res. 2020, 238, 126506. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, S.; Banerjee, S.; Mukherjee, S.; Ganguli, A.; Mondal, S. Heavy metals bio-removal potential of the isolated Klebsiella sp TIU20 strain which improves growth of economic crop plant (Vigna radiata L.) under heavy metals stress by exhibiting plant growth promoting and protecting traits. Biocatal. Agric. Biotechnol. 2021, 38, 102204. [Google Scholar] [CrossRef]
- Benizri, E.; Kidd, P.S. The Role of the Rhizosphere and Microbes Associated with Hyperaccumulator Plants in Metal Accumulation. In Agromining, Farming for Metals; Springer International Publishing: Cham, Switzerland, 2018; pp. 157–188. [Google Scholar]
- Widdig, M.; Schleuss, P.M.; Weig, A.; Guhr, A.; Spohn, M. Nitrogen and Phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front. Environ. Sci. 2019, 7, 00158. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Kaur, S.; Ubeyitogullari, A. Extraction of phenolic compounds from rice husk via ethanol-water-modified supercritical carbon dioxide. Heliyon 2023, 9, e14196. [Google Scholar] [CrossRef]
- Alitaleshi, F.; Daghbandan, A.; Pendashteh, A. Performance of rice husk biocarrier on ammonia nitrogen removal in the MBBR treating aquaculture wastewater using biological attached growth process: Performance and kinetic study. J. Environ. Chem. Eng. 2023, 12, 111446. [Google Scholar] [CrossRef]
- Saqib Rashid, M.; Liu, G.; Yousaf, B.; Song, Y.; Ahmed, R.; Rehman, A.; Arif, M.; Irshad, S.; Cheema, A.I. Efficacy of rice husk biochar and compost amendments on the translocation, bioavailability, and heavy metals speciation in contaminated soil: Role of free radical production in maize (Zea mays L.). J. Clean. Prod. 2022, 330, 129805. [Google Scholar] [CrossRef]
- Duan, H.; Ji, M.; Chen, A.; Zhang, B.; Shi, J.; Liu, L.; Li, X.; Sun, J. Evaluating the impact of rice husk on successions of bacterial and fungal communities during cow manure composting. Environ. Technol. Innov. 2021, 24, 102084. [Google Scholar] [CrossRef]
- Montañez, A.; Blanco, A.R.; Barlocco, C.; Beracochea, M.; Sicardi, M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl. Soil. Ecol. 2012, 58, 21–28. [Google Scholar] [CrossRef]
- Li, Q.; Koyama, M.; Nakasaki, K. Effect of storage time on organic matter decomposition during composting by inoculating enriched microorganisms. Environ. Technol. Innov. 2023, 29, 102984. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, D.; Song, Z.; Ren, L.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A. Organic fertilizer activates soil beneficial microorganisms to promote strawberry growth and soil health after fumigation. Environ. Pollut. 2022, 295, 118653. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Ma, J.; Jiang, J.; You, X.; Lv, R.; Zhou, S.; Pan, C.; Liu, B.; Xu, Q.; et al. How does biochar influence soil nitrification and nitrification-induced N2O emissions? Sci. Total Environ. 2023, 908, 168530. [Google Scholar] [CrossRef]
- Iboko, M.P.; Dossou-Yovo, E.R.; Obalum, S.E.; Oraegbunam, C.J.; Diedhiou, S.; Brümmer, C.; Témé, N. Paddy rice yield and greenhouse gas emissions: Any trade-off due to co-application of biochar and nitrogen fertilizer? A systematic review. Heliyon 2023, 9, e22132. [Google Scholar] [CrossRef]
- Lee, S.I.; Park, H.J.; Jeong, Y.J.; Seo, B.S.; Kwak, J.H.; Yang, H.I.; Xu, X.; Tang, S.; Cheng, W.; Lim, S.S.; et al. Biochar-induced reduction of N2O emission from East Asian soils under aerobic conditions: Review and data analysis. Environ. Pollut. 2021, 291, 118154. [Google Scholar] [CrossRef]

| OTUs | Chao1 | PD Whole Tree | Shannon | Simpson | ||
|---|---|---|---|---|---|---|
| Soil | T1 | 5234.50 ± 25.50 c | 5889.93 ± 20.49 c | 418.11 ± 57.77 c | 9.45 ± 0.41 c | 0.99 ± 0.00 b |
| T2 | 6700.33 ± 427.10 b | 7536.67 ± 400.84 b | 545.94 ± 40.10 b | 10.13 ± 0.13 b | 1.00 ± 0.00 a | |
| T3 | 6338.50 ± 76.50 b | 7285.38 ± 43.22 b | 501.63 ± 6.93 b | 10.59 ± 0.17 a | 1.00 ± 0.00 a | |
| T4 | 7801.67 ± 395.32 a | 8471.72 ± 321.61 a | 653.56 ± 30.22 a | 10.68 ± 0.01 a | 1.00 ± 0.00 a | |
| Root | T1 | 544.50 ± 42.50 c | 530.56 ± 122.39 c | 61.32 ± 9.24 b | 4.60 ± 0.20 a | 0.90 ± 0.03 a |
| T2 | 720.67 ± 35.92 b | 830.55 ± 39.66 b | 82.07 ± 1.49 a | 5.56 ± 0.52 a | 0.94 ± 0.03 a | |
| T3 | 543.67 ± 80.75 c | 728.38 ± 7.68 bc | 64.08 ± 0.30 b | 4.22 ± 1.19 a | 0.90 ± 0.04 a | |
| T4 | 1113.50 ± 57.50 a | 1084.01 ± 168.38 a | 84.40 ± 7.60 a | 5.43 ± 0.36 a | 0.88 ± 0.07 a |
| pH | SOM | TN | AP | AK | |
|---|---|---|---|---|---|
| soil | |||||
| Nitrogen fixation | −0.14 | −0.39 | −0.05 | −0.57 | −0.52 |
| Assimilatory nitrate reduction | 0.19 | −0.38 | 0.28 | −0.34 | 0.189 |
| Dissimilatory nitrate reduction | 0.69 * | −0.38 | 0.67 * | −0.01 | 0.67 * |
| Denitrification | 0.61 * | −0.24 | 0.62 * | 0.14 | 0.66 * |
| Nitrification | 0.28 | −0.08 | 0.16 | 0.05 | 0.59 * |
| Complete nitrification | 0.88 ** | −0.59 * | 0.86 ** | −0.08 | 0.62 * |
| root | |||||
| Nitrogen fixation | −0.02 | −0.48 | 0.03 | −0.82 ** | −0.45 |
| Assimilatory nitrate reduction | −0.33 | −0.18 | −0.18 | −0.43 | −0.57 |
| Dissimilatory nitrate reduction | −0.11 | −0.38 | −0.10 | −0.48 | −0.69 * |
| Denitrification | −0.32 | 0.03 | −0.43 | −0.13 | −0.22 |
| Nitrification | 0.02 | −0.03 | −0.06 | 0.18 | −0.11 |
| Complete nitrification | −0.40 | 0.69 * | −0.46 | 0.49 | −0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, S.; Wang, D.; Li, Q.; Wang, C.; Wu, L. Comparative Study on the Effects of Different Soil Improvement Methods in Blueberry Soil. Agronomy 2024, 14, 125. https://doi.org/10.3390/agronomy14010125
Li Y, Liu S, Wang D, Li Q, Wang C, Wu L. Comparative Study on the Effects of Different Soil Improvement Methods in Blueberry Soil. Agronomy. 2024; 14(1):125. https://doi.org/10.3390/agronomy14010125
Chicago/Turabian StyleLi, Yanan, Shuxia Liu, Dongmei Wang, Qi Li, Chengyu Wang, and Lin Wu. 2024. "Comparative Study on the Effects of Different Soil Improvement Methods in Blueberry Soil" Agronomy 14, no. 1: 125. https://doi.org/10.3390/agronomy14010125





